Abstract
Background
NaV1.7 is preferentially expressed, at relatively high levels, in peripheral neurons, and is often referred to as a “peripheral” sodium channel, and NaV1.7-specific blockers are under study as potential pain therapeutics which might be expected to have minimal CNS side effects. However, occasional reports of patients with NaV1.7 gain-of-function mutations and apparent hypothalamic dysfunction have appeared. The two sodium channels previously studied within the rat hypothalamic supraoptic nucleus, NaV1.2 and NaV1.6, display up-regulated expression in response to osmotic stress.
Results
Here we show that NaV1.7 is present within vasopressin-producing neurons and oxytocin-producing neurons within the rat hypothalamus, and demonstrate that the level of Nav1.7 immunoreactivity is increased in these cells in response to osmotic stress.
Conclusions
NaV1.7 is present within neurosecretory neurons of rat supraoptic nucleus, where the level of immunoreactivity is dynamic, increasing in response to osmotic stress. Whether NaV1.7 levels are up-regulated within the human hypothalamus in response to environmental factors or stress, and whether NaV1.7 plays a functional role in human hypothalamus, is not yet known. Until these questions are resolved, the present findings suggest the need for careful assessment of hypothalamic function in patients with NaV1.7 mutations, especially when subjected to stress, and for monitoring of hypothalamic function as NaV1.7 blocking agents are studied.
Keywords: Hypothalamus, Nav1.7, Salt-loading, Supraoptic nucleus
Background
Gain-of-function mutations of the NaV1.7 sodium channel, which is preferentially expressed at relatively high levels within peripheral (dorsal root ganglion and sympathetic ganglion) neurons [1-3] produce several syndromes associated with severe pain, including inherited erythromelalgia [4-8] and paroxysmal extreme pain disorder [9,10] as well as painful small-fiber neuropathy [11,12], while loss-of-function mutations of NaV1.7 cause channelopathy-associated insensitivity to pain [13-15]. In contrast with the severe pain associated with gain-of-function mutations of NaV1.7 and loss of pain sensitivity associated with loss-of-function mutations of NaV1.7, abnormalities of CNS function have in general not been reported in these disorders, consistent with preferential expression of NaV1.7 within peripheral neurons. NaV1.7-specific blockers are being studied as potential therapies for pain, with the rationale that they would be expected to have few, if any, CNS-related side-effects. Nevertheless, there have been reports of hypothermia, possibly due to an abnormality of central (hypothalamic) thermoregulation [16-18] in patients with NaV1.7 mutations and erythromelalgia. The syndrome of inappropriate release of antidiuretic hormone, SIADH, without any structural cause, recently developed in a patient carrying a gain-of-function mutation of NaV1.7, G856D, within a kindred with painful small-fiber neuropathy (Hoeijmakers et al, personal communication). Affected family members, all of whom carry the G856D mutation, display small-fiber neuropathy characterized by severe pain and vasomotor dyscontrol in their distal extremities, small hands and feet, and autonomic dysfunction. The G856D mutation enhances channel activation, impairs fast-inactivation, and markedly enhances the channel’s persistent current and response to slow ramp stimuli. The occurrence of SIADH in this patient suggested the possibility that the gain-of-function mutation in NaV1.7 might have contributed to hyperexcitability of vasopressin-releasing (magnocellular neurosecretory) neurons in the supraoptic nucleus within the hypothalamus.
Vasopressin release by supraoptic magnocellular neurons can be triggered by osmotic stress and depends on bursting activity in these cells [19]. It is known that tetrodotoxin-sensitive sodium channels contribute to this bursting [20-22]. While high levels of expression of NaV1.7 have been reported in hypothalamic nuclei including the supraoptic nucleus in rodents [13,23], only weak levels of NaV1.7 expression were detected within the primate supraoptic nucleus [13]. In the present study, we have built upon earlier studies in rodents which showed that the deployment of sodium channels in the hypothalamus is dynamic, with levels of expression of the two sodium channel subtypes that were previously studied, NaV1.2 and NaV1.6, and of sodium channel beta-1 and beta-2 subunits and sodium currents, displaying up-regulation within supraoptic magnocellular neurons exposed to osmotic stress via salt-loading [24] and as a result of the hyperosmolar state associated with experimental diabetes [25]. Reasoning that Nav1.7 expression within supraoptic magnocellular neurons might be subject to similar plasticity, we exposed rats to salt-loading and assessed the level of NaV1.7 immunoreactivity within these neurons. We demonstrate here that NaV1.7 is present within vasopressin- and oxytocin-producing neurons of the supraoptic nucleus, and show that the level of NaV1.7 protein in these cells in not static but, on the contrary, is increased in response to salt-loading.
Results
Previous work from our laboratory has demonstrated the expression of the tetrodotoxin-sensitive (TTX-S) sodium channels, NaV1.2 and NaV1.6, but not NaV1.1 and NaV1.3, and of TTX-S sodium currents in magnocellular neurosecretory cells (MSC) of the hypothalamic supraoptic nucleus [24]. This early study also showed that the expression of NaV1.2 and NaV1.6 channels are upregulated and amplitude of the sodium current increased following salt-loading challenge [24]. To determine whether NaV1.7 is expressed and upregulated in magnocellular neurosecretory cells of the supraoptic nucleus, we assessed the supraoptic nucleus of control and salt-loaded (2% NaCl in drinking water) rats using immunocytochemistry. Measurement of plasma osmotic pressure confirmed the presence of hyperosmolarity in the salt-loaded rats: control, 323.3 ± 4.8 mOsm; salt-loaded, 353.2 ± 3.3 mOsm (p < 0.05).
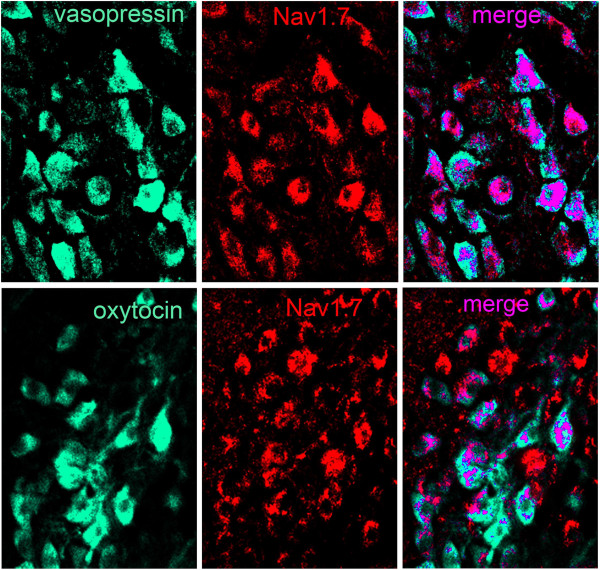
Magnocellular neurosecretory cells in the supraoptic nucleus of control rats exhibited distinct NaV1.7 immunolabeling (Figure 1). Some magnocellular neurosecretory cells displayed moderate levels of NaV1.7 immunosignal, while other magnocellular neurosecretory cells exhibited a low level or no NaV1.7 immunofluorescence. Two types of magnocellular neurosecretory cells exist within the supraoptic nucleus, oxytocin-producing and vasopressin-producing, with little co-expression of these hormones in individual magnocellular neurosecretory cells. Both oxytocin- and vasopressin-producing magnocellular neurosecretory cells displayed NaV1.7 immunolabeling (Figure 1). Approximately 72% (33 of 46) of oxytocin-producing and 53% (59 of 112) of vasopressin-producing MSC expressed NaV1.7 labeling above background levels.
Figure 1.
Nav1.7 expression in vasopressin- and oxytocin-producing magnocellular neurosecretory cells in supraoptic nucleus. Magnocellular neurosecretory neurons (MSN) of the supraoptic nucleus (SON) exhibit robust vasopressin and oxytocin immunolabeling (green). MSN of the SON display Nav1.7 immunoreactivity (red). Double-immunocytochemical studies with antibodies to vasopressin or oxytocin and Nav1.7 demonstrate that both peptide-producing cell-types exhibit co-localization (magenta) with Nav1.7. Merged image of vasopressin or oxytocin with Nav1.7 is presented as magenta to enhance visualization of co-localization.
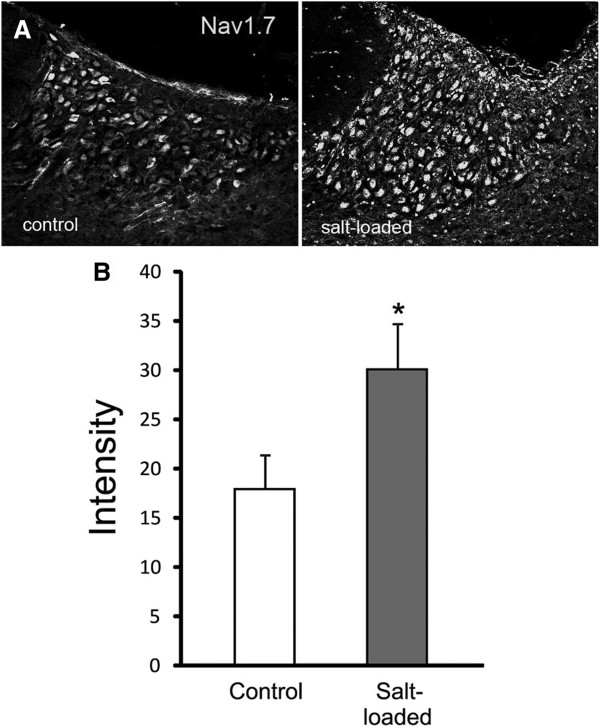
Salt-loading induced a substantial increase in the level of NaV1.7 immunoreactivity in magnocellular neurosecretory cells of the supraoptic nucleus compared to magnocellular neurosecretory cells in control rats (Figure 2A). In addition to the detection of greater numbers of magnocellular neurosecretory cells that displayed NaV1.7 immunolabeling, the intensity of NaV1.7 signal in some magnocellular neurosecretory neurons was markedly greater than that observed in magnocellular neurosecretory cells from control rats. Quantification of the mean intensity of NaV1.7 signal within the circumscribed supraoptic nucleus demonstrated a significant up regulation of NaV1.7 in response to salt-loading challenge (Figure 2B). These observations demonstrate that, in addition to up regulation of the TTX-S sodium channels NaV1.2 and NaV1.6 within magnocellular neurosecretory cells of the supraoptic nucleus with salt-loading, the level of NaV1.7 protein in these cells is significantly increased in osmotically-challenged rats.
Figure 2.
Nav1.7 is upregulated in magnocellular neurosecretory neurons of supraoptic nucleus following salt loading. (A) Magnocellular neurosecretory neurons (MSN) within supraoptic nucleus (SON) of control rats exhibit Nav1.7 immunolabeling. Following salt-loading, MSN display more prominent Nav1.7 immunoreactivity compared to control rats. (B) Quantification of Nav1.7 immunosignal within the SON demonstrates an approximately two-fold increase in Nav1.7 immunofluorescence in SON of salt-loaded rats compared to control rats.
Discussion
In this study we have demonstrated that NaV1.7 is present within neurons within the hypothalamic supraoptic nucleus, specifically within vasopressin- and oxytocin-producing magnocellular neurosecretory neurons. We also show that the level of NaV1.7 protein in these cells is not fixed but, on the contrary, is dynamic, increasing as a result of salt-loading.
A role NaV1.7 in electrogenesis in DRG neurons is well-established, and it is clear that NaV1.7 functions as a threshold channel in these neurons, amplifying small depolarizing inputs to bring the cell to threshold for action potential generation [26,27] and possibly facilitating invasion into, and/or transmitter release from, preterminal axons within the spinal cord dorsal horn [1,28]. In contrast, a functional role of NaV1.7 within supraoptic neurons is less well understood. Action potential bursts, triggered by osmotic changes, lead to release of vasopressin by supraoptic magnocellular neurons [19] and it is known that tetrodotoxin-sensitive sodium channels contribute to this bursting [20-22].
Supraoptic magnocellular neurons are known to be highly dynamic. It is known that, in response to changes in osmolality, the expression of peptides within these cells changes, and they change in size [29]. In parallel, it has been shown that in response to increased osmolarity there are changes in deployment of sodium channels, with up-regulated expression of the NaV1.2 and NaV1.6 alpha subunits, and of the sodium channel beta-1 and beta-2 subunits [24,25]. The present results show that the level of NaV1.7 protein, like that of NaV1.2 and NaV1.6 [24,25], is dynamic, and is up-regulated within supraoptic magnocellular neurons exposed to osmotic stress via salt-loading. A previous study [24] demonstrated an increase in the amplitude of the transient Na+ current, and an even greater increase in the amplitude and density of the Na+ currents evoked by slow ramp stimuli in supraoptic neurons following salt-loading. While definitive identification of the current as NaV1.7 current would require specific blockade or knockout, both of these types of current have been observed to be produced by NaV1.7 [26]. Because NaV1.7 is present within vasopressin neurons, it seems likely that this sodium channel isoform plays some role in vasopressin release in response to the osmotic stress imposed by salt-loading.
Although only low levels of NaV1.7 have been reported in the hypothalamus in primates [13], it is possible that the density of NaV1.7 channels within magnocellular neurons of the human supraoptic nucleus, like that in rodents, is subject to up-regulation in response to some forms of stress. NaV1.7 blockers are currently under development as potential pharmacotherapeutics for pain [30-34]. Hypothalamic dysfunction has not been observed thus far in families with channelopathy-associated insensitivity to pain due to null mutations in the gene encoding NaV1.7. However, functional NaV1.7 channels are absent beginning in early embryogenesis in affected individuals in these families, and the possibility that there might be compensatory changes in hypothalamic neurons which maintain relatively normal function in these cells cannot be excluded. Whether levels of NaV1.7 are increased in response to environmental factors or stress within the human hypothalamus, and whether NaV1.7 plays a functional role in hypothalamic neurons in humans, is not known. Until these questions are resolved, the present findings suggest the need for assessment of hypothalamic function in patients carrying NaV1.7 mutations especially when subjected to stress, and for monitoring of hypothalamic function as NaV1.7 blocking agents are studied.
Conclusions
In summary, our results demonstrate that sodium channel NaV1.7 is expressed in vasopressin-producing and oxytocin-producing magnocellular neurosecretory neurons of the rat hypothalamic supraoptic nucleus. The level of NaV1.7 immunoreactivity in the supraoptic nucleus is significantly increased following salt-loading, suggesting a contribution of NaV1.7 in the response of magnocellular neurosecretory neurons to osmotic stress. While it is not yet known whether levels of expression of NaV1.7 are increased in response to environmental factors or stress within the human hypothalamus, or whether NaV1.7 plays a functional role in hypothalamic neurons in humans, the present findings suggest the need for assessment of hypothalamic function in patients carrying NaV1.7 mutations, especially when subjected to stress, and for monitoring of hypothalamic function as NaV1.7 blocking agents are studied.
Methods
Salt loading
Adult male Sprague-Dawley rats (200-220 g), housed under a 12 h-12 h dark-light cycle, were salt-loaded with 2% NaCl (ad libitum) in their drinking water and unlimited access to food. Rats were sacrificed for immunocytochemical investigation 7 days following salt loading. All experiments were approved by the VA Connecticut Healthcare System Institutional Animal Care and Use Committee. To confirm the extent of salt loading, plasma osmotic pressure of the rats was measured (vapor pressure osmometer model 5500, Wescor, USA). Body weights were significantly (p < .05) lower in salt-loaded (186.6 ± 4.7 g) compared to control (248.6 ± 1.9) rats. Six control and 6 salt-loaded rats were used for the immunocytochemistry studies.
Immunocytochemistry
Rats were perfused with 4% paraformaldehyde in 0.14 M Sorensen’s phosphate buffer, pH 7.4, and the brain removed and postfixed for 25-30 minutes. After cryoprotection in 30% sucrose in 0.01 M PBS for 24 h, the brains were blocked, frozen and coronal cryosections (16 μm) containing SON and optic chiasm were cut. Sections were incubated in blocking solution (PBS containing 3% normal donkey serum, 3% fish skin gelatin, 0.1% Triton X-100 and 0.02% sodium azide) for 15 min at room temperature, primary antibody(ies) [rabbit anti- NaV1.7, 1:200, Y083 (Black et al. 2012); guinea pig anti-vasopressin, 1:100, Peninsula Lab, San Carlos, CA); mouse anti-oxytocin, 1:500, Abcam, Cambridge, MA)] in blocking solution 2-4 days at 4°C, rinsed in PBS, incubated 1-2 days at 4°C with secondary antibody(ies) [donkey anti-rabbit Alexa Fluor-488-congugated F(ab’)2 fragment, donkey anti-rabbit Alexa Fluor-Cy3, donkey anti-guinea pig Alexa Fluor-488; donkey anti-mouse Alexa Fluor-488; all secondary antibodies from Jackson Immuno Research, West Grove, PA], rinsed with PBS and mounted on glass slides with Aqua-polymount (Polyscience, Warrington, PA). Control experiments in which the primary antibody was omitted exhibited only background levels of labeling.
Quantification
Tissue sections were examined with a Nikon C1 confocal microscope (Nikon USA, Melville, NY), using a 20× objective and operating in single mode for detection of NaV1.7 alone or in frame lamba (sequential) mode for detection of NaV1.7 and vasopressin or oxytocin to prevent possible bleed-through between 488 and Cy3 channels.
For detection of NaV1.7 in the supraoptic nucleus (SON), images were acquired from 6 control and 6 salt-loaded rats, utilizing the same confocal settings for acquisition of NaV1.7 immunofluorescent signals. Images were opened in Nikon Elements and the mean signal intensity of the circumscribed SON was calculated by the software.
For co-localization of NaV1.7 and vasopressin or oxytocin in SON neurons, signals for NaV1.7 and vasopressin were thresholded at intensities 20% above background levels, and the percentage of vasopressin neurons expressing NaV1.7 was calculated. Data are presented as mean ± SEM and statistical analysis was performed with Excel Student’s t-test, with p < 0.05 considered significant.
Abbreviations
DRG: Dorsal root ganglion; MSN: Magnocellular neurosecretory neurons; SIADH: Syndrome of inappropriate release of anti-diuretic hormone; SON: Supraoptic nucleus.
Competing interests
The authors declare no competing interests.
Authors’ contributions
JAB designed immunocytochemical experiments, acquired, analyzed and interpreted data, and participated in writing manuscript. JGJH participated in conception and design of experiments and to editing the manuscript. CGF participated in conception and design of experiments and to editing the manuscript. ISJM participated in conception and design of experiments and to editing the manuscript. SGW participated in design and interpretation of experiments and in writing the manuscript. All authors read and approved the final manuscript.
Contributor Information
Joel A Black, Email: joel.black@yale.edu.
Janneke GJ Hoeijmakers, Email: j.hoeijmakers@mumc.nl.
Catharina G Faber, Email: c.faber@mumc.nl.
Ingemar SJ Merkies, Email: isjmerkies@planet.nl.
Stephen G Waxman, Email: stephen.waxman@yale.edu.
Acknowledgements
The authors thank Shujun Liu and Pamela Zwinger for excellent technical assistance. This work was supported by the Medical Research Service and Rehabilitation Research Service, Department of Veterans Affairs. The Center for Neuroscience and Regeneration Research is a Collaboration of the Paralyzed Veterans of America with Yale University.
References
- Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: from molecule to man. Nat Rev Neurosci. 2013;14(1):49–62. doi: 10.1038/nrn3404. [DOI] [PubMed] [Google Scholar]
- Rush AM, Dib-Hajj SD, Liu S, Cummins TR, Black JA, Waxman SG. A single sodium channel mutation produces hyper- or hypo excitability in different types of neurons. Proc Natl Acad Sci USA. 2006;103(21):8245–8250. doi: 10.1073/pnas.0602813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Moss BL, He ZJ, Koszowski AG, Whisenand T, Levinson SR, Wolf JJ, Silos-Santiago I, Halegoua S, Mandel G. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Natl Acad Sci USA. 1997;94(4):1527–1532. doi: 10.1073/pnas.94.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Dib-Hajj SD, Waxman SG. Inherited erythermalgia: limb pain from an S4 charge-neutral Na channelopathy. Neurology. 2006;67(9):1563–1567. doi: 10.1212/01.wnl.0000231514.33603.1e. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Waxman SG. Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J Neurosci. 2004;24(38):8232–8236. doi: 10.1523/JNEUROSCI.2695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Rush AM, Cummins TR, Hisama FM, Novella S, Tyrrell L, Marshall L, Waxman SG. Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain. 2005;128(Pt 8):1847–1854. doi: 10.1093/brain/awh514. [DOI] [PubMed] [Google Scholar]
- Han C, Dib-Hajj SD, Lin Z, Li Y, Eastman EM, Tyrrell L, Cao X, Yang Y, Waxman SG. Early- and late-onset inherited erythromelalgia: genotype-phenotype correlation. Brain. 2009;132(Pt 7):1711–1722. doi: 10.1093/brain/awp078. [DOI] [PubMed] [Google Scholar]
- Han C, Rush AM, Dib-Hajj SD, Li S, Xu Z, Wang Y, Tyrrell L, Wang X, Yang Y, Waxman SG. Sporadic onset of erythermalgia: a gain-of-function mutation in Nav1.7. Ann Neurol. 2006;59(3):553–558. doi: 10.1002/ana.20776. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Estacion M, Jarecki BW, Tyrrell L, Fischer TZ, Lawden M, Cummins TR, Waxman SG. Paroxysmal extreme pain disorder M1627K mutation in human Nav1.7 renders DRG neurons hyperexcitable. Mol Pain. 2008;4:37. doi: 10.1186/1744-8069-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, Ostman J, Klugbauer N, Wood JN, Gardiner RM. et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron. 2006;52(5):767–774. doi: 10.1016/j.neuron.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM. et al. Gain of function Nav1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71(1):26–39. doi: 10.1002/ana.22485. [DOI] [PubMed] [Google Scholar]
- Han C, Hoeijmakers JG, Ahn HS, Zhao P, Shah P, Lauria G, Gerrits MM, Te Morsche RH, Dib-Hajj SD, Drenth JP. et al. Nav1.7-related small fiber neuropathy: Impaired slow-inactivation and DRG neuron hyper excitability. Neurology. 2012;78(21):1635–1643. doi: 10.1212/WNL.0b013e3182574f12. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Dahllund L, Eriksson AB, Hellgren D, Karlsson U, Lund PE, Meijer IA, Meury L, Mills T, Moody A. et al. A stop codon mutation in SCN9A causes lack of pain sensation. Hum Mol Genet. 2007;16(17):2114–2121. doi: 10.1093/hmg/ddm160. [DOI] [PubMed] [Google Scholar]
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y. et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444(7121):894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg YP, MacFarlane J, MacDonald ML, Thompson J, Dube MP, Mattice M, Fraser R, Young C, Hossain S, Pape T. et al. Loss-of-function mutations in the Nav1.7 gene underlie congenital indifference to pain in multiple human populations. Clin Genet. 2007;71(4):311–319. doi: 10.1111/j.1399-0004.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- Misery L, Greco M, Fleuret C, Firmin D, Mocquard Y, Renault A, Roguedas AM. Severe neurological complications of hereditary erythermalgia. J Eur Acad Dermatol Venereol. 2007;21(10):1446–1447. doi: 10.1111/j.1468-3083.2007.02265.x. [DOI] [PubMed] [Google Scholar]
- Seneschal J, Sole G, Taieb A, Ferrer X. A case of primary erythermalgia with encephalopathy. J Neurol. 2009;256(10):1767–1768. doi: 10.1007/s00415-009-5188-1. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Saitoh M, Hoshino H, Mimaki M, Yokoyama Y, Takamizawa M, Mizuguchi M, Lin ZM, Yang Y, Igarashi T. A case of primary erythermalgia, wintry hypothermia and encephalopathy. Neuropediatrics. 2007;38(3):157–159. doi: 10.1055/s-2007-990265. [DOI] [PubMed] [Google Scholar]
- Voisin DL, Bourque CW. Integration of sodium and osmosensory signals in vasopressin neurons. Trends Neurosci. 2002;25(4):199–205. doi: 10.1016/S0166-2236(02)02142-2. [DOI] [PubMed] [Google Scholar]
- Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221(4615):1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- Inenaga K, Nagatomo T, Kannan H, Yamashita H. Inward sodium current involvement in regenerative bursting activity of rat magnocellular supraoptic neurones in vitro. J Physiol. 1993;465:289–301. doi: 10.1113/jphysiol.1993.sp019677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Oscillatory bursting of phasically firing rat supraoptic neurones in low-Ca2+ medium: Na + influx, cytosolic Ca2+ and gap junctions. J Physiol. 1996;496(Pt 2):379–394. doi: 10.1113/jphysiol.1996.sp021692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Fundin B, Meury L, Jureus A, Sandberg K, Krupp J, Ahmad S, O’Donnell D. Distribution of the voltage-gated sodium channel Na(v)1.7 in the rat: expression in the autonomic and endocrine systems. J Comp Neurol. 2007;504(6):680–689. doi: 10.1002/cne.21484. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cummins TR, Ishikawa K, Black JA, Ibata Y, Waxman SG. Molecular and functional remodeling of electrogenic membrane of hypothalamic neurons in response to changes in their input. Proc Natl Acad Sci USA. 1999;96(3):1088–1093. doi: 10.1073/pnas.96.3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JP, Craner MJ, Cummins TR, Black JA, Waxman SG. Sodium channel expression in hypothalamic osmosensitive neurons in experimental diabetes. Neuroreport. 2002;13(11):1481–1484. doi: 10.1097/00001756-200208070-00027. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Howe JR, Waxman SG. Slow closed-state inactivation: a novel mechanism underlying ramp current in cells expressing the hNE/PN1 sodium channel. J Neurosci. 1998;18(23):9607–9619. doi: 10.1523/JNEUROSCI.18-23-09607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RI, Cummins TR, Ghassemi F, Dib-Hajj SD, Waxman SG. Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons. J Physiol. 2003;551(Pt 3):741–750. doi: 10.1113/jphysiol.2003.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minett MS, Nassar MA, Clark AK, Passmore G, Dickenson AH, Wang F, Malcangio M, Wood JN. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nat Commun. 2012;3:791. doi: 10.1038/ncomms1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Bourque CW. Mechanisms of rhythmogenesis: insights from hypothalamic vasopressin neurons. Trends Neurosci. 2006;29(2):108–115. doi: 10.1016/j.tins.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Chakka N, Bregman H, Du B, Nguyen HN, Buchanan JL, Feric E, Ligutti J, Liu D, McDermott JS, Zou A. et al. Discovery and hit-to-lead optimization of pyrrolopyrimidines as potent, state-dependent Na(v)1.7 antagonists. Bioorg Med Chem Lett. 2012;22(5):2052–2062. doi: 10.1016/j.bmcl.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. From genes to pain: Nav1.7 and human pain disorders. Trends Neurosci. 2007;30(11):555–563. doi: 10.1016/j.tins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Goldberg YP, Price N, Namdari R, Cohen CJ, Lamers MH, Winters C, Price J, Young CE, Verschoof H, Sherrington R. et al. Treatment of Na(v)1.7-mediated pain in inherited erythromelalgia using a novel sodium channel blocker. Pain. 2012;153(1):80–85. doi: 10.1016/j.pain.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Klement G, Babich O, Larsson O, Lund PE, Malmberg A, Sandberg L, Sands ZA, Dabrowski M. Identification of novel Nav1.7 antagonists using high throughput screening platforms. Comb Chem High Throughput Screen. 2012;15(9):713–720. doi: 10.2174/138620712803519680. [DOI] [PubMed] [Google Scholar]
- Liu M, Wood JN. The roles of sodium channels in nociception: implications for mechanisms of neuropathic pain. Pain Med. 2011;12(Suppl 3):S93–S99. doi: 10.1111/j.1526-4637.2011.01158.x. [DOI] [PubMed] [Google Scholar]