Abstract
Leukotoxin is a protein that is secreted by Aggregatibacter actinomycetemcomitans and that primarily targets the active form of leukocyte function associated antigen 1 (LFA1) on WBC. Because of its specificity for WBC, leukotoxin is being developed as a novel biologic treatment for hematologic malignancies and autoimmune–inflammatory diseases. Early studies indicated that leukotoxin is specific for WBC from humans and Old World primates. In the current study, we used in vivo and in vitro assays to show that leukotoxin has a wider host range than previously believed and can kill rodent WBC. Administration of leukotoxin to rats and mice resulted in a rapid drop in WBC number but had no effect on RBC or platelet counts. Using LFA1-knockout mice, we showed that leukotoxin-mediated depletion of WBC is dependent on LFA1. In addition, similar to its effect on human monocytes, leukotoxin kills murine myeloid leukemia via a lysosome-mediated pathway that is dependent on cathepsin D. This newly described broader host range of leukotoxin enables the biology of the protein to be studied in rodent species and offers the possibility of using rodent models for evaluating the therapeutic efficacy of leukotoxin in various diseases.
Abbreviations: 7AAD, 7-amino-actinomycin D; LFA1, leukocyte function associated antigen 1; MLE, mouse lung epithelium
The protein leukotoxin is a potential therapeutic agent that is produced by Aggregatibacter actinomycetemcomitans and binds to leukocyte function associated antigen 1 (LFA1) on WBC (reviewed in reference 23). LFA1 is a β2 integrin that is expressed exclusively on WBC and is involved in WBC adhesion to endothelial cells and migration into tissue (reviewed in reference 26). In addition, LFA1 plays an important role in immunologic signaling and synapse. LFA1 exists in 3 structural conformations (resting, intermediate, and active) and the active, or extended state, is the form that is specifically involved in cellular binding and migration through its interaction with intercellular adhesion molecules on vascular endothelial cells. Our recent studies on the interaction between leukotoxin and LFA1 have revealed that leukotoxin preferentially interacts with LFA1 in the active configuration.9,18,24 Therefore, leukotoxin primarily affects activated WBC and spares resting WBC and cells that do not express LFA1.
Numerous diseases of WBC are characterized by the increased expression of active LFA1 on diseased cells. These diseases include hematologic malignancies, autoimmune and inflammatory diseases, and HIV infection.12,15,18-21,28,32,35,39,52 Due to its preference for WBC that express activated LFA1, leukotoxin is being developed as an agent for the treatment of WBC diseases. We have shown in vitro that leukotoxin kills malignant monocytes via a novel cathepsin-D–dependent, lysosome-mediated pathway that requires internalization of the leukotoxin–LFA1 complex and delivery of leukotoxin to the lysosome.9 This mechanism is similar to that of therapeutic type II monoclonal antibodies, such as the antiCD20 compound tositumomab and GA101, which have been shown to cause direct cell death by inducing the release of lysosomal contents into the cytosol.1,29 Leukotoxin-mediated killing of lymphocytes appears to favor a caspase-dependent apoptotic pathway rather than a lysosomal pathway.9,50 In addition, using ex vivo experiments, we have shown that leukotoxin efficiently depletes HIV-infected cells, which express higher levels of LFA1 than do noninfected cells.18 Therefore, leukotoxin may have the capacity to target the HIV reservoir, a feat that current antiretroviral therapy does not accomplish.
Preclinical studies have shown that leukotoxin possesses noteworthy therapeutic efficacy in humanized mouse models for leukemia24 and psoriasis41 and that leukotoxin is highly synergistic with conventional chemotherapeutic agents.14 In addition, in nonhuman primates, leukotoxin is active, specific, and well-tolerated in the μg/kg range.24 Rhesus macaques (Macaca mulatta) treated with leukotoxin showed an initial drop in the WBC count after the first several hours, but after 12 h, counts had returned to levels comparable to those of saline-treated control monkeys. Blood data from the same study24 indicated no liver or kidney toxicity.
Early studies of leukotoxin indicated that the protein is highly species-specific, affecting WBC from human and Old World primates only.40,44 This specificity has hampered the ability to easily test the in vivo effects of leukotoxin in rodent models for hematologic and autoimmune–inflammatory diseases. Although our studies and the literature regarding the LFA1 expression levels of diseased WBC support the use of leukotoxin for the treatment of numerous diseases such as multiple sclerosis, lupus, type I diabetes, rheumatoid arthritis, Crohn disease, and ulcerative colitis, humanized rodent models are not yet available for these diseases. As part of the developmental plan to obtain Investigational New Drug status for leukotoxin, we have been running pharmacokinetic analyses in rats and mice. These studies have led to the surprising find that rodent WBC are indeed sensitive to leukotoxin and that killing is dependent on LFA1. The observed effects of leukotoxin on rodent WBC parallel those seen with monkey WBC in vivo and parallels the mechanism of action observed in human leukemias and lymphomas in vitro. In the current report, we further characterize the in vitro and in vivo effects of leukotoxin on mouse and rat WBC and propose that rodent disease models would be highly useful for testing the therapeutic efficacy and understanding the biology of leukotoxin from A. actinomycetemcomitans.
Materials and Methods
Animal studies.
We used Sprague–Dawley rats (Charles River, Wilmington, MA) with femoral vein catheters to allow for easy intravenous injections and blood draws. Control blood samples were drawn from the catheter before leukotoxin injection. Leukotoxin then was injected either intravenously or subcutaneously, and blood samples were collected from catheters at various time points as indicated for CBC analysis. Mouse studies to examine the pharmacodynamics of leukotoxin were carried out using CD1 mice (Charles River, Wilmington, MA). For the LFA1 knockout study, B6.129S7-Itgaltm1Bll/J (CD11a knockout) and C57BL/6J (wildtype control) mice were purchased from The Jackson Laboratory (Bar Harbor, ME).10,49 Leukotoxin was injected subcutaneously into the mice, and blood samples were withdrawn by cardiocentesis at various time points as indicated for complete blood count analysis. Rodents were anesthetized with isoflurane during injections and blood draws. Animals were euthanized by CO2 asphyxiation or cardiocentesis.
These studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals.22 The protocols were approved by the IACUC of the University of Medicine and Dentistry of New Jersey (protocol numbers 11016 and 10018).
Cell lines and culture conditions.
Murine cells lines (M1, A20) were purchased from ATCC (Manassas, VA) and grown in RPMI 1640 supplemented with 10% FBS. Mouse lung epithelium (MLE) cells were a generous gift from Dr Gill Diamond (originally purchased from ATCC) and were grown in HITES (hydrocortisone, insulin, transferrin, estradiol, selenium) medium supplemented with 2% FBS and 2 mM L-glutamine. All cell lines were grown at 37 °C, 5% CO2.
Purification of leukotoxin.
Leukotoxin was purified as described previously.8
Flow cytometry.
Cell viability was measured by using annexin V–FITC and 7-amino-actinomycin D (7AAD) (Biolegend, San Diego, CA). Lysosomal acidity was determined by incubating the cells with the stain LysoTracker Green DND-26 (Invitrogen, Carlsbad, CA) for 1 h Samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ).
Cellular viability assays.
Cell death of murine cell lines was determined by plating 5 × 105 cells/mL, treating with leukotoxin for 24 h, and staining the cells with both annexin V and 7AAD. When determining the mechanism of cell death, M1 cells were pretreated with 10 µM pepstatin A (Sigma-Aldrich, St Louis, MO.) for 1 h before leukotoxin treatment. To measure cellular depletion after leukotoxin treatment, 5 × 105 cells/mL A20 cells were plated and treated with various concentrations of leukotoxin for 24 h. The cell density of samples was measured automatically (ViCell, Beckman-Coulter, Fullerton, CA).
Statistical analyses.
Data were evaluated by the unpaired Student t test by using Prism (GraphPad software, La Jolla, CA). A P value less than 0.05 was considered to be statistically significant.
Results
In vivo effects of leukotoxin in rats.
To test the in vivo effects of leukotoxin, we injected the agent intravenously and subcutaneously into rats and then performed CBC analyses over time (Figure 1). Leukotoxin had no effect on RBC and platelet counts (data not shown). However, total WBC, neutrophil, lymphocyte, and monocyte counts dropped after leukotoxin administration. Monocyte and neutrophil counts subsequently increased to levels higher than preinjection values.
Figure 1.
In vivo effect of leukotoxin in rats. Leukotoxin was injected intravenously (0.34 mg/kg) or subcutaneously (0.80 mg/kg) into Sprague–Dawley rats, blood samples were withdrawn at the various times noted, and CBC analyses were performed. Data are given as mean ± SE (n = 4). No significant changes in WBC counts were noted after injection of the vehicle control (data not shown). *, Value significantly (P ≤ 0.05) different from the corresponding preinjection value.
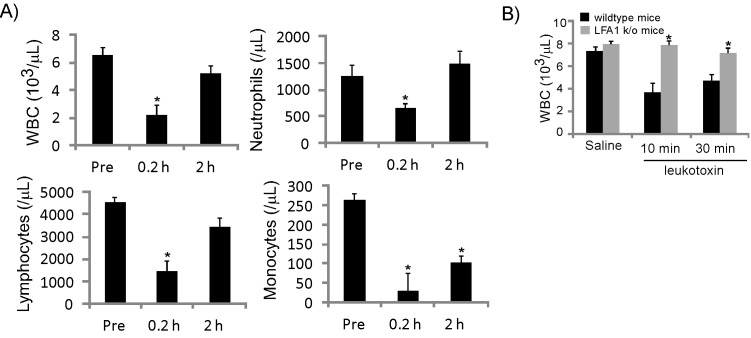
In vivo effect of leukotoxin on mouse cells.
To determine whether leukotoxin affects murine WBC in vivo, leukotoxin was injected subcutaneously into mice, and CBC analyses were performed (Figure 2 A). Similar to findings from the rat experiments, mouse WBC, neutrophil, lymphocyte, and monocyte counts dropped within several minutes after the administration of leukotoxin, whereas RBC and platelet counts did not change.
Figure 2.
In vivo effect of leukotoxin in mice. (A) Leukotoxin was injected subcutaneously (4 mg/kg) into mice, which then were euthanized at various time points for blood collection by cardiocentesis. Blood samples underwent CBC analysis. Data are given as mean ± SEM (n = 4); *, value significantly (P ≤ 0.05) different from the corresponding preinjection value. (B) Leukotoxin was injected subcutaneously (4 mg/kg) into wildtype mice and mice that do not express CD11a (LFA1 k/o), which then were euthanized at various time points for blood collection by cardiocentesis. Blood samples underwent CBC analysis. Data are given as mean ± 1 SD (n = 3); *, value significantly (P ≤ 0.05) different from the corresponding saline value.
To confirm that LFA1 is required for leukotoxin-mediated toxicity of rodent cells, we examined the effects of leukotoxin in CD11a (LFA1) knockout mice10,49 (Figure 2 B). Although leukotoxin depleted WBC in wildtype C57BL mice, WBC count was not affected after administration of leukotoxin to CD11a knockout mice. These results demonstrate that leukotoxin specifically targets mouse WBC in an LFA1-dependent manner.
In vitro effect of leukotoxin on mouse epithelial and WBC.
To determine whether leukotoxin specifically targets WBC in rodents, M1 myeloblast and MLE cells were treated with various concentrations of leukotoxin for 24 h and evaluated for cell death by annexin V–7AAD staining and flow cytometry (Figure 3 A). At 0.1 µg/mL, leukotoxin killed M1 cells. However, MLE cells were not susceptible to leukotoxin-mediated cell death, even at concentrations as high as 40 μg/mL, highlighting the drug's sensitivity for WBC. After confirmation of WBC specificity, M1 cells were treated with diverse leukotoxin concentrations and tested alongside the human monocytic leukemia cell line THP1, to compare differences in susceptibility to leukotoxin (Figure 3 B). Cell death was first observed in M1 cells at leukotoxin concentrations as low as 10 ng/mL. However, unlike in THP1 cells, complete cell death of M1 cells could not be achieved, even at the highest concentrations. The maximal amount of cell death that could be achieved by using leukotoxin with M1 cells was approximately 60%. Even when a concentration as high as 40 μg/mL was used, no more than 62% cell death was observed (data not shown). According to IC50 values (THP1, 25 ng/mL; M1, 1.2 μg/mL), THP1 cells were approximately 50 times more sensitive to leukotoxin than were M1 cells.
Figure 3.
Effect of leukotoxin on murine cells. (A) M1 and MLE cells were treated with various concentrations of leukotoxin and incubated for 24 h. Cell death was measured as the total number of annexin V+7AAD– and annexin V+7AAD+ cells. The experiment was performed in triplicate, and error bars represent 1 SD. Values significantly (*, P ≤ 0.05; †, P ≤ 0.01) different from those for untreated cells are indicated. (B) M1 and THP1 cells were treated with various concentrations of leukotoxin for 24 h. Cytotoxcity was measured as the sum of annexin V+7AAD– and annexin V+7AAD+ cells. Data are representative of 3 independent experiments, and error bars represent 1 SD. (C) A20 cells were treated with various concentrations of leukotoxin for 24 h, and cell density was assessed by using an automated cell-density analyzer. Data are representative of 3 independent experiments, and error bars represent 1 SD. †, Value is significantly (P ≤ 0.01) different from that for untreated cells.
Because leukotoxin successfully killed M1 cells, we tested a second mouse cell line, A20 (B-cell lymphoma), for leukotoxin-mediated killing. Although A20 cells were susceptible to leukotoxin, the mechanism of action appeared to be different from that in M1 cells. Instead of undergoing apoptosis and staining positively for annexin V (like the M1 cells did), A20 cells were depleted by leukotoxin. Depletion was measured on the basis of the concentration of cells remaining after leukotoxin treatment, and cells were depleted after leukotoxin concentrations as low as 1 ng/mL (Figure 3 C). The cells remaining were viable, in light of the lack of staining by annexin V. M1 cells showed no depletion after leukotoxin treatment (data not shown).
leukotoxin induces loss of lysosomal pH and release of cathepsin D in M1 cells.
We recently showed that leukotoxin kills human monocytes by a novel lysosome-mediated mechanism.9 To determine whether leukotoxin intoxicates mouse and human monocytes via similar mechanisms, M1 cells were treated with leukotoxin and evaluated for loss of lysosomal integrity by using the stain LysoTracker Green, which fluoresces green in the presence of acidic compartments. Using flow cytometry, we noted that leukotoxin caused an increase in lysosomal pH (Figure 4 A), similar to the situation in THP1 cells.9 Because of the lysosomal involvement, we tested whether the mechanism of death involved the release of lysosomal protease cathepsin D. Using the cathepsin D inhibitor pepstatin A, we found that cell death was significantly (P < 0.05) inhibited after 24 h of treatment with 0.1, 0.2, and 1.0 μg/mL leukotoxin (Figure 4 B), indicating that lysosomal permeabilization is vital for leukotoxin-mediated cell death in mouse M1 cells.
Figure 4.
Leukotoxin causes a decrease in acidity and an increase in cathepsin D release in M1 cells. (A) Cells were treated with 10 µg/mL leukotoxin for 4 h, stained with LysoTracker Green (75 nM), and analyzed by flow cytometry. (B) M1 cells were pretreated with the cathepsin D inhibitor pepstatin A (10 µM) for 1 h and treated with various concentrations of leukotoxin for 24 h. Cell death was measured as the sum of annexin V+7AAD– and annexin V+7AAD+ cells. Data are representative of 3 independent experiments, and error bars represent 1 SD. †, Value is significantly (P ≤ 0.01) different from that for untreated cells.
Discussion
Previous studies reported that A. actinomycetemcomitans leukotoxin was specific for WBC from humans and Old World primates.40,44 Our current results contradict this previous finding. Although rodent cells were less sensitive to leukotoxin than were human cells, rodent WBC were clearly susceptible to leukotoxin-mediated killing in an LFA1-specific manner. We noted that leukotoxin killed mouse WBC more rapidly than rat cells. We believe that this difference in kinetics of killing is due to a variation in the sensitivity of the cells to leukotoxin. Therefore, we selected blood sampling time points that reflected the reproducible changes in WBC counts after leukotoxin administration. A possible explanation for differences in sensitivity is that the amino acid sequence of mouse CD11a is more similar to human CD11a than is that of rats. We showed that LFA1 is required for leukotoxin-mediated killing of mouse cells, given the resistance of WBC to leukotoxin in CD11a knockout mice. This finding was confirmed in vitro by showing that MLE cells were not susceptible to leukotoxin.
The observed increase in neutrophil and monocyte counts above baseline levels after 24 h is likely due to the rapid migration of the cells from the tissue back into the peripheral blood, as has been reported for other therapeutic toxins.34,48 Although an increase in peripheral blood leukocytes may appear counterproductive in the treatment of an autoimmune disease, we believe that this mechanism will be beneficial, because the drug draws inflammatory WBC from the inflamed tissue and back into the blood stream. WBC levels eventually plateaued (by 7 d), similar to the findings in nonhuman primate studies.24 Furthermore, the animals treated with leukotoxin do not experience any long-term adverse events such as myelosuppression. We also noted a difference in the patterns of WBC depletion between the different administration routes (intravenous compared with subcutaneous) and attribute this difference to the slower absorption rate of drugs injected subcutaneously.
There are several possibilities that could explain the differences between the results of the current and prior studies. First, the toxin that was used previously was not a highly purified, secreted version and could have been less active than were our leukotoxin preparations.39,43 However, explanations more likely to account for the observed differences are the incubation times used and the ways used to measure cell death. Earlier studies incubated cells with leukotoxin for only 1 h and used the chromium release assay to detect cell death.39 Chromium release is a marker of late apoptosis–necrosis, and we found that rodent cells had to be incubated for at least 3 h with leukotoxin before death could be measured with annexin V, which detects early cell death. Therefore, the chromium release assay would not have detected cell death in 1 h in cells that are less susceptible to leukotoxin than are primate cells.
Because of the similarities between the rodent and primate in vivo data, we decided to test whether the mechanism in rodent cell death was the same as in humans. We recently showed that a human monocytic leukemia cell line, THP1, undergoes caspase-independent cell death through a mechanism that relies on lysosomal components.9 In THP1 cells, interaction between leukotoxin and LFA1 resulted in the internalization of the complex and delivery to lysosomes, leading to the disruption of the organelles and release of cathepsin D. Analysis of leukotoxin-mediated cell death of mouse M1 cells revealed that lysosome-associated disruption occurs and is dependent on cathepsin D release. Therefore, the mechanism of action of leukotoxin on rodent cells closely resembles that of human cells. We suggest that the killing of rodent cells by leukotoxin represents a specific and biologically relevant interaction. Interestingly, the mechanism of death differed between the 2 mouse cell lines. After treatment with leukotoxin, M1 cells underwent a controlled mechanism of cell death that could be detected by annexin V staining. In contrast, A20 cells were only depleted by leukotoxin, and the remaining cells were viable. This difference in mechanism most likely is due to the type of cell being analyzed; a similar phenomenon is observed in human cell lines. These data further justify the testing of leukotoxin in rodents because of similar mechanisms across species.
We observed that after killing of M1 and A20 cells by leukotoxin, a population of cells existed that were not killed by the toxin. In addition, this remaining population of cells was not killed after additional incubations with leukotoxin. Therefore, the viable cells appeared to be resistant to leukotoxin. What makes these cells resistant is unknown, but they may represent a genetically heterogeneous population of cells. Some of these cells may have lost properties during laboratory subculture that are required for leukotoxin to kill cells.
Leukotoxin is being developed as a first-in-class biologic agent for the treatment of diseases and disorders that affect WBC because of its specificity for active LFA1. LFA1 has been identified as an important target for the treatment of many diseases, including hematologic malignancies,2,6,19,21,31,35,36,38 autoimmune–inflammatory diseases,4,5,7,11,15,20,25,27,28,30,32,39,46,51-53 and HIV infection,12,16-18,42,43 and for preventing tissue transplant rejection and graft-versus-host disease.3,37,45
Until now, preclinical efficacy studies with leukotoxin have been restricted to humanized animal models. For autoimmune diseases, the only available humanized model is for psoriasis, in which skin from patients with psoriasis is grafted onto SCID mice.13,33,41,47 Although SCID mice have provided useful information, a major drawback has been the inability to observe the toxin's effect on the immune system, given that SCID mice lack the cells that are the toxin's primary targets. Numerous widely accepted rodent models exist for other autoimmune–inflammatory diseases, including rheumatoid arthritis, lupus, multiple sclerosis, and gastrointestinal diseases. Therefore, the work we present here justifies the evaluation of leukotoxin in rodent models for many other autoimmune and inflammatory diseases and establishes rodents as valuable tools in which to study the biology and physiologic properties of leukotoxin and its role in disease. In addition, it is noteworthy that the administration of leukotoxin to rodents did not result in any adverse reactions or affect survival, suggesting that the agent was well-tolerated. Furthermore, having the ability to test the experimental agent in rodents likely will minimize the number of nonhuman primates that will be needed for preclinical evaluation of leukotoxin.
Acknowledgments
We thank Rahee Shah and Laxita Gupta for assistance with animal experiments and Amy Le for purification of leukotoxin. We thank Gill Diamond for the mouse lung epithelial cells. SCK declares competing interests in the form of stock ownership in the company (Actinobac Biomed) that has licensed the use of leukotoxin. In addition, SCK has received consulting fees from this company.
References
- 1.Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH, Shimada K, Chan CH, Tutt A, Beers SA, Glennie MJ, Cragg MS, Illidge TM. 2011. Novel type II antiCD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 117:4519–4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelopoulou MK, Kontopidou FN, Pangalis GA. 1999. Adhesion molecules in B-chronic lymphoproliferative disorders. Semin Hematol 36:178–197 [PubMed] [Google Scholar]
- 3.Cavazzana-Calvo M, Jabado N, Bordigoni P, Michel G, Haddad E, Mechinaud F, Landman-Parker J, Leblanc T, Plouvier E, Baruchel A, Stephan JL, Souillet G, Vilmer E, Wijdenes J, Le Deist F, Fischer A. 1997. In vivo infusion of antiLFA1 and antiCD2 antibodies prevents graft failure after HLA partially incompatible bone marrow transplantation in children with high-risk acute lymphoblastic leukaemia. Leuk Lymphoma 28:103–112 [DOI] [PubMed] [Google Scholar]
- 4.Connolly MK, Kitchens EA, Chan B, Jardieu P, Wofsy D. 1994. Treatment of murine lupus with monoclonal antibodies to lymphocyte function-associated antigen 1: dose-dependent inhibition of autoantibody production and blockade of the immune response to therapy. Clin Immunol Immunopathol 72:198–203 [DOI] [PubMed] [Google Scholar]
- 5.Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de Fougerolles A, Kotelianski V, Gardner H, Nestle FO. 2007. α1β1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med 13:836–842 [DOI] [PubMed] [Google Scholar]
- 6.Csanaky G, Matutes E, Vass JA, Morilla R, Catovsky D. 1997. Adhesion receptors on peripheral blood leukemic B cells. A comparative study on B cell chronic lymphocytic leukemia and related lymphoma–leukemias. Leukemia 11:408–415 [DOI] [PubMed] [Google Scholar]
- 7.de Boer OJ, Wakelkamp IM, Pals ST, Claessen N, Bos JD, Das PK. 1994. Increased expression of adhesion receptors in both lesional and nonlesional psoriatic skin. Arch Dermatol Res 286:304–311 [DOI] [PubMed] [Google Scholar]
- 8.Diaz R, Ghofaily LA, Patel J, Balashova NV, Freitas AC, Labib I, Kachlany SC. 2006. Characterization of leukotoxin from a clinical strain of Actinobacillus actinomycetemcomitans. Microb Pathog 40:48–55 [DOI] [PubMed] [Google Scholar]
- 9.DiFranco KM, Gupta A, Galusha LE, Perez J, Nguyen TV, Fineza CD, Kachlany SC. 2012. Leukotoxin (Leukothera®) targets active leukocyte function antigen 1 (LFA1) protein and triggers a lysosomal mediated cell death pathway. J Biol Chem 287:17618–17627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. 1999. Relative contribution of LFA1 and Mac1 to neutrophil adhesion and migration. J Immunol 163:5029–5038 [PubMed] [Google Scholar]
- 11.Elovaara I, Lalla M, Spare E, Lehtimaki T, Dastidar P. 1998. Methylprednisolone reduces adhesion molecules in blood and cerebrospinal fluid in patients with MS. Neurology 51:1703–1708 [DOI] [PubMed] [Google Scholar]
- 12.Fortin JF, Cantin R, Tremblay MJ. 1998. T cells expressing activated LFA1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM1. J Virol 72:2105–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilhar A, David M, Ullmann Y, Berkutski T, Kalish RS. 1997. T-lymphocyte dependence of psoriatic pathology in human psoriatic skin grafted to SCID mice. J Invest Dermatol 109:283–288 [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Le A, Belinka BA, Kachlany SC. 2011. In vitro synergism between LFA1 targeting leukotoxin (LeukotheraTM) and standard chemotherapeutic agents in leukemia cells. Leuk Res 35:1498–1505 [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa Y, Yokono K, Taki T, Amano K, Tominaga Y, Yoneda R, Yagi N, Maeda S, Yagita H, Okumura K, Kasuga M. 1994. Prevention of autoimmune insulin-dependent diabetes in nonobese diabetic mice by antiLFA1 and antiICAM1 mAb. Int Immunol 6:831–838 [DOI] [PubMed] [Google Scholar]
- 16.Hioe CE, Bastiani L, Hildreth JE, Zolla-Pazner S. 1998. Role of cellular adhesion molecules in HIV type 1 infection and their impact on virus neutralization. AIDS Res Hum Retroviruses 14 Suppl 3:S247–S254 [PubMed] [Google Scholar]
- 17.Hioe CE, Chien PC, Jr, Lu C, Springer TA, Wang XH, Bandres J, Tuen M. 2001. LFA1 expression on target cells promotes human immunodeficiency virus type 1 infection and transmission. J Virol 75:1077–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hioe CE, Tuen M, Vasiliver-Shamis G, Alvarez Y, Prins KC, Banerjee S, Cho MW, Dustin ML, Kachlany SC. 2011. HIV envelope gp120 activates LFA1 on CD4+ T lymphocytes and increases cell susceptibility to LFA1-targeting leukotoxin (LtxA). PLoS ONE 6:e23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horst E, Radaszkiewicz T, Hooftman-den Otter A, Pieters R, van Dongen JJ, Meijer CJ, Pals ST. 1991. Expression of the leucocyte integrin LFA1 (CD11a/CD18) and its ligand ICAM1 (CD54) in lymphoid malignancies is related to lineage derivation and stage of differentiation but not to tumor grade. Leukemia 5:848–853 [PubMed] [Google Scholar]
- 20.Hu X, Wohler JE, Dugger KJ, Barnum SR. 2010. β2 integrins in demyelinating disease: not adhering to the paradigm. J Leukoc Biol 87:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inghirami G, Wieczorek R, Zhu BY, Silber R, Dalla-Favera R, Knowles DM. 1988. Differential expression of LFA1 molecules in nonHodgkin's lymphoma and lymphoid leukemia. Blood 72:1431–1434 [PubMed] [Google Scholar]
- 22.Institute for Laboratory Animal Research . 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 23.Kachlany SC. 2010. Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. J Dent Res 89:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kachlany SC, Schwartz AB, Balashova NV, Hioe CE, Tuen M, Le A, Kaur M, Mei Y, Rao J. 2010. Antileukemia activity of a bacterial toxin with natural specificity for LFA1 on white blood cells. Leuk Res 34:777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kevil CG, Hicks MJ, He X, Zhang J, Ballantyne CM, Raman C, Schoeb TR, Bullard DC. 2004. Loss of LFA1, but not Mac1, protects MRL/MpJ-Fas(lpr) mice from autoimmune disease. Am J Pathol 165:609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinashi T. 2005. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol 5:546–559 [DOI] [PubMed] [Google Scholar]
- 27.Koszik F, Stary G, Selenko-Gebauer N, Stingl G. 2010. Efalizumab modulates T cell function both in vivo and in vitro. J Dermatol Sci 60:159–166 [DOI] [PubMed] [Google Scholar]
- 28.Kretowski A, Mysliwiec J, Kinalska I. 1999. The alterations of CD11A expression on peripheral blood lymphocytes–monocytes and CD62L expression on peripheral blood lymphocytes in Graves disease and type 1 diabetes. Rocz Akad Med Bialymst 44:151–159 [PubMed] [Google Scholar]
- 29.Kroemer G, Jaattela M. 2005. Lysosomes and autophagy in cell death control. Nat Rev Cancer 5:886–897 [DOI] [PubMed] [Google Scholar]
- 30.Martin S, Hibino T, Faust A, Kleemann R, Kolb H. 1996. Differential expression of ICAM1 and LFA1 versus L-selectin and VCAM1 in autoimmune insulitis of NOD mice and association with both Th1- and Th2-type infiltrates. J Autoimmun 9:637–643 [DOI] [PubMed] [Google Scholar]
- 31.Mengarelli A, Zarcone D, Caruso R, Tenca C, Rana I, Pinto RM, Grossi CE, De Rossi G. 2001. Adhesion molecule expression, clinical features, and therapy outcome in childhood acute lymphoblastic leukemia. Leuk Lymphoma 40:625–630 [DOI] [PubMed] [Google Scholar]
- 32.Mysliwiec J, Kretowski A, Kinalski M, Kinalska I. 1999. CD11a expression and soluble ICAM-1 levels in peripheral blood in high-risk and overt type 1 diabetes subjects. Immunol Lett 70:69–72 [DOI] [PubMed] [Google Scholar]
- 33.Nickoloff BJ. 2000. Characterization of lymphocyte-dependent angiogenesis using a SCID mouse: human skin model of psoriasis. J Investig Dermatol Symp Proc 5:67–73 [DOI] [PubMed] [Google Scholar]
- 34.Perentesis JP, Gunther R, Waurzyniak B, Yanishevski Y, Myers DE, Ek O, Messinger Y, Shao Y, Chelstrom LM, Schneider E, Evans WE, Uckun FM. 1997. In vivo biotherapy of HL60 myeloid leukemia with a genetically engineered recombinant fusion toxin directed against the human granulocyte macrophage colony-stimulating factor receptor. Clin Cancer Res 3:2217–2227 [PubMed] [Google Scholar]
- 35.Phongpradist R, Chittasupho C, Okonogi S, Siahaan T, Anuchapreeda S, Ampasavate C, Berkland C. 2010. LFA1 on leukemic cells as a target for therapy or drug delivery. Curr Pharm Des 16:2321–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinto A, Carbone A, Gloghini A, Marotta G, Volpe R, Zagonel V. 1993. Differential expression of cell adhesion molecules in B-zone small lymphocytic lymphoma and other well-differentiated lymphocytic disorders. Cancer 72:894–904 [DOI] [PubMed] [Google Scholar]
- 37.Posselt AM, Bellin MD, Tavakol M, Szot GL, Frassetto LA, Masharani U, Kerlan RK, Fong L, Vincenti FG, Hering BJ, Bluestone JA, Stock PG. 2010. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the antiLFA1 antibody efalizumab. Am J Transplant 10:1870–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reuss-Borst MA, Klein G, Waller HD, Muller CA. 1995. Differential expression of adhesion molecules in acute leukemia. Leukemia 9:869–874 [PubMed] [Google Scholar]
- 39.Shiina M, Kobayashi K, Mano Y, Ueno Y, Ishii M, Shimosegawa T. 2002. Upregulation of CD11a (LFA1) expression on peripheral CD4+ T cells in primary biliary cirrhosis. Dig Dis Sci 47:1209–1215 [DOI] [PubMed] [Google Scholar]
- 40.Simpson DL, Berthold P, Taichman NS. 1988. Killing of human myelomonocytic leukemia and lymphocytic cell lines by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun 56:1162–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stenderup K, Rosada C, Dam TN, Salerno E, Belinka BA, Kachlany SC. 2011. Resolution of psoriasis by a leukocyte-targeting bacterial protein in a humanized mouse model. J Invest Dermatol 131:2033–2039 [DOI] [PubMed] [Google Scholar]
- 42.Tardif MR, Gilbert C, Thibault S, Fortin JF, Tremblay MJ. 2009. LFA1 antagonists as agents limiting human immunodeficiency virus type 1 infection and transmission and potentiating the effect of the fusion inhibitor T20. Antimicrob Agents Chemother 53:4656–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tardif MR, Tremblay MJ. 2005. LFA1 is a key determinant for preferential infection of memory CD4+ T cells by human immunodeficiency virus type 1. J Virol 79:13714–13724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai CC, McArthur WP, Baehni PC, Hammond BF, Taichman NS. 1979. Extraction and partial characterization of a leukotoxin from a plaque-derived gram-negative microorganism. Infect Immun 25:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turgeon NA, Avila JG, Cano JA, Hutchinson JJ, Badell IR, Page AJ, Adams AB, Sears MH, Bowen PH, Kirk AD, Pearson TC, Larsen CP. 2010. Experience with a novel efalizumab-based immunosuppressive regimen to facilitate single donor islet cell transplantation. Am J Transplant 10:2082–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valdimarsson H, Thorleifsdottir RH, Sigurdardottir SL, Gudjonsson JE, Johnston A. 2009. Psoriasis—an autoimmune disease caused by molecular mimicry. Trends Immunol 30:494–501 [DOI] [PubMed] [Google Scholar]
- 47.Villadsen LS, Schuurman J, Beurskens F, Dam TN, Dagnaes-Hansen F, Skov L, Rygaard J, Voorhorst-Ogink MM, Gerritsen AF, van Dijk MA, Parren PW, Baadsgaard O, van de Winkel JG. 2003. Resolution of psoriasis upon blockade of IL15 biological activity in a xenograft mouse model. J Clin Invest 112:1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walz G, Zanker B, Brand K, Waters C, Genbauffe F, Zeldis JB, Murphy JR, Strom TB. 1989. Sequential effects of interleukin 2–diphtheria toxin fusion protein on T-cell activation. Proc Natl Acad Sci USA 86:9485–9488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu H, Rodgers JR, Perrard XY, Perrard JL, Prince JE, Abe Y, Davis BK, Dietsch G, Smith CW, Ballantyne CM. 2004. Deficiency of CD11b or CD11d results in reduced staphylococcal enterotoxin-induced T cell response and T cell phenotypic changes. J Immunol 173:297–306 [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi N, Kieba IR, Korostoff J, Howard PS, Shenker BJ, Lally ET. 2001. Maintenance of oxidative phosphorylation protects cells from Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Cell Microbiol 3:811–823 [DOI] [PubMed] [Google Scholar]
- 51.Yonekawa K, Harlan JM. 2005. Targeting leukocyte integrins in human diseases. J Leukoc Biol 77:129–140 [DOI] [PubMed] [Google Scholar]
- 52.Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. 2002. Inhibition of LFA1–ICAM1 and VLA4–VCAM1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev 22:146–167 [DOI] [PubMed] [Google Scholar]
- 53.Zeigler M, Chi Y, Tumas DB, Bodary S, Tang H, Varani J. 2001. Anti-CD11a ameliorates disease in the human psoriatic skin–SCID mouse transplant model: comparison of antibody to CD11a with Cyclosporin A and clobetasol propionate. Lab Invest 81: 1253–1261 [DOI] [PubMed] [Google Scholar]