Abstract
We report a case of brain abscess after craniotomy and the placement of a recording chamber for electrophysiologic records in an adult rhesus macaque (Macaca mulatta) enrolled in visual research. Approximately 2 wk after surgery, the macaque presented with nonspecific gastrointestinal signs and showed no evidence of fever, neurologic deficits, increased intracranial pressure, suggestive alterations in the CBC, or abnormal changes in the recording chamber. The macaque responded to symptomatic and antibiotic treatment and showed no behavioral or abnormal clinical signs for 3 wk before collapsing suddenly. The macaque was euthanized, and pathologic evaluation revealed a large brain abscess immediately under the original craniotomy.
Brain abscess is a serious, potentially fatal condition that can arise from direct implantation of an infectious organism (for example, trauma, wound or surgical complication), local extension from an adjacent structure (for example, infection of the cribriform plate, inner ear, paranasal sinus), or hematogenous spread (usually from a primary site in the heart, lungs or tooth extraction by bacterial embolism).9,14 In humans, most brain abscesses are due to direct or indirect cranial infection from the paranasal sinus, middle ear, or teeth. An increasing proportion is secondary to head trauma or complications from neurosurgeries, whereas as many as 20% to 30% of cases have no obvious or identified cause.6 The condition is relatively uncommon in nonhuman primates because of the lack of foci of infection that can lead to direct extension to the brain.19 The hematogenous spread of bacteria or fungi is considered the most common route,2 whereas brain abscesses from direct implantation of a pathogen are seldom reported in the literature despite the numerous nonhuman primates involved in neuroscience research and undergoing regular electrophysiologic recordings in the brain. Most importantly, a brain abscess can be difficult to diagnose, given its nonspecific and variable presentation in both humans and nonhuman primates.13,19
Here we describe the clinical case of an adult rhesus macaque (Macaca mulatta) involved in neuroscience research who presented with nonspecific gastrointestinal signs 2 wk after cephalic implantation and craniotomy surgery. The macaque responded to symptomatic treatment, but the condition recurred several weeks later and led to acute circulatory shock. During necropsy, the condition was diagnosed as a brain abscess.
Case Report
An adult rhesus macaque (Macaca mulatta; age, 17 y; weight, 11.2 kg) was examined in late April 2012 for signs of acute vomiting 2 wk after the placement of a left recording chamber and craniotomy over the middle temporal visual area.8 Historically, this macaque had been implanted with a recording chamber in 2003 and underwent several electrophysiologic recordings in the right middle temporal area for a year before all the implants were removed. In addition, this macaque had a history of recurrent tooth-root abscesses of the left upper canine, which completely resolved in 2010 after apicoectomy by a dentist. The macaque was part of the Salk Institute primate colony, which is composed of captive born macaques acquired from approved United States institutions and is held indoors under standard husbandry conditions. All primates were sero-negative for SIV, simian retrovirus type D, and simian T-cell leukemia virus and were free of tuberculosis. According to standard operating procedures, recording chambers of implanted macaques were monitored routinely and cleaned under sterile conditions at least 3 times weekly by the laboratory staff using successive rinses of sterile saline and Dakin solution (0.25% to 1% sodium hypochlorite). If a recording chamber showed signs of infection, the chamber was cultured and either treated or removed according to veterinary recommendation to minimize the risks of osteomyelitis and implant instability or meningoencephalitis (especially associated with electrode penetration). All procedures were approved by the Salk Institute IACUC and performed in accordance with all applicable national and local laws, regulations, and policies.
The macaque underwent craniotomy and placement of a recording chamber over the middle temporal area by an experienced surgical team. The surgical site was shaved, depilated, disinfected by using a 2-stage surgical scrub with 70% ethanol and povidone–iodine, and covered with a sterile surgical drape. All procedures were performed under strict sterile conditions, and there was no breach in sterility reported by the surgical team. During the craniotomy, the surgeon reported a potential minor dural tear, which was treated postoperatively with prophylactic antibiotics (enrofloxacin 5 mg/kg IM then PO daily for 7 d). The macaque also received standard pre- and perioperative antibiotherapy (cefazolin 25 mg/kg IV preoperative and every 2 h during surgery) and postoperative analgesia (buprenorphine 0.01 mg/kg IM preoperatively followed by 0.03 mg/kg IM every 12 h; carprofen 2 mg/kg SC once daily for 3 d postoperatively). The macaque recovered slowly from surgery, taking 5 d to return to baseline food and water consumption, but showed no abnormal behavioral or clinical manifestation. After full recovery, the macaque was chaired 3 times over a 10-d period for weighing and awake cleaning of the recording chamber. During that period, the macaque was bright, alert, and responsive; appetite and water consumption were within normal limits; and body weight was stable. Based on reports from the laboratory, the recording chamber and dura were normal.
Sixteen days after the original surgery, the monkey was sedated (ketamine 4 mg/kg IM, acepromazine 0.04 mg/kg IM, and atropine 0.02 mg/kg IM) to remove the surgical staples and clean the wound margins. The macaque recovered completely but vomited 3 times in the evening. He was treated conservatively for 72 h with a 12-h fast followed by ranitidine (1 mg/kg SC every 12 h for 3 d), metoclopramide (0.3 mg/kg IM once daily for 3 d), and subcutaneous fluids (lactated Ringers solution 125 mL SC every 12 h for 3 d). The clinical manifestations of postsurgical meningitis are often nonspecific during the immediate postoperative period, and the interval between surgery and clinical onset can take over 12 d.23 As such, considering the history of dural tear, prophylactic antibiotherapy (enrofloxacin 5 mg/kg IM or PO once daily for 7 d) was started as a precaution, even though the chamber appeared normal and the macaque did not show other signs of meningitis such as headache (signs include the animal holding its head, avoiding movement, and moving slowly when prompted), meningeal stiffness, and overt neurologic deficits. Over the next few days, the macaque showed nonspecific gastrointestinal clinical signs, with occasional vomiting of small amounts of bile and nonproductive retching, tooth grinding, mild ptyalism, decreased appetite, and episodes of lying down in the cage with abdominal discomfort. There was no fever, alteration in consciousness, obvious neurologic deficits or signs of increased intracranial pressure, or bulging of the dura in the recording chamber.
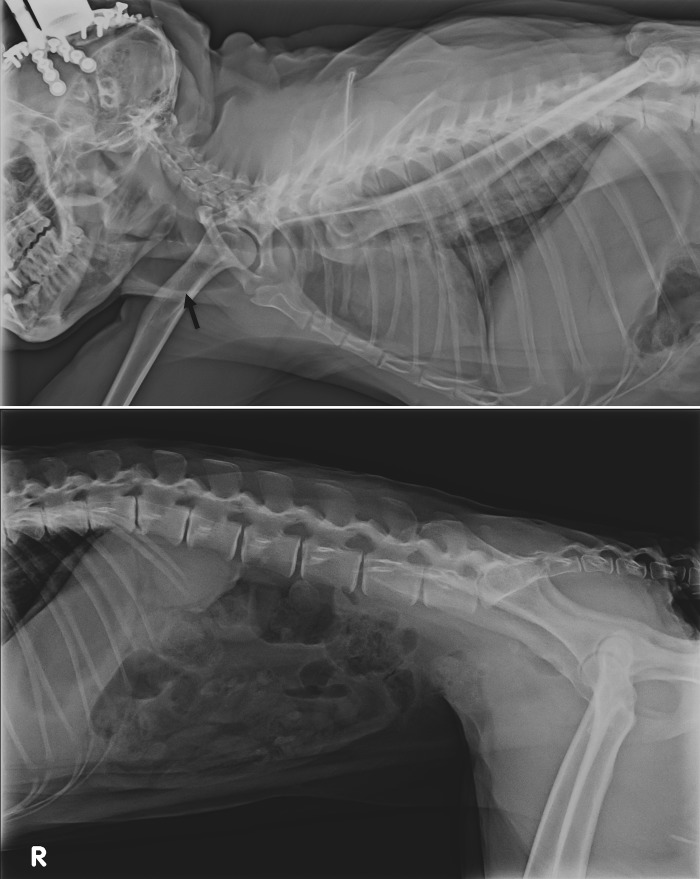
In the absence of clinical improvement, the macaque was sedated to perform a full physical examination, CBC, serum biochemistry, and abdominal and chest radiographs. The macaque received lactated Ringers solution supplemented with 2.5% dextrose IV during the procedure. Except for dehydration (5% to 7%), the macaque's physical examination was unremarkable. The abdominal radiographs were within normal limits, whereas the chest films showed marked distension of the cervical esophagus (Figure 1), which we further investigated by endoscopy. The endoscopy did not reveal any esophageal stricture or dilation or any signs of inflammation, erosion, ulceration, or trauma of the esophagus or stomach. CBC revealed moderate neutrophilic leukocytosis with no left shift but a monocytosis associated with lymphopenia and eosinopenia, which were attributed to stress. Serum biochemistry showed marginal hypochloremia and hypokalemia and increased creatine phosphokinase (Table 1). On the basis of these results, the macaque was prescribed maropitant (1 mg/kg SC once daily) and the same regimen of ranitidine and subcutaneous fluids as previously for 7 d as well as enrofloxacin (5 mg/kg IM or PO once daily) for 12 additional days. The macaque was encouraged to eat a bland diet and fruit-flavored gelatin (JELL-O, Kraft Foods, Northfield, IL). CBC and serum biochemistry with amylase and lipase were repeated 2 d later and revealed mild monocytosis, hypochloremia, and hypokalemia (Table 1). Clinically, the macaque improved progressively over the next 7 d, with no more vomiting and gradual return to baseline food and water consumption. The recording chamber was cleaned regularly by the laboratory personnel and was reported as normal with no evidence of increased intracranial pressure or infection.
Figure 1.
Survey radiographs. The thoracic radiographs (upper panel) shows marked distention of the cervical esophagus (arrow).
Table 1.
CBC and serum biochemistry at initial presentation and during the terminal circulatory collapse
| Date | ||||
| Reference range | 02 May 2012 | 04 May 2012 | 03 June 2012 | |
| Total protein | 5.0–7.0 g/dL | 7.0 | 7.0 | 7.5 |
| Albumin | 2.4–3.3 g/dL | 3.1 | 3.3 | 3.7 |
| Globulin | 2.0–4.0 g/dL | 3.9 | 3.7 | 3.8 |
| AST | 10–100 IU/L | 81 | 46 | 49 |
| ALT | 10–100 IU/L | 43 | 52 | 30 |
| ALP | 100–250 IU/L | 110 | 109 | 132 |
| Total bilirubin | 0.0–1.0 mg/dL | 0.3 | 0.2 | 0.1 |
| BUN | 10–22 mg/dL | 27 | 20 | 26 |
| Creatinine | 0.1–2.0 mg/dL | 0.7 | 0.6 | 0.8 |
| Phosphorus | 3.0–5.5 mg/dL | 3.3 | 3.5 | 5.8 |
| Glucose | 70–120 mg/dL | 133 | 106 | 95 |
| Calcium | 7.2–11.5 mg/dL | 8.9 | 9.4 | 9.5 |
| Sodium | 138–150 mEq/L | 146 | 146 | 151 |
| Potassium | 3.5–5.5 mEq/L | 3.3 | 3.0 | 3.5 |
| Chloride | 109–115 mEq/L | 104 | 95 | 105 |
| Creatinine phosphokinase | 100–400 IU/L | 2268 | 1165 | 449 |
| Amylase | 1000–2500 IU/L | — | 388 | — |
| Lipase | 10–700 IU/L | — | 25 | — |
| WBC | 8.0–16.0 × 103/μL | 16.3 | 13.4 | 14.0 |
| RBC | 5.5–8.5 × 106/μL | 5.5 | 5.3 | 6.2 |
| Hct | 38.0% to 50.0% | 43 | 40 | 48 |
| Platelets | ×103/μL | 395 | 342 | 427 |
| Neutrophils | 4800–12000/μL | 13040 | 10452 | 10640 |
| Lymphocytes | 1600–4800/μL | 978 | 1876 | 1820 |
| Monocytes | 0–480/μL | 2282 | 938 | 1260 |
| Eosinophils | 80–800/μL | 0 | 134 | 280 |
Values outside the reference range are bolded. Antech Diagnostics (Irvine, CA) performed the analysis and provided the reference range values.
After recovery, the macaque showed no abnormal behavior or clinical signs for 3 wk. There was no penetration of the dura throughout that period. In late May, the macaque was fluid-restricted prior to performing a behavioral task in the chair. On return to the cage, the macaque ate and vomited twice small amounts of bile and food. The animal was bright, alert, and responsive and treated conservatively for 48 h with a 12-h fast followed by bismuth subsalicylate (5.5 mL PO every 12 h), famotidine (0.5 mg/kg PO once daily), and fluids (lactated Ringers solution 300 mL SC daily). The next day, the macaque ate a small amount of fruit-flavored gelatin (JELL-O) and sat on his perch but appeared to be in pain, which was treated with buprenorphine (0.03 mg/kg IM every 12 h). The macaque's condition deteriorated rapidly over the next 24 h. He became weak and depressed and went rapidly into circulatory shock. The macaque became nonresponsive, hypotensive, hypothermic, and tachypneic. Shock was treated with 100% oxygen by mask, bolus administration of warm intravenous fluids (lactated Ringers solution 60 mL/kg), corticosteroids (dexamethasone 4 mg/kg IV), and antibiotics (enrofloxacin 5 mg/kg IV).
Vital signs markedly improved during the next hour, but the macaque went into sudden cardiopulmonary arrest. The animal was intubated immediately and ventilated with 100% oxygen by using a ventilator (16 breaths per minute, 15 mL/kg tidal volume, with peak inflation pressure of 20 cm H20). Chest compressions were started (100 compressions per minute), and epinephrine (0.01 mg/kg) given intravenously. Although cardiac resuscitation was successful, the macaque was comatose, could not be weaned off the ventilator, and was euthanized for humane reasons. CBC and serum biochemistry taken before the macaque died revealed monocytosis indicative of chronic inflammation, evidence of dehydration, and mild hyperphosphatemia, hypernatremia, and hypochloremia.
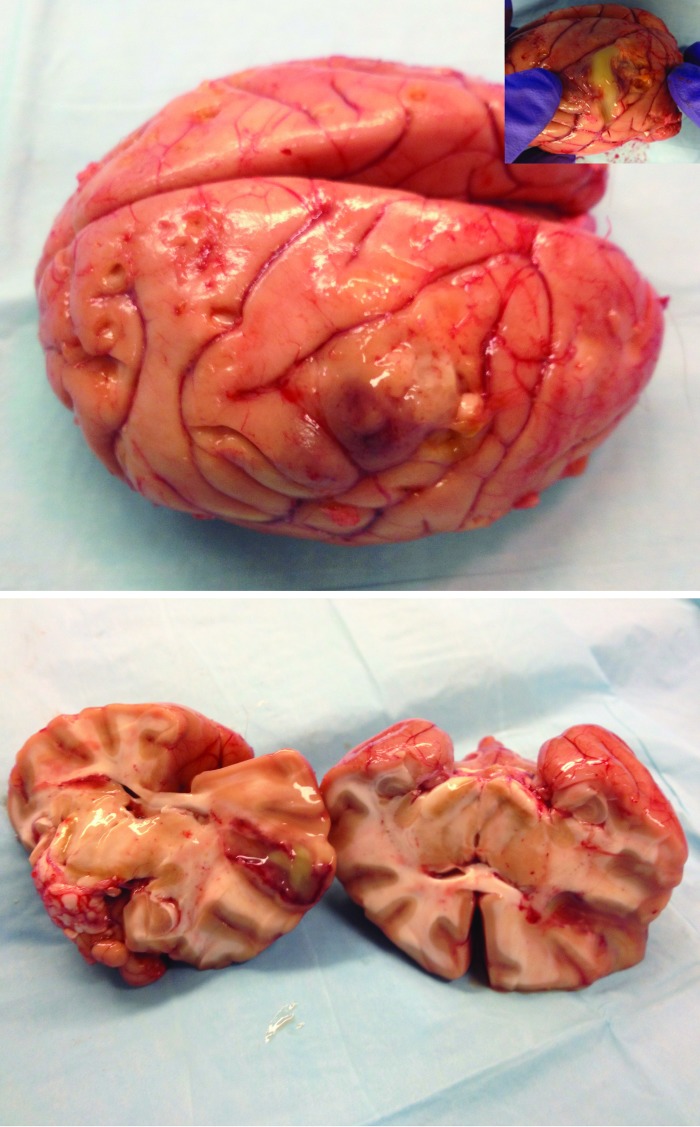
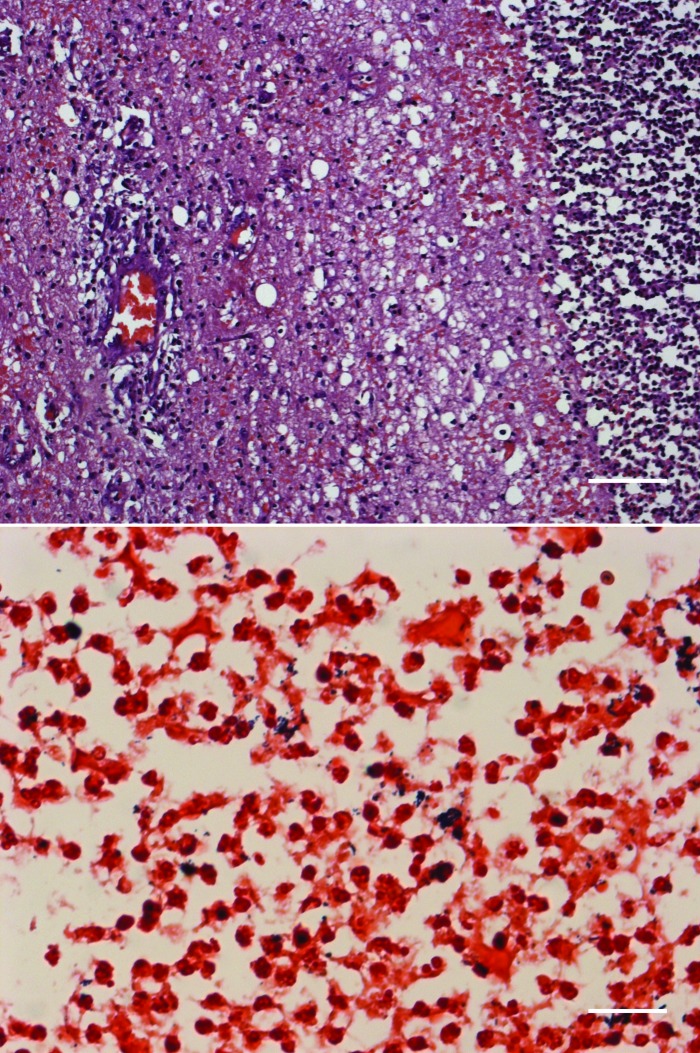
A complete necropsy was performed. On gross examination, the macaque had a low body condition score and was dehydrated (3% to 5%). The trachea contained a moderate amount of spumous liquid tinted with blood, and there was moderate pulmonary edema. The recording chamber contained a moderate amount of fibrinopurulent exudate, and the exposed dura was thick, opaque, and adhered to the brain parenchyma. Dissection of the underlying brain revealed a brain abscess (2 × 3.5 cm) filled with purulent material; the abscess spanned from the gray matter of the visual cortex to deeper structures and white matter (Figure 2). There was no clear herniation. Aerobic cultures taken from the brain showed abundant growth of β-hemolytic Streptococcus spp., Corynebacterium spp., and a few colonies of Serratia marcescens. On histopathology, there was a large ovoid abscess in the gray and white matter that had a necropurulent center containing numerous gram-positive cocci and coccobacili (Figure 3). The surrounding parenchyma was edematous and hemorrhagic, with degenerated myelin fibers, numerous neutrophils, gitter cells, and reactive astrocytes. The outer zone contained condensed reactive vessels, with several neutrophilic vasculitis, occasional lymphoplasmacytic perivascular cuffing, marked gliosis, and some proliferating fibroblasts. Over and around the abscess, moderate subacute fibrinopurulent meningitis with several gram-positive coccoid colonies was present.
Figure 2.
Brain abscess in the left middle temporal area is filled with purulent exudate.
Figure 3.
Large brain abscess (upper panel; hematoxylin and eosin stain; scale bar, 100 μm) contains abundant gram-positive cocci and coccobacilli (lower panel; Gram stain; scale bar, 100 μm).
Discussion
Brain abscesses in humans and nonhuman primates are relatively uncommon and can be clinically challenging to diagnose. Presenting clinical signs are often nonspecific and depend on the origin, size, and location of the abscess; infectious pathogen; and presence of underlying conditions. Human patients typically present with one or more symptoms including fever, headache, altered level of consciousness, and focal neurologic signs.6 Headache is the most common presenting sign, occurring in about 50% of patients. The headache associated with a brain abscess is dull aching and poorly localized, as compared with the more severe and abrupt headache observed in bacterial meningitis. Fever is present in fewer than 50% of patients, variable, and cannot be used to exclude the diagnosis.13 The presence of focal neurologic deficits can be observed in about one-third to one-half of patients and varies greatly with the location of the abscess. Other clinical signs include alterations of mental status, nausea, vomiting, seizures, neck stiffness, and photophobia. Papilledema, which denotes increased intracranial pressure, is present in fewer than 25% of cases.6,13 Clinical signs in nonhuman primates are similarly nonspecific, or directly related to the location of the abscess, or mostly associated with the primary pathologic process.19-22 In addition, changes in laboratory data are often nondiagnostic or nonspecific in humans and nonhuman primates; both can have normal CBC with no evidence of neutrophilic leukocytosis or presence of left shift.13,21 CSF taps have poor diagnostic value and actually are contraindicated due to the risk of brainstem herniation.1,13 Villano and colleagues21 reported a slightly elevated WBC, unremarkable liver and kidney biochemistry, and increased ammonia in a cynomolgus macaque with an abscess in the cerebellopontine angle. In other reports,19,20 changes in WBC probably were associated with the primary process rather than the brain abscess. The overall consensus is that the infrequency and nonspecific presentation of brain abscess account for delays in diagnosis and potential fatal outcomes.
Similarly, the macaque described in this case report did not show any signs of acute meningitis, such as fever, neck stiffness, altered mental status, and other neurologic deficits. In fact, he recuperated normally from surgery and presented, more than 2 wk after the original surgery, with nonspecific acute gastrointestinal signs after sedation, with no febrile episode, altered level of consciousness, neurologic deficits, or evidence of increased intracranial pressure. Importantly, laboratory personnel evaluated the cephalic chamber throughout the period and did not report any evidence of infection or abnormal changes in the dura or the underlying brain parenchyma. CBC showed marginal changes, and the macaque responded to symptomatic treatment.
The macaque's chest radiograph revealed dilation of the proximal esophagus, but there was no evidence of megaesophagus or other esophageal lesions on endoscopy or gross necropsy. Air in the esophagus is a frequent radiographic finding on normal thoracic radiographs.18 In humans, esophageal dysmotility with abnormal manometry is associated with an air column extending over 2 thirds of the esophagus.11 In small animals, transient dilation of the esophagus can be observed in sedated or anesthetized patients or due to esophagitis. Overall, radiographic evidence of air collection in the esophagus, especially when localized, is neither sensitive nor specific of esophageal dysmotility or megaesophagus.11 The observed esophageal dilation in our macaque was likely an incidental finding. The motor innervation of the esophagus is predominantly via the vagus nerve. The dorsal nucleus of the vagus nerve lies in the lower half of the brainstem, in the medulla oblongata under the floor of the fourth ventricle. The vagus nerve exits from the medulla oblongata in the groove between the olive and the inferior cerebellar peduncle. As such, it would be surprising that a localized lesion in the visual cortex could affect the central or peripheral innervation of the esophagus causing transient hypomotility and localized dilation of the proximal esophagus with no other signs of increased intracranial pressure or brain stem and vagal involvement. The macaque fully recovered from the first clinical episode and showed no behavioral or abnormal clinical signs for 3 wk before a sudden and acute collapse. This clinically silent period followed by sudden deterioration likely coincided with the development of the abscess despite antibiotic treatment until the space-occupying lesion caused increased intracranial pressure and progressive fatal herniation.
Because no penetration of the dura occurred from the time of the original surgery until the death of animal, the most probable etiology of the brain abscess in this monkey was the minor dural tear that occurred during surgery. These tears can result in acute bacterial meningitis and typically are treated with postoperative antibiotics. Although third-generation cephalosporins, such as ceftriaxone, remain the first choice for treatment of meningitis in humans,4 these antibiotics can have significant side effects in nonhuman primates, including diarrhea and vomiting. In contrast, fluoroquinolone antibiotics, such as enrofloxacin, have broad-spectrum activity against many gram-negative bacilli and cocci, can penetrate into inflamed CSF, are well tolerated in nonhuman primates, and have been used successfully to treat bacterial meningitis in both humans and animal models.7,15
Overall, brain abscess should remain high on the differential diagnosis list of any NHP with a cephalic implant and craniotomy even if the clinical presentation and laboratory data remain nonspecific and the animal responds to symptomatic treatment. MRI is the imaging procedure of choice in virtually all CNS infections, and is typically more sensitive than CT for identifying infection-associated CNS tissue changes including brain abscesses.1 Unless contraindicated (for example: ferromagnetic implants or equipment), an MRI should be performed early on NHPs with suspected brain abscess and before any attempt of lumbar puncture for CSF analysis to minimize the risks of herniation.1 Unfortunately, modern imaging modalities for NHPs are often unavailable in research facilities or require close collaborations with referral human or veterinary hospitals. As such, early broad-spectrum empirical antibiotherapy might be the best solution before an MRI can be performed. In humans, the most common bacteria present in brain abscesses following penetrating trauma and postneurosurgical complications include Staphylococcus species, Clostridium species, Enterobacteriaceae, Pseudomonaceae, and anaerobes.1,6,13 Based on our experience and past studies,3,10 prevalent species in wound margins and cephalic implants of NHPs are Staphylococcus, Streptococcus, and Corynebacterium species as well as Enterobacteriaceae such as Enterococcus, E. coli, Klebsiella, Proteus, and Serratia. As observed in our primate, brain abscesses are commonly associated with mixed bacterial infections,17 including anaerobic negative-rods and, thus, should be treated initially with a combination of antibiotics to cover a wide range of potential bacterial contaminants. Standard recommendations in human medicine include a combination of a third generation cephalosporin and metronidazole to cover for anaerobes.1 With the exception of cefuroxime, no first or second generation cephalosporin enters the CSF at therapeutic levels even when meninges are inflamed.16 Considering the side effects of third generation cephalosporin in primates, one may consider the combination of metronidazole with newer fluoroquinolones that possess a broader activity against Gram-negative and positive aerobic bacteria causing meningo-encephalitis, and enter readily into the CSF.7 Antibiotherapy should be modified based on culture and sensitivity after sampling of the purulent material to account for potentially resistant bacterial strains. In general, human patients require 4 to 8 wk of antibiotic therapy but the duration may vary based on the type of bacteria, the extent of the abscess, the health status of the patient and the surgical management of the disease.13 Several reports have demonstrated successful treatment of brain abscess with antibiotics alone, especially for small lesions (less 2 cm) located in a well vascularized cortical area.5,12 This type of medical management may require prolonged therapy as well as careful clinical and imagery monitoring while surgical options (drainage, excision) would likely lead to a shorter recovery and better outcome.13
In summary, a brain abscess was found in an adult macaque several weeks after a reported minor dural tear during cephalic implantation surgery and despite antibiotic treatment. The original clinical presentation and laboratory data were nonspecific, and the macaque completely recovered with symptomatic treatment for a few weeks before sudden collapse. Retrospectively, we believe that, with the limited diagnostic and therapeutic tools available in some research institution, any dural tear should warrant aggressive antibiotic treatment to prevent further complications. Brain abscess, in addition to bacterial meningitis, should be included in the differential diagnosis of an implanted primate showing signs of disease. To our knowledge, this report is the first description of a brain abscess in a macaque with a cephalic implant, despite the fact that these implants can easily get infected and are commonly used for electrophysiologic recording of neuronal activity in conscious animals.3,10 Although the brain typically is resistant to bacterial infection, future studies could explore the true prevalence of clinical and subclinical brain abscesses and meningoencelphalitis in nonhuman with cephalic implants and the potential effects on animal wellbeing and research data.
References
- 1.Beckham DJ, Tyler KL. 2012. Neurointensive care of patients with acute CNS infections. Neurotherapeutics 9:124–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennet TB, Abee CR, Henrickson R. 1998. Nonhuman primates in biomedical research. Diseases. San Diego (CA): Academic Press. [Google Scholar]
- 3.Bergin LI, Chien CC, Marini RP, Fox J. 2000. Isolation and characterization of Corynebacterium ulcerans from cephalic implants in macaques. Comp Med 50:530–535 [PubMed] [Google Scholar]
- 4.Bhimraj A. 2012. Acute community-acquired bacterial meningitis in adults: an evidence-based review. Cleve Clin J Med 79:393–400 [DOI] [PubMed] [Google Scholar]
- 5.Carpenter J. 1994. Brain stem abscesses: cure with medical therapy, case report, and review. Clin Infect Dis 18:219–226 [DOI] [PubMed] [Google Scholar]
- 6.Carpenter J, Stapleton S, Hollisman R. 2007. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis 26:1–11 [DOI] [PubMed] [Google Scholar]
- 7.Cottagnoud P, Tauber MG. 2003. Fluoroquinolones in the treatment of meningitis. Curr Infect Dis Rep 5:329–336 [DOI] [PubMed] [Google Scholar]
- 8.Krekelberg B, Albright TD. 2005. Motion mechanisms in macaque MT. J Neurophysiol 93:2908–2921 [DOI] [PubMed] [Google Scholar]
- 9.Kumar V, Abbas AK, Fausto N. 2005. Robbins and Cotran pathologic basis of disease, 7th ed. New York (NY): Elsevier. [Google Scholar]
- 10.Lee GE, Danneman PJ, Rufo RD, Kalesnykas RP, Eng VM. 1998. Use of chlorine dioxide for antimicrobial prophylactic maintenance of cephalic recording devices in rhesus macaques. Contemp Top Lab Anim Sci 37:59–63 [PubMed] [Google Scholar]
- 11.Lock G, Strotzer M, Straub RH, Scholmerich J, Feuerbach S, Holstege A, Lang B. 1998. Air oesophagram: a frequent, but not a specific, sign of oesophageal involvement in connective tissue diseases. Br J Rheumatol 37:1011–1014 [DOI] [PubMed] [Google Scholar]
- 12.Mamelak AN, Mampalam TH, Obana WG, Rosemblum ML. 1995. Improved management of multiple brain abscesses: a combined surgical and medical approach. Neurosurgery 36:76–85 [DOI] [PubMed] [Google Scholar]
- 13.Mathisen GE, Johnson PJ. 1997. Brain abscess. Clin Infect Dis 25:763–779 [DOI] [PubMed] [Google Scholar]
- 14.Maxie MG. 2007. Jubb, Kennedy and Palmer's pathology of domestic animals, volume 1, 5th ed. New York (NY): Elsevier. [Google Scholar]
- 15.Nau R, Schmidt T, Kay K, Froula JL, Tauber M. 1995. Quinolone antibiotics in the therapy of experimental pneumococcal meningitis in rabbits. Antimicrob Agents Chemother 39:593–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plumb DC. 2005. Plumb's veterinary drug handbook, 5th ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 17.Prasad KN, Mishra AM, Gupta D, Husain N, Husain M, Gupta RK. 2006. Analysis of microbial etiology and mortality in patients with brain abscess. J Infect 53:221–227 [DOI] [PubMed] [Google Scholar]
- 18.Proto AV, Lane EJ. 1977. Air in the esophagus: a frequent radiographic finding. AJR Am J Roentgenol 129:433–440 [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg DP, Lowenstine LJ. 1981. Brain abscess in a rhesus monkey. J Am Vet Med Assoc 179:1299–1303 [PubMed] [Google Scholar]
- 20.Sakakibara I, Sugimoto Y, Minato H, Takasaka M, Honjo S. 1984. Spontaneous nocardiosis with brain abscess caused by Nocardia asteroides in a cynomolgus monkey. J Med Primatol 13:89–95 [PubMed] [Google Scholar]
- 21.Villano JS, Ogden B, Goh A, Hui LS, Chow PKH. 2008. Cerebellar abscess in a cynomolgus macaque (Macaca fascicularis). J Med Primatol 37 Suppl. 1:82–87 [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson LM, Wallace JM, Cline JM. 1999. Disseminated blastomycosis in a rhesus monkey (Macaca mulatta). Vet Pathol 36:460–462 [DOI] [PubMed] [Google Scholar]
- 23.Zarrouk V, Vassor I, Bert F, Bouccara D, Kalamarides M, Bendersky N, Redondo A, Sterkers O, Fantin B. 2007. Evaluation of the management of postoperative aseptic meningitis. Clin Infect Dis 44:1555–1559 [DOI] [PubMed] [Google Scholar]