Abstract

Among different classes of psychotropic drugs, hallucinogenic agents exert one of the most prominent effects on human and animal behaviors, markedly altering sensory, motor, affective, and cognitive responses. The growing clinical and preclinical interest in psychedelic, dissociative, and deliriant hallucinogens necessitates novel translational, sensitive, and high-throughput in vivo models and screens. Primate and rodent models have been traditionally used to study cellular mechanisms and neural circuits of hallucinogenic drugs’ action. The utility of zebrafish (Danio rerio) in neuroscience research is rapidly growing due to their high physiological and genetic homology to humans, ease of genetic manipulation, robust behaviors, and cost effectiveness. Possessing a fully characterized genome, both adult and larval zebrafish are currently widely used for in vivo screening of various psychotropic compounds, including hallucinogens and related drugs. Recognizing the growing importance of hallucinogens in biological psychiatry, here we discuss hallucinogenic-induced phenotypes in zebrafish and evaluate their potential as efficient preclinical models of drug-induced states in humans.
Keywords: Hallucinogenic drugs, zebrafish, animal models, neurobehavioral in vivo screens
Understanding normal and pathological brain functions is an important challenge in biomedical research that requires extensive clinical and preclinical investigation.1,2 Animal (experimental) models have long been used in translational neuroscience to study brain mechanisms and their effects on behavior.3,4 Environmental, genetic, and pharmacological manipulations are widely used to probe brain mechanisms and circuits in various animal models.5,6 Among different classes of psychotropic drugs, hallucinogenic agents (Table 1) occupy a unique niche, underlying the growing interest of clinical and basic scientists in these compounds.7,8 For example, hallucinogens exert profound effects on human and animal behaviors, sensory perception and processing.9,10 Thus, increased understanding of the effects of drugs that induce one of the most potent CNS effects, may provide critical insights into normal and pathological brain functioning.7,11 Furthermore, hallucinogens have very “rich” pharmacology and act via multiple receptors, which remain poorly understood in terms of both their mechanisms and the resultant behavioral responses.12−14
Table 1. Summary of Psychopharmacological Profiles of Major Hallucinogenic Substances in Human and Animal Models.
| drugs | profile(s) | clinical effects | effects in rodent models | ref |
|---|---|---|---|---|
| psychedelic hallucinogens | ||||
| LSD | nonselective 5HT agonist | perceptual distortions, depersonalization, visual/auditory hallucinations, slowing of time | altered agonistic behaviors, increased exploration | (182) |
| mescaline | 5H2A/2C agonist | perceptual distortions, depersonalization, agitation, slowing of time | altered agonistic behaviors, hyperlocomotion, anxiety | (183) |
| MDMAa | monoamine transporter blocker | euphoria, sense of intimacy, antianxiety, empathogenic action | hyperlocomotion, hypersocial behavior | (184) |
| ibogaineb | 5-HT and opioidergic agonist; NMDA antagonist (also possesses some anticholinergic activity) | introspection, dream-like state, oneirophrenic action | decreased drug-induced hypelocomotion | (185) |
| dissociative hallucinogens | ||||
| PCP | NMDA antagonist | vivid dreams, out-of-body feelings, aggression, anxiety | psychoses-like behavior, hyper-locomotion, motor incoordination, stereotypies, circling, altered pain sensitivity | (186) |
| ketamine | NMDA antagonist | dissociation, depersonalization and derealization | antidepressant effect, circling, reduced anxiety, altered pain sensitivity | (187) |
| MK-801 | NMDA antagonist | long-lasting hallucinations, amnesia | psychoses-like behavior, circling, anxiety behavior | (188) |
| salvinorin Ac | κ-opioid agonist | depersonalization, distortion of reality, hyperactivation, sensation of motion and revisitation of memories | hypolocomotion, altered pain sensitivity | (38) |
| deliriants | ||||
| atropine | antagonist of M-cholinoreceptors | confusion/disorientation, hallucinations and delusions | hypolocomotion, decreased exploratory action | (189) |
| scopolamine | antagonist of M-cholinoreceptors | confusion/disorientation, increased paranoia and agitation | hypolocomotion, memory loss | (190) |
Psychostimulant and mild psychedelic drug.
Mixed profile (psychedelic and dissociative), antagonist of NMDA receptor, and 5-HT/opioidergic agonist.
Mixed profile (dissociative and psychedelic), opioidergic agonist.
In clinical and experimental models, hallucinogens evoke responses that strikingly resemble (and/or are highly relevant to) certain human brain disorders, such as substance abuse,15,16 psychoses,17,18 affective disorders,19,20 and cognitive deficits.21,22 Thus, the use of hallucinogenic drugs can lead to new sensitive experimental models of brain disorders.23 Moreover, mounting evidence indicates that hallucinogens may have some clinical value in treating selected brain disorders,24 including obsessive compulsive disorder,25,26 post-traumatic stress disorder,27,28 and depression.29−31 Therefore, this line of research can lead to widening and innovating the spectrum of potential therapeutic approaches to some serious debilitating pathological conditions (see refs (10, 24, and 32) for discussion).
Evoking strong subjective changes in perception, emotion, and behavior,9,10 hallucinogens include several classes of psychotropic compounds, such as psychedelic, dissociative, and deliriant agents.33,34 Typical psychedelic (mind-altering) agents include serotonergic drugs, such as lysergic acid diethylamide (LSD), mescaline, psilocybin, and its biologically active form psilocin. These drugs modulate various serotonin (5-HT) receptors and evoke distortion, depersonalization, somatic symptoms, and sensory hallucinations (Table 1). Albeit not a typical hallucinogen per se, 3,4-methylenedioxy-N-methylamphetamine (MDMA) also acts in a similar manner, evoking mild psychedelic effects35,36 associated with the inhibition of monoamine transporters.
The dissociative hallucinogens, such as ketamine, phencyclidine (PCP), and MK-801 (dizocilpine), act as noncompetitive antagonists of the N-methyl-d-aspartic acid (NMDA) receptors, clinically evoking depersonalization, derealization, and dissociation with self and reality (Table 1). Opioidergic agent salvinorin A (acting as a kappa opioid receptor agonist) also possesses strong dissociative (and some psychedelic) hallucinogenic properties in humans,37,38 and affects behavior in animals.39−41 Some hallucinogenic agents, such as psychedelic drug ibogaine, have mixed pharmacological profiles, combining antagonism of NMDA receptors with serotonergic and opioidergic agonism (Table 1). Finally, the clinical effect of deliriant hallucinogenic drugs, acting as antimuscarinic cholinergic agents, includes delirium (confusion/agitation and disorganization of behavior), which differs markedly from psychedelic and dissociative states produced by the two other classes of hallucinogens (Table 1). Overall, the resurgence of clinical and preclinical interest in hallucinogenic drug research is encouraging,24 and may help us understand normal and pathological brain mechanisms modulated by these drugs.
Developing Complementary Nonmammalian Models for Hallucinogenic Drug Action
Various primate and rodent models have been used extensively to study mechanisms and neural circuits that underlie neurobehavioral effects of hallucinogenic drugs;7,42,43 also see ref (14) for review. Typical behavioral responses evoked by hallucinogens in rodent models include serotonin-like behavioral syndrome (“serotonin behavior”), head twitching, behavioral stereotypies, altered startle or prepulse inhibition, and drug discrimination.14
Despite their wide use in neuroscience research, mammalian models of hallucinogenic drug action also have their own conceptual and practical limitations.14 Recently, both translational “cross-species” modeling and increasing the spectrum of model organisms have been recognized as key strategies for biological psychiatry research.44 Reflecting the importance of evolutionarily conserved traits in translational neuroscience,45 optimal animal models of brain disorders must be evolutionarily relevant, that is, targeting common behavioral and physiological phenotypes, neural pathways and circuits in a similar manner across various species.46 In our view, this approach enables a good focus on ancient, conserved (and, therefore, fundamental and translationally relevant) aspects of brain pathology,46 including hallucinogenic drug-related phenomena. Collectively, this suggests that developing novel nonmammalian models of hallucinogenic drug action (in addition to the existing mammalian paradigms) is a necessary goal for making further progress in this field.
In line with this, the growing practical need to develop efficient high-throughput in vivo screens for novel psychotropic compounds requires alternative, sensitive, and time/cost-efficient models of psychotropic drug action.46 Recent evidence indicates that various novel model species, including invertebrates, are emerging as useful tools to target various aspects of hallucinogen-induced CNS phenomena.47−50 For example, Drosophila melanogaster has been successfully used to study visual processing, locomotor activity, and the modulation of gene expression within the brain in response to LSD exposure, suggesting that the fruit fly can serve as a genetically tractable model system to define molecular events leading from hallucinogenic receptor activation to behavior.48 Among several relatively new model species, zebrafish (Danio rerio) offer a unique combination of features, rapidly emerging as high-throughput and sensitive screens to complement existing rodent models of brain disorders.46
Importantly, unlike invertebrate models, zebrafish show high physiological and genetic homology to humans, are easy to manipulate genetically, and display robust behavioral phenotypes.51−58 Possessing a fully characterized genome,59,60 with functional domains of many key proteins nearly 100% identical to their human homologues,58,61,62 zebrafish may also be used for in vivo screening of various psychotropic compounds, including hallucinogens.46,63 Zebrafish behavior has been recently thoroughly described,64 making neurophenotypic screens based on this model particularly attractive. In addition, zebrafish testing is very cost-efficient, as it may reduce a research budget by 500–1000-fold, compared to rodent testing with similar study designs.46 Zebrafish are also characterized by well-developed monoaminergic, glutamatergic, opioid, and cholinergic neurotransmitter systems,54,55,65 all relevant to psychotropic action of hallucinogenic drugs (Table 1). Although fish models will not be able to fully recapitulate all aspects of a complex brain disorder, their attributes make zebrafish aquatic experimental paradigms a valuable tool in psychopharmacological research.46 Collectively, this renders zebrafish models particularly suitable for drug discovery and in vivo screening of hallucinogens and related compounds (see ref (64) for a comprehensive catalog of hallucinogenic drug-evoked behaviors in zebrafish).
Emphasizing the role of specific receptor systems in the drug-induced phenotypes, recent studies have examined the effects of various hallucinogenic compounds, including LSD, mescaline, MDMA, PCP, MK-801, ketamine, ibogaine, salvinorin A, atropine, and scopolamine in adult zebrafish66−73 (Tables 2 and 3). The recognized strength of adult zebrafish models is their high relevance of adult fish physiology to human brain disorders, well-developed physiological systems, sensitivity to environmental challenges, and a rich spectrum of quantifiable neurobehavioral phenotypes.46,64,66,74−78
Table 2. Summary of Neurobehavioral Alterations Evoked in Zebrafish by Selected Hallucinogenic Agentsa.
| hallucinogenic
drugs |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| serotonergic |
mixed | glutamatergic |
opioid | cholinergic |
||||||||
| phenotypes and domains | test | LSD | mescaline | psilocybin | MDMA | ibogaineb | PCP | ketamine | MK-801 | salvinorin A | atropine | scopolamine |
| motor activity | ||||||||||||
| distance traveled | NTT | 0 | 0 | 0 | 0 | ↑ | 0 | 0 | ↑ | ↑0c | 0 | |
| velocity | NTT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ↑ | ↑ | 0 | |
| anxiety-related behavior | ||||||||||||
| latency to top | NTT | ↓ | ↓ | 0 | ↓ | ↓ | ↓ | ↓ | ||||
| transitions/time in top | NTT | ↑ | ↑ | 0 | ↑ | 0 | 0 | ↑↓ | 0↑ | 0↑c | 0 | |
| erratic movements | NTT | 0 | 0 | 0 | ↓ | ↑ | ↑ | 0 | 0 | |||
| freezing duration/bouts | NTT | ↑ | 0 | 0 | ↓ | ↑0 | 0 | 0 | 0 | |||
| latency to white | LDB | 0 | 0 | ↓ | 0 | |||||||
| transitions/time in white | LDB | 0 | 0 | ↑ | ↑0 | |||||||
| social behavior | ||||||||||||
| shoaling behavior | SBT | ↓ | ↑ | ↑0c | ↓ | ↓ | 0↓c | ↓ | ↓ | 0* | ||
| social preference | SPT | 0 | 0 | 0 | ↑ | ↓ | ||||||
| cognitive function | ||||||||||||
| habituation | NTT | ↑ | ↑ | ↑d | ↑ | 0 | ↓ | |||||
| homebase formation | NTT | ↑ | ||||||||||
| learning | SCT | ↓ | ||||||||||
| memory | YMT | ↓ | ↓ | |||||||||
| neurological phenotypes | ||||||||||||
| circling behavior | NTT | 0c | 0c | 0c | 0c | 0 | ↑ | ↑ | ↑ | |||
| organization of behaviore | NTT | ↓ | ↓ | ↓ | ↓ | |||||||
| reward | ||||||||||||
| time in drug-paired sector | CPP | ↑ | ||||||||||
| physiological biomarkers | ||||||||||||
| whole-brain c-fos expression | ↑ | ↑ | 0 | ↑ | 0c | |||||||
| whole-body cortisol | ↑ | 0 | ↑ | 0 | ↑ | ↓ | 0c | |||||
| ref | (66, 126) | (97, 101) | c | (100, 126) | (70) | (101) | (69, 144) | (191−194) | (41, 97) | (83) | (72, 73, 154, 191) | |
0, no effects; ↑, activated/increased; ↓, reduced/inhibited/impaired; empty cell, not assessed. Tests: NTT, novel tank test; LDB, light-dark box test; SBT, shoaling behavior test; SCT, shuttle chamber test; SPT, social preference test; CPP, conditioned place preference test; YMT, Y-maze test.
Pharmacological profile of ibogaine is mixed, and combines serotonergic activity with glutamatergic, opioidergic and cholinergic effects.
Own unpublished observations (Kalueff et al., 2011–2012, also see Kyzar et al., 2012a).
Inverted habituation profile for ibogaine.
Behavioral sequencing assessed in the NTT by the ethograms method.
Table 3. Summary of Studies Investigating Psychopharmacological/Behavioral Profiles of Selected Hallucinogenic Substances in Adult and Larval Zebrafish Modelsa.
| larval zebrafish |
adult
zebrafish |
|||
|---|---|---|---|---|
| drugs | effects | ref | effects | ref |
| LSD | + | (66, 126) | ||
| mescaline | + | (97, 101) | ||
| psilocybin | +0 | b | ||
| MDMA | + | (100, 126) | ||
| ibogaine | + | (70) | ||
| PCP | + | (101) | ||
| ketamine | + | (158) | + | (69, 144) |
| MK-801 | + | (191−194)b | ||
| salvinorin A | + | (41, 97)b | ||
| atropine | + | (84, 153) | ||
| scopolamine | + | (72, 73, 154, 191, 195) | ||
+, reported effects; 0, no effects. Note the prevalence of adult fish models and studies, suggesting that larval paradigms may need further attention from zebrafish neuropharmacologists working with hallucinogenic substances.
Own unpublished observations (Kalueff et al., 2011–2012, also see Kyzar et al., 2012a).
In addition to adult fish, larval zebrafish are also widely used to study brain functions and disorders, becoming indispensable models for genetic research and drug discovery.79−82 The established strength of larval fish models is in their high-throughput nature, ease of genetic manipulations, and well-defined behavioral and neurological phenotypes.46,64,74 Several studies using larvae (e.g., refs (83 and 84)) have already tested hallucinogens, such as a deliriant agent atropine and a dissociative drug ketamine, in zebrafish behavioral models (Tables 2 and 3). Recognizing the growing importance of hallucinogenic drugs as key modulators and potential drug targets in biological psychiatry,7,24,85 as well as robust behavioral phenotypes of zebrafish highly sensitive to various neuropharmacological manipulations,46,78,86−88 here we discuss hallucinogenic-induced behaviors in zebrafish, and evaluate their potential as preclinical models of such effects in humans.
Modeling Hallucinogenic Drug Action in Zebrafish
As already mentioned, hallucinogenic drugs potently affect human perception, emotionality, and cognitive functions.9,10 Table 2 summarizes current data on responses to major hallucinogenic drugs in zebrafish, revealing both common and unique drug-induced phenotypes in various “key” neurobehavioral domains: locomotor, affective, cognitive, social, and neurological phenotypes. Behavioral experiments to assess these domains in zebrafish typically involve manual observations with computer-aided video-tracking in the novel open arenas (e.g., the novel tank test, NTT or the light-dark box test, LDB), exposure to various cognitive tasks (e.g., the Y-maze test, YMT), as well as assessing individual or group-tested fish (e.g., in the shoaling behavior test, SBT); see refs (89−95) for review of zebrafish behavioral models and testing approaches. Despite the growing utility of zebrafish in neuroscience research, developing aquatic models of hallucinogenic drugs is a challenging task, and may benefit from a detailed analysis of several major neurobehavioral domains.
Motor Activity
In-depth evaluation of locomotor effects of various psychotropic drugs in animal models is a key question in drug screening studies. Analyses of zebrafish locomotor responses shows that some serotonergic (e.g., LSD, mescaline and MDMA) and glutamatergic (e.g., PCP and ketamine) drugs do not significantly alter the “main” zebrafish motor activity indices, such as distance traveled and swimming velocity (Table 2). While glutamatergic agents PCP and ketamine (similarly to the serotonergic agents) did not affect zebrafish locomotion, treatment with MK-801 increased both velocity and distance traveled, showing the hyperactivity that may be attributed to a dose-dependent response, similar to that observed in mice following MK-801 administration.96 Ibogaine-treated zebrafish exhibited an increase in velocity but unaltered distance traveled, illustrating a mild increase in motor activity. Salvinorin A also elevated motor activity by increasing distance traveled, while its effects on velocity were not reported (also see the lack of effects of various acute doses in Table 2, as assessed in ref (97) and our own unpublished studies). Likewise, a deliriant scopolamine had no effects on distance traveled and velocity in zebrafish (Table 2). At the same time, larval zebrafish locomotor data are mostly lacking for main classes of hallucinogenic drugs, representing an essential knowledge gap to be filled by future studies.
Collectively, our analysis shows that serotonergic, glutamatergic, opioidergic, and anticholinergic drugs either do not affect, or only mildly alter, zebrafish motor activity (Table 2). This suggests that despite being under the influence of hallucinogenic agents (as assessed by other overt behavioral effects of these drugs), motor activity remains mainly intact in zebrafish models following exposure to different classes of hallucinogenic drugs. This observation may be particularly interesting because it is in line with the well-known Hollister’s criteria of hallucinogenic drugs as mind-altering agents without major effects on locomotion.98 Thus, zebrafish appear to show a similar pattern of responsivity to locomotor action of hallucinogens, suggesting the utility of these fish as a relevant translational model of drug action.
Anxiety-Related Behavior
Affective action of various hallucinogenic drugs has long been reported in clinical and preclinical literature.14,23,36,99 Robust anxiety-related behaviors have also been well-established in zebrafish, complementing other important phenotypes, such as social behavior, cognitive function, and reward-related responses89−95 (also see ref (64) for a comprehensive catalog of zebrafish anxiety-related behaviors). Analysis of the effects of hallucinogenic drugs on zebrafish anxiety-like behavior was based on several key phenotypes assessed in two common and highly sensitive aquatic models of anxiety, the NTT and LDB tests (Table 2). Overall, zebrafish treated with behaviorally active doses of LSD display predominantly anxiolytic-like responses, including increased time spent in top half of the tank, more transitions to the top, shorter latency to the top, as well as fewer freezing bouts and lower freezing duration. The increased exploration of the top portion of the tank observed and the greatly reduced freezing behaviors suggest LSD-induced anxiolytic-like state. Similarly, mescaline also evoked anxiolytic-like behavior in zebrafish (although not as pronounced as LSD), causing increased transitions to the top, increased time spent in the top, and decreased latency to the top (Table 2). While no changes were observed in erratic movements (similar to the effects of LSD), treatment with mescaline yielded no change in freezing bouts and freezing duration, collectively suggesting that mescaline at doses tested does possesses anxiolytic properties, but not at the same magnitude as does LSD.
Like LSD and mescaline, MDMA also reduced anxiety-like behaviors in zebrafish, increasing time in top and reduced latency to top (Table 2; ref (100)). However, transitions to the top were also reduced in a dose-dependent manner, likely due to a global increase in top dwelling, as fish spent more time in top and displayed fewer visits to the bottom part of the tank. Interestingly, while LSD and mescaline produced no change in erratic movements (Table 2), MDMA-treated zebrafish exhibited fewer erratic movements, another behavior consistent with lowered anxiety.100 As with mescaline, administration of MDMA produced no overt changes in freezing frequency or duration. Overall, albeit showing varying degrees of behavioral effects, the serotonergic hallucinogenic agents tested (Table 2) appear to reduce anxiety in zebrafish without inducing anxiogenic-like or overt psychostimulant effects,66,100,101 the observation that appears to be consistent with the known profile of these compounds in humans and rodents (Table 1).
Among the glutamatergic hallucinogenic agents assessed in zebrafish, ketamine produced strong anxiolytic-like action, including a marked increase in time spent in the top part of the tank, reduced latency to the top as well as unaltered erratic movement, freezing bouts, and duration (Table 2). Ketamine treatment, however, did reduce transitions to the top (which, together with increased time spent in the top, may be attributed to the tranquilizing effect of the drug). Treatment with PCP did not alter time in top, transitions to the top, freezing bouts and freezing duration, but evoked anxiolytic-like decreased latency to the top (also increasing erratic movements). The effects of MK-801, PCP, and ketamine on anxiety-like behavior seem to be in line with clinical and rodent literature that generally shows anxiolytic-like effects evoked by NMDA antagonism;102−115 also see our recent data on anxiolytic-like profile in zebrafish evoked by another NMDA antagonist, kynurenic acid.116 Interestingly, while ibogaine-treated zebrafish demonstrated no significant changes in time in the top, transitions to the top, and freezing duration, the drug elicited an anxiolytic-like phenotype in zebrafish by decreasing latency to the top (also note increased erratic movement and freezing bouts70). Since ibogaine possesses “mixed” serotonergic and glutamatergic properties, this observation is generally consistent with behavioral effects in zebrafish exposed to other serotonergic and glutamatergic drugs discussed above (Table 2).
The effect of salvinorin A on anxiety-like behavior merits further in-depth investigation using this aquatic model and a wide range of acute and chronic doses. Interestingly, despite prominent acute effects on humans,37 our pilot studies with a wide dose range of this compound failed to detect consistent anxiety-related effects (ref (97); Kalueff et al., 2009–2012 unpublished observations), suggesting that in vivo screening using a wider spectrum of tests and paradigms may be needed to address complex psychopharmacology of this hallucinogenic agent. To the best of our knowledge, there was only one published study on the effects of deliriants (scopolamine) in adult zebrafish, showing no effects on time in the top sector (Table 2). Likewise, larval zebrafish anxiety-related data are currently lacking all four main classes of hallucinogenic drugs, representing other knowledge gaps meriting future studies.
Overall, our analyses of zebrafish phenotypes (Table 2) reveal strong effects of various hallucinogenic drugs on zebrafish affective-related parameters. These observations may be particularly in line with the well-accepted Hollister’s criteria of hallucinogenic drugs as agents strongly affecting mind/thought, perception, and mood/affect.98 From this point of view, zebrafish appear to show a significant translational validity as a model of hallucinogenic drugs’ action on affective behavior.
Social Behavior
In both humans and animals, social behaviors are strongly affected by various hallucinogenic drugs.117−121 For example, social withdrawal is commonly seen in response to hallucinogenic agents in both primate43 and rodent tests.122 Since zebrafish are highly social animals, they may be a good model organism to study the effects of various experimental manipulations in the domain of social behavior.123−126 Thus, an in-depth evaluation of social behaviors, empowered by the newest developments in automated neurophenotyping technology (e.g., monitoring fish shoals),126 becomes an important direction of research using zebrafish models and tests to study hallucinogenic drugs.
Analysis of zebrafish social behaviors summarized in Table 2 shows that serotonergic agent LSD (while not affecting social preference) markedly decreases shoaling, which may reflect reduced social motivation and anxiety, or altered perception of the environment. For example, although decreased shoaling may parallel the drug’s anxiolytic effects described previously, changes in visual perception (e.g., of neighboring zebrafish) may also contribute to reduced shoaling behavior observed, requiring further investigation to examine sensory function in LSD-treated fish. Interestingly, zebrafish observed after acute treatment with mescaline showed increased shoaling, which may be interpreted as increased anxiety. However, given the observed anxiolytic-like effects of this drug in zebrafish in NTT, the increased shoaling suggests that mescaline may, in fact, stimulate social behavior. The effect of mescaline on social preference (e.g., in the three-chamber social preference test, SPT) has not yet been investigated, and may shed light on whether mescaline can promote sociability in zebrafish. An alternative explanation, albeit based on indirect evidence and qualitative observation of zebrafish behavior, may involve “energizing” effects of mescaline (Kalueff et al., 2011–2013, own unpublished observation), which can contribute to higher probability of individual fish running into each other when assessed together in a relatively small and narrow SBT tank. Analyses of the effect of MDMA on shoaling behavior126 reveal a marked reduction of shoal cohesion, resembling the behavioral effects previously reported for LSD.66 While the latter effect strikingly differs from well-known pro-social “empathogenic” action of MDMA in humans,127−129 it resembles the lack of consistent effects of MDMA on social behavior in rodents,130−135 raising the possibility that the empathogenic action of MDMA may represent a species-specific human phenotype, which is difficult to mimic in animal models. For example, given the role of oxytocin on facilitating the pro-social effects of MDMA in several species, including humans,136−138 differing degrees of the hormone’s functional roles among fish139,140 vs mammals141−143 may account for some discrepancies.
As shown in Table 2, treatment with PCP evokes no changes in zebrafish shoaling,101 while its effect on social preference has not yet been tested. Ketamine showed conflicting effects on social behavior, impairing shoaling but increasing social preference.144 While reduced shoaling may indicate lower sociability, increased social preference suggests that ketamine may affect social behavior or, alternatively, increase motor activity in the preference test. However, MK-801 impairs both social preference and shoaling, implying a reduction in sociability of zebrafish (Table 2), which parallels rodent data on social withdrawal induced by acute administration of this drug.145,146
Zebrafish exposed to behaviorally active doses of ibogaine show effects similar to those produced by LSD, including unaltered social preference but markedly reduced shoaling.70 To the best of our knowledge, there are no published studies on the effects of salvinorin A and deliriants on adult zebrafish shoaling (Tables 2 and 3), representing an aspect to be addressed further. However, the frequently occurring decrease in zebrafish shoaling produced by both serotonergic (LSD, MDMA, ibogaine) and glutamatergic (ketamine, PCP, MK-801, ibogaine) agents, coupled with unclear effects on social preference, may reflect a global change in social behavior and/or sensory perception of environment and conspecifics, clearly warranting further investigation in zebrafish aquatic tests.
Notably, while social preference tests have long been developed in rodents,147 shoaling represents a zebrafish-specific behavior that is difficult to adapt to rodent tests. Indeed, adult zebrafish shoaling is not only sensitive to various psychotropic drugs124,125,148 and can be easily performed in automated high-throughput manner,126 but is also highly relevant to social “group” behaviors likely to be affected by hallucinogenic drugs in humans (which, like zebrafish, are highly social species). Given the growing importance of social context in hallucinogenic drug use and abuse,129,149 the latter aspect can be of particular interest to assess using zebrafish models. Overall, the prevalence of a disruption of shoaling behaviors in zebrafish exposed to acute doses of hallucinogenic drugs (Table 2) suggests that the social domain is affected by hallucinogenic drugs in zebrafish, similar to other species, including humans. Thus, zebrafish models appear to show some translational validity as a potential model of hallucinogenic drugs’ action on social behavior.
Cognitive Function
Overall, zebrafish possess excellent cognitive phenotypes, as assessed in various experimental models adapted from rodent cognitive tasks.14,74,89,150−152 The effects of hallucinogenic drugs on zebrafish cognition have not been extensively investigated, and caution is needed in addressing this aspect, until additional tests are conducted on more specific and/or complex cognitive tasks. However, several studies using hallucinogenic agents have indirectly assessed cognitive phenotypes in this species. For example, LSD and MDMA both reduced intrasession habituation in adult zebrafish, suggesting their altered spatial working memory (Table 2). In line with this, ibogaine reversed normal habituation responses in zebrafish.70 The anticholinergic agent atropine had no effects on habituation in larval zebrafish,153 scopolamine impaired startle habituation,154 and the effects of several glutamatergic agents and salvinorin A have not yet been tested. The latter aspect may be particularly interesting to assess in future studies, given rodent evidence that some agents, such as glutamatergic antagonists, can impair reference and working memory in rats.155
Notably, ibogaine produced a dramatic increase in homebase formation, raising the possibility of altered spatial memory and/or awareness in zebrafish (note, however, the lack of published data on homebase formation produced by other common hallucinogenic agents; Table 2). Furthermore, as already mentioned, data are scarce or lacking on the effects of major hallucinogenic drugs in more specialized memory or learning tests in adult or larval zebrafish (Table 2), meriting future studies. Likewise, zebrafish evidence is lacking on other critical cognitive tests highly relevant to hallucinogenic drug action, such as prepulse inhibition of startle, time perception tasks and impulsivity/response inhibition (see ref (14) for a detailed review of rodent cognitive paradigms in hallucinogenic research). Given robust startle responses reported in both adult and larval zebrafish,153,156 the possibility of modeling such responses in specific novel zebrafish cognitive tasks becomes particularly promising. Taken together, data on zebrafish exposed to acute doses of hallucinogenic drugs (Table 2) suggest that cognitive domain is not most strongly impaired by hallucinogens in zebrafish, which is in line with Hollister’s criteria of hallucinogenic drugs as agents potently altering mind, perception, and affect, but not human intelligence or memory.98
Neurological and Reward-Related Phenotypes
In addition to motor, affective, social, and cognitive behaviors, zebrafish neurological and reward-related phenotypes are highly relevant to hallucinogenic drug action and merit further scrutiny. For example, while serotonergic drugs do not generally evoke circling behavior in adult zebrafish, glutamatergic NMDA antagonists ketamine, MK-801, and PCP all induce overt circling in both zebrafish and rodent models (see refs (71, 101, and 157) for details). Although ibogaine was not active on zebrafish circling,70 salvinorin A was reported to produce circling-like behaviors,41 suggesting that opiodiergic mechanisms may also be involved in hallucinogenic-induced circling behavior in zebrafish models. There are almost no published studies on the effects of the main classes of hallucinogenic drugs on circling behavior in larval zebrafish, necessitating future testing. The only study screening ketamine effects in larval zebrafish,158 however, did report aberrant circling-like (looplike) swimming, further paralleling adult fish findings discussed above. Given the similarity of observed drug-induced “circling” behavior across various species (see ref (70) for discussion), zebrafish appear to mimic specific neurological phenotypes evoked by selected hallucinogenic drugs in various nonfish studies.
While some potent hallucinogenic drugs (e.g., LSD, MDMA) have relatively low addictive potential, others (e.g., ketamine, salvinorin A) are frequently abused, justifying the inclusion of most hallucinogenic agents in the control substance lists worldwide.24 Regardless of their abuse potential, virtually all hallucinogenic drugs evoke reward-like action in both human and animal subjects.9,159 Recently, the reward properties of salvinorin A have been reported in zebrafish,41 generally paralleling clinical and rodent data on this drug of abuse.37,160,161 However, there are no currently available data on the reward effects of any of other classes of hallucinogenic drugs in either adult or larval zebrafish, representing the critical area for future extensive investigation. Similarly, zebrafish data are lacking on other critical reward-related tests relevant to hallucinogenic action, such as drug discrimination and fixed-ratio reinforcement (see ref (14) for detailed review of rodent literature). Therefore, given the well-established zebrafish reward neural pathways,77,162 the possibility of developing novel aquatic models to target such phenotypes (and their modulation by hallucinogens) becomes timely.
Physiological Biomarkers
In addition to behavioral responses to hallucinogens in zebrafish models, it is critical to link the observed drug-induced phenotypes with other well-validated physiological biomarkers, such as brain c-fos and whole-body cortisol,46,66,70 relevant to the effects of hallucinogenic drugs. As shown in Table 2, selected hallucinogens drugs affect both c-fos and cortisol levels in adult zebrafish. Notably, altered c-fos brain expression and cortisol/corticosterone levels have been reported in various mammalian models following hallucinogenic drugs, including LSD,163,164 MDMA,165,166 ketamine,167,168 and PCP.113,169 Zebrafish display a well-developed neuroendocrine system74,170 and show high sensitivity of their CNS proto-oncogene expression to various experimental manipulations.87,144,171 From this point of view, the sensitivity of zebrafish physiological biomarkers to hallucinogenic drugs (Table 2) supports the utility of such aquatic models to parallel behavioral phenotypes with physiological responses.
Critical Synthesis: The Lessons from Zebrafish Models
Like any experimental model, zebrafish have both strengths and limitations, some of which have already been discussed, and others will be addressed further. What “big” lessons can be learned from zebrafish models of hallucinogenic drug action? First, zebrafish appear to be highly sensitive to hallucinogenic drugs (Table 2), demonstrating striking parallels with humans in relative behavioral efficacy of various hallucinogens (see refs (66−73) for details). Taken together, this suggests a high predictive validity of zebrafish models for this area of research. Second, zebrafish show behavioral and physiological responses that parallel rodent and human findings in important, clinically relevant neurobehavioral domains (such as locomotor, affective, cognitive, social and reward; Tables 2 and 3), thereby suggesting high construct and face validity for hallucinogenic drug research. Third, there are methodological and conceptual problems with using animal models to study complex drug-evoked diseases in humans. For example, there is a growing research need in using traditional (e.g., rodent) experimental models to address these challenges. However, given the complexity of zebrafish behavior, aquatic models discussed here seem to provide valuable exploratory insights into complex behavioral and physiological phenotypes underlying hallucinogenic drug action. We also recognize the limitations on time and efficacy imposed by the complex and multifaceted nature of zebrafish paradigms. At the same time, as advanced video-analysis software and detailed zebrafish ethograms become available, research laboratories are now able to rapidly obtain reliable behavioral data, supplemented with video capture and video tracking, and followed by a multistep assays of physiological biomarkers.63 Several other potential problems exist. For example, the dissection between hallucinogenic behaviors per se and additional domains (likely to be affected by these drugs, e.g., anxiety, activity, and cognition) requires a thorough investigation using more specific behavioral tests. Thus, a proper selection of zebrafish behavioral end points, as well as applying additional tools (e.g., movement pattern analyses) and pharmacological agents (e.g., anxiolytics or antipsychotics), may help such dissection. For example, several antipsychotics showed attenuation of drug-evoked responses in zebrafish,172−175 suggesting their utility for antipsychotic drug discovery and modeling hallucinogenic-induced psychoses. Furthermore, as polymodal dose-dependent responses and nonspecific sedation are commonly seen for various drugs of abuse, testing a wide spectrum of doses may be needed to fully explore complex drug-evoked behaviors in zebrafish.
Recent advances in molecular biology of zebrafish176−178 enable precise mapping of neural pathways activated in zebrafish during selected behaviors, and similar neurophysiological approaches can be applied to the effects of various hallucinogenic drugs. Furthermore, in several assays, treatment with hallucinogens was also accompanied by parasympathetic responses (e.g., altered heart rate84), strongly supporting the utility of zebrafish models to study additional physiological biomarkers of hallucinogenic drug action, paralleling their strong behavioral effects discussed above in detail. Likewise, gene transcriptomic analyses in relation to hallucinogenic drug action (similar to those already successfully applied to other drugs of abuse; e.g., refs (77 and 179)) can enable a better dissection of common (vs drug-specific) gene expression profiles evoked by these agents. Application of receptorome and other omics-based approaches46 to zebrafish may further characterize the diverse patterns of hallucinogenic drugs action, as well as drug interactions, pharmacogenetic mechanisms, and toxicities. In line with this, using transgenic larval zebrafish as biosensors for hallucinogenic drugs’ behavioral and genomic effects158 further supports promising applications of zebrafish-based screens discussed here.
Future Directions: Where Next?
Several existing knowledge gaps in zebrafish responses to hallucinogenic drugs have already been discussed above. Along other available experimental models in biomedicine, the use of zebrafish tests and screens becomes an invaluable tool in studying neurobehavioral disorders. Considering multiple research-driven limitations in this field, one of the main challenges is the development of satisfactory animal models of psychiatric disorders with a high construct, predictive, face and population validity.14,17,44,180,181 This also coincides with another challenge, a global reduction of research budgets worldwide, necessitating in better and lower-cost options for in vivo drug screening. From this practical point of view, larval and adult zebrafish models offer excellent opportunities to foster innovative high-throughput drug discovery, yet preserving the benefits of utilizing vertebrate model species for such screening. Furthermore, given the ease of genetic manipulation in the species sharing 80–85% homology with humans, the possibility of developing pharmacogenetic zebrafish models for hallucinogenic drug action becomes another promising and feasible direction of research. Finally, although many models can quantify the behavioral responses induced by psychotropic drugs, there is a growing need in molecular biomarkers of hallucinogenic action on various neurotransmitter pathways, including serotonin, dopamine, glutamate, cannabinoid, opioid, and acetylcholine receptors.14,21 The effects of hallucinogens on these signaling pathways and receptors are only partially understood, and future research is required to establish the precise neuronal signaling mechanism underlying hallucinogenic drug action.14
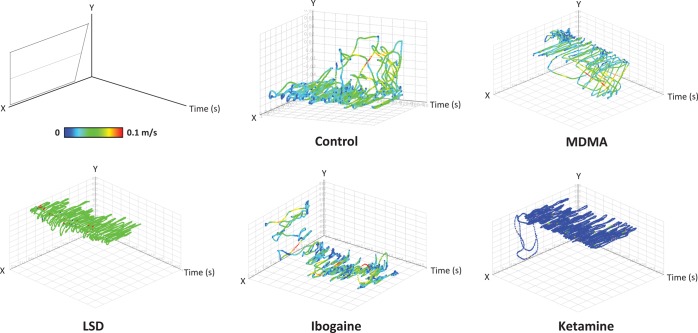
Discussing the advantages of zebrafish neurobehavioral models, we should also note that zebrafish locomotion (unlike rodent paradigms) occurs in both horizontal (X, Z) and vertical (Y) dimensions, involving swimming in a truly three-dimensional (3D) space. Can we capitalize on the rich neurobehavioral data offered by the 3D nature of zebrafish locomotion? Recently, we have introduced a novel automated neurophenotyping methodology based on analyses of 3D reconstructions of swimming paths, and have applied and validated this method for various experimental manipulations.63 These studies have developed detailed 3D-based approaches to phenotyping of zebrafish motor and anxiety-related behaviors, offering an innovative high-throughput data-dense methodology for automated visualization and quantification of fish swimming activity in both X, Y, Z (spatial) and X, Y, Time (spatiotemporal) coordinates. The use of 3D reconstruction of movement patterns (see Figure 1 for example) to study hallucinogenic drugs66,70 enables a more precise deconstruction of zebrafish behavior affected by various agents. As rodent models used in neurobehavioral research are mainly based on 2D movement, zebrafish paradigms offer an increased dimensionality of behavioral phenotyping. Therefore, our growing understanding of zebrafish 3D behavior enhances neurobehavioral research using these models,63 including their application to studying hallucinogenic drug action (see Figure 1 for examples) and targeting several key, translationally relevant domains (Table 4).
Figure 1.
Three-dimensional (3D) spatiotemporal reconstructions (in X, Y, Time coordinates) of adult zebrafish swim paths63 reveal marked phenotypical differences between representative hallucinogenic drugs, including LSD (250 ug/L), ketamine (20 mg/L), ibogaine (20 mg/L), MDMA (120 mg/L) vs control (drug-free) fish. Following acute 20-min exposure, zebrafish novel tank behavior was video-tracked using EthoVision XT7-8.5 program, and raw tracks were processed, formatted, and visualized in a 3D scatter plot (X, Y, Time) using RapidMiner 5.0 software, according to ref (63). Representative 3D reconstructions were selected on a consensus basis by 2–3 independent highly trained observers, comparing swim paths of all subjects within a cohort, ranking them from 1 to n based on similarity to each other (no/low to high activity) and choosing the middle for the illustrations. For a more detailed analysis of 3D reconstructions, the average velocity (m/s) of each fish was reflected by the changes in color (from blue, green, yellow, to red) as the velocity increases.63 In particular, LSD and ketamine evoked a clear top dwelling, with ketamine exposed fish also exhibiting some decrease in velocity relative to controls. Similarly, MDMA also reduced anxiety-like behaviors in zebrafish, increasing time in top and reduced latency to top. Ibogaine-treated fish demonstrated a mild increase in motor activity, and a reversal of their natural diving response (geotaxis), inducing initial top swimming followed by bottom dwelling. Overall, these 3D traces reveal overt behavioral effects on zebrafish exposed to different hallucinogenic agents, thereby enabling a rapid visualization and interpretation of the observed drug-induced phenotypes (see ref (63) for details).
Table 4. Summary of Major Zebrafish Neurobehavioral Domains That Parallel Targeted Human Brain Disorders Relevant to Hallucinogenic Drug Actiona.
| domain | relevant human brain disorder | currently available zebrafish models | ref |
|---|---|---|---|
| motor activity | psychoses, ADHD | drug-induced hyperlocomotion, antipsychotic drug action, genetic models of ADHD, motor retardation | (174, 175, 196) |
| mood | depression | chronic unpredictable stress, anhedonic behavior, motor retardation, genetic models of depression, antidepressant action | (152, 197)b |
| anxiety | anxiety | reduced exploration, increased anxiety-like responses | (66, 100, 144) |
| social behavior | social withdrawal | reduced shoaling behavior | (123, 126, 198) |
| cognition | cognitive deficits | affected habituation, memory performance, altered homebase formation | (191, 192, 199) |
| neurological phenotypes | behavioral stereotypies, OCD | stereotypic circling | (144, 194) |
| reward | addiction | conditioned place preference (CPP), drug discrimination, genetic models of reward | (77, 200, 201) |
ADHD, attention deficit/hyperactivity disorder; OCD, obsessive-compulsive disorder.
Own unpublished observations (Kyzar et al., 2012b).
Concluding Remarks
We have previously discussed the unique advantage of zebrafish neurobehavioral research based on the availability of both larval and adult zebrafish models.46 Mounting evidence, summarized in Tables 3 and 4, indicates that both these models can be sensitive to various hallucinogenic drugs, and may therefore have the potential to foster further high-throughput drug discovery, neurodevelopmental studies, and substance abuse research using zebrafish.46,86 In summary, this report outlines the rationale, current successes, and conceptual framework for developing novel efficient models of hallucinogenic drug action in zebrafish. The growing utility of such aquatic models to study hallucinogen-induced phenotypes will foster future experimental studies in this field, markedly enhancing both mechanistically driven top-down translational research, as well as bottom-up high-throughput screening for novel biomarkers and drug targets.
Acknowledgments
The authors thank Marion Fossum, BS for his help with this study. Assistance of Jonathan M. Cachat, Ph.D., Michael Gebhardt, B.S., and Kyle S.L. Robinson, M.S. with generating 3D images for selected hallucinogenic drugs (Figure 1) is also acknowledged. Selected pilot data mentioned in this study were presented as abstracts at the following meetings: Kalueff et al. (2012), Novel experimental models of hallucinogenic drug action, anxiety and depression (17th International Neuroscience Stress and Behavior Conference, St. Petersburg, Russia); Kyzar et al. (2012a), Effects of the hallucinogenic drug psilocybin on zebrafish behavior and physiology (Behavior, Biology, and Chemistry: Translational Research in Addiction Conference, San Antonio, TX) and Kyzar et al. (2012b), Developing zebrafish models of depression?: Effects of reserpine on zebrafish behavior and physiology (Experimental Biology EB-2012 Conference, San Diego, USA). The authors are grateful to the National Institutes of Health’s NIDA Drug Supply Program, which continues to enable them and other investigators to study various biomedical aspects of hallucinogenic drugs.
Author Contributions
N.N., A.M., and A.M.S. have contributed equally to this paper. N.N., A.M., R.A., V.G., D.K., A.M.S., M.K.P., and A.V.K. searched the literature, wrote parts of the manuscript, and participated in conceptual discussion of this study. A.M.S. searched the literature, prepared graphs, wrote, and edited the manuscript. A.V.K. coordinated the study and literature search, conceived the topic of the review, as well as wrote and edited the manuscript.
This project was partly supported by the Zebrafish Neurophenome Project (ZNP), ZNRC, the ZENEREI Institute, LA Board of Regents OPT-IN, and NIDA/NIH SOAR R03 DA030900-02 grants to A.V.K.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Abu-Asab M. S.; Chaouchi M.; Alesci S.; Galli S.; Laassri M.; Cheema A. K.; Atouf F.; Vanmeter J.; Amri H. (2011) Biomarkers in the age of omics: time for a systems biology approach. OMICS 15, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agid Y.; Buzsaki G.; Diamond D. M.; Frackowiak R.; Giedd J.; Girault J. A.; Grace A.; Lambert J. J.; Manji H.; Mayberg H.; Popoli M.; Prochiantz A.; Richter-Levin G.; Somogyi P.; Spedding M.; Svenningsson P.; Weinberger D. (2007) How can drug discovery for psychiatric disorders be improved?. Nat. Rev. Drug Discovery 6, 189–201. [DOI] [PubMed] [Google Scholar]
- van der Staay F. J.; Arndt S. S.; Nordquist R. E. (2009) Evaluation of animal models of neurobehavioral disorders. Behav. Brain Funct. 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorte J. L.; Egan R. J.; Hart P. C.; Bergner C. L.; Cachat J. M.; Canavello P. R.; Kalueff A. V. (2010) Qui non proficit, deficit: experimental models for ’integrative’ research of affective disorders. J. Affective Disord. 121, 1–9. [DOI] [PubMed] [Google Scholar]
- Bountra C.; Oppermann U.; Heightman T. D. (2011) Animal models of epigenetic regulation in neuropsychiatric disorders. Curr. Top. Behav. Neurosci. 7, 281–322. [DOI] [PubMed] [Google Scholar]
- Kas M. J.; Krishnan V.; Gould T. D.; Collier D. A.; Olivier B.; Lesch K. P.; Domenici E.; Fuchs E.; Gross C.; Castren E. (2011) Advances in multidisciplinary and cross-species approaches to examine the neurobiology of psychiatric disorders. Eur. Neuropsychopharmacol. 21, 532–544. [DOI] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Murnane K. S.; Reissig C. J. (2008) The behavioral pharmacology of hallucinogens. Biochem. Pharmacol. 75, 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M. A. (1998) Why Study Hallucinogenic Drugs in Animal?. Heffter Rev. Psychedelic Res. 1, 33–38. [Google Scholar]
- Nichols D. E. (2004) Hallucinogens. Pharmacol. Ther. 101, 131–181. [DOI] [PubMed] [Google Scholar]
- Winkelman M. (1991) Therapeutic Effects of Hallucinogens. Anthropol. Conscious. 2, 15–19. [Google Scholar]
- Moghaddam B.; Krystal J. H. (2012) Capturing the angel in “angel dust”: Twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr. Bull. 38, 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. S. (2010) Psychedelics and the human receptorome. PLoS One 5, e9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitler M.; Leonhardt S.; Appel N. M.; De Souza E. B.; Glennon R. A. (1990) Receptor pharmacology of MDMA and related hallucinogens. Ann. N.Y. Acad. Sci. 600, 626–638discussion 638–629.. [DOI] [PubMed] [Google Scholar]
- Hanks J. B.; Gonzalez-Maeso J. (2013) Animal models of serotonergic psychedelics. ACS Chem. Neurosci. 4, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. J.; McFie L.; Fleetwood H.; Robinson J. A. (2002) Ecstasy (MDMA): are the psychological problems associated with its use reversed by prolonged abstinence?. Psychopharmacology (Berlin, Ger.) 159, 294–303. [DOI] [PubMed] [Google Scholar]
- Ratzenboeck E.; Saria A.; Kriechbaum N.; Zernig G. (2001) Reinforcing effects of MDMA (″ecstasy″) in drug-naive and cocaine-trained rats. Pharmacology 62, 138–144. [DOI] [PubMed] [Google Scholar]
- Geyer M. A., and Moghaddam B. (2002) Animal models relevant to schizophrenia disorders In Neuropsychopharmacology: The Fifth Generation of Progress (Davis K. L., Charney D., Coyle J. T., and Nemeroff C., Eds.), pp 689–701, Lippincott Williams & Wilkins, Philidelphia. [Google Scholar]
- Houenou J.; Homri W.; Leboyer M.; Drancourt N. (2011) Ibogaine-associated psychosis in schizophrenia: a case report. J. Clin. Psychopharmacol. 31, 659. [DOI] [PubMed] [Google Scholar]
- Thomasius R.; Zapletalova P.; Petersen K.; Buchert R.; Andresen B.; Wartberg L.; Nebeling B.; Schmoldt A. (2006) Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. J. Psychopharmacol. 20, 211–225. [DOI] [PubMed] [Google Scholar]
- Eveloff H. H. (1968) The LSD syndrome. A review. Calif. Med. 109, 368–373. [PMC free article] [PubMed] [Google Scholar]
- Geyer M. A.; Vollenweider F. X. (2008) Serotonin research: contributions to understanding psychoses. Trends Pharmacol. Sci. 29, 445–453. [DOI] [PubMed] [Google Scholar]
- McCann U. D.; Eligulashvili V.; Ricaurte G. A. (2000) (±)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: clinical studies. Neuropsychobiology 42, 11–16. [DOI] [PubMed] [Google Scholar]
- Adams L. M.; Geyer M. A. (1985) A proposed animal model for hallucinogens based on LSD’s effects on patterns of exploration in rats. Behav. Neurosci. 99, 881–900. [DOI] [PubMed] [Google Scholar]
- Nutt D. J.; King L. A.; Nichols D. E. (2013) Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nat. Rev. Neurosci. 14, 577–585. [DOI] [PubMed] [Google Scholar]
- Moreno F. A.; Wiegand C. B.; Taitano E. K.; Delgado P. L. (2006) Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J. Clin. Psychiatry 67, 1735–1740. [DOI] [PubMed] [Google Scholar]
- Zghoul T.; Blier P. (2003) Enhancing action of LSD on neuronal responsiveness to serotonin in a brain structure involved in obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 6, 13–21. [DOI] [PubMed] [Google Scholar]
- Doblin R. (2002) A clinical plan for MDMA (Ecstasy) in the treatment of posttraumatic stress disorder (PTSD): partnering with the FDA. J. Psychoact. Drugs 34, 185–194. [DOI] [PubMed] [Google Scholar]
- Mithoefer M. C.; Wagner M. T.; Mithoefer A. T.; Jerome L.; Doblin R. (2011) The safety and efficacy of {±}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J. Psychopharmacol. 25, 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R. M.; Cappiello A.; Anand A.; Oren D. A.; Heninger G. R.; Charney D. S.; Krystal J. H. (2000) Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354. [DOI] [PubMed] [Google Scholar]
- Kavalali E. T.; Monteggia L. M. (2012) Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am. J. Psychiatry 169, 1150–1156. [DOI] [PubMed] [Google Scholar]
- Majumder I.; White J. M.; Irvine R. J. (2012) Antidepressant-like effects of ecstasy in subjects with a predisposition to depression. Addict. Behav. 37, 1189–1192. [DOI] [PubMed] [Google Scholar]
- Parrott A. C. (2007) The psychotherapeutic potential of MDMA (3,4-methylenedioxymethamphetamine): an evidence-based review. Psychopharmacology (Berlin, Ger.) 191, 181–193. [DOI] [PubMed] [Google Scholar]
- Smith K. M.; Larive L. L.; Romanelli F. (2002) Club drugs: methylenedioxymethamphetamine, flunitrazepam, ketamine hydrochloride, and gamma-hydroxybutyrate. Am. J Health Syst. Pharm. 59, 1067–1076. [DOI] [PubMed] [Google Scholar]
- Miller F. P., Vandome A. F., and McBrewster J., Eds. (2009) Psychedelics, Dissociatives and Deliriants, Alphascript Publishing, Saarbrücken. [Google Scholar]
- Verheyden S. L.; Henry J. A.; Curran H. V. (2003) Acute, sub-acute and long-term subjective consequences of ‘ecstasy’ (MDMA) consumption in 430 regular users. Hum. Psychopharmacol. 18, 507–517. [DOI] [PubMed] [Google Scholar]
- Vollenweider F. X.; Gamma A.; Liechti M.; Huber T. (1998) Psychological and cardiovascular effects and short-term sequelae of MDMA (“ecstasy”) in MDMA-naive healthy volunteers. Neuropsychopharmacology 19, 241–251. [DOI] [PubMed] [Google Scholar]
- Babu K. M.; McCurdy C. R.; Boyer E. W. (2008) Opioid receptors and legal highs: Salvia divinorum and Kratom. Clin. Toxicol. 46, 146–152. [DOI] [PubMed] [Google Scholar]
- Roth B. L.; Baner K.; Westkaemper R.; Siebert D.; Rice K. C.; Steinberg S.; Ernsberger P.; Rothman R. B. (2002) Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc. Natl. Acad. Sci. U.S.A. 99, 11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowry M.; Mosher M.; Briner W. (2003) Acute physiologic and chronic histologic changes in rats and mice exposed to the unique hallucinogen salvinorin A. J. Psychoact. Drugs 35, 379–382. [DOI] [PubMed] [Google Scholar]
- Seeman P.; Guan H. C.; Hirbec H. (2009) Dopamine D2High receptors stimulated by phencyclidines, lysergic acid diethylamide, salvinorin A, and modafinil. Synapse 63, 698–704. [DOI] [PubMed] [Google Scholar]
- Braida D.; Limonta V.; Pegorini S.; Zani A.; Guerini-Rocco C.; Gori E.; Sala M. (2007) Hallucinatory and rewarding effect of salvinorin A in zebrafish: kappa-opioid and CB1-cannabinoid receptor involvement. Psychopharmacology (Berlin, Ger.) 190, 441–448. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J.; Weisstaub N. V.; Zhou M.; Chan P.; Ivic L.; Ang R.; Lira A.; Bradley-Moore M.; Ge Y.; Zhou Q.; Sealfon S. C.; Gingrich J. A. (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53, 439–452. [DOI] [PubMed] [Google Scholar]
- Schlemmer R. F. Jr.; Davis J. M. (1986) A primate model for the study of hallucinogens. Pharmacol., Biochem. Behav. 24, 381–392. [DOI] [PubMed] [Google Scholar]
- Kalueff A. V.; Wheaton M.; Murphy D. L. (2007) What’s wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav. Brain Res. 179, 1–18. [DOI] [PubMed] [Google Scholar]
- Kas M. J.; Fernandes C.; Schalkwyk L. C.; Collier D. A. (2007) Genetics of behavioural domains across the neuropsychiatric spectrum; of mice and men. Mol. Psychiatry 12, 324–330. [DOI] [PubMed] [Google Scholar]
- Stewart A. M.; Cachat J.; Gaikwad S.; Robinson K. S.; Gebhardt M.; Kalueff A. V. (2013) Perspectives on experimental models of serotonin syndrome in zebrafish. Neurochem. Int. 62, 893–902. [DOI] [PubMed] [Google Scholar]
- Nichols C. D. (2006) Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol. Ther. 112, 677–700. [DOI] [PubMed] [Google Scholar]
- Nichols C. D.; Ronesi J.; Pratt W.; Sanders-Bush E. (2002) Hallucinogens and Drosophila: linking serotonin receptor activation to behavior. Neuroscience 115, 979–984. [DOI] [PubMed] [Google Scholar]
- Dasari S.; Viele K.; Turner A. C.; Cooper R. L. (2007) Influence of PCPA and MDMA (ecstasy) on physiology, development and behavior in Drosophila melanogaster. Eur. J. Neurosci. 26, 424–438. [DOI] [PubMed] [Google Scholar]
- Kendall D., Alexander S., and Soderstrom K. (2009) Lessons from Nonmammalian Species. In Behavioral Neurobiology of the Endocannabinoid System, pp 173–198, Springer, Berlin, Heidelberg. [Google Scholar]
- Alsop D.; Vijayan M. (2009) The zebrafish stress axis: molecular fallout from the teleost-specific genome duplication event. Gen. Comp. Endrocrinol. 161, 62–66. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nunez V.; Barrallo A.; Traynor J. R.; Rodriguez R. E. (2006) Characterization of opioid-binding sites in zebrafish brain. J. Pharmacol. Exp. Ther. 316, 900–904. [DOI] [PubMed] [Google Scholar]
- Lillesaar C. (2011) The serotonergic system in fish. J. Chem. Neuroanat. 41, 294–308. [DOI] [PubMed] [Google Scholar]
- Panula P.; Chen Y. C.; Priyadarshini M.; Kudo H.; Semenova S.; Sundvik M.; Sallinen V. (2010) The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 40, 46–57. [DOI] [PubMed] [Google Scholar]
- Panula P.; Sallinen V.; Sundvik M.; Kolehmainen J.; Torkko V.; Tiittula A.; Moshnyakov M.; Podlasz P. (2006) Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish 3, 235–247. [DOI] [PubMed] [Google Scholar]
- Sundvik M.; Panula P. (2012) The organization of the histaminergic system in adult zebrafish (Danio rerio) brain: neuron number, location and co-transmitters. J. Comp. Neurol. 520, 3287–3845. [DOI] [PubMed] [Google Scholar]
- Tay T. L.; Ronneberger O.; Ryu S.; Nitschke R.; Driever W. (2011) Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat. Commun. 2, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey U. B.; Nichols C. D. (2011) Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 63, 411–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliaeva N. F.; Kashirtseva V. N.; Medvedeva N. V.; Khudoklinova I.; Ipatova O. M.; Archakov A. I. (2010) Zebrafish as a model organism for biomedical studies. Biomed. Khim. 56, 120–131. [DOI] [PubMed] [Google Scholar]
- Cheng K. C.; Xin X.; Clark D. P.; La Riviere P. (2011) Whole-animal imaging, gene function, and the Zebrafish Phenome Project. Curr. Opin. Genet. Dev. 21, 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods I. G.; Kelly P. D.; Chu F.; Ngo-Hazelett P.; Yan Y. L.; Huang H.; Postlethwait J. H.; Talbot W. S. (2000) A comparative map of the zebrafish genome. Genome Res. 10, 1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langheinrich U. (2003) Zebrafish: a new model on the pharmaceutical catwalk. Bioessays 25, 904–912. [DOI] [PubMed] [Google Scholar]
- Cachat J.; Stewart A.; Utterback E.; Hart P.; Gaikwad S.; Wong K.; Kyzar E.; Wu N.; Kalueff A. V. (2011) Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS One 6, e17597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff A. V.; Gebhardt M.; Stewart A. M.; Cachat J.; Brimmer M.; Chawla J. S.; Craddock C.; Roth A.; Landsman S.; Gaikwad S.; Baatrup E.; Tierney K.; Shamchuk A.; Norton W.; Miller N.; Nicolson T.; Braubach O.; Gilman C. P.; Pittman J.; Rosemberg D. B.; Gerlai R.; Echevarria D.; Lamb E.; Neuhauss S. C. F.; Wang W.; Bally-Cuif L.; ZNRC (2013) Towards a comprehensive catalog of zebrafish behavior 1.0, and beyond. Zebrafish 10, 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslin J.; Panula P. (2001) Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio). J. Comp. Neurol. 440, 342–377. [DOI] [PubMed] [Google Scholar]
- Grossman L.; Utterback E.; Stewart A.; Gaikwad S.; Chung K. M.; Suciu C.; Wong K.; Elegante M.; Elkhayat S.; Tan J.; Gilder T.; Wu N.; Dileo J.; Cachat J.; Kalueff A. V. (2010) Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav. Brain Res. 214, 277–284. [DOI] [PubMed] [Google Scholar]
- Stewart A. M.; Cachat J.; Green J.; Gaikwad S.; Kyzar E.; Roth A.; Davis A.; Collins C.; El-Ounsi M.; Pham M.; Kalueff A. V. (2012) Constructing the habituome for phenotype-driven zebrafish research. Behav. Brain Res. 236C, 110–117. [DOI] [PubMed] [Google Scholar]
- Sison M.; Gerlai R. (2011) Behavioral performance altering effects of MK-801 in zebrafish (Danio rerio). Behav. Brain Res. 220, 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhary S. M.; Ayubcha D.; Ansari F.; Kamran K.; Karim M.; Leheste J. R.; Horowitz J. M.; Torres G. (2011) A behavioral and molecular analysis of ketamine in zebrafish. Synapse 65, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachat J.; Kyzar E. J.; Collins C.; Gaikwad S.; Green J.; Roth A.; El-Ounsi M.; Davis A.; Pham M.; Landsman S.; Stewart A. M.; Kalueff A. V. (2013) Unique and potent effects of acute ibogaine on zebrafish: the developing utility of novel aquatic models for hallucinogenic drug research. Behav. Brain Res. 236, 258–269. [DOI] [PubMed] [Google Scholar]
- Ewald H. S. (2009) A zebrafish model of schizophrenia and sickness behavior: MK-801 and endogenous NMDAR antagonism, p 107, University of Louisville, Louisville, KY. [Google Scholar]
- Kim Y. H.; Lee Y.; Kim D.; Jung M. W.; Lee C. J. (2010) Scopolamine-induced learning impairment reversed by physostigmine in zebrafish. Neurosci. Res. 67, 156–161. [DOI] [PubMed] [Google Scholar]
- Richetti S. K.; Blank M.; Capiotti K. M.; Piato A. L.; Bogo M. R.; Vianna M. R.; Bonan C. D. (2011) Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav. Brain Res. 217, 10–15. [DOI] [PubMed] [Google Scholar]
- Stewart A.; Kadri F.; DiLeo J.; Chung K.; Cachat J.; Goodspeed J.; Suciu C.; Roy S.; Gaikwad S.; Wong K.; Elegante M.; Elkhayat S.; Wu N.; Gilder T.; Tien D.; Kalueff A. V. (2010) The Developing Utility of Zebrafish in Modeling Neurobehavioral Disorders. Int. J. Comp. Psychol. 23, 104–121. [Google Scholar]
- Egan R. J.; Bergner C. L.; Hart P. C.; Cachat J. M.; Canavello P. R.; Elegante M. F.; Elkhayat S. I.; Bartels B. K.; Tien A. K.; Tien D. H.; Mohnot S.; Beeson E.; Glasgow E.; Amri H.; Zukowska Z.; Kalueff A. V. (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 205, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachat J. M., Canavello P. R., Elegante M. F., Bartels B. K., Elkhayat S. I., Hart P. C., Tien A. K., Tien D. H., Beeson E., Mohnot S., Laffoon A. L., Haymore W., and Kalueff A. V. (2010) Modeling Stress and Anxiety in Zebrafish. In Zebrafish Models in Neurobehavioral Research (Kalueff A. V., and Cachat J., Eds.), Humana Press, New York. [Google Scholar]
- Webb K. J.; Norton W. H.; Trumbach D.; Meijer A. H.; Ninkovic J.; Topp S.; Heck D.; Marr C.; Wurst W.; Theis F. J.; Spaink H. P.; Bally-Cuif L. (2009) Zebrafish reward mutants reveal novel transcripts mediating the behavioral effects of amphetamine. Genome Biol 10, R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A.; Wu N.; Cachat J.; Hart P.; Gaikwad S.; Wong K.; Utterback E.; Gilder T.; Kyzar E.; Newman A.; Carlos D.; Chang K.; Hook M.; Rhymes C.; Caffery M.; Greenberg M.; Zadina J.; Kalueff A. V. (2011) Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1421–1431. [DOI] [PubMed] [Google Scholar]
- van der Zwaag B.; Franke L.; Poot M.; Hochstenbach R.; Spierenburg H. A.; Vorstman J. A. S.; van Daalen E.; de Jonge M. V.; Verbeek N. E.; Brilstra E. H.; van ’t Slot R.; Ophoff R. A.; van Es M. A.; Blauw H. M.; Veldink J. H.; Buizer-Voskamp J. E.; Beemer F. A.; van den Berg L. H.; Wijmenga C.; van Amstel H. K. P.; van Engeland H.; Burbach J. P. H.; Staal W. G. (2009) Gene-Network Analysis Identifies Susceptibility Genes Related to Glycobiology in Autism. PLoS One 4, e5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihel J.; Prober D. A.; Arvanites A.; Lam K.; Zimmerman S.; Jang S.; Haggarty S. J.; Kokel D.; Rubin L. L.; Peterson R. T.; Schier A. F. (2010) Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 327, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. L. (1992) Prenatal stress exposure alters postnatal behavioral expression under conditions of novelty challenge in rhesus monkey infants. Dev. Psychobiol. 25, 529–540. [DOI] [PubMed] [Google Scholar]
- Yonan A. L.; Palmer A. A.; Smith K. C.; Feldman I.; Lee H. K.; Yonan J. M.; Fischer S. G.; Pavlidis P.; Gilliam T. C. (2003) Bioinformatic analysis of autism positional candidate genes using biological databases and computational gene network prediction. Genes Brain Behav. 2, 303–320. [DOI] [PubMed] [Google Scholar]
- Best J. D.; Alderton W. K. (2008) Zebrafish: An in vivo model for the study of neurological diseases. Neuropsychiatr. Dis. Treat. 4, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. D.; Hoyt C.; Feldman S.; Blunt L.; Raymond A.; Page-McCaw P. S. (2010) Cardiac response to startle stimuli in larval zebrafish: sympathetic and parasympathetic components. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 298, R1288–1297. [DOI] [PubMed] [Google Scholar]
- O’Connor K. A.; Roth B. L. (2005) Screening the receptorome for plant-based psychoactive compounds. Life Sci. 78, 506–511. [DOI] [PubMed] [Google Scholar]
- Stewart A.; Wong K.; Cachat J.; Gaikwad S.; Kyzar E.; Wu N.; Hart P.; Piet V.; Utterback E.; Elegante M.; Tien D.; Kalueff A. (2010) Zebrafish models to study drug abuse-related phenotypes. Rev. Neurosci. 22, 95–105. [DOI] [PubMed] [Google Scholar]
- Stewart A. M.; Desmond D.; Kyzar E.; Gaikwad S.; Roth A.; Riehl R.; Collins C.; Monnig L.; Green J.; Kalueff A. V. (2012) Perspectives of zebrafish models of epilepsy: what, how and where next?. Brain Res. Bull. 87, 135–143. [DOI] [PubMed] [Google Scholar]
- Stewart A. M.; Kalueff A. V. (2012) The developing utility of zebrafish models for cognitive enhancers research. Curr Neuropharmacol 10, 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser R.; Gerlai R. (2006) Behavioral phenotyping in zebrafish: comparison of three behavioral quantification methods. Behav. Res. Methods 38, 456–469. [DOI] [PubMed] [Google Scholar]
- Blaser R. E.; Rosemberg D. B. (2012) Measures of anxiety in zebrafish (Danio rerio): dissociation of black/white preference and novel tank test. PLoS One 7, e36931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximino C.; de Brito T. M.; da Silva Batista A. W.; Herculano A. M.; Morato S.; Gouveia A. Jr. (2010) Measuring anxiety in zebrafish: a critical review. Behav. Brain Res. 214, 157–171. [DOI] [PubMed] [Google Scholar]
- Kalueff A. V., and Cachat J. M. (2010) Zebrafish Models in Neurobehavioral Research, Humana Press, New York. [Google Scholar]
- Kalueff A. V., and Cachat J. M. (2010) Zebrafish Neurobehavioral Protocols, Humana Press, New York. [Google Scholar]
- Kalueff A. V., and Stewart A. M. (2012) Zebrafish Protocols for Neurobehavioral Research, Humana Press, New York. [Google Scholar]
- Norton W.; Bally-Cuif L. (2010) Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 11, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R. J.; Dai H.; Gui J. (1998) Effects of dizocilpine (MK-801) on motor activity and memory. Psychopharmacology (Berlin, Ger.) 137, 241–246. [DOI] [PubMed] [Google Scholar]
- Collins C. J. (2012) The zebrafish (Danio rerio) as a highly sensitive tool for screening hallucinogenic drug action: Lessons from mescaline and salvanorin A. In Neuroscience Program, Tulane University, New Orleans, LA. [Google Scholar]
- Hollister L. E. (1968) Chemical psychoses: LSD and related drugs, Thomas, Springfield. [Google Scholar]
- Maldonado E.; Navarro J. F. (2000) Effects of 3,4-methylenedioxy-methamphetamine (MDMA) on anxiety in mice tested in the light-dark box. Prog. Neuropsychopharmacol. Biol. Psychiatry 24, 463–472. [DOI] [PubMed] [Google Scholar]
- Stewart A.; Riehl R.; Wong K.; Green J.; Cosgrove J.; Vollmer K.; Kyzar E.; Hart P.; Allain A.; Cachat J.; Gaikwad S.; Hook M.; Rhymes K.; Newman A.; Utterback E.; Chang K.; Kalueff A. (2011) Behavioral effects of MDMA (“Ecstasy”) on adult zebrafish. Behav. Pharmacol. 22, 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar E. J.; Collins C.; Gaikwad S.; Green J.; Roth A.; Monnig L.; El-Ounsi M.; Davis A.; Freeman A.; Capezio N.; Stewart A. M.; Kalueff A. V. (2012) Effects of hallucinogenic agents mescaline and phencyclidine on zebrafish behavior and physiology. Prog. Neuropsychopharmacol. Biol. Psychiatry 37, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugeray A.; Launay J. M.; Callebert J.; Surget A.; Belzung C.; Barone P. R. (2011) Evidence for a key role of the peripheral kynurenine pathway in the modulation of anxiety- and depression-like behaviours in mice: focus on individual differences. Pharmacol., Biochem. Behav. 98, 161–168. [DOI] [PubMed] [Google Scholar]
- Louzada-Junior P.; Dias J. J.; Santos W. F.; Lachat J. J.; Bradford H. F.; Coutinho-Netto J. (1992) Glutamate release in experimental ischaemia of the retina: an approach using microdialysis. J. Neurochem. 59, 358–363. [DOI] [PubMed] [Google Scholar]
- Bubser M.; Keseberg U.; Notz P. K.; Schmidt W. J. (1992) Differential behavioural and neurochemical effects of competitive and non-competitive NMDA receptor antagonists in rats. Eur. J. Pharmacol. 229, 75–82. [DOI] [PubMed] [Google Scholar]
- Corbett R.; Camacho F.; Woods A. T.; Kerman L. L.; Fishkin R. J.; Brooks K.; Dunn R. W. (1995) Antipsychotic agents antagonize non-competitive N-methyl-d-aspartate antagonist-induced behaviors. Psychopharmacology (Berlin, Ger.) 120, 67–74. [DOI] [PubMed] [Google Scholar]
- Plaznik A.; Palejko W.; Nazar M.; Jessa M. (1994) Effects of Antagonists at the Nmda Receptor Complex in 2 Models of Anxiety. Eur. Neuropsychopharm. 4, 503–512. [DOI] [PubMed] [Google Scholar]
- Irwin S. A.; Iglewicz A. (2010) Oral Ketamine for the Rapid Treatment of Depression and Anxiety in Patients Receiving Hospice Care. J. Palliative Med. 13, 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. L.; Li M.; Dang X. R.; Wang Z. H.; Rao Z. R.; Wu S. X.; Li Y. Q.; Wang W. (2009) A NMDA receptor antagonist, MK-801 impairs consolidating extinction of auditory conditioned fear responses in a Pavlovian model. PloS One 4, e7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loss C. M.; Cordova S. D.; de Oliveira D. L. (2012) Ketamine reduces neuronal degeneration and anxiety levels when administered during early life-induced status epilepticus in rats. Brain Res. 1474, 110–117. [DOI] [PubMed] [Google Scholar]
- Garcia L. S. B.; Comim C. M.; Valvassori S. S.; Reus G. Z.; Stertz L.; Kapczinski F.; Gavioli E. C.; Quevedo J. (2009) Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog. Neuro-Psychopharmacol.h 33, 450–455. [DOI] [PubMed] [Google Scholar]
- Engin E.; Treit D.; Dickson C. T. (2009) Anxiolytic- and Antidepressant-Like Properties of Ketamine in Behavioral and Neurophysiological Animal Models (Vol 161, Pg 359, 2009). Neuroscience 162, 1438–1439. [DOI] [PubMed] [Google Scholar]
- Inta D.; Filipovic D.; Lima-Ojeda J. M.; Dormann C.; Pfeiffer N.; Gasparini F.; Gass P. (2012) The mGlu5 receptor antagonist MPEP activates specific stress-related brain regions and lacks neurotoxic effects of the NMDA receptor antagonist MK-801: Significance for the use as anxiolytic/antidepressant drug. Neuropharmacology 62, 2034–2039. [DOI] [PubMed] [Google Scholar]
- Turgeon S. M.; Kim D.; Pritchard M.; Salgado S.; Thaler A. (2011) The effects of phencyclidine (PCP) on anxiety-like behavior in the elevated plus maze and the light-dark exploration test are age dependent, sexually dimorphic, and task dependent. Pharmacol., Biochem. Behav. 100, 191–198. [DOI] [PubMed] [Google Scholar]
- Kehne J. H.; McCloskey T. C.; Baron B. M.; Chi E. M.; Harrison B. L.; Whitten J. P.; Palfreyman M. G. (1991) NMDA receptor complex antagonists have potential anxiolytic effects as measured with separation-induced ultrasonic vocalizations. Eur. J. Pharmacol. 193, 283–292. [DOI] [PubMed] [Google Scholar]
- Riaza Bermudo-Soriano C.; Perez-Rodriguez M. M.; Vaquero-Lorenzo C.; Baca-Garcia E. (2012) New perspectives in glutamate and anxiety. Pharmacol., Biochem. Behav. 100, 752–774. [DOI] [PubMed] [Google Scholar]
- Robinson K. S.; Stewart A. M.; Cachat J.; Landsman S.; Gebhardt M.; Kalueff A. V. (2013) Psychopharmacological effects of acute exposure to kynurenic acid (KYNA) in zebrafish. Pharmacol., Biochem. Behav. 108C, 54–60. [DOI] [PubMed] [Google Scholar]
- Bedi G.; Phan K. L.; Angstadt M.; de Wit H. (2009) Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berlin, Ger.) 207, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley K. C.; Arnold J. C.; McGregor I. S. (2005) Serotonin (1A) receptor involvement in acute 3,4-methylenedioxymethamphetamine (MDMA) facilitation of social interaction in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 648–657. [DOI] [PubMed] [Google Scholar]
- Rewerski W.; Kostowski W.; Piechocki T.; Rylski M. (1971) The effects of some hallucinogens on aggressiveness of mice and rats. I. Pharmacology 5, 314–320. [DOI] [PubMed] [Google Scholar]
- Solowij N.; Hall W.; Lee N. (1992) Recreational MDMA use in Sydney: a profile of ’Ecstacy’ users and their experiences with the drug. Br. J. Addict. 87, 1161–1172. [DOI] [PubMed] [Google Scholar]
- McCardle L.; Fishbein D. H. (1989) The self-reported effects of PCP on human aggression. Addict. Behav. 14, 465–472. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. (1999) Phencyclidine in the social interaction test: an animal model of schizophrenia with face and predictive validity. Rev. Neurosci. 10, 59–90. [DOI] [PubMed] [Google Scholar]
- Miller N.; Gerlai R. (2007) Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav. Brain Res. 184, 157–166. [DOI] [PubMed] [Google Scholar]
- Saverino C.; Gerlai R. (2008) The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav. Brain Res. 191, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N. Y.; Gerlai R. (2008) Oscillations in shoal cohesion in zebrafish (Danio rerio). Behav. Brain Res. 193, 148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J.; Collins C.; Kyzar E. J.; Pham M.; Roth A.; Gaikwad S.; Cachat J.; Stewart A. M.; Landsman S.; Grieco F.; Tegelenbosch R.; Noldus L. P.; Kalueff A. V. (2012) Automated high-throughput neurophenotyping of zebrafish social behavior. J. Neurosci. Methods 210, 266–271. [DOI] [PubMed] [Google Scholar]
- Adamson S., Ed. (1985) Through the Gateway of the Heart: Accounts of Experiences With MDMA and Other Empathogenic Substances, Four Trees Publications, San Francisco. [Google Scholar]
- Tancer M. E.; Johanson C. E. (2001) The subjective effects of MDMA and mCPP in moderate MDMA users. Drug Alcohol Depend. 65, 97–101. [DOI] [PubMed] [Google Scholar]
- Bedi G.; Hyman D.; de Wit H. (2010) Is ecstasy an “empathogen”? Effects of ±3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol. Psychiatry 68, 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens K. J.; Cornish J. L.; Hunt G. E.; McGregor I. S. (2007) Repeated weekly exposure to MDMA, methamphetamine or their combination: long-term behavioural and neurochemical effects in rats. Drug Alcohol Depend. 86, 183–190. [DOI] [PubMed] [Google Scholar]
- Daza-Losada M.; Rodriguez-Arias M.; Maldonado C.; Aguilar M. A.; Guerri C.; Minarro J. (2009) Acute behavioural and neurotoxic effects of MDMA plus cocaine in adolescent mice. Neurotoxicol. Teratol. 31, 49–59. [DOI] [PubMed] [Google Scholar]
- Maldonado E.; Navarro J. F. (2001) MDMA (“ecstasy”) exhibits an anxiogenic-like activity in social encounters between male mice. Pharmacol. Res. 44, 27–31. [DOI] [PubMed] [Google Scholar]
- Morley K. C.; McGregor I. S. (2000) (±)-3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) increases social interaction in rats. Eur. J. Pharmacol. 408, 41–49. [DOI] [PubMed] [Google Scholar]
- Navarro J. F.; Maldonado E. (1999) Behavioral profile of 3,4-methylenedioxy-methamphetamine (MDMA) in agonistic encounters between male mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 23, 327–334. [DOI] [PubMed] [Google Scholar]
- Thompson M. R.; Callaghan P. D.; Hunt G. E.; McGregor I. S. (2008) Reduced sensitivity to MDMA-induced facilitation of social behaviour in MDMA pre-exposed rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1013–1021. [DOI] [PubMed] [Google Scholar]
- Thompson M. R.; Callaghan P. D.; Hunt G. E.; Cornish J. L.; McGregor I. S. (2007) A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4-methylenedioxymethamphetamine (“ecstasy”). Neuroscience 146, 509–514. [DOI] [PubMed] [Google Scholar]
- Dumont G. J.; Sweep F. C.; van der Steen R.; Hermsen R.; Donders A. R.; Touw D. J.; van Gerven J. M.; Buitelaar J. K.; Verkes R. J. (2009) Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc. Neurosci. 4, 359–366. [DOI] [PubMed] [Google Scholar]
- Hunt G. E.; McGregor I. S.; Cornish J. L.; Callaghan P. D. (2011) MDMA-induced c-Fos expression in oxytocin-containing neurons is blocked by pretreatment with the 5-HT-1A receptor antagonist WAY 100635. Brain Res. Bull. 86, 65–73. [DOI] [PubMed] [Google Scholar]
- Braida D.; Donzelli A.; Martucci R.; Capurro V.; Busnelli M.; Chini B.; Sala M. (2012) Neurohypophyseal hormones manipulation modulate social and anxiety-related behavior in zebrafish. Psychopharmacology (Berlin, Ger.) 220, 319–330. [DOI] [PubMed] [Google Scholar]
- Godwin J.; Thompson R. (2012) Nonapeptides and social behavior in fishes. Horm. Behav. 61, 230–238. [DOI] [PubMed] [Google Scholar]
- Carter C. S.; Grippo A. J.; Pournajafi-Nazarloo H.; Ruscio M. G.; Porges S. W. (2008) Oxytocin, vasopressin and sociality. Prog. Brain Res. 170, 331–336. [DOI] [PubMed] [Google Scholar]
- Arletti R.; Benelli A.; Bertolini A. (1989) Influence of oxytocin on feeding behavior in the rat. Peptides 10, 89–93. [DOI] [PubMed] [Google Scholar]
- Camerino C. (2009) Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity 17, 980–984. [DOI] [PubMed] [Google Scholar]
- Riehl R.; Kyzar E.; Allain A.; Green J.; Hook M.; Monnig L.; Rhymes K.; Roth A.; Pham M.; Razavi R.; DiLeo J.; Gaikwad S.; Hart P.; Kalueff A. V. (2011) Behavioral and physiological effects of acute ketamine exposure in adult zebrafish. Neurotoxicol. Teratol. 33, 658–667. [DOI] [PubMed] [Google Scholar]
- Rung J. P.; Carlsson A.; Ryden Markinhuhta K.; Carlsson M. L. (2005) (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 29, 827–832. [DOI] [PubMed] [Google Scholar]
- Gururajan A.; Taylor D. A.; Malone D. T. (2012) Cannabidiol and clozapine reverse MK-801-induced deficits in social interaction and hyperactivity in Sprague-Dawley rats. J. Psychopharmacol. 26, 1317–1332. [DOI] [PubMed] [Google Scholar]
- Moy S. S.; Nadler J. J.; Perez A.; Barbaro R. P.; Johns J. M.; Magnuson T. R.; Piven J.; Crawley J. N. (2004) Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 3, 287–302. [DOI] [PubMed] [Google Scholar]
- Gerlai R.; Lee V.; Blaser R. (2006) Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio). Pharmacol., Biochem. Behav. 85, 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancer M.; Johanson C. E. (2003) Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug Alcohol Depend. 72, 33–44. [DOI] [PubMed] [Google Scholar]
- Blaser R. E.; Chadwick L.; McGinnis G. C. (2010) Behavioral measures of anxiety in zebrafish (Danio rerio). Behav. Brain Res. 208, 56–62. [DOI] [PubMed] [Google Scholar]
- Levin E. D. (2011) Zebrafish assessment of cognitive improvement and anxiolysis: filling the gap between in vitro and rodent models for drug development. Rev. Neurosci. 22, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piato A. L.; Capiotti K. M.; Tamborski A. R.; Oses J. P.; Barcellos L. J.; Bogo M. R.; Lara D. R.; Vianna M. R.; Bonan C. D. (2011) Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 561–567. [DOI] [PubMed] [Google Scholar]
- Best J. D.; Berghmans S.; Hunt J. J.; Clarke S. C.; Fleming A.; Goldsmith P.; Roach A. G. (2008) Non-associative learning in larval zebrafish. Neuropsychopharmacology 33, 1206–1215. [DOI] [PubMed] [Google Scholar]
- Levin E. D., and Cerutti D. T. (2009) Behavioral Neuroscience of Zebrafish. In Methods of Behavior Analysis in Neuroscience (Buccafusco J. J., Ed.), 2nd ed., Chapter 15, CRC Press, Boca Raton, FL. [PubMed] [Google Scholar]
- Klein S.; Hadamitzky M.; Koch M.; Schwabe K. (2004) Role of glutamate receptors in nucleus accumbens core and shell in spatial behaviour of rats. Neuroscience 128, 229–238. [DOI] [PubMed] [Google Scholar]
- Chanin S., Fryar C., Varga D., Raymond J., Kyzar E., Enriquez J., Bagawandoss S., Gaikwad S., Roth A., Pham M., Zapolsky I., Bruce I., Hester J., Green J., Desmond D., Stewart A. M., and Kalueff A. V. (2012) Assessing startle responses and their habituation in adult zebrafish. In Zebrafish Protocols for Neurobehavioral Research (Kalueff A. V., and Stewart A. M., Eds.), pp 287–300, Humana Press, New York. [Google Scholar]
- Riehl R.; Kyzar E.; Allain A.; Green J.; Hook M.; Monnig L.; Rhymes K.; Roth A.; Pham M.; Razavi R.; Dileo J.; Gaikwad S.; Hart P.; Kalueff A. V. (2011) Behavioral and physiological effects of acute ketamine exposure in adult zebrafish. Neurotoxicol. Teratol. 33, 658–667. [DOI] [PubMed] [Google Scholar]
- Suen M. F.; Chan W. S.; Hung K. W.; Chen Y. F.; Mo Z. X.; Yung K. K. (2013) Assessments of the effects of nicotine and ketamine using tyrosine hydroxylase-green fluorescent protein transgenic zebrafish as biosensors. Biosens. Bioelectron. 42, 177–185. [DOI] [PubMed] [Google Scholar]
- Hyman S. E. (1994) Why Does the Brain Prefer Opium to Broccoli?. Harvard Rev. Psychiatry 2, 43–46. [DOI] [PubMed] [Google Scholar]
- Braida D.; Limonta V.; Capurro V.; Fadda P.; Rubino T.; Mascia P.; Zani A.; Gori E.; Fratta W.; Parolaro D.; Sala M. (2008) Involvement of kappa-opioid and endocannabinoid system on Salvinorin A-induced reward. Biol. Psychiatry 63, 286–292. [DOI] [PubMed] [Google Scholar]
- Singh S. (2007) Adolescent salvia substance abuse. Addiction 102, 823–824. [DOI] [PubMed] [Google Scholar]
- Ninkovic J.; Bally-Cuif L. (2006) The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods 39, 262–274. [DOI] [PubMed] [Google Scholar]
- Erdtmann-Vourliotis M.; Mayer P.; Riechert U.; Hollt V. (1999) Acute injection of drugs with low addictive potential (δ-(9)-tetrahydrocannabinol, 3,4-methylenedioxymethamphetamine, lysergic acid diamide) causes a much higher c-fos expression in limbic brain areas than highly addicting drugs (cocaine and morphine). Brain Res. Mol. Brain Res. 71, 313–324. [DOI] [PubMed] [Google Scholar]
- Gresch P. J.; Strickland L. V.; Sanders-Bush E. (2002) Lysergic acid diethylamide-induced Fos expression in rat brain: role of serotonin-2A receptors. Neuroscience 114, 707–713. [DOI] [PubMed] [Google Scholar]
- Navarro J. F.; Rivera A.; Maldonado E.; Cavas M.; de la Calle A. (2004) Anxiogenic-like activity of 3,4-methylenedioxy-methamphetamine (“Ecstasy”) in the social interaction test is accompanied by an increase of c-fos expression in mice amygdala. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 249–254. [DOI] [PubMed] [Google Scholar]
- Salzmann J.; Marie-Claire C.; Le Guen S.; Roques B. P.; Noble F. (2003) Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br. J. Pharmacol. 140, 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulom V.; Lee H. W.; Zhao L.; Eghbali-Webb M. (2002) Stimulation of DNA synthesis, activation of mitogen-activated protein kinase ERK2 and nuclear accumulation of c-fos in human aortic smooth muscle cells by ketamine. Cell Proliferation 35, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao S.; Nagata A.; Miyamoto E.; Masuzawa M.; Murayama T.; Shingu K. (2003) Inhibitory effect of propofol on ketamine-induced c-Fos expression in the rat posterior cingulate and retrosplenial cortices is mediated by GABAA receptor activation. Acta Anaesthesiol. Scand. 47, 284–290. [DOI] [PubMed] [Google Scholar]
- Gao X. M.; Hashimoto T.; Tamminga C. A. (1998) Phencyclidine (PCP) and dizocilpine (MK801) exert time-dependent effects on the expression of immediate early genes in rat brain. Synapse 29, 14–28. [DOI] [PubMed] [Google Scholar]
- Canavello P. R., Cachat J. M., Beeson E., Laffoon A. L., Grimes C., Haymore W., Elegante M. F., Bartels B. K., Hart P. C., Elkhayat S., Tien D. H., Mohnot S., Amri H., and Kalueff A. V. (2010) Measuring endocrine (cortisol) responses of zebrafish to stress. In Zebrafish Neurobehavioral Protocols (Kalueff A. V., and Cachat J., Eds.), Humana Press, New York. [Google Scholar]
- Hawrylycz M.; Baldock R. A.; Burger A.; Hashikawa T.; Johnson G. A.; Martone M.; Ng L.; Lau C.; Larson S. D.; Nissanov J.; Puelles L.; Ruffins S.; Verbeek F.; Zaslavsky I.; Boline J. (2011) Digital atlasing and standardization in the mouse brain. PLoS Comput. Biol. 7, e1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess H. A.; Granato M. (2007) Sensorimotor gating in larval zebrafish. J. Neurosci. 27, 4984–4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savio L. E.; Vuaden F. C.; Piato A. L.; Bonan C. D.; Wyse A. T. (2012) Behavioral changes induced by long-term proline exposure are reversed by antipsychotics in zebrafish. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 36, 258–263. [DOI] [PubMed] [Google Scholar]
- Seibt K. J.; Oliveira Rda L.; Zimmermann F. F.; Capiotti K. M.; Bogo M. R.; Ghisleni G.; Bonan C. D. (2010) Antipsychotic drugs prevent the motor hyperactivity induced by psychotomimetic MK-801 in zebrafish (Danio rerio). Behav. Brain Res. 214, 417–422. [DOI] [PubMed] [Google Scholar]
- Seibt K. J.; Piato A. L.; da Luz Oliveira R.; Capiotti K. M.; Vianna M. R.; Bonan C. D. (2011) Antipsychotic drugs reverse MK-801-induced cognitive and social interaction deficits in zebrafish (Danio rerio). Behav. Brain Res. 224, 135–139. [DOI] [PubMed] [Google Scholar]
- Ahrens M. B.; Li J. M.; Orger M. B.; Robson D. N.; Schier A. F.; Engert F.; Portugues R. (2012) Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature 485, 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. A.; Livet J.; Sanes J. R.; Lichtman J. W.; Schier A. F. (2011) Multicolor Brainbow imaging in zebrafish. Cold Spring Harbor Protoc. 10.1101/pdb.prot5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Bene F.; Wyart C. (2012) Optogenetics: a new enlightenment age for zebrafish neurobiology. Dev. Neurobiol. 72, 404–414. [DOI] [PubMed] [Google Scholar]
- Kily L. J.; Cowe Y. C.; Hussain O.; Patel S.; McElwaine S.; Cotter F. E.; Brennan C. H. (2008) Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro-adaptation pathways. J. Exp. Biol. 211, 1623–1634. [DOI] [PubMed] [Google Scholar]
- Manger P. R.; Cort J.; Ebrahim N.; Goodman A.; Henning J.; Karolia M.; Rodrigues S. L.; Strkalj G. (2008) Is 21st century neuroscience too focussed on the rat/mouse model of brain function and dysfunction?. Front. Neuroanat. 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. (1984) The validity of animal models of depression. Psychopharmacology (Berlin, Ger.) 83, 1–16. [DOI] [PubMed] [Google Scholar]
- Grailhe R.; Waeber C.; Dulawa S. C.; Hornung J. P.; Zhuang X.; Brunner D.; Geyer M. A.; Hen R. (1999) Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron 22, 581–591. [DOI] [PubMed] [Google Scholar]
- Kovacic P.; Somanathan R. (2009) Novel, unifying mechanism for mescaline in the central nervous system: electrochemistry, catechol redox metabolite, receptor, cell signaling and structure activity relationships. Oxid. Med. Cell. Longevity 2, 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M. H.; Wang X.; Rothman R. B. (2007) 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology 189, 407–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao-Yao He N. N. H. M.; Ravindranathan A.; Jeanblanc J.; Logrip M. L.; Phamluong K.; Janak P. H.; Ron D. (2005) Glial Cell Line-Derived Neurotrophic Factor Mediates the Desirable Actions of the Anti-Addiction Drug Ibogaine against Alcohol Consumption. J. Neurosci. 25, 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick D. A., and Balster R. L. (1995) Phencyclidine (PCP). In Psychopharmacology: the fourth generation of progress (Bloom F. E., and Kupfer D. J., Eds.), Raven Press, New York. [Google Scholar]
- Krystal J. H.; Karper L. P.; Seibyl J. P.; Freeman G. K.; Delaney R.; Bremner J. D.; Heninger G. R.; Bowers M. B. Jr.; Charney D. S. (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 51, 199–214. [DOI] [PubMed] [Google Scholar]
- Ennaceur A.; Michalikova S.; van Rensburg R.; Chazot P. L. (2011) MK-801 increases the baseline level of anxiety in mice introduced to a spatial memory task without prior habituation. Neuropharmacology 61, 981–991. [DOI] [PubMed] [Google Scholar]
- Ahtee L.; Shillito E. (1970) The effect of benzodiazepines and atropine on exploratory behaviour and motor activity of mice. Br. J. Pharmacol. 40, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. J.; Kim D. H.; Jung J. M.; Kim J. M.; Cai M.; Liu X.; Hong J. G.; Lee C. H.; Lee K. R.; Ryu J. H. (2012) The ameliorating effects of stigmasterol on scopolamine-induced memory impairments in mice. Eur. J. Pharmacol. 676, 64–70. [DOI] [PubMed] [Google Scholar]
- Cognato Gde P.; Bortolotto J. W.; Blazina A. R.; Christoff R. R.; Lara D. R.; Vianna M. R.; Bonan C. D. (2012) Y-Maze memory task in zebrafish (Danio rerio): the role of glutamatergic and cholinergic systems on the acquisition and consolidation periods. Neurobiol. Learn. Mem 98, 321–328. [DOI] [PubMed] [Google Scholar]
- Echevarria D. J.; Hammack C. M.; Pratt D. W.; Hosemann J. D. (2008) A Novel Behavioral Test Battery to Assess Global Drug Effects Using the Zebrafish. Int. J. Comp. Psychol. 21, 19–34. [Google Scholar]
- Sison M.; Gerlai R. (2011) Behavioral performance altering effects of MK-801 in zebrafish (Danio rerio). Behav. Brain Res. 220, 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain H. A.; Sigstad C.; Scalzo F. M. (2004) Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio). Neurotoxicol. Teratol. 26, 725–729. [DOI] [PubMed] [Google Scholar]
- Cerutti D. T., and Levin E. D. (2006) Cognitive Impairment Models Using Complementary Species. In Animal Models of Cognitive Impairment (Levin E. D., and Buccafusco J. J., Eds.), CRC Press, Boca Raton, FL. [PubMed] [Google Scholar]
- Lange M.; Norton W.; Coolen M.; Chaminade M.; Merker S.; Proft F.; Schmitt A.; Vernier P.; Lesch K. P.; Bally-Cuif L. (2012) The ADHD-susceptibility gene lphn3.1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol. Psychiatry 17, 946–954. [DOI] [PubMed] [Google Scholar]
- Ziv L.; Muto A.; Schoonheim P. J.; Meijsing S. H.; Strasser D.; Ingraham H. A.; Schaaf M. J.; Yamamoto K. R.; Baier H. (2013) An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol. Psychiatry 18, 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham M., Raymond J., Hester J., Kyzar E., Gaikwad S., Bruce I., Fryar C., Chanin S., Enriquez J., Bagawandoss S., Zapolsky I., Green J., Stewart A. M., Robison B. D., and Kalueff A. V. (2012) Assessing social behavior phenotypes in adult zebrafish: shoaling, social preference and mirror biting tests. In Zebrafish Protocols for Neurobehavioral Research (Kalueff A. V., and Stewart A. M., Eds.), Humana Press, New York. [Google Scholar]
- Stewart A.; Cachat J.; Wong K.; Gaikwad S.; Gilder T.; DiLeo J.; Chang K.; Utterback E.; Kalueff A. V. (2010) Homebase behavior of zebrafish in novelty-based paradigms. Behav. Processes 85, 198–203. [DOI] [PubMed] [Google Scholar]
- Mathur P.; Lau B.; Guo S. (2011) Conditioned place preference behavior in zebrafish. Nat. Protoc. 6, 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B.; Bretaud S.; Huang Y.; Lin E.; Guo S. (2006) Dissociation of food and opiate preference by a genetic mutation in zebrafish. Genes, Brain Behav. 5, 497–505. [DOI] [PubMed] [Google Scholar]