Abstract
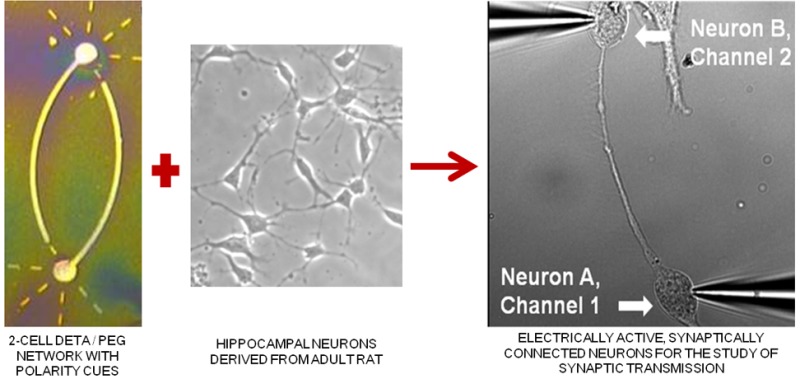
In this study, we demonstrate the directed formation of small circuits of electrically active, synaptically connected neurons derived from the hippocampus of adult rats through the use of engineered chemically modified culture surfaces that orient the polarity of the neuronal processes. Although synaptogenesis, synaptic communication, synaptic plasticity, and brain disease pathophysiology can be studied using brain slice or dissociated embryonic neuronal culture systems, the complex elements found in neuronal synapses makes specific studies difficult in these random cultures. The study of synaptic transmission in mature adult neurons and factors affecting synaptic transmission are generally studied in organotypic cultures, in brain slices, or in vivo. However, engineered neuronal networks would allow these studies to be performed instead on simple functional neuronal circuits derived from adult brain tissue. Photolithographic patterned self-assembled monolayers (SAMs) were used to create the two-cell “bidirectional polarity” circuit patterns. This pattern consisted of a cell permissive SAM, N-1[3-(trimethoxysilyl)propyl] diethylenetriamine (DETA), and was composed of two 25 μm somal adhesion sites connected with 5 μm lines acting as surface cues for guided axonal and dendritic regeneration. Surrounding the DETA pattern was a background of a non-cell-permissive poly(ethylene glycol) (PEG) SAM. Adult hippocampal neurons were first cultured on coverslips coated with DETA monolayers and were later passaged onto the PEG-DETA bidirectional polarity patterns in serum-free medium. These neurons followed surface cues, attaching and regenerating only along the DETA substrate to form small engineered neuronal circuits. These circuits were stable for more than 21 days in vitro (DIV), during which synaptic connectivity was evaluated using basic electrophysiological methods.
Keywords: Adult neurons, hippocampus, polarity, electrophysiological characterization networks, self-assembled monolayers
Most drugs that are in development by pharmaceutical companies for neurological deficits are targeting diseases in which the phenotype develops only at an older age. In contrast, most of the established cell based screens use embryonic cells due to the difficulties in obtaining viable cultures from adult animals. Thus, there is a clear need for new in vitro methods to enable the utilization of adult neurons in high-information content, functional screening assays. The ability to control cell placement and location of synapse formation in adult cultures would greatly enhance the ability to create functional systems for these assays.
Conduction of action potentials in the central nervous system (CNS) depends upon the formation of synapses between the axon of one neuron and the dendrite or soma of another.1,2 At a synapse, the plasma membrane of the presynaptic terminal, or synaptic bouton, comes in close contact with the membrane of the target postsynaptic cell, with extensive arrays of molecular machinery present in each to link the two membranes together.3 Excitatory neurons in the hippocampus release the neurotransmitter glutamate into the synaptic cleft which binds to ligand-gated ion channels in the postsynaptic membrane, producing an influx of ions to create an excitatory postsynaptic potential (EPSP).4−6 If the EPSP is strong enough to reach the threshold, an action potential is triggered in the postsynaptic cell.7
Synaptogenesis, synaptic communication, and synaptic plasticity have been extensively studied using hippocampal brain slice or dissociated cell cultures in a variety of serum containing or serum-free in vitro systems.8 Hippocampal slice cultures, where thin slices of intact hippocampal tissue are removed from adult brain tissue, are most widely used for electrophysiological studies into neuronal communication, long-term potentiation (LTP), and pathophysiology of brain disease.9−11 Studies of communication in dissociated cultures typically rely upon hippocampal neurons extracted from the brains of embryonic day 18 rat/mouse embryos.12 Methods have been developed to reintroduce and control complex interconnectivity in dissociated cultures through patterning. Some of the methods used were surface chemistry and photolithography,13−15 microcontact printing,16,17 printing,18,19 microfluidics,20 atomic force microscopy,21 or other chemical and topographical cues.22,23
Previously, we developed an adult hippocampal culture system utilizing a novel culture method, new serum-free media formulation, and the nonbiological substrate N-1[3-(trimethoxysilyl)propyl] diethylenetriamine (DETA).24 This substrate forms self-assembled monolayers (SAMs) on any hydroxylated surface, is nondegradable by cells, promotes both the attachment and regeneration of neurons in vitro, and, through photolithographic patterning, can be used to control neuronal attachment and direct axonal outgrowth.25,26 Laser ablation photolithography has been used to generate patterned surfaces with regions that support cell adhesion and regeneration, and others that do not.27 PEG SAMs prevent the adsorption of proteins on glass surfaces by the entropy/hydrated surface hypothesis.28−30 Therefore, a surface composed of small connected regions of DETA surrounded by PEG would facilitate the adhesion of small numbers of neurons and direct regeneration to facilitate the formation of small neuronal circuits.
In this study, we demonstrate the formation of small circuits of electrically active, synaptically connected neurons derived from the hippocampus of adult rats. Neuronal attachment and directed bidirectional neurite polarity outgrowth was controlled using a photolithographically patterned combination of PEG-DETA SAMs. Serum-free media formulations, antimitotic factors, and the neurotransmitter glutamate supported the formation of small synaptically connected circuits composed of mature, terminally differentiated glutamatergic neurons that were stable for long physiologically significant periods in vitro. These findings fill a void in the study of synaptogenesis, synaptic communication, synaptic plasticity, neuropharmacology, and brain disease pathophysiology in mature neurons by allowing for easy identification of connected neurons and by providing a stable culture system by which the same neurons can be studied over time.
Results
Surface Modification to Create Cell-Adhesive Bidirectional Polarity Patterns of DETA against a Background of Noncell Supporting PEG
The masks were derived from previous work that demonstrated geometric control of neuronal polarity31 and network formation32 that were demonstrated using different SAM combinations for embryonic neurons.33 The bidirectional polarity patterns have two somal adhesion sites (SAS) of 30 μm diameter approximately 150 μm apart connected by 5 μm lines of DETA. The dimensions of the patterns promoted the attachment of neurons onto the SAS, regrowth of axons along the connecting lines, and dendritic branching along the dotted strips of DETA. Copper deposition results were consistent between pattern batches, indicating the reproducibility of the patterning process. The DETA foreground surrounded by PEG background provided a pattern that supported the attachment of neurons and directed regeneration of dendrites and axons along surface cues in order to promote the formation of small functional circuits of neurons (Figure 1). In contrast to earlier experiments, where a fluorinated silane, 13F, was used as the cell attachment inhibiting surface,31 here PEG was used to inhibit not only cell attachment, but the adsorption of proteins still present from adult tissue dissociation.34 Control coverslips were used in order to test the quality of the PEG-DETA patterns: (1) one PEG coverslip was ablated without a photomask followed by DETA deposition, and (2) a DETA monolayer was deposited upon a second PEG without prior ablation. Laser irradiation and DETA deposition were done in the same conditions as for the PEG-DETA patterns.
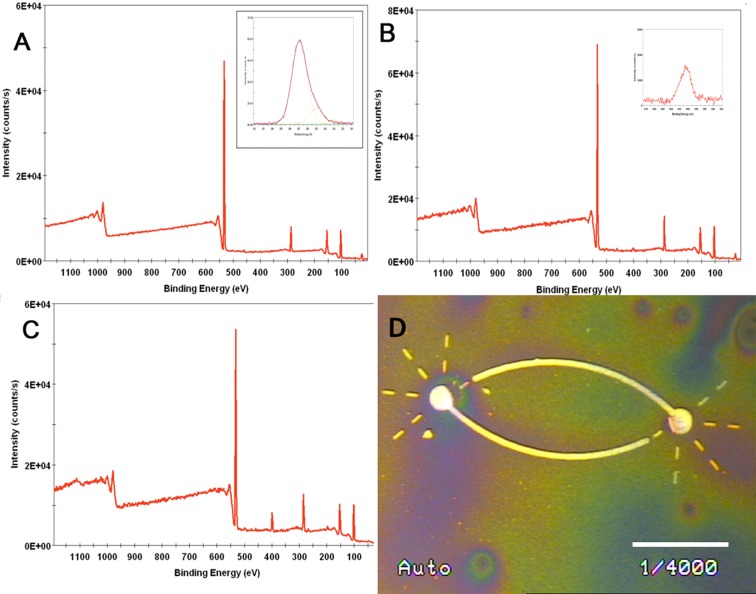
Figure 1.
XPS analysis and metallization reaction for PEG-DETA patterns. (A) XPS survey spectrum of PEG-coated glass coverslip (inset shows high resolution C1s spectrum). (B) XPS survey spectrum of DETA on PEG-coated glass coverslip (inset shows high resolution N1s spectrum) analysis of the two layers. (C) XPS survey spectrum of DETA on ablated PEG-coated glass coverslip. (D) Image of the two-cell circuit bidirectional polarity pattern visualized using palladium catalyzed copper reduction metallization (light lines indicate the DETA regions). Scale bar = 75 μm, line width = 5.5 μm, and somal adhesion site (SAS) = 25 μm.
The XPS measurements of the control coverslips indicate that PEG formed a SAM on glass coverslips (Figure 1A). Additionally, DETA formed a SAM on ablated PEG, but was not incorporated (or only incorporated in traces amounts) in the unexposed PEG regions (Figure 1B, C). Further, static water contact angle measurements of 49 ± 2° validated the hydrophilicity of the laser exposed PEG after DETA rederivatization. However, the nonablated PEG monolayer was not affected by the reaction with DETA, as was revealed by the contact angle value of 49 ± 2° for a nonradiated PEG control coverslip(s), values that are close to the ones for pure PEG (37 ± 2°). The pattern uniformity was verified by copper reduction metallization (Figure 1D), with the light regions representing the cell-adhesive DETA regions of the patterns.
Attachment and Regeneration of Neurons on Patterns
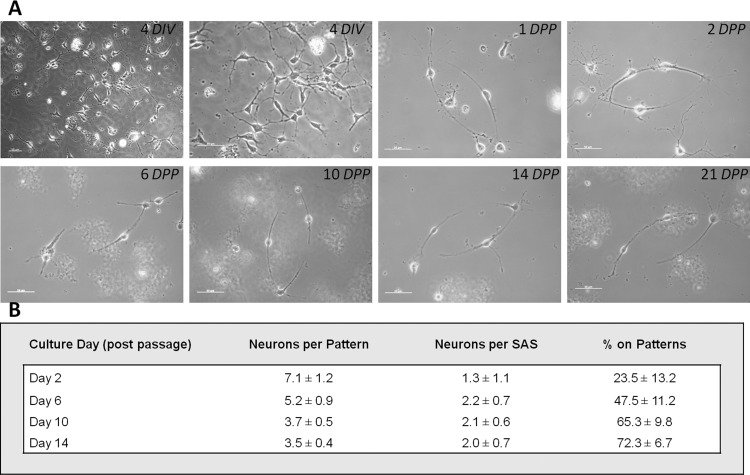
Small patterns of adult hippocampal neurons were prepared using defined media formulations and a two-step culture process that allowed the optimal number of neurons to be deposited for proper in vitro neuronal network formation (Figure 2). In an earlier paper,24 a direct one-step plating was utilized to create functional adult rat hippocampal cultures. To increase the probability of the formation of correct, functional cellular patterns, a second purification step was introduced. This step involved passaging neurons from unpatterned DETA coverslips to patterned DETA/PEG coverslips, to eliminate cells damaged by the dissociation of adult tissue (which would not form functional patterns) as well as contaminating proteins (which could mask the positive features of surface patterns). Thus, in the first step, the hippocampus of adult rats was processed to dissociate the neurons. These neurons were plated on DETA coverslips (Figure 3, 4 DIV). After 4 days in vitro (DIV), a period during which the neurons recovered and regenerated, the neurons were passaged from the DETA coverslip(s), counted, and plated at 50 cells/mm2 on PEG-DETA bidirectional polarity patterned coverslips. Neuronal conformity to the patterns was analyzed throughout the study, with the optimum conformity being two neurons per pattern, each on opposite SASs, with 100% of the neurons found on the DETA patterns versus the PEG background. As shown in Figure 3, pattern conformity improves over time, from 7.1 ± 1.2 neurons per pattern after 2 DPP (days on polarity patterns) to 3.5 ± 0.4 after 14 DPP. Similar improvements to pattern conformity were seen in neurons per SAS and the percentage of neurons on the patterns versus the background (72.3 ± 6.7% on 14 DPP). Pattern conformity improved over time in part due to the nonadhesive nature of the PEG background, with neurons either migrating to the more adhesive DETA regions or washing off the coverslip(s). Additionally, adult neurons on the patterned regions continued to regenerate neurites and to migrate along the patterned area (see video 1).
Figure 2.
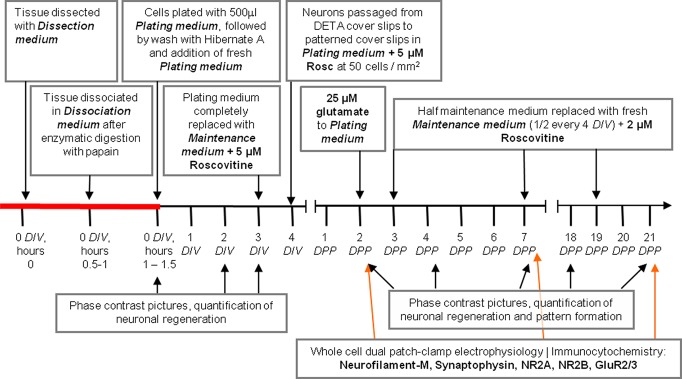
Time line of the adult hippocampal cell culture process and passage onto PEG-DETA bidirectional polarity patterned coverslips. DIV, days in vitro; DPP, days on polarity patterns.
Figure 3.
(A) Time-course pictures of neurons on culture after 4 DIV, 1 DPP, 2 DPP, 6 DPP, 10 DPP, 14 DPP, and 21 DPP. Scale bar = 50 μm. (B) Neuronal conformity to PEG-DETA bidirectional polarity pattern(s). Attachment and regeneration of neurons on the bidirectional polarity patterns was quantified by counting the number of neurons (1) attached to any part of the two-cell circuit pattern and (2) specifically to the SASs. The percentage of neurons attached to the DETA patterns versus the PEG background was quantified (n > 15, where n is the number of patterned coverslips evaluated).
Formation and Maintenance of Synaptic Connections
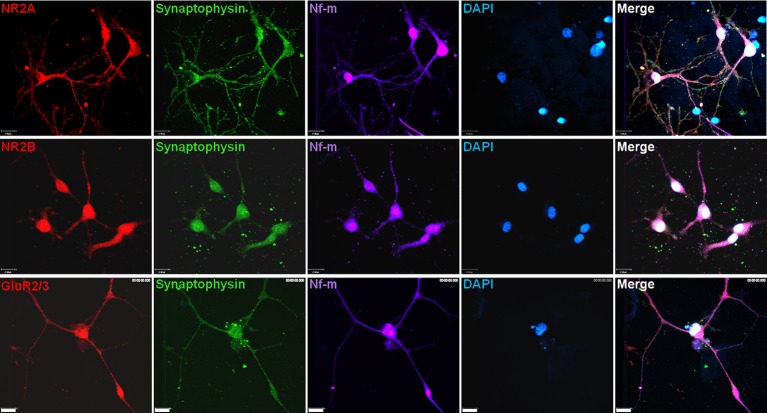
While the adult neurons physically connected their axons and dendrites along the guided DETA path of the pattern, testing was needed to determine whether functional synaptic connections had formed. First, unpatterned control coverslips with passaged neurons were examined immunocytochemically 14 days after passage to determine the expression of pre- and postsynaptic markers. The presynaptic marker synaptophysin and the postsynaptic receptor subunits for AMPA and NMDA channels were present (Figure 4). These pre- and postsynaptic proteins indicated the potential for the neurons to regenerate functional synapses.33,35−38
Figure 4.
Functional two-cell circuits. NR2A, NR2B, or GluR2/3 (red); synaptophysin (green); neurofilament-M (far-red); and DAPI (blue) expression 14 days after passage in adult neurons on unpatterned DETA-coated control coverslips. Scale bars = 17 μm.
Dual-patch clamp electrophysiology was performed on neurons on patterns after 14 DPP in order to establish the function of the synaptic connections between neurons (n = 8, two-cell circuits analyzed). By monitoring the postsynaptic neuron while stimulating an action potential in the presynaptic neuron, the presence of functional synapses was able to be measured.
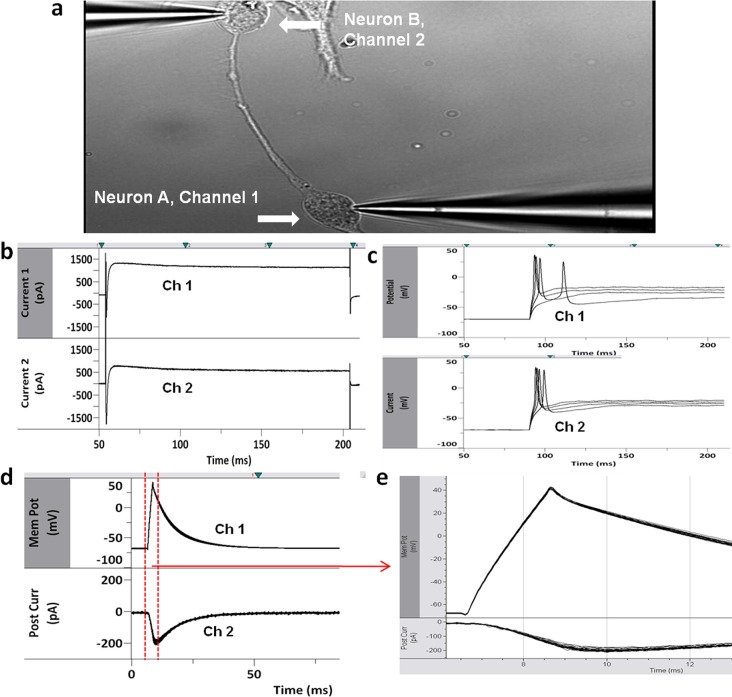
Both neurons on the pattern were simultaneously patched (Figure 5a), and electrophysiological recordings were performed to indicate the functional recovery of these cells. The channel conductance of each of the cells was measured in voltage clamp mode, with both cells showing normal inward and outward sodium and potassium-dependent current flow (Figure 5b). The electrical ability of each cell, as a function of ability to fire action potentials, was measured in current clamp mode. Each cell generated a single action potential upon stimulation, consistent with adult hippocampal neurons in dissociated cell culture (Figure 5c). Next, neuron A, the presynaptic neuron, was held at −70 mV holding potential, and action potentials were evoked. The postsynaptic currents were measured in neuron B (Figure 5d, e). The large inward current response in the postsynaptic neurons when the presynaptic neuron was held at −70 mV holding was consistent with excitatory synaptic connections.
Figure 5.
Dual patch clamp recordings were performed on neurons on bidirectional polarity patterns (n = 8, two-cell circuits analyzed) (a). Representative electrophysiological recordings showed both cells were neurons (b): voltage-gated sodium and potassium channels in voltage-clamp experiments. (c) Action potentials generated upon stimulation in current clamp mode where the cells were held at −70 mV. Neuron A, channel 1; neuron B, channel 2. Synaptic connections between the neurons were measured. (d, e) Presynaptic neurons (channel 1) were held at −70 mV, and action potentials were evoked. Postsynaptic currents (channel 2) were measured.
Discussion
The development of an in vitro system, where attachment and regeneration of adult hippocampal neurons was guided using photolithography to form small synaptically connected neuronal circuits, represents a significant technological advancement for basic and applied neuroscience. PEG-DETA surfaces prepared by laser ablation photolithography were sufficient to direct the attachment of cells specifically to bidirectional polarity patterns while restricting attachment to the PEG background. Mature, terminally differentiated neurons derived from hippocampal brain tissue of adult rats attached, adhered, and regenerated functional neurites along the guided DETA cues of the bidirectional polarity pattern. The polarity pattern was initially designed to determine and guide the longest neurite, which became the axon, toward the predetermined synapse zone of the other cell.31 The design for dendritic morphology was a generic one, mimicking four neurite-branches originating from the cell body.33 Adult hippocampal neurons followed both the axonal and dendritic cues, although the dendritic attachment area seemed to be too restrictive, as the neurites tended to overgrow that portion of the pattern. The neurons were found to be both electrically active and synaptically connected, and displayed synaptic connectivity characteristic of excitatory glutamatergic neurons.
In previous experiments, we developed a disorganized culture system that supported the attachment, survival, and regeneration of electrically active neurons derived from the hippocampus of adult rats.24 Neurons cultured utilizing the protocol outlined here were passaged from unpatterned DETA coverslips to PEG-DETA bidirectional polarity patterned coverslips (n > 15, where n is the number of patterned coverslips evaluated). These cells reacted to the guidance cues provided by the DETA substrate to attach and regenerate along the DETA lines to form engineered neuronal circuits. Initially the majority of cells attached to the PEG background but over time fewer cells were found on the PEG background versus the DETA patterns (23.5 ± 13.2% on DETA patterns on 2 DPP, improving to 72.3 ± 6.7% on DETA patterns on 14 DPP). Additionally, pattern fidelity improved over time, improving from 7.1 ± 1.2 neurons per DETA pattern on 2 DPP to 3.5 ± 0.4 on 14 DPP, with the ideal number being 2 to correspond to the two somal adhesion sites.
The electrical characteristics of individual adult hippocampal neurons were identified in earlier experiments.24,39 Individual neurons on DETA bidirectional polarity patterns have distinctive adult neuronal sodium and potassium currents and fire action potentials when stimulated (Figure 5b, c). Using dual whole cell patch-clamp electrophysiology, the function and properties of synaptic connections between neurons on these patterns were measured. Excitatory synapses, with large inward current response in the postsynaptic neuron in response to action potential in the presynaptic neurons at −70 mV holding, were identified.
The ability to culture adult neurons with oriented connections in a defined, serum-free culture system has tremendous applications for basic and applied neuroscience. While patterning of neurons has been accomplished by this group previously, it had been with embryonic phenotypes.40,41 Adult neurons express many ion channels not found in embryonic culture, even after 14–21 days, so it more accurately represents the phenotype of interest for comparison to whole animal studies. Expression of the machinery required for excitatory synaptic connections was also evaluated in the adult hippocampal neurons. Neurons expressed synaptophysin and the presynaptic vesicle glycoprotein was used to quantify the synapses.37 The NMDA and AMPA channel subunits NR2A, NR2B, and GluR2/3, the distinctive postsynaptic ligand-gated ion channels that control EPSP,33,35,36,38 were all present in the adult hippocampal neurons. The presence of both presynaptic and postsynaptic EPSP proteins and characteristic excitatory synaptic electrophysiological parameters confirmed the presence of excitatory synapses between neurons on the patterned coverslips.
DETA’s efficacy as a biological substrate for bioengineering applications is founded in its reproducible nature and its ability to be patterned using photolithography.32 Its role in patterning applications as the cell-permissive substrate that is surrounded by noncell permissive SAMs is further strengthened because it is not degraded by the cells.31,42 This characteristic of DETA allows it to form sharp patterns that do not blend with the noncell permissive PEG background and promotes pattern stability under long-term culture conditions.25,31 PEG SAMs prevent the adsorption of proteins on glass surfaces by the entropy/hydrated surface hypothesis.28−30 Therefore, a surface composed of small connected regions of DETA surrounded by PEG facilitates the adhesion of small numbers of neurons and directs regeneration to form circuits of synaptically connected neurons. These SAMs can also be applied to any hydroxylated surface or material, meaning these bidirectional polarity patterns can be applied to microelectrode array (MEA) devices for high content electrical studies of neuronal circuits. This system provides a unique tool that can be used for studies into LTP.43−45 In addition, with adaptation to extracellular multielectrode array (MEA) technology, this functional in vitro system would enable high-content neuropharmacology studies, facilitating drug development and furthering research into different neurological disorders, such as ALS, AD and Parkinson’s disease.
Methods
PEG Surface Modification
Glass coverslips (VWR cat. no. 48366067, 22 × 22 mm2 No. 1) were cleaned using 1:1 HCl/methanol followed by a concentrated H2SO4 soak for 2 h. The coverslips were then coated with a PEG-terminated silane following a modified protocol from Papra et al.46 Dry toluene was prepared by distillation over metallic sodium to remove any water or other contaminants. The alkylsilane (2-[methoxypol(ethyleneoxy)proply]trimethoxysilane, Gelest, Tullytown, PA) was added to the toluene to a final concentration of 0.1% by volume in an MBraun glovebox (MBraun, Stratham, NH). Concentrated HCl was added to a final volume of 0.08% (0.8 mL HCl/L) to the PEG/toluene solution, and the solution briefly stirred. The coverslips were then incubated in the PEG/toluene solution for 1 h at room temperature. After 1 h, the samples were removed and rinsed in serial washes of toluene (1×), ethanol (2×), and diH2O (1×). The washed samples were blown dry under a stream of ultrapure nitrogen and were used immediately or stored in a desiccator until needed.
Deep-UV Photolithography of PEG-Silane Monolayers
PEG-silane modified silica substrates were patterned using deep ultraviolet (DUV) photolithography. The samples were patterned in a photolithography system of our own design, which was based on a mask aligner, 193 nm ArF excimer laser (Lambda Physik, Santa Clara, CA) with an in-line beam homogenizer. Samples were placed on the stage of the mask aligner under a 5 × 5 in. chrome plated photomask, which contained the pattern to be ablated. The masks were written in dark-field polarity such that the areas corresponding to the ablated pattern were transparent and the remaining areas were opaque. When necessary, the substrate was aligned using the aligner stage to ensure micrometer precision placement of the pattern. The substrate was then brought into contact with the mask and a vacuum applied between the stage and mask to ensure a hard contact to minimize the gap between the substrate and mask to ensure a high contrast pattern with minimal edge effects due to refraction of the laser light. The substrates were then exposed to 193 nm ultraviolet laser light for 30 s with a pulse intensity of 200 mJ and a frequency of 10 Hz. After ablation the samples were removed from the aligner stage and stored for subsequent processing.
Backfill of Patterned PEG-Silane Monolayers with DETA-Silane
After ablation the patterned PEG-silane substrates were backfilled with the alkylsilane (3-trimethoxysilyl propyl)diethylenetriamine (DETA). Fresh distilled dry toluene was prepared as described above. DETA was added to the toluene to achieve a final concentration of 0.1% (vol:vol) inside a nitrogen atmosphere glovebox. The DETA/toluene solution was removed from the glovebox and transferred to a beaker and the previously patterned samples were immersed in the solution. To drive the reaction forward, the solution was gently heated to no more than 65 °C for 30 min. After reaction with DETA, the samples were allowed to cool to room temperature, washed three times with dry toluene, and heated to 65 °C for 30 more minutes.
DETA Control Surface Modification and Characterization
Glass coverslips (VWR 48366067, 22 × 22 mm2 No. 1) were cleaned by acid washing using a 50:50 mixture of concentrated hydrochloric acid and methanol. The coverslips were washed three times, 30 min per wash, and were rinsed in distilled deionized water between each washing. The DETA (United Chemical Technologies Inc., Bristol, PA, T2910) monolayer was formed by the reaction of the cleaned surface with a 0.1% (v/v) mixture of the organosilane in freshly distilled toluene (VWR BDH1151). The DETA-coated coverslips were heated to just below the boiling point of toluene, rinsed with toluene, reheated to just below the boiling temperature, and then oven-dried. The DETA formed a reaction site limited monolayer on the surface of the coverslip.
Surface Characterization
Contact Angle Goniometry Analysis
The surface contact angle of unmodified (clean glass coverslips) and modified (PEG or DETA) substrates was measured by contact angle goniometry using a Ramé Hart (Netcong, NJ) contact angle goniometer. In all cases, the contact angle of a static sessile drop (5 μL) of water placed on the sample was measured three times and averaged. A contact angle of less than 5° was obtained for clean substrates. An average of 37 ± 2° was measured for PEG substrates, and of 49 ± 2° for DETA control slides.
X-ray Photoelectron Spectroscopy
In order to authenticate the monolayer formation, both PEG and DETA control surfaces were characterized by X-ray photoelectron spectroscopy (XPS) using a VG ESCALAB 220i-XL spectrometer equipped with an aluminum anode and a quartz monochromator. The spectrometer was calibrated against the reference binding energies of clean Cu, Ag and Au samples. XPS survey scans were recorded in order to determine the relevant elements (pass energy of 50 eV, step size of 1 eV). Si 2p, C 1s, N 1s, and O 1s high resolution spectra were recorded to determine the quality of the surfaces (pass energy of 20 eV, step size of 0.1 eV). The fitting of the peaks was performed with Avantage version 3.25 software provided by Thermo Electron Corporation. The quality of the surfaces was in agreement with previously reported results.32,46,47
Palladium-Catalyzed Metallization of Patterned Silane Monolayers
Patterned samples were visualized using a palladium-catalyzed copper reduction reaction, modified from Kind et al.48 In this reaction, copper is deposited in regions containing the amine terminated silane DETA.
Adult Rat Hippocampal Dissociated Cell Culture Methodology
Adult neurons are extracted, dissociated, cultured, and maintained using the following protocol and medium formulation. Briefly, the hippocampus of adult rats (Charles River, age 6–12 months) were dissected and homogenized into small tissue fragments in cold medium (∼4 °C) consisting of Hibernate-A calcium free (500 mL, Brain Bits, cat.# HA-Ca), and antibiotic-antimycotic (1%, Gibco, cat.# 15240-096). The tissue was digested for 30 min at 37 °C in calcium-free Hibernate-A (HA) containing 6 mg papain/12 mL (HA no calcium). Following digestion, the tissue was washed three times with cold HA media to remove any active enzyme. Next, the tissue was suspended in Dissociation Medium containing a Base Medium: B27 (2%, Gibco, cat.# 17504–044), Glutamax (2 mM, Gibco, cat.# 35050-061) and an antibiotic/antimycotic (1%, Gibco, cat.# 15240-096); plus Hibernate-A (500 mL, Brain Bits, cat.# HA), Z-Asp(OMe)-Gln-Met-Asp(OMe) fluoromethyl ketone (4 mM, Sigma, cat.# C0480), Z-Val-Ala-Asp fluoromethyl ketone (5 mM, Sigma, cat.# C2105), dextrose-coated cerium oxide nanoparticles (100 nM), and (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (70 nM, Sigma, cat.# 238813), and broken apart into individual cells through mechanical dissociation with fire-polished Pasteur pipettes. The dissociated cells were suspended in Plating Medium containing the Base Medium plus Neurobasal-A (500 mL, Gibco, cat.# 10888), recombinant human brain-derived neurotrophic factor (20 ng/mL, Cell Sciences, cat.# CRB600B), NT-3, recombinant human (20 ng/mL, Cell Sciences, cat.# CRN500B), bFGF, recombinant human (5 ng/mL, Invitrogen, cat.# 13256–029), insulin-like growth factor-I (E3R) human (20 ng/mL, Sigma, cat.# I2656), and two components to reduce free radical damage, dextrose-coated cerium oxide nanoparticles (100 nM) and (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (70 nM). The cells were then deposited onto DETA-coated glass coverslips for 30–45 min. The coverslips were washed with warm HA by gently swirling the medium to remove tissue debris. Following this washing step, fresh Plating Medium was applied and remained for the first 3 DIV. On 3 DIV, the medium was removed and replaced by Maintenance Medium which was composed of the Base Medium plus Neurobasal-A (500 mL, Gibco, cat.# 10888), recombinant human brain-derived neurotrophic factor (20 ng/mL, Cell Sciences, cat.# CRB600B), NT-3, recombinant human (20 ng/mL, Cell Sciences, cat.# CRN500B), bFGF, recombinant human (5 ng/mL, Invitrogen, cat.# 13256–029), and insulin-like growth factor-I (E3R) human (20 ng/mL, Sigma, cat.# I2656), with 5 μM Roscovitine (Rosc, Sigma, R7772). All research was approved by the Institutional Animal Care and Use Committee at the University of Central Florida and conformed to NIH guidelines.
Adult Neuronal Network Formation on Patterned PEG-DETA Surfaces
After 4 DIV, the adult hippocampal neurons on the DETA coverslips were passaged to PEG-DETA patterned coverslips. Briefly, neurons were dislodged from the DETA with trypsin (0.05% trypsin/EDTA in HBSS, Gibco, 25200). Trypsin inhibitor (trypsin inhibitor, soybean, Gibco, 17075–029) in Dissociation medium at 0.5 mg per ml deactivated the trypsin. The dislodged neurons were collected and spun at 500g for 5 min. The supernatant of deactivated trypsin in HBSS was discarded, and the neuronal cell pellet was suspended in 1 mL Plating medium. The neurons in suspension were counted using a Bright-Line hemacytometer, and neurons were plated onto the PEG-DETA patterned coverslips at 50 cells/mm2 in Plating medium supplemented with 5 μM Rosc. After two days postplating (DPP), glutamate (N-acetyl-l-glutamic acid, Aldrich, 855642) was added to the plating medium to a final concentration of 25 μM. On 3 DPP and again every 4 day after, half of the medium was removed and replaced with fresh Maintenance Medium supplemented with 2 μM Rosc.
Time-Lapse Microscopy
Time-lapse recording was performed immediately after the cells were plated onto PEG-DETA bidirectional polarity patterns. Living cells were observed under an inverted microscope (Zeiss-Axiovert 100) equipped with Plan-Neofluar 40× objective (Zeiss, Oberkochen, Germany) and a humidified incubation chamber for constant temperature at 37 °C and 5% CO2. Pictures were captured with a Hamamatsu C8484-05G digital charge-coupled device camera (Hamamatsu Photonics, Shizuoka, Japan). Experiments were run under the control of Okolab software (OKO-lab, Ottaviano, NA, Italy), and pictures were taken every 5 min. Live cell image sequences were compiled to create videos, 12 images per second (see video 1).
Immunocytochemistry and Laser Scanning Confocal Microscopy
To prepare cells for immunocytochemical characterization, coverslips were rinsed twice with phosphate buffered saline (PBS). Cells were fixed with 4% paraformaldehyde for 10 min at room temperature and subsequently rinsed three times with PBS. Cells were permeabilized for 5 min with 0.5% Triton X-100 in PBS and then blocked for 2 h in 5% normal goat serum in PBS. Anti-neurofilament-M (Chemicon, AB5735, 1:500), anti-synaptophysin (Chemicon, MAB368, 1:300), and either anti-NMDAR2A (Chemicon, AB1555P, 1:200), anti-NMDAR2B (Chemicon, AB15557P, 1:200), or anti-glutamate receptor 2 and 3 (Chemicon, AB1506, 1:50) were added in blocking solution for 12 h at 4 °C. After three washes with PBS, fluorescently labeled secondary antibodies (Invitrogen, A11011 (594 nm), A21449 (647 nm), and A11029 (488 nm), 1:200) in blocking buffer were applied for 2 h. Vectashield mounting medium with DAPI (H1200, Vector Laboratories, Burlingame, CA) was used to mount the coverslips onto slides. Fluorescent images were acquired with the UltraView spinning disc confocal system (PerkinElmer) with an AxioObserver.Z1 (Carl Zeiss) stand, and a Plan-Apochromat 40×/1.4 Oil DIC plan-apochromat objective with 26 μm resolution. Z-Stack projections of the scanned images were generated and modified within the Volocity image processing program (PerkinElmer).
Dual Whole-Cell Patch Clamp Electrophysiology
Extracellular recording solution comprised Neurobasal-A medium containing 130 mM NaCl, 1.8 mM CaCl2, 5.2 mM KCl, 1 mM MgCl2, 2.2 mM NaHCO3, and 10 mM HEPES (pH 7.3) (300 mOsm). Patch pipettes (4–8 MΩ) were filled with intracellular solution (K-gluconate 140 mM, EGTA 1 mM, MgCl2 2 mM, Na2ATP 5 mM, HEPES 10 mM; pH 7.2). Cells were visualized on the stage with a Zeiss Axioscope, 2 FS Plus, upright microscope in Maintenance Medium. Voltage clamp and current clamp experiments were performed with a Multiclamp 700A (MDS Analytical Devices) amplifier. Signals were low-pass filtered at 3 kHz and digitized at 20 kHz with an Axon Digidata 1322A interface. Data recordings and analysis were performed with Clampex software. Whole-cell capacitance and series resistance were compensated electronically. Only cells with access resistance less than 22 MΩ were analyzed. Inward currents that had the characteristics of fast sodium currents, and outward currents that had the characteristics of potassium currents, were measured in voltage clamp mode. Voltage step length was 50 ms, incremented at 20 mV per step, 1 s between each step, with a holding potential of −70 mV. The action potential threshold was measured in current-clamp mode with increasing 1 s depolarizing current injections. The protocol for determining the presence and type of neuronal synapses has been described in detail previously.33
Glossary
Abbreviations
- CNS
central nervous system
- DETA
N-1[3-(trimethoxysilyl)propyl]diethylenetriamine
- DIV
days in vitro
- DPP
days postplating
- DUV
deep ultraviolet
- EPSP
excitatory postsynaptic potential
- HA
Hibernate-A
- LTP
long-term potentiation
- PEG
poly(ethylene glycol)
- PBS
phosphate buffered saline
- SAMs
self-assembled monolayers
- SAS
somal adhesion sites
- XPS
X-ray photoelectron spectroscopy
A video showing a sequence of live cell images (12 images per second) is available in the HTML version of the paper.
Author Contributions
D.E. performed the cell biology research and analyzed the cell culture and electrophysiology data and was the primary writer for the paper. M.S. conducted the surface chemistry modification and analysis. P.M. assisted with the electrophysiology experiments and their interpretation. J.J.H. was PI on the grant that funded the project, helped design the experiments, and completed the writing and editing of the manuscript for submission.
This work was supported by NIH Grant Number R01NS050452 and from a gift from Unither Neurosciences, Inc.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Rapport R. L. (2005) Nerve Endings: The Discovery of the Synapse, pp 1–37, W. W. Norton & Company: New York, NY. [Google Scholar]
- Squire L. R., Bloom F., and Spitzer N. (2008) Fundamental Neuroscience, pp 425–426, Academic Press: San Diego, CA. [Google Scholar]
- Toni N.; Teng E.; Bushong E.; Aimone J.; Zhao C.; Consiglio A.; van Praag H.; Martone M.; Ellisman M.; Gage F. H. (2007) Synapse formation on neurons born in the adult hippocampus. Nat. Neurosci. 10, 727–734. [DOI] [PubMed] [Google Scholar]
- Calabrese B.; Wilson M. S.; Halpain S. (2006) Development and Regulation of Dendritic Spine Synapses. Physiology 21, 38–47. [DOI] [PubMed] [Google Scholar]
- Daoudal G.; Hanada Y.; Debanne D. (2002) Bidirectional plasticity of excitatory postsynaptic potential (EPSP)-spike coupling in CA1 hippocampal pyramidal neurons. Proc. Natl. Acad. Sci. U.S.A. 99, 14512–14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R.; Steinberg I.; Walton K. (1981) Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys. J. 33, 323–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett M.; Larkman P. (2007) The action potential. Pract. Neurol. 7, 192–197. [PubMed] [Google Scholar]
- Bliss T.; Collingridge G. (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39. [DOI] [PubMed] [Google Scholar]
- Cho S.; Wood A.; Bowlby M. (2007) Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Curr. Neuropharmacol. 5, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen I. (2005) Organotypic hippocampal slice cultures: a model system to study basic cellular and molecular mechanisms of neuronal cell death, neuroprotection, and synaptic plasticity. Neurochem. Res. 30, 1521–1528. [DOI] [PubMed] [Google Scholar]
- Noraberg J.; Poulsen F.; Blaabjerg M.; Kristensen B.; Bonde C.; Montero M.; Meyer M.; Gramsbergen J.; Zimmer J. (2005) Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection, and neurorepair. Curr. Drug Targets 4, 435–452. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C. (2004) Long-term culture of hippocampal neurons. In Current Protocols in Neuroscience, John Wiley and Sons, Inc.: Hoboken, NJ; Chapter 3: Unit 3.2. [DOI] [PubMed] [Google Scholar]
- Fromherz P.; Schaden H.; Vetter T. (1991) Guided outgrowth of leech neurons in culture. Neurosci. Lett. 129, 77–80. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D.; Kahler K. H.; Hockberger P. E. (1988) Controlled outgrowth of dissociated neurons on patterned substrates. J. Neurosci. 8, 4098–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler B. C.; Brewer G. J. (2010) Designing neural networks in culture. Proc. IEEE 98, 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane R. S.; Takayama S.; Ostuni E.; Ingber D. E.; Whitesides G. M. (1999) Patterning proteins and cells using soft lithography. Biomaterials 20, 2363–2376. [DOI] [PubMed] [Google Scholar]
- Vogt A. K.; Wrobel G.; Meyer W.; Knoll W.; Offenhausser A. (2005) Synaptic plasticity in micropatterned neuronal networks. Biomaterials 26, 2549–2557. [DOI] [PubMed] [Google Scholar]
- Gustavsson P.; Johansson F.; Kanje M.; Wallman L.; Linsmeier C. E. (2007) Neurite guidance on protein micropatterns generated by a piezoelectric microdispenser. Biomaterials 28, 1141–1151. [DOI] [PubMed] [Google Scholar]
- Xu T.; Gregory C. A.; Molnar P.; Cui X.; Jalota S.; Bhaduri S. B.; Boland T. (2006) Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 27, 3580–3588. [DOI] [PubMed] [Google Scholar]
- Morin F.; Nishimura N.; Griscom L.; Lepioufle B.; Fujita H.; Takamura Y.; Tamiya E. (2006) Constraining the connectivity of neuronal networks cultured on microelectrode arrays with microfluidic techniques: a step towards neuron-based functional chips. Biosens. Bioelectron. 21, 1093–1100. [DOI] [PubMed] [Google Scholar]
- Staii C.; Viesselmann C.; Ballweg J.; Shi L.; Liu G.-Y.; Williams J. C.; Dent E. W.; Coppersmith S. N.; Eriksson M. A. (2009) Positioning and guidance of neurons on gold surfaces by directed assembly of proteins using Atomic Force Microscopy. Biomaterials 30, 3397–3404. [DOI] [PubMed] [Google Scholar]
- Li N.; Folch A. (2005) Integration of topographical and biochemical cues by axons during growth on microfabricated 3-D substrates. Exp. Cell Res. 311, 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Venkataramani S.; Xu H.; Song Y.-K.; Song H.-K.; Palmore G. T. R.; Fallon J.; Nurmikko A. V. (2006) Combined topographical and chemical micropatterns for templating neuronal networks. Biomaterials 27, 5734–5739. [DOI] [PubMed] [Google Scholar]
- Edwards D.; Das M.; Molnar P.; Hickman J. J. (2010) Addition of glutamate to serum-free culture promotes recovery of electrical activity in adult hippocampal neurons in vitro. J. Neurosci. Methods 190, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A. E.; Barker J. L.; Stenger D. A.; Hickman J. J. (1995) Investigation of the factors necessary for growth of hippocampal neurons in a defined system. J. Neurosci. Methods 62, 111–119. [DOI] [PubMed] [Google Scholar]
- Stenger D. A.; Pike C. J.; Hickman J. J.; Cotman C. W. (1993) Surface determinants of neuronal survival and growth on self-assembled monolayers in culture. Brain Res. 630, 136–147. [DOI] [PubMed] [Google Scholar]
- Dulcey C.; Georger J.; Krauthamer V.; Stenger D.; Fare T.; Calvert J. (1991) Deep UV photochemistry of chemisorbed monolayers: patterned coplanar molecular assemblies. Science 252, 551–554. [DOI] [PubMed] [Google Scholar]
- Nogaoka S., Mori Y., Takiuchi H., Yokota K., Tanizawa H., and Nishiumi S. (1984) Biocompatible materials. In Polymers as Biomaterials (Shalaby S. W., Hoffman A. S., Ratner B. D., and Horbett T. A., Eds.), p 361, Plenum Press, New York. [Google Scholar]
- Prime K.; Whitesides G. (1993) Adsorption of proteins onto surface containing end attached oligo(ethylene oxide): a model system using self-assembled monolayers. J. Am. Chem. Soc. 115, 10714–10721. [Google Scholar]
- Yang Z.; Galloway J.; Yu H. (1999) Protein interactions with poly(ethylene glycol) self assembled monolayers on glass substrates: diffusion and adsorption. Langmuir 15, 8405–8411. [Google Scholar]
- Stenger D. A.; Hickman J. J.; Bateman K. E.; Ravenscroft M. S.; Ma W.; Pancrazio J. J.; Shaffer K.; Schaffner A. E.; Cribbs D. H.; Cotman C. W. (1998) Microlithographic determination of axonal/dendritic polarity in cultured hippocampal neurons. J. Neurosci. Methods 82, 167–173. [DOI] [PubMed] [Google Scholar]
- Ravenscroft M. S.; Bateman K. E.; Shaffer K. M.; Schessler H. M.; Jung D. R.; Schneider T. W.; Montgomery C. B.; Custer T. L.; Schaffner A. E.; Liu Q. Y.; Li Y. X.; Barker J. L.; Hickman J. J. (1998) Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane-modified surfaces. J. Am. Chem. Soc. 120, 12169–12177. [Google Scholar]
- Molnar P.; Kang J. F.; Bhargava N.; Das M.; Hickman J. J. (2007) Synaptic Connectivity in Engineered Neuronal Networks. Methods Mol. Biol. 403, 165–173. [DOI] [PubMed] [Google Scholar]
- Wilson K. A.; Finch C. A.; Anderson P.; Vollmer F.; Hickman J. J. (2012) Whispering gallery mode biosensor quantification of fibronectin adsorption kinetics onto alkylsilane monolayers and interpretation of resultant cellular response. Biomaterials 33, 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin R. P.; Martin L. J.; MacDonald J. F.; Orser B. A. (2007) alpha-5-GABA A Receptors Regulate the Intrinsic Excitability of Mouse Hippocampal Pyramidal Neurons. J. Neurophysiol. 98, 2244–2254. [DOI] [PubMed] [Google Scholar]
- Cady C.; Evans M. S.; Brewer G. J. (2001) Age-related differences in NMDA responses in cultured rat hippocampal neurons. Brain Res. 921, 1–11. [DOI] [PubMed] [Google Scholar]
- Calhoun M.; Jucker M.; Martin L.; Thinakaran G.; Price D.; Mouton P. (1996) Comparative evaluation of synaptophysin-based methods for quantification of synapses. J. Neurocytol. 25, 821–828. [DOI] [PubMed] [Google Scholar]
- Liu X.; Murray K.; Jones E. (2004) Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J. Neurosci. 24, 8885–8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. S.; Collings M. A.; Brewer G. J. (1998) Electrophysiology of embryonic, adult and aged rat hippocampal neurons in serum-free culture. J. Neurosci. Methods 79, 37–46. [DOI] [PubMed] [Google Scholar]
- Das M.; Molnar P.; Devaraj H.; Poeta M.; Hickman J. J. (2003) Electrophysiological and morphological characterization of rat embryonic motoneurons in a defined system. Biotechnol. Prog. 19, 1756–1761. [DOI] [PubMed] [Google Scholar]
- Das M.; Rumsey J. W.; Gregory C. A.; Bhargava N.; Kang J. F.; Molnar P.; Riedel L.; Guo X.; Hickman J. J. (2007) Embryonic motoneuron-skeletal muscle co-culture in a defined system. Neuroscience 146, 481–488. [DOI] [PubMed] [Google Scholar]
- Spargo B. J.; Testoff M. A.; Nielsen T. B.; Stenger D. A.; Hickman J. J.; Rudolph A. S. (1994) Spatially controlled adhesion, spreading, and differentiation of endothelial cells on self-assembled molecular monolayers. Proc. Natl. Acad. Sci. U.S.A. 91, 11070–11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Rios M.; Miller M. (2006) Target-specific regulation of synaptic efficacy in the feeding central pattern generator of Aplysia: potential substrates for behavioral plasticity. Biol. Bull. 210, 215–229. [DOI] [PubMed] [Google Scholar]
- Ginsberg S. D. (2005) Glutamatergic Neurotransmission Expression Profiling in the Mouse Hippocampus After Perforant-Path Transection. Am. J. Geriatr. Psychiatry 13, 1052–1061. [DOI] [PubMed] [Google Scholar]
- Tominaga-Yoshino K.; Urakubo T.; Okada M.; Matsuda H.; Ogura A. (2008) Repetitive induction of late-phase LTP produces long-lasting synaptic enhancement accompanied by synaptogenesis in cultured hippocampal slices. Hippocampus 18, 281–293. [DOI] [PubMed] [Google Scholar]
- Papra A.; Gadegaard N.; Larsen N. B. (2001) Characterization of ultrathin poly(ethylene glycol) monolayers on silicon substrates. Langmuir 17, 1457–1460. [Google Scholar]
- Das M.; Bhargava N.; Gregory C.; Riedel L.; Molnar P.; Hickman J. J. (2005) Adult rat spinal cord culture on an organosilane surface in a novel serum-free medium. In Vitro Cell. Dev. Biol.: Anim. 41, 343–348. [DOI] [PubMed] [Google Scholar]
- Kind H.; Bittner A.; Cavalleri O.; Kern K.; Greber T. (1998) Electroless deposition of metal nanoislands on aminothiolate-functionalized Au(111) electrodes. J. Phys. Chem. B 102, 7582–7589. [Google Scholar]