Abstract
The morning blood pressure surge (MBPS) has been shown to be an independent predictor of cardiovascular events. There is insufficient evidence on the effect of nebivolol, a vasodilating β1-receptor blocker, on the MBPS when given in the morning or the evening. This is a prospective, randomized, double-blind, crossover study designed to test morning vs. evening dosing of nebivolol in nondiabetic, hypertensive patients. Patients received nebivolol 5 mg/day (force-titrated to 10 mg/day after 1 week) in the morning or evening and corresponding placebos. Patients underwent ambulatory BP monitoring at baseline and after each treatment phase. Forty-two patients were randomized, of whom 38 completed both study periods. Both morning and evening dosed nebivolol significantly lowered daytime, nighttime, and 24-hour BP after 3 weeks of treatment. Evening (but not morning) dosing significantly reduced prewaking systolic BP from baseline (8.64 ± 26.46 mm Hg, P = .048). Nebivolol given in the morning or the evening significantly reduces 24-hour BP parameters. Evening dosed nebivolol may confer some advantage over morning dosing in reducing prewaking systolic BP.
Keywords: Hypertension, morning blood pressure surge, nebivolol
Introduction
The circadian variation in blood pressure (BP) is well described, and is characterized by a 10%–20% nighttime dip in systolic and diastolic BP and a moderate rise in BP before arousal, culminating in a BP spike on awakening.1 These effects have been shown to relate to clinical events. Adverse cardiovascular (CV) outcomes, such as myocardial infarction, stroke, and sudden cardiac death, are known to follow circadian patterns, peaking in incidence in the morning, typically between 6 AM and 12 noon.2–5 This has been attributed, in part, to the morning BP surge (MBPS), which also most commonly occurs during these hours.2
The time of day at which an antihypertensive medication is taken has been shown to affect the diurnal pattern of BP.6,7 The time of day in which a medication is given can differentially affect both the morning surge and nocturnal BP.8 Thus, it is important to characterize the effects of new antihypertensive therapies with these variables in mind.
Nebivolol is a third-generation lipophilic β1-selective blocker with direct vasodilator properties that are mediated by a direct stimulatory effect on endothelial nitric oxide synthase (eNOS).9 The antihypertensive efficacy of nebivolol is well established, and it is approved in the United States for the treatment of hypertension. Given its pharmacological profile (half-life: 10–30 hours), it is a true once-per-day preparation.10 However, most studies have assessed the efficacy of nebivolol after morning dosing, and there is little information on whether effects of nebivolol on the morning surge and nocturnal dipping in BP vary with times of dosing.11,12
The primary objective of this study is to determine the effect on BP and the morning surge in BP when nebivolol is taken in the morning vs. the evening. Secondary outcomes of interest are the effect of nebivolol on nocturnal dipping and the 24-hour BP effect with morning vs. evening dosing.
Methods and Study Population
Study Design
This was a single center, prospective, randomized, double-blind, placebo-controlled, crossover study using 24-hour ambulatory BP monitoring (ABPM). The study was approved by and conducted according to the guidelines of the Institutional Review Board of the University of Alabama at Birmingham.
Study Participants
Patients were 19–76 years old with mild to moderate hypertension (defined as office systolic BP ≥140 and ≤180 mm Hg at the time of randomization). Excluded were patients with diabetes, heart failure, symptomatic coronary artery disease, bradycardia, history of bronchospastic disease, or cancer (within the last 5 years), elevated transaminases (aspartate transaminase or alanine transaminase >2× the upper limit of normal), and those taking cardio-active medications (excluding diuretics). All participants gave written informed consent.
Study Procedures
Patients who were taking antihypertensive medications underwent a washout period before randomization, until office BP was stable (two consecutive assessments within 15% of one another and at least 5 days apart). Patients who were taking a β-blocker underwent a detitration schedule before the washout. Patients who were treatment naive were required to meet the same BP stability criteria. The washout period typically lasted about 2 weeks.
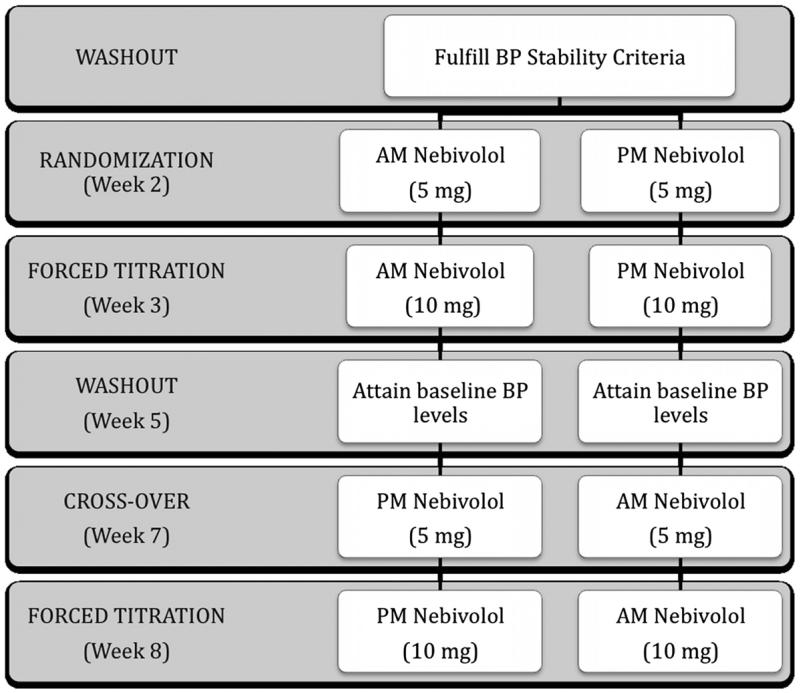
Patients whose systolic BP was stable at ≥140 and ≤180 mm Hg were randomly allocated into one of two arms (ie, morning-dosed nebivolol or evening-dosed nebivolol; Figure 1), using an allocation table produced by a computerized random number generator. Corresponding placebos were given to the study patients (those receiving nebivolol in the morning received placebo in the evening and vice versa). Patients were instructed to take the medicines 12 hours apart, without food. Nebivolol was started at a dose of 5 mg/day and force-titrated to 10 mg/day after 1 week. At the end of 3 weeks of treatment, patients then underwent a detitration schedule and a second washout (during which they were given placebo tablets in the morning and evening) until the office BP returned to baseline BP (Figure 1). The patients subsequently crossed over to the alternate time of dosing; with nebivolol being given at 5 mg/day initially then force titrated to 10 mg/day after 1 week (given for 2 weeks).
Figure 1.
Study Design.
BP Measurement
Using the auscultatory technique, seated office BPs were obtained weekly or more often as needed by a single trained physician who was blinded to the treatment allocation,. The arm that had the higher systolic BP measurement at the time of randomization was used for all office BP measurements throughout the study.
Four sets of 24-hour ABPMs were performed using a properly calibrated noninvasive cuff oscillometric recorder (Oscar-2 device, SunTech Medical, Morrisville, NC) and an appropriately sized arm cuff: 1) before randomization, 2) after 3 weeks of nebivolol (first phase), 3) after the second washout, and 4) at the end of the second phase of nebivolol therapy. Systolic and diastolic BP and heart rate were measured every 15 minutes during the day and every 30 minutes during the sleeping hours. Patients were instructed to maintain their usual activity and keep a log of their sleeping and waking up times while undergoing ABPM. ABPM reports that were missing more than 20% of the 24-hour readings, or missing data for an interval of >2 hours were repeated as necessary.
Ambulatory BP Parameters
For this study, the following definitions are used: BP on awakening is the average of the ambulatory BP readings in the first 2 hours after awakening; lowest nighttime BP is the average of the lowest BP attained at night and the readings before and after this value (average of three readings); prewaking BP is the average of BP readings 2 hours before awakening (average of three or four readings) and presleep BP is the average of BP readings 2 hours before sleep time (as declared by the patient, and the average of four to eight readings). Based on these definitions, we used the following formulas:
Trough to morning MBPS = (BP on awakening – lowest nighttime BP)
Prewaking MBPS = (BP on awakening – prewaking BP)
Nocturnal BP fall = (Presleep BP – lowest nighttime BP)
Morning BPS = (24 hour BP – BP on awakening)
Nocturnal BP dip = (Daytime ambulatory BP – night-time ambulatory BP)/daytime ambulatory BP × 100%
We also computed for the cardiac workload (systolic BP × heart rate) based on values obtained on ambulatory BP monitoring.
Statistical Analysis
Data were analyzed using paired t-test between the ABPM parameters before and after treatment. The two groups were also compared using t-test to detect a difference between the groups after treatment. The data were analyzed using intention-to-treat.
Results
Patient Characteristics
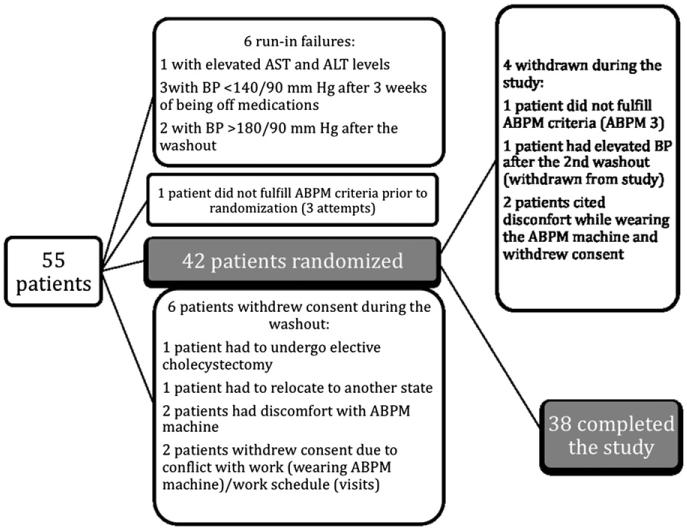
Fifty-five patients were screened, of whom six did not meet the eligibility criteria before randomization (Figure 2). One patient signed the consent form but withdrew consent the next day, before the baseline visit. Three patients withdrew consent during washout, before randomization. Two patients refused to participate after the first ABPM measurement, citing discomfort while wearing the ABPM machine as the reason. One patient did not fulfill the ABPM criteria after three attempts before randomization and was dropped from the study.
Figure 2.
Patient Exclusionary Cascade.
Of the 42 that were randomized, 4 were withdrawn from the study: 1 patient was withdrawn because of SBP >180 mm Hg during the washout before the crossover, 1 patient did not fulfill the ABPM criteria after three attempts for ABPM number 3 and was dropped from the study, and 2 patients withdrew consent after randomization because of discomfort from wearing the ABPM machine (Figure 2). Thirty-eight patients completed the study (both phases).
Table 1 lists the baseline characteristics of the randomized patients. The mean age was 52±12 years. Half of the patients (52%) were African American, and 46% were female. Patients were obese (body mass index 31.8 ± 5.9 kg/m2), taking an average of 1.4 antihypertensive medications, and with a mean blood pressure of 152.4 ± 11.3/92.8 ± 7.5 mm Hg at the time of randomization.
Table 1.
Baseline characteristics of randomized patients
| Parameter (Units) n = 42 | Value |
|---|---|
| Age (y) | 51.7 ± 11.6 |
| Race (% African Americans) | 52% |
| Gender (% females) | 46% |
| Weight (kg) | 92.3 ± 19.7 |
| Height (meters) | 1.70 ± 0.11 |
| Body mass index (kg/m2) | 31.8 ± 5.9 |
| Waist circumference (cm) | 105.7 ± 15.1 |
| Smoker (current or past) | 37% |
| Years smoked | 7.6 ± 10.4 |
| Years hypertensive | 10.5 ± 8.6 |
| Baseline systolic blood pressure (mm Hg) | 152.4 ± 11.3 |
| Baseline diastolic blood pressure (mm Hg) | 92.8 ± 7.5 |
| Number of antihypertensive medications | 1.4 ± 0.9 |
| ● No medications | 4 |
| ● 1 medication | 18 |
| ● 2 medications | 21 |
| ● 3 medications | 2 |
Two out of the 38 completers had a washout period >2 weeks, as the BP did not yet fulfill the stability criteria after that period. These same patients had a washout period >1 week after the first phase of nebivolol treatment was completed (before the crossover). Exclusion of these two patients from the analysis did not significantly affect the results.
Ambulatory BP Monitoring Parameters
On randomization, the mean daytime BP was 162 ± 14/95 ± 9 mm Hg, the mean nighttime BP was 150 ± 17/84 ± 11 mm Hg, and the mean 24-hour BP was 160 ± 13/93 ± 9 mm Hg. There were 13 dippers (where the nocturnal BP decline was 10%–20% of the daytime BP values) at baseline, and 6 patients had the reverse dipping pattern (where nocturnal BP was higher than daytime BP).
Morning- and evening-dosed nebivolol produced significant reductions in daytime, nighttime, and 24-hour systolic and diastolic BP, and the degree of BP reduction was similar between the two treatment allocations (Table 2). Nebivolol given in the morning or evening reduced the mean daytime BP by 12/9 mm Hg, the mean nighttime BP by 11/8 mm Hg, and the mean 24 hour BP by 12/8 mm Hg from baseline. There were more patients with a dipping status when nebivolol was dosed in the morning (15 vs. 11 with evening dosing, P = .373).
Table 2.
Ambulatory blood pressure parameters at baseline and after 3 weeks of AM and PM dosed nebivolol
| Parameter | Baseline (mm Hg) | AM Nebivolol (mm Hg) | PM Nebivolol (mm Hg) | P Value (AM vs. PM) |
|---|---|---|---|---|
| Daytime SBP | 162.49 | 150.44* | 150.82* | .915 |
| Daytime DBP | 95.15 | 86.23* | 86.32* | .975 |
| Nighttime SBP | 150.46 | 139.67* | 139.84* | .962 |
| Nighttime DBP | 84.39 | 76.92* | 77* | .977 |
| 24 hr SBP | 159.67 | 147.97* | 148.24* | .940 |
| 24 hr DBP | 92.56 | 84.13* | 84.16* | .991 |
| SBP on awakening | 168.82 | 153.33* | 148.42* | .373 |
| DBP on awakening | 97.74 | 87.92* | 87.42* | .854 |
| Pre-awake SBP | 151.56 | 139.74† | 139.58* | .967 |
| Pre-awake DBP | 87.08 | 74.18* | 77.92* | .140 |
AM, morning; DBP, diastolic blood pressure; PM, evening; SBP, systolic blood pressure.
P < .001 vs. baseline.
P < .005 vs. baseline.
The mean BP on awakening was 153.3 ± 20.5/87.9 ± 11.5 mm Hg when nebivolol was taken in the morning, and 148.4 ± 27.3/87.4 ± 11.3 mm Hg when nebivolol was dosed at night (NS; P = .854; Table 3). The systolic trough to morning MBPS was significantly reduced from baseline in both treatment schedules. The evening dose of nebivolol resulted in greater reduction of systolic trough to morning MBPS than nebivolol given in the morning (11.8 vs. 7.54 mm Hg), but the difference was not statistically significant (P = .497) (Table 3). The slope of the early-morning BP was decreased (flattened curve) following the administration of nebivolol (both morning and evening dosing), but the change did not reach statistical significance (Table 4). Evening dosing of nebivolol also significantly reduced prewaking systolic BP from baseline.
Table 3.
Change in computed ambulatory blood pressure parameters after 3 weeks of AM or PM dosed nebivolol
| Parameter | AM Nebivolol (mm Hg) | PM Nebivolol (mm Hg) | P Value (AM vs. Baseline) | P Value (PM vs. Baseline) | P Value (AM vs. PM Nebivolol) |
|---|---|---|---|---|---|
| Trough MSBPS | −7.54 ± 18.23 | −11.08 ± 31.41 | .014 | .034 | .497 |
| Trough MDBPS | −3.64 ± 15.33 | −4.03 ± 13.70 | .146 | .074 | .879 |
| Prewaking SBP | −1.62 ± 18.32 | −8.64 ± 26.46 | .585 | .048 | .137 |
| Prewaking DBP | 3.08 ± 14.29 | −1.41 ± 10.88 | .187 | .423 | .022 |
| Nocturnal SBP fall | −9.69 ± 20.54 | −4.69 ± 25.29 | .005 | .254 | .130 |
| Nocturnal DBP fall | −4.49 ± 15.20 | −3.23 ± 17.32 | .073 | .251 | .623 |
| Nocturnal SBP dip | −2.07% ± 9.56% | −0.12% ± 8.25% | .893 | .928 | .974 |
| Nocturnal DBP dip | −0.6% ± 11.1% | −0.48% ± 9.2% | .726 | .751 | .986 |
AM, morning; DBP, diastolic blood pressure; MDBPS, mean diastolic blood pressure surge; PM, evening; MSBPS, mean systolic blood pressure surge; SBP, systolic blood pressure.
All values (except prewaking DBP for AM nebivolol) reflect a decline from baseline levels.
Table 4.
Blood pressure slope in the early morning and cardiac workload with morning vs. evening dosing of nebivolol
| Parameter | Baseline | AM Nebivolol | PM Nebivolol | P Value (AM vs. PM) |
|---|---|---|---|---|
| SBP slope | 4.31 ± 2.33 | 3.91 ± 3.85 | 2.21 ± 6.29 | .155 |
| DBP slope | 2.67 ± 2.50 | 3.44 ± 2.35 | 2.38 ± 1.86 | .031 |
| Daytime cardiac workload | 12.8 ± 1.9 × 103 | 10.4 ± 1.8 × 103* | 10.3 ± 1.7 × 103* | .812 |
| Nighttime cardiac workload | 10.6 ± 1.8 × 103 | 8.98 ± 1.8 × 103* | 8.8 ± 1.5 × 103* | .617 |
| 24-hour cardiac workload | 12.3 ± 1.7 × 103 | 10.1 ± 1.8 × 103* | 9.9 ± 1.6 × 103* | .747 |
AM, morning; DBP, diastolic blood pressure; PM, evening; SBP, systolic blood pressure.
P < .001 from baseline.
There were two patients who were taking nebivolol in the morning who had diastolic BP <60 mm Hg while asleep (43 and 47 mm Hg). Systolic BP was between 94 and 98 mm Hg for these patients. These same patients had a lowest asleep BP of 118/64 and 104/63 when they were taking nebivolol in the evening. In contrast, two patients who were taking evening-dosed nebivolol had a lowest asleep BP of 94/46 and 93/47 mm Hg, which was again not noted while they were taking morning-dosed nebivolol. There were no episodes of a low systolic BP (<90 mm Hg) while asleep in any of the study patients.
Cardiac workload was reduced significantly following morning or evening administration of nebivolol, and the difference between the two dosage schedules was not significant (Table 4).
Adverse Events
Nebivolol was well tolerated with no serious adverse drug reactions reported by the study patients. One patient had a gout flare during the washout period: one patient had diarrhea that resolved spontaneously and did not recur even when nebivolol was continued, and a third patient had right leg cramps, which resolved spontaneously after 1 day and were not associated with electrolyte abnormalities. There was no report of bronchospastic events, sexual dysfunction, or fatigue.
Discussion
The primary objective of this study was to determine the effect of the timing (morning vs. evening) of nebivolol administration on the diurnal pattern of BP, particularly the morning surge and nocturnal BP. Our results show that nebivolol was effective in reducing daytime, nighttime, 24-hour ambulatory BP and systolic trough to morning MBPS, and there was no difference between morning and evening dosing. This signifies that nebivolol consistently lowers the daytime, nighttime, and 24-hour BP irrespective of the time of day when it was taken, supporting its use as a true once-per-day antihypertensive agent without any requirements for specific timing of dosing.
Although it has been used in Europe for almost 10 years, nebivolol was only recently introduced to the US market. Like carvedilol, nebivolol belongs to the third-generation of β-blockers, which possess direct vasodilator properties in addition to their adrenergic blocking characteristics.9,10 Nebivolol has the highest β1-receptor affinity among β-blockers and, most interestingly, substantially improves endothelial dysfunction via its strong stimulatory effects on the eNOS, resulting in nitric oxide release and via its anti-oxidant properties.10 Because impaired endothelial function plays a major role in the pathophysiology of hypertension, atherosclerotic vascular disease, and congestive heart failure, the endothelial effects of nebivolol suggest that this drug might provide additional benefit beyond β1-receptor blockade. Clinically, this compound has been proven to have antihypertensive and anti-ischemic effects as well as beneficial effects on hemodynamics and prognosis in patients with chronic congestive heart failure.10 Our data show decreased cardiac workload following nebivolol administration. Further studies to evaluate nebivolol for other properties that might be clinically beneficial are warranted.
Hypertension is associated with increased cardio- and cerebrovascular morbidity and mortality, and antihypertensive drugs have been shown to reduce the risk of adverse cardiovascular events. Both myocardial infarction and stroke are more common during the morning hours; a time when both normo- and hypertensives show a circadian peak in BP.2 Although clinicians have a number of safe and well-tolerated antihypertensive agents in various classes and formulations at their disposal, few are designed to specifically attenuate the morning BP surge while maintaining 24-hour efficacy. Whereas some once-per-day anti-hypertensive drugs/drug preparations have been studied with morning and evening dosing, most have not. It was in this setting that we tested the efficacy of nebivolol when dosed in the morning vs. the evening in regard to its 24-hour efficacy and its effect on the MBPS and nocturnal BP.
In our study, the morning rise in systolic BP was 17.2 ± 9.3 mm Hg (as reflected by prewaking MBPS, or the difference between BP on awakening and the prewaking BP), comparable to the findings of Gosse and colleagues, where the morning BP rose by 14 ± 16 mm Hg on rising.13 In their cohort, which included 507 initially untreated hypertensive patients, the mean systolic BP change on rising was correlated with the incidence of cardiovascular events and left ventricular hypertrophy, but the study was not powered to show a decrease in risk following treatment. In our study, evening dosing of nebivolol significantly decreased the prewaking systolic MBPS by 8.64 ± 26.46 mm Hg (P = .048), an effect that was not seen with morning dosing. This was driven mostly by a lower (non-statistically significant) systolic BP on awakening with evening-dosed nebivolol compared with nebivolol given in the morning (148 vs. 153 mm Hg, P = .373), because the preawake systolic BP values were similar in the two groups (Table 2). While the difference between the two treatment schedules was not statistically significant, the trend in the finding that nebivolol given in the evening produces a significant decrease in both prewaking and trough to morning systolic MBPS may argue that evening dosing of nebivolol may be better than morning dosing in reducing cardiovascular risk associated with a higher MBPS. This would need to be explored in an appropriately powered larger trial.
Limitations to this study include its exploratory nature, its short duration of treatment, and reliance on patient self-report with regards to sleeping and waking up times. Nevertheless, nebivolol (at 10 mg/day) was shown to be effective in reducing 24-hour BP in as early as 2 weeks of treatment. Further studies are needed to elucidate the impact of morning vs. evening dosing of nebivolol on cardiovascular outcomes.
Conclusions
Evening-dosed nebivolol may offer a therapeutic advantage over morning-dosed nebivolol in reducing the prewaking systolic BP.
Acknowledgments
Funded by an investigator-initiated grant provided by Forest Laboratories.
References
- 1.Staessen JA, Bieniaszewski L, O'Brien E, Gosse P, Hayashi H, Imai Y, et al. Nocturnal blood pressure fall on ambulatory monitoring in a large international database. The “Ad Hoc” Working Group. Hypertension. 1997;29:30–9. doi: 10.1161/01.hyp.29.1.30. [DOI] [PubMed] [Google Scholar]
- 2.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–6. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 3.Polonia J, Amado P, Barbosa L, Nazaré J, Silva JA, Bertoquine S, et al. Morning rise, morning surge and daytime variability of blood pressure and cardiovascular target organ damage. A cross-sectional study in 743 subjects. Rev Port Cardiol. 2005;24:65–78. [PubMed] [Google Scholar]
- 4.Tofler GH, Muller JE, Stone PH, Forman S, Solomon RE, Knatterud GL, et al. Modifiers of timing and possible triggers of acute myocardial infarction in the Thrombolysis in Myocardial Infarction Phase II (TIMI II) Study Group. J Am Coll Cardiol. 1992;20:1049–55. doi: 10.1016/0735-1097(92)90356-r. [DOI] [PubMed] [Google Scholar]
- 5.Thakur RK, Hoffman RG, Olson DW, Joshi R, Tresch DD, Aufderheide TP, et al. Cardiac variation in sudden cardiac death: effects of age, sex and initial cardiac rhythm. Ann Emerg Med. 1996;27:29–34. doi: 10.1016/s0196-0644(96)70292-5. [DOI] [PubMed] [Google Scholar]
- 6.Glasser SP, Neutel JM, Gana TJ, Albert KS. Efficacy and safety of a once daily graded-release diltiazem formulation in essential hypertension. Am J Hypertens. 2003;16:51–8. doi: 10.1016/s0895-7061(02)03153-9. [DOI] [PubMed] [Google Scholar]
- 7.White WB, Sica DA, Calhoun D, Mansoor GA, Anders RJ. Preventing increases in early-morning blood pressure, heart rate, and the rate pressure product with controlled onset extended release verapamil at bedtime vs. enalapril, losartan, and placebo on arising. Am Heart J. 2002;144:657–65. doi: 10.1067/mhj.2002.124866. [DOI] [PubMed] [Google Scholar]
- 8.De la Sierra A, Redon J, Banegas JR, Sequra J, Parati G, Gorostidi M, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466–72. doi: 10.1161/HYPERTENSIONAHA.108.124008. [DOI] [PubMed] [Google Scholar]
- 9.Munzel T, Gori T. Nebivolol. The somewhat-different β-adrenergic receptor blocker. J Am Coll Cardiol. 2009;54:1491–9. doi: 10.1016/j.jacc.2009.05.066. [DOI] [PubMed] [Google Scholar]
- 10.Veverka A, Salinas JL. Nebivolol in the treatment of chronic heart failure. Vasc Health Risk Manage. 2007;3:647–54. [PMC free article] [PubMed] [Google Scholar]
- 11.Cockcroft JR. A review of the safety and efficacy of nebivolol in the mildly hypertensive patient. Vasc Health Risk Manage. 2007;3:909–17. [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen ME, Cockcroft JR. The latest generation of beta-blockers: new pharmacologic properties. Curr Hypertens Rep. 2006;8:279–86. doi: 10.1007/s11906-006-0065-0. [DOI] [PubMed] [Google Scholar]
- 13.Gosse P, Lasserre R, Minifie C, Lemetayer P, Clementy J. Blood pressure surge on rising. J Hypertens. 2004;22:1113–8. doi: 10.1097/00004872-200406000-00011. [DOI] [PubMed] [Google Scholar]