Abstract
Glyceollins are stress-induced compounds in soybeans with bioactive properties distinct from parent soy isoflavones. The goals of this study were to evaluate effects of dietary glyceollin-enriched and standard soy protein isolates and identify candidate target pathways of glyceollins on transcriptional profiles within mammary gland tissue. Thirty female postmenopausal cynomolgus monkeys were randomized to diets containing one of three protein sources for 3 weeks: (1) control casein / lactalbumin (C/L); (2) standard soy protein containing 194 mg/day isoflavones (SOY); and (3) glyceollin-enriched soy protein containing 189 mg/day isoflavones + 134 mg/day glyceollins (GLY). All diets contained a physiologic dose of estradiol (E2) (1 mg/day). All doses are expressed in human equivalents scaled by caloric intake. Relative to the control C/L diet, the GLY diet resulted in greater numbers of differentially regulated genes which showed minimal overlap with those of SOY. Effects of GLY related primarily to pathways involved in lipid and carbohydrate metabolism, including peroxisome proliferator-activated receptor (PPAR)-gamma and AMP-activated protein kinase (AMPK) signaling, adipocytokine expression, triglyceride synthesis, and lipase activity. Notable genes upregulated by the GLY diet included PPAR-gamma, adiponectin, leptin, lipin 1, and lipoprotein lipase. The GLY diet also resulted in lower serum total cholesterol, specifically non-high-density lipoprotein cholesterol, and increased serum triglycerides compared to the C/L diet. No effects of GLY or SOY were seen on serum insulin, adipocytokines, or vascular and bone turnover markers. These preliminary findings suggest that glyceollin-enriched soy protein has divergent effects from standard soy with some specificity for adipocyte activity and nutrient metabolism.
Keywords: glyceollin, soy, isoflavone, estrogen receptor, metabolism
Introduction
Diet is a key factor in the etiology of many chronic diseases, including cardiovascular disease, osteoporosis, diabetes, and cancer. Much recent interest has focused on the role of specific bioactive components, particularly from dietary plants, in the prevention and management of these conditions (1). Isoflavonoids are an important class of bioactive phytochemicals widely consumed as part of soy-based foods. Soy protein is rich in the glycosylated forms of the isoflavones genistein and daidzein, which have structural similarities to endogenous estrogens and exhibit a variety of biological functions relevant to human health (2, 3).
Recent evidence indicates that isoflavone metabolites may also mediate certain health-related effects of soy foods. The best-studied example is equol, which is formed from daidzein by gut bacteria in a subset of human soy consumers (4) and various non-human species (5). Under the influence of stressors such as trauma or infection, daidzein may also act as a precursor in soybeans to a unique class of defensive compounds called glyceollins (6, 7). Prior studies have shown that glyceollins exhibit distinct properties compared to genistein and daidzein, including inhibition of estrogen receptor (ER) signaling (7-9), which correlated with a comparable suppression of estrogen–induced proliferation of breast cancer cells. Glyceollins have also been characterized for their ability to inhibit fungal growth (10, 11). More recently, glyceollins have been shown to help normalize glucose homeostasis in vitro by potentiating β-cell function and survival and to improve glucose utilization in 3T3-L1 adipocytes (12). Glyceollins have also demonstrated anti-inflammatory effects (13-14). These results suggest that the glyceollins have unique effects potentially relevant to human health (15).
Although much research has focused on the anticancer ability of the glyceollins, the effects of glyceollins on other biological pathways and systems are less known. Of particular importance is the in vivo activity of the glyceollins when consumed at dietary levels. In the current study we evaluated the short-term effects of glyceollin-enriched soy protein on gene expression profiles in mammary gland adipose tissue. Also, serum lipids, vascular and bone markers, and metabolic markers were examined in each dietary group. Our goals were to identify candidate target pathways of glyceollins and evaluate comparative effects of glyceollin-enriched and standard soy protein isolates.
Materials and Methods
Study design and diets
Subjects for this study were 30 adult female surgically postmenopausal cynomolgus macaques (Macaca fascicularis) with an average age of 17.8 ± 0.5 years. All animals had been ovariectomized for 4 years and housed in stable social groups of 3-4 animals each. Animals were randomized by social group to receive one of three diets in which the protein source varied as follows: (1) casein / lactalbumin (C/L, n = 9); (2) soy protein isolate containing 193.6 mg/1800 kcal isoflavones (SOY, n = 11); and (3) glyceollin-enriched soy protein containing 188.5 mg/1800 kcal isoflavones and 134.1 mg/1800 kcal glyceollins (GLY, n = 10). All isoflavone doses are expressed in aglycone equivalents. Each diet also included a physiologic dose of micronized 17beta-estradiol (E2, 1 mg/1800 kcal), as described previously (16). Additional details regarding diet production, composition, and analysis are also provided in this prior report (16).
Briefly, the GLY diet contained 959.5 μg of unconjugated glyceollins per gram of product (76.8% glyceollin I, 9.9% glyceollin II, and 13.6% glyceollin III), as determined by high-pressure liquid chromatography (HPLC) and UV-monitoring (visible spectrophotometry). Relative isoflavone content in aglycone units was 61.5% genistein, 34.6% daidzein, and 3.8% glyceitin for SOY and 52.6% genistein, 43.0% daidzein, and 4.4% glycitein for GLY. Diets were isocaloric and similar in macronutrients, cholesterol, calcium, and phosphorus. The soy protein isolate was generously provided by The Solae Company (St. Louis, MO), while the glyceollin-enriched protein was provided through collaborative efforts of The Solae Company, the Southern Regional Research Center of the United States Department of Agriculture (USDA), and the Tulane University School of Medicine. Estradiol tablets were obtained from Mylan Pharmaceuticals (Morganton, WV).
Animals were fed 120 kcal per kg body weight (BW) once daily. Daily doses of isoflavones, glyceollins, and E2 were scaled to 1800 kcal of diet (rather than BW) to account for differences in metabolic rates between the monkeys and human subjects (17). Monkeys were thus given 0.44 mg (C/L), 12.91 mg (SOY), or 12.57 mg (GLY) of isoflavones/kg BW; 8.94 mg glyceollins/kg BW (GLY); and 66.7 μg of E2/kg BW (all groups) each day. All procedures involving animals were conducted in compliance with State and Federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by the Wake Forest Institutional Animal Care and Use Committee. The facilities and laboratory animal program of Wake Forest University are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Gene microarrays and quantitative gene expression assays
For microarray analyses, total RNA was extracted from frozen mammary biopsies using Tri Reagent (Molecular Research Center, Cincinnati, OH), purified using RNeasy Mini kit (QIAGEN, Valencia, CA), and quantitated using a NanoDrop ND-1000 UV-vis spectrophotometer (NanoDrop, Wilmington, DE). Biopsy collection has been described previously (18). RNA intactness and quality were confirmed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Wilmington, DE). Four samples from each group (n = 12 total) were used for microarray analysis. RNA was hybridized to GeneChip Rhesus Macaque Genome Arrays (Affymetrix, Santa Clara, CA), washed, and scanned at Cogenics® (now Beckman-Coulter Genomics, Morrisville, NC). Intensity data were extracted from scanned images using GeneChip Operating Software (Affymetrix).
Expression of selected gene targets related to lipid and glucose metabolism pathways (identified on microarray analysis) were determined using quantitative real-time polymerase chain reaction (qRT-PCR). Macaque-specific qRT-PCR primer-probe sets were generated for the internal control genes GAPDH and ACTB, while rhesus macaque or human ABI Taqman primer-probe sets were used for target assays (see Supporting Information, Table S1). Total RNA was extracted, quantitated, and reverse-transcribed as above from all mammary samples (n = 30). Real-time PCR reactions were performed on an Applied Biosystems ABI PRISM® 7500 Fast Sequence Detection System using Taqman reagents and a standard thermocycling protocol. Relative expression was determined using the Δ ΔCt method calculated by ABI Relative Quantification 7500 Software v2.0.1. Stock mammary tissue was run in triplicate on each plate as an external calibrator.
Microarray data were analyzed using the GeneSifter® software program (Geospiza, Seattle, WA). Intensity data were RMA-normalized, converted to a log2 scale, screened for heterogeneity among samples and groups, and evaluated using supervised analysis of variance (ANOVA) and pairwise comparisons between treatments. Principal components analysis (PCA), pattern navigation, cluster analysis, heatmapping, and KEGG pathway analyses were performed on filtered data subsets, as described in results. Differences in gene numbers altered by each treatment were compared using a Chi-Square Test. Euclidean distances (representing the numeric difference between treatment vectors) were calculated as part of hierarchical clustering dendrograms using average linkage. Pathways were evaluated via KEGG analyses in which a z-score > 2.0 was considered significant overrepresentation of genes in a particular pathway. Representation of differentially expressed genes within specific canonical and functional categories was evaluated using Ingenuity Pathway Analysis (IPA) software v8.0 (Ingenuity Systems, Redwood City, CA). Significance of gene numbers within a given category was determined in IPA using a Fisher's Exact Test with Benjamini and Hochberg correction and expressed as -log10 (P value) for each treatment group.
Serum markers
Blood was collected at baseline and post-treatment for measurement of serum markers. Serum concentrations of total glyceollins (I-III) and soy isoflavonoids were measured by liquid chromatographic-photodiode array mass spectrometric analysis, as described previously (16, 18). Serum concentrations of E2, vascular and bone turnover markers [monocyte chemoattractant protein (MCP)-1, endothelin (ET)-1, and collagen degradation products (C-terminal crosslink of type 1 collagen, Ctx)], and metabolic markers [insulin, glucagon-like peptide (GLP)-1), adiponectin, and leptin] were measured using commercially available kits and protocols for radioimmunoassay (E2, DSL-4800 ultra-sensitive from Diagnostic Systems Laboratories, Webster, TX) (12) or enzyme-linked immunosorbent assays [MCP-1 and ET-1 from R&D Systems, Minneapolis, MN; Ctx (Crosslaps®) from Osteometer Biotech A/S, Herlev, Denmark; GLP-1 (Total), leptin, and insulin from ALPCO Diagnostics, Salem, NH; and adiponectin from Mercodia, Winston-Salem, NC] (19-21). Total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, and triglyceride (TG) concentrations were measured using enzymatic methods on a COBAS FARA II analyzer (Roche Diagnostics, Montclair, NJ) with standard protocols and reagents. Serum assays were run in a fully standardized clinical chemistry laboratory at Wake Forest School of Medicine (WFSM). HDL concentrations were measured using the heparin-manganese precipitation procedure (20). Low-density lipoprotein cholesterol (LDL) plus very low-density lipoprotein cholesterol (VLDL) was calculated as the difference between TC and HDL. Samples from baseline and post-treatment timepoints were run at the same time for all serum measures.
Statistical analysis
Non-microarray data were analyzed using the SAS statistical package (version 9.1, SAS Institute; Cary, NC). A general linear model (ANOVA) was used to determine mean values and calculate group differences. All data were evaluated for normal distribution and homogeneity of variances among groups. Gene expression and serum marker data were log-transformed to improve distribution, and data were then retransformed to original scale and reported as fold-change of control with 90% confidence interval. One animal in the SOY group was excluded from gene expression analyses based on poor RNA quality. Final group sizes were thus n = 9 for C/L and n = 10 for SOY and GLY for qRT-PCR data. Post-treatment serum lipid and marker data were covaried by respective baseline values. All pairwise P-values were adjusted for the number of pairwise tests using a Bonferroni correction. A two-tailed significance level of 0.05 was chosen for all comparisons.
Results
Dietary intake
Body weight, serum E2, and serum isoflavonoids were measured as indicators of diet intake. Treatment groups did not differ significantly in mean BW at baseline or post-treatment, in BW change, or in serum E2 concentrations (ANOVA P > 0.05 for all) (16). Mean serum glyceollin concentrations were negligible in the SOY group and 134.2 ± 34.6 nmol/L in the GLY group at 4 hrs post-feeding (P < 0.001 compared to SOY), while total serum isoflavonoid concentrations were significantly higher in the SOY and GLY groups compared to C/L group at 4 hrs post-feeding (P < 0.001 for both) and 24 hrs post-feeding (P < 0.05 for both) (16). The SOY and GLY groups did not differ in serum isoflavonoid concentrations at either 4 hrs post-feeding (P = 0.59) or 24 hrs post-feeding (P = 0.73). Total serum isoflavonoids for the SOY and GLY diets at 4 hrs post-feeding were comparable to those reported in human soy intervention studies following a high-soy meal (22).
Gene expression profiles
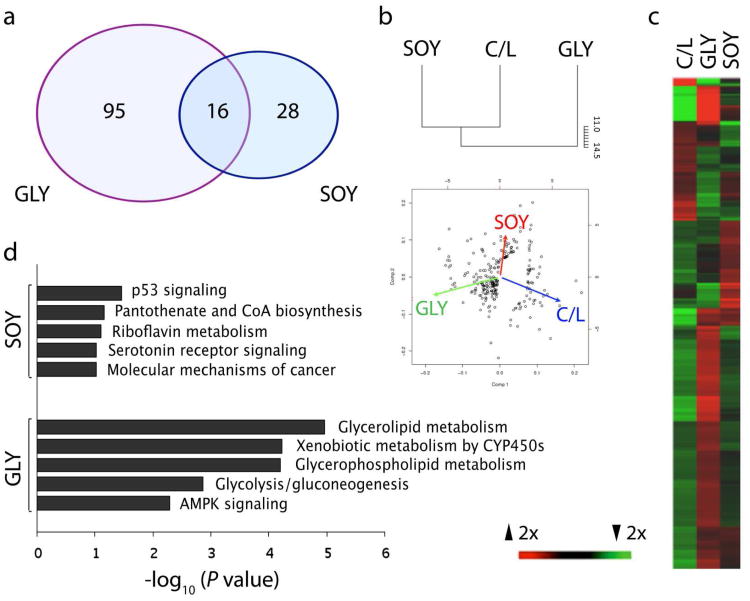
Global expression profiling of mammary tissues showed that relative to the C/L reference group, greater numbers of genes were differentially regulated by GLY compared to SOY. For example, among 139 total (named) genes with FC > 1.5 and ANOVA P < 0.05, a greater number were differentially regulated in the GLY group (n = 111) than in the SOY group (n = 44) (P < 0.001 by Chi-square test) with only 12% overlap between GLY and SOY genes (Figure 1a). Supervised hierarchical clustering showed that C/L and SOY (rather than GLY and SOY) were the most closely associated groups with a Euclidean distance of ∼11 for genes significantly altered at FC > 1.5 (Figure 1b). The distinction in profiles for GLY from SOY was also evident qualitatively from PCA vectors (Figure 1b) and heatmaps (Figure 1c) for genes altered at FC > 1.5. A complete list of all significantly altered genes at FC > 1.5 by ANOVA and by supervised pairwise comparisons is provided in Supporting information, Table S2.
Figure 1.
Gene expression profiles in mammary fat for diets containing casein / lactalbumin (C/L), standard soy protein (SOY), and glyceollin-enriched soy protein (GLY). a, Venn diagram showing total number of genes (with GenBank identifiers) with FC > 1.5, ANOVA P < 0.05, quality > 2, and t-test P < 0.05 compared to C/L group. b-c, Hierarchical clustering dendrogram and principal component analysis (b) and corresponding heat map (c) for gene probes with FC > 1.5 and ANOVA P < 0.05 (n = 252). Euclidean distance and average linkage were used for dendrogram and clustering. Red and green colors on heatmap indicate increased and decreased expression, respectively. d, Top canonical pathways among genes significantly altered at FC > 1.5 by GLY (n= 129 mapped genes) and SOY (n= 129 mapped genes) diets identified by Ingenuity pathway analysis.
Pathway analyses
Pathway analysis was used to sort altered genes by canonical and functional categories. The most overrepresented canonical pathways for altered genes in the GLY group all related to lipid, carbohydrate, and/or energy metabolism. These pathways included glycerophospholipid and glycerolipid metabolism, cytochrome p450 metabolism, and AMPK signaling (P < 0.01 for all) (Figure 1d). Overrepresentation of these pathways or related pathways was not seen for the SOY group. The most overrepresented functional pathways in IPA for GLY genes were lipid metabolism, small molecule biochemistry, and carbohydrate metabolism (P < 0.05 for all). The most significant subcategory within lipid metabolism was triacylglycerol biosynthesis (−log10P value = 6.9). Similar patterns were seen with KEGG pathway analysis, which revealed significant overrepresentation of altered genes (z-score > 2) for the GLY group related to lipid, glucose, and energy metabolism (Table 1). Notable pathways here included glycerolipid metabolism, peroxisome proliferator-activating receptor (PPAR) signaling, and cytochrome p450 metabolism.
Table 1.
Pathway analysis of transcripts significantly altered in mammary tissue by GLY and SOY diets relative to the control C/L diet.
| Pathway | list | # ↑ | # ↓ | geneset | z-score ↑ | z-score ↓ |
|---|---|---|---|---|---|---|
| GLY (z-score > 2) | ||||||
|
|
||||||
| Glycerophospholipid metabolism | 5 | 5 | 0 | 67 | 5.17 | -0.38 |
| Glycerolipid metabolism | 4 | 4 | 0 | 41 | 5.47 | -0.29 |
| Metabolism of xenobiotics by cytochrome P450 | 4 | 4 | 0 | 59 | 4.33 | -0.35 |
| ECM-receptor interaction | 4 | 3 | 1 | 76 | 2.49 | 2.15 |
| Malaria | 3 | 3 | 0 | 48 | 3.54 | -0.32 |
| Drug metabolism - cytochrome P450 | 3 | 3 | 0 | 57 | 3.13 | -0.35 |
| PPAR signaling pathway | 3 | 3 | 0 | 58 | 3.09 | -0.35 |
| SOY (z-score > 2) | ||||||
|
|
||||||
| ECM-receptor interaction | 4 | 3 | 1 | 76 | 5.01 | 3.12 |
| Malaria | 4 | 3 | 1 | 48 | 6.54 | 4.05 |
| Focal adhesion | 4 | 3 | 1 | 184 | 2.75 | 1.75 |
| Hypertrophic cardiomyopathy | 3 | 3 | 0 | 76 | 5.01 | -0.30 |
| Dilated cardiomyopathy | 3 | 3 | 0 | 82 | 4.79 | -0.31 |
Pathways were identified by KEGG analysis from gene probes with FC > 1.5, P < 0.05, and at least 3 genes altered in the same direction within a pathway. Only pathways with significant z-score (> 2) are shown.
Quantitative gene expression
To further examine these findings, select gene targets related to lipid and carbohydrate metabolism, PPAR signaling, and/or adipocytokine activity were evaluated by qRT-PCR. Nine out of the 16 targets evaluated were upregulated in the GLY group compared to C/L (P < 0.05 for all) while none of the 16 differed between SOY and C/L groups (Table 2). Targets increased in the GLY group included genes related to adipocytokine signaling (adiponectin and leptin), carbohydrate metabolism (glycerol-3-phosphate dehydrogenase and glycogen synthase), PPAR signaling (PPAR-gamma and lipin 1), and lipid metabolism (lipoprotein lipase and perilipin).
Table 2.
Dietary protein effects on relative expression of select genes related to lipid and glucose metabolism, PPAR signaling, and adipocytokine activity within mammary adipose tissue, as determined by qRT-PCR.
| Gene | C/L | SOY | GLY | Pathways |
|---|---|---|---|---|
| ADIPOQ | 1.0 (0.7-1.3) | 1.5 (1.2-1.9) | 3.2 (2.6-3.8)**,# | adipocytokine & PPAR signaling; type II diabetes |
| DGAT2 | 1.0 (0.7-1.4) | 2.6 (2.1-3.2) | 3.0 (2.5-3.6)* | glycerolipid metabolism, triglyceride biosynthesis |
| GPAM | 1.0 (0.8-1.2) | 1.1 (0.9-1.3) | 1.8 (1.6-2.1) | glycerolipid, glycerophospholipid, & fatty acid metabolism, triglyceride biosynthesis |
| GPD1 | 1.0 (0.7-1.3) | 2.0 (1.6-2.5) | 3.4 (2.8-4.2)** | glycerolipid, glycerophospholipid, & carbohydrate metabolism, gluconeogenesis |
| GYS1 | 1.0 (0.8-1.2) | 1.4 (1.2-1.6) | 1.9 (1.6-2.1)** | starch metabolism, insulin signaling |
| LASS6 | 1.0 (0.8-1.2) | 0.6 (0.4-0.7) | 0.5 (0.4-0.7) | lipid biosynthesis |
| LEP | 1.0 (0.7-1.4) | 2.4 (1.8-3.3) | 4.4 (3.4-5.9)** | adipocytokine, Jak-STAT, & cytokine-cytokine signaling |
| LPIN1 | 1.0 (0.8-1.3) | 1.9 (1.6-2.4) | 2.7 (2.2-3.2)* | PPAR & insulin signaling; triglyceride & nutrient metabolism |
| LPL | 1.0 (0.8-1.3) | 1.4 (1.1-1.7) | 2.7 (2.2-3.3)** | glycerolipid metabolism; PPAR signaling |
| PGC1A | 1.0 (0.7-1.3) | 0.6 (0.4-0.8) | 0.9 (0.7-1.1) | adipocytokine & insulin signaling pathways; glucose homeostasis; fatty acid oxidation; gluconeogenesis |
| PGC1B | 1.0 (0.8-1.2) | 0.9 (0.7-1.1) | 1.1 (1.0-1.3) | PPAR, estrogen receptor, & |
| glucocorticoid signaling | ||||
| PLIN | 1.0 (0.8-1.3) | 1.6 (1.3-1.9) | 2.7 (2.2-3.2)** | PPAR signaling |
| PPARA | 1.0 (0.8-1.2) | 1.0 (0.8-1.2) | 1.1 (0.9-1.3) | adipocytokine & PPAR signaling |
| PPARG | 1.0 (0.7-1.5) | 2.4 (1.7-3.5) | 5.6 (4.0-7.8)** | adipocytokine & PPAR signaling |
| SCD | 1.0 (0.6-1.5) | 2.2 (1.6-3.0) | 2.3 (1.6-3.1) | fatty acid biosynthesis, PPAR signaling |
| SORBS1 | 1.0 (0.8-1.2) | 1.2 (1.0-1.5) | 1.8 (1.5-2.1) | PPAR & insulin signaling |
Values represent mean fold-change relative to C/L diet with 90% confidence interval.
P < 0.05 vs C/L;
P < 0.01 vs C/L;
P < 0.05 vs SOY.
Serum markers
Serum measures did not differ significantly among groups at baseline (ANOVA P > 0.05 for all). Following treatment, the GLY group had lower TC and LDL+VLDL compared to C/L and SOY groups and greater TG compared to C/L (P < 0.01 for all) (Table 3). The SOY group also had greater TG compared to the C/L group (P = 0.02). No significant group differences were seen for serum HDL or TC to HDL ratio. Similarly, no group differences were observed for vascular (MCP-1, ET-1), bone (Ctx), or metabolic markers at baseline or post-treatment (ANOVA P > 0.05 for all).
Table 3.
| C/L | SOY | GLY | |
|---|---|---|---|
| Lipids | |||
|
|
|||
| TC (mg/dl) | 337 (311-364) | 322 (300-345) | 225 (210-242)**,## |
| TG (mg/dl) | 53 (45-62) | 97 (85-112)* | 108 (93-126)** |
| HDL (mg/dl) | 43 (37-50) | 46 (40-53) | 49 (42-57) |
| VLDL+LDL (mg/dl) | 282 (257-309) | 262 (241-284) | 171 (157-186)**,## |
| TC/HDL | 7.4 (6.3-8.8) | 7.1 (6.1-8.2) | 4.7 (4.1-5.5) |
| Vascular and bone markers | |||
|
|
|||
| MCP-1 (pg/ml) | 194 (178-212) | 206 (189-223) | 189 (174-205) |
| ET-1 (pg/ml) | 1.76 (1.53-2.03) | 1.38 (1.21-1.56) | 1.30 (1.13-1.49) |
| Ctx (ng/ml) | 0.92 (0.82-1.02) | 0.91 (0.82-1.01) | 0.84 (0.75-0.94) |
| Metabolic markers | |||
|
|
|||
| Insulin (mU/L) | 16.7 (14.0-19.8) | 20.0 (17.1-23.4) | 19.0 (16.1-22.4) |
| GLP-1 (pmol/L) | 4.1 (3.1-5.3) | 2.7 (2.1-3.4) | 2.8 (2.1-3.5) |
| Adiponectin (ug/ml) | 4.7 (4.0-5.5) | 3.1 (2.6-3.6) | 4.8 (4.1-5.5) |
| Leptin (ng/ml) | 1.1 (0.9-1.3) | 1.4 (1.2-1.6) | 0.9 (0.7-1.0) |
C, total cholesterol; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein; HDL, high-density lipoprotein; GLP-1, glucagon-like peptide-1.
Values represent mean (90% confidence interval) at post-treatment covaried by baseline measures. P values were corrected for multiple pairwise comparisons.
For conversion of lipid values to SI units (mmol/l), divide by 38.67 for TC, LDL+VLDL, and HDL, and by 88.57 for TG.
Symbols indicate significant differences with C/L group
P < 0.05
P < 0.01
or with SOY group (P < 0.01).
Discussion
Glyceollins are a novel class of phytoalexin compounds produced as defense molecules in response to stress by certain types of leguminous plants, most notably soy (8). In this study we evaluated transcriptional profiles in mammary adipose tissue resulting from glyceollin-enriched soy protein in comparison with a standard soy protein isolate. We identified distinct gene expression effects for GLY that showed little overlap with those of SOY. Effects of GLY related primarily to pathways involved in lipid and carbohydrate metabolism, including PPAR and adipocytokine signaling, lipoprotein lipase activity, and TG synthesis. The GLY diet also resulted in lower serum total cholesterol, specifically LDL and VLDL, and higher serum TGs compared to the C/L diet. These preliminary findings suggest that glyceollin-enriched soy protein has divergent effects from standard soy related to adipocyte activity and nutrient metabolism.
Prior studies investigating glyceollin effects on metabolic pathways are limited. In a recent cell culture study, glyceollins improved insulin-stimulated glucose uptake and decreased TG accumulation in 3T3-L1 adipocytes, inhibited apoptosis in beta-cells, and potentiated GLP-1 secretion in enteroendocrine cells, suggesting that glyceollins may exert beneficial effects on glucose and lipid homeostasis (12). Results here show a mixed pattern of metabolic effects. At the transcriptional level, the GLY group had increased lipoprotein lipase, adiponectin, and PPAR-gamma expression, which may be associated with a more favorable metabolic profile, while also having increased expression of TG synthesis markers such as diacylglycerol acyltransferase 2. At a systemic level, GLY effects were limited to lower VLDL+LDL cholesterol and higher TGs. The latter pattern is similar to that reported for estrogens as well as tamoxifen, a pharmacologic selective ER modulator (SERM) that lowers VLDL+LDL cholesterol while inducing fatty acid synthesis and TG formation (23-27). Other related SERM effects include induction of adiponectin and lipoprotein lipase gene expression in adipocytes (28), also consistent with GLY effects.
Mechanisms underlying transcriptional effects of GLY are unclear at this time. The SERM-like pattern noted above suggests that some GLY effects may be mediated via glyceollin interactions with ERs. Prior studies show that glyceollins competitively bind ERs and elicit ER-dependent effects distinct from the soy isoflavones daidzein and genistein (7-9). Glyceollin I in particular has ER binding characteristics and antiestrogenic properties in human breast cancer cells comparable to tamoxifen (7, 9). Adipose cells express functional ERs and respond to both estrogen and antiestrogen influences (29), suggesting that they are plausible targets for SERMs and SERM-like dietary agents. Given that GLY contains a complex protein mixture that may contain elicited bioactive compounds apart than glyceollins, other non-ER-dependent mechanisms are also possible. Further investigation is needed to better characterize GLY protein components and evaluate effects following longer-term exposure to both GLY and purified glyceollins. It is also important to note that we found no evidence for antagonism of classical estrogen effects by GLY on biomarkers related to bone resorption (Ctx) (30), vasoconstriction (ET-1) (20), or inflammation (MCP-1) (19), suggesting that short-term effects of GLY or dietary levels of glyceollins on these systems are minimal.
Dietary interventions that alter nutrient metabolism may have an important influence on risk for metabolic syndrome and related comorbid conditions. Recent evidence indicates that glyceollins are a unique class of phytoalexins in elicited or “activated” soy. Results of this study suggest that glyceollin-enriched soy protein has a mixed pattern of effects on pathways related to lipid, carbohydrate, and energy metabolism. Our findings demonstrate that soybean treatment prior to processing may alter the profile of bioactive constituents in soy protein, leading to distinct transcriptional effects from standard soy protein isolates. This concept may have implications for the identification of bioactive components in other plant-based foods.
Supplementary Material
Acknowledgments
C.E.W., J.M.C., and M.E.B. designed the study; S.M.B. provided the glyceollin-elicited soy; C.E.W. and J.M.C. conducted the research; C.E.W. and F.N.D. generated gene expression data; T.C.R. generated serum marker data; and C.E.W. analyzed data and drafted the manuscript. C.E.W. and M.E.B. had primary responsibility for final content. All authors read and approved the final manuscript. The investigators thank Maryanne Post, Lisa O'Donnell, and Jean Gardin for technical assistance.
Funding Sources. This study was supported by funds from the WFSM Department of Pathology. The parent study was supported in part by grants from the National Institutes of Health (NIH) (R01-AT00639 and K01-RR021322), the USDA, and the American Cancer Society (ACS). The SOY diet protein isolate was generously provided by The Solae Company (St. Louis, MO), while the glyceollin-enriched soy protein was supported by collaborative efforts of The Solae Company, the USDA, and the Tulane University School of Medicine. The contents are solely the responsibility of the authors and do not necessarily represent the view of the NIH, USDA, ACS, or Solae.
Abbreviations Used
- ACS
American Cancer Society
- ACTB
beta-actin
- ADIPOQ
adiponectin
- AMPK
AMP-activated protein kinase
- ANOVA
analysis of variance
- BW
body weight
- C/L
casein / lactalbumin control diet
- DGAT2
diacylglycerol O-acyltransferase homolog 2
- ECM
extracellular matrix
- ET
endothelin
- E2
17beta-estradiol
- ER
estrogen receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- FC
fold change
- GLP
glucagon-like peptide
- GLY
glyceollin-enriched soy protein diet
- GPAM
glycerol-3-phosphate acyltransferase, mitochondrial
- GPD1
glycerol-3-phosphate dehydrogenase
- GLP
glucagon-like peptide
- GYS1
glycogen synthase 1
- HDL
high-density lipoprotein cholesterol
- HPLC
high-pressure liquid chromatography
- IPA
Ingenuity Pathway Analysis
- LASS6
LAG1 homolog, ceramide synthase 6
- LDL
low-density lipoprotein cholesterol
- LEP
leptin
- LPIN1
lipin 1
- LPL
lipoprotein lipase
- MCP
monocyte chemoattractant protein
- NIH
National Institutes of Health
- PLIN
perilipin
- PPAR
peroxisome proliferator-activating receptor
- PPAR-alpha
peroxisome proliferator-activating receptor alpha
- PPAR-gamma
peroxisome proliferator-activated receptor gamma
- PGC1A
PPAR-gamma coactivator-1alpha
- PGC1B
PPAR-gamma coactivator-1 beta
- PCA
principal components analysis
- qRT-PCR
quantitative real-time polymerase chain reaction
- SCD
stearoyl-CoA desaturase
- SERM
selective estrogen receptor modulator
- SORBS1
sorbin and SH3 domain containing 1
- SOY
standard soy protein diet
- TC
total serum cholesterol
- TG
triglyceride
- VLDL
very low-density lipoprotein cholesterol
- Ctx
CrossLaps collagen degradation products
Footnotes
Author disclosures: C.E.W., S.M.B., and M.E.B. have served on a scientific advisory board for NuMe Health, LLC, and have intellectual property rights related to glyceollins. Other authors declare no conflicts of interest.
Supporting Information. Primer / probe sets for target genes evaluated by qRT-PCR (Table S1); a complete list of all significantly altered genes at FC > 1.5 by ANOVA and by supervised pairwise comparisons (Table S2). Toxicological mouse data for glyceollins is provided in Table S3. This material is available free of charge via the Internet at http://pubs.acs.org.
Literature Cited
- 1.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 2.Duncan AM, Phipps WR, Kurzer MS. Phyto-oestrogens. Best Pract Res Cloin Endocrinol Metab. 2003;17:253–271. doi: 10.1016/s1521-690x(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 3.Setchell KDR. Soy isoflavones: benefits and risk from nature's selective estrogen receptor modulators (SERMs) J Am Coll Nutr. 2001;20:354S–362S. doi: 10.1080/07315724.2001.10719168. [DOI] [PubMed] [Google Scholar]
- 4.Duncan AM, Merz-Demlow BE, Xu X, Phipps WR, Kurzer MS. Premenopausal equol excretors show plasma hormone profiles associated with lowered risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:581–586. [PubMed] [Google Scholar]
- 5.Gu L, House SE, Prior RL, Fang N, Ronis MJ, Clarkson TB, Wilson ME, Badger TM. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J Nutr. 2006;136:1215–1221. doi: 10.1093/jn/136.5.1215. [DOI] [PubMed] [Google Scholar]
- 6.Lozovaya VV, Lygin AV, Zernova OV, Li S, Hartman GL, Widholm JM. Isoflavonoid accumulation in soybean hairy roots upon treatment with Fusarium solani. Plant Physiol Biochem. 2004;42:671–679. doi: 10.1016/j.plaphy.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann MC, Tilghman SL, Boué SM, Salvo VA, Elliott S, Williams KY, Skripnikova EV, Ashe H, Payton-Stewart F, Vanhoy-Rhodes L, Fonseca JP, Corbitt C, Collins-Burow B, Howell MS, Lacey M, Shih BY, Carter-Wientjes C, Beckman B, Wiese TE, McLachlan JA, Cleveland TE, Burow ME. Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy. J Pharmacol Exp Ther. 2010;332:35–45. doi: 10.1124/jpet.109.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burow ME, Boue SM, Collins-Burow BM, Melnik LI, Duong BN, Carter-Wientjes CH, Li S, Wiese TE, Cleveland TE, McLachlan JA. Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor alpha and beta. J Clin Endocrinol Metab. 2001;86:1750–1758. doi: 10.1210/jcem.86.4.7430. [DOI] [PubMed] [Google Scholar]
- 9.Payton-Stewart F, Khupse RS, Boué SM, Elliott S, Zimmermann MC, Skripnikova EV, Ashe H, Tilghman SL, Beckman BS, Cleveland TE, McLachlan JA, Bhatnagar D, Wiese TE, Erhardt P, Burow ME. Glyceollin I enantiomers distinctly regulate ER-mediated gene expression. Steroids. 2010;75:870–878. doi: 10.1016/j.steroids.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Suh HJ, Lee CH, Kim JH, Kang SC, Park S, Kim JS. Antifungal activity of glyceollins isolated from soybean elicited with Aspergillus sojae. J Agric Food Chem. 2010;58:9483–9487. doi: 10.1021/jf101694t. [DOI] [PubMed] [Google Scholar]
- 11.Ng TB, Ye XJ, Wong JH, Fang EF, Chan YS, Pan W, Ye XY, Sze SC, Zhang KY, Liu F, Wang HX. Glyceollin, a soybean phytoalexin with medicinal properties. Appl Microbiol Biotechnol. 2011;90:59–68. doi: 10.1007/s00253-011-3169-7. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Ahn I, Kim JH, Lee MR, Kim JS, Kim HJ. Glyceollins, one of the phytoalexins from soybeans under fungal stress, enhance insulin sensitivity and exert insulinotropic actions. J Agric Food Chem. 2010;58:1551–1557. doi: 10.1021/jf903432b. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Sung MK, Kim JS. Anti-inflammatory effects of glyceollins derived from soybean by elicitation with Aspergillus sojae. Inflamm Res. 2011 doi: 10.1007/s00011-011-0351-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Cha BY, Choi B, Lim JS, Woo JT, Kim JS. Glyceollins inhibit platelet-derived growth factor-mediated human arterial smooth muscle cell proliferation and migration. British J Nutr. 2011 doi: 10.1017/S0007114511002571. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Boué SM, Cleveland TE, Shih BY, Carter-Wientjes C, McLachlan JA, Burow ME. Phytoalexin-enriched functional foods. J Agric Food Chem. 2009;57:2614–2622. doi: 10.1021/jf8040403. [DOI] [PubMed] [Google Scholar]
- 16.Wood CE, Clarkson TB, Appt SE, Franke AA, Boue SM, Burow ME, McCoy T, Cline JM. Effects of soybean glyceollins and estradiol in postmenopausal female monkeys. Nutr Cancer. 2006;56:74–81. doi: 10.1207/s15327914nc5601_10. [DOI] [PubMed] [Google Scholar]
- 17.Schneider K, Oltmanns J, Hassauer M. Allometric principles for interspecies extrapolation in toxicological risk assessment--empirical investigations. Regul Toxicol Pharmacol. 2004;39:334–347. doi: 10.1016/j.yrtph.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Franke AA, Custer LJ, Wilkens LR, Le Marchand LL, Nomura AM, Goodman MT, Kolonel LN. Liquid chromatographic-photodiode array mass spectrometric analysis of dietary phytoestrogens from human urine and blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:45–59. doi: 10.1016/s1570-0232(02)00216-7. [DOI] [PubMed] [Google Scholar]
- 19.Register TC, Cann JA, Kaplan JR, Williams JK, Adams MR, Morgan TM, Anthony MS, Blair RM, Wagner JD, Clarkson TB. Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. J Clin Endocrinol Metab. 2005;90:1734–1740. doi: 10.1210/jc.2004-0939. [DOI] [PubMed] [Google Scholar]
- 20.Register TC, Wagner JD, Zhang L, Hall J, Clarkson TB. Effects of tibolone and conventional hormone replacement therapies on arterial and hepatic cholesterol accumulation and on circulating endothelin-1, vascular cell adhesion molecule-1, and E-selectin in surgically menopausal monkeys. Menopause. 2002;9:411–421. doi: 10.1097/00042192-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Lees CJ, Register TC, Turner CH, Wang T, Stancill M, Jerome CP. Effects of raloxifene on bone density, biomarkers, and histomorphometric and biomechanical measures in ovariectomized cynomolgus monkeys. Menopause. 2002;9:320–328. doi: 10.1097/00042192-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Setchell KD, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, Creutzinger V, Heubi JE. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr. 2003;133:1027–1035. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 23.Love RR, Wiebe DA, Feyzi JM, Newcomb PA, Chappell RJ. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women after 5 years of treatment. J Natl Cancer Inst. 1994;86:1534–1539. doi: 10.1093/jnci/86.20.1534. [DOI] [PubMed] [Google Scholar]
- 24.Ntukidem NI, Nguyen AT, Stearns V, Rehman M, Schott A, Skaar T, Jin Y, Blanche P, Li L, Lemler S, Hayden J, Krauss RM, Desta Z, Flockhart DA, Hayes DF. Consortium on Breast Cancer Pharmacogenomics. Estrogen receptor genotypes, menopausal status, and the lipid effects of tamoxifen. Clin Pharmacol Ther. 2008;83:702–710. doi: 10.1038/sj.clpt.6100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole LK, Jacobs RL, Vance DE. Tamoxifen induces triacylglycerol accumulation in the mouse liver by activation of fatty acid synthesis. Hepatology. 2010;52:1258–1265. doi: 10.1002/hep.23813. [DOI] [PubMed] [Google Scholar]
- 26.Hozumi Y, Suemasu K, Takei H, Aihara T, Takehara M, Saito T, Ohsumi S, Masuda N, Ohashi Y. The effect of exemestane, anastrozole, and tamoxifen on lipid profiles in Japanese postmenopausal early breast cancer patients: final results of National Surgical Adjuvant Study BC 04, the TEAM Japan sub-study. Ann Oncol. 2011;22:1777–1782. doi: 10.1093/annonc/mdq707. [DOI] [PubMed] [Google Scholar]
- 27.Gudbrandsen OA, Rost TH, Berge RK. Causes and prevention of tamoxifen-induced accumulation of triacylglycerol in rat liver. J Lipid Res. 2006;47:2223–2232. doi: 10.1194/jlr.M600148-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Murase Y, Kobayashi J, Nohara A, Asano A, Yamaaki N, Suzuki K, Sato H, Mabuchi H. Raloxifene promotes adipocyte differentiation of 3T3-L1 cells. Eur J Pharmacol. 2006;538:1–4. doi: 10.1016/j.ejphar.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 29.Dieudonne MN, Leneveu MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol. 2004;286:C655–C661. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- 30.Register TC, Jayo MJ, Anthony MS. Soy phytoestrogens do not prevent bone loss in postmenopausal monkeys. J Clin Endocrinol Metab. 2003;88:4362–4370. doi: 10.1210/jc.2003-030493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.