Abstract
Purpose
Interstitial cystitis (IC) is a painful bladder syndrome associated with urinary frequency and urgency. Elusive etiology of IC makes its diagnosis only possible by exclusion in many cases. In the present study, we used proteomics for identifying disease-associated proteins in rat model of chronic bladder irritation.
Materials & Methods
Chronic irritation of rat bladder was caused by a brief (90 secs) intravesical instillation of 0.2ml of 0.4N HCl. Whole bladders were collected at different time points after treatment, snap frozen and nuclear and cytosolic protein extracts were obtained. Samples were resolved in standard 2D-gels stained with an improved Coomasie Stain or by Differential Gel Electrophoresis (DIGE). Differentially expressed spots were excised and identified by MALDI-TOF MS/MS. Histological and Western blot analysis were also performed.
Results
Bladder morphology and histologic appearance of bladder sections following HCl treatment reflected hemorrhage, edema, epithelial denudation, detrusor mastocytosis and eosinophilia. Proteomic analysis of irritated rat bladder revealed marked overexpression of four nuclear proteins and marked underexpression of one nuclear protein compared to normal rat bladders. Among these proteins, inflammation-associated Calgranulin A (over) and smooth muscle protein-22/transgelin (under) showed opposed expression patterns following bladder irritation.
Conclusions
Presence of mast cells and eosinophils and overexpression of calgranulin A confirm the inflammatory component of HCl-irritated bladder. Altered expression of nuclear proteins is of particular interest because of their possible role as a prognostic marker in inflammatory bladder disorders. However, more studies are needed before clinical application of these findings can be established.
Keywords: Interstitial Cystitis, Proteomics, Biomarkers, 2-D Gel
Introduction
Identification of disease-associated proteins in tissues that can serve as disease-specific biomarkers has been the goal of traditional proteomic studies 1. Interstitial cystitis (IC) is a painful bladder syndrome of unclear etiology, associated with urinary frequency and urgency. The National Institutes of Health (NIH) have established a diagnostic criteria for IC based on the presence of irritative voiding symptoms in the absence of other identifiable pathology 2.
Currently, the diagnosis of IC in most cases is done by exclusion mainly because of the lack of adequate biological markers in blood or urine 3. Although exact etiology of IC is unknown, bladder inflammation appears to be common in many patients, with increased number of mast cells in bladder and increased afferent activity 4. Previous attempts for identifying molecular markers of IC used immunohistochemical labeling for proteins in bladder biopsies of IC patients. One study revealed abnormal expression of uroplakin, E-cadherin and tight junction protein ZO-1 in bladder of IC patients 5. In another study, cell proliferation assay and enzyme-linked immunosorbent assays of urine specimens from IC patients showed increased levels of antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor6.
Recently, proteomic approaches have been successful in identifying biological markers for inflammatory diseases of unclear etiology 7. Proteomic investigation of nuclear matrix (NM) in normal and cancerous bladders has led to the identification of key nuclear matrix proteins (NMPs) that now serve as biomarkers for bladder cancer 8.
We hypothesized that IC induces unique changes in the nuclear proteome of the bladder that can help fingerprint the disease and serve as biomarkers. In the present study, we applied the tools of proteomics to identify key nuclear proteins in bladder tissue obtained from a rat model of chronic irritation.
Materials & Methods
Adult Sprague-Dawley female rats (160~240g) were used for this study. All animal experimentation was previously approved by the Institutional Animal Care and Use Committee.
Induction of chronic bladder irritation
Under ketamine (50 mg/kg)+xylazine (5 mg/kg) i.m. anesthesia, rats were intravesically instilled for 90 seconds with 0.2ml of 0.4 N HCl using PE50 polyethylene catheters (Clay-Adams, Parsippany, NJ,). Bladders were subsequently rinsed with buffered saline. Rats were then kept with standard chow and water access. The bladders were removed on days 1, 4, 8, 10, 14 and 28.
Procedures for protein extraction
Tissue samples were snap frozen, crushed and solubilized according to ReadyPrep® (Cytoplasmic/Nuclear) Protein Extraction Kit (Bio-Rad, Hercules, CA). Protein concentration in the nuclear and cytoplasmic extracts was determined with a Bradford assay (Bio-Rad Protein Assay kit), and the samples were aliquoted and stored at −70°C.
Two-dimensional electrophoresis
First-dimension separation was carried out with 17 cm pH 5–8 immobile pH gradient strips (IPG, Bio-Rad). Samples containing up to 1 mg of protein were loaded and isoelectric focusing (IEF) was performed for 145 kV/hr at 20°C. Equilibrated IPG strips were transferred onto 15% uniform 18.5 × 16 cm polyacrylamide gels and run at 30 mA. Protein spots in the gels were visualized using improved Coomassie Blue staining (Imperial Stain, Pierce) and images collected in a Versa-Doc 4000 Imaging System (Bio-Rad). Images were analyzed using DeCyder software (GE Healthcare). All samples were processed at least three times to confirm reproducibility.
Nuclear extracts from control and HCl-treated bladders were also analyzed by reciprocal labeling Differential 2-D gel electrophoresis (DIGE) as described10.
Protein identification
Protein spots whose expression was highly up- or down-regulated in HCl-treated bladders were excised from the gels, destained, enzymatically digested and identified by matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry in a ABI 4700 MALDI-TOF.
Western Blot Analysis
Nuclear extracts (75 μg total protein) from control and HCl-irritated bladders were separated on 16.5% Tris-Tricine or 15% Tris-Glycine gels, semi-dry transferred to nitrocellulose membranes (Bio-Rad) and probed with primary antibodies against calgranulin A (goat polyclonal, 1 μg/ml, Santa Cruz Biotechnologies, Santa Cruz, CA) or transgelin (goat polyclonal, 0.25 μg/ml, Abcam, Cambridge, MA). Enhanced chemiluminescence (ECL Plus, GE Healthcare) images were collected with a VersaDoc 4000 Imaging System.
Alterations at Histological Level
Formalin fixed rat bladders were cryo-sectioned serially into 5-μm sections to be stained with Hematoxilin and Eosin, Luna and Alcian blue-safranin to evaluate gross histology, eosinophil infiltrates and number of mast cells, respectively. Digitized images were analyzed with Image Pro software (Media Cybernetics). Differences were analyzed using non-parametric Wilcoxon ranks sum test.
Results
Time course of gross pathological changes in the rat bladder after the HCl treatment are depicted in Figure 1. Severe hemorrhage at day 1–4 seems to partially resolve on the 7th day after the treatment. Histology on these bladders showed edema in the lamina propria and epithelial denudation shortly after HCl treatment and massive cellular infiltration in the lamina propria and re-epithelization at day 8 after HCl irritation (Fig. 2)
Figure 1.
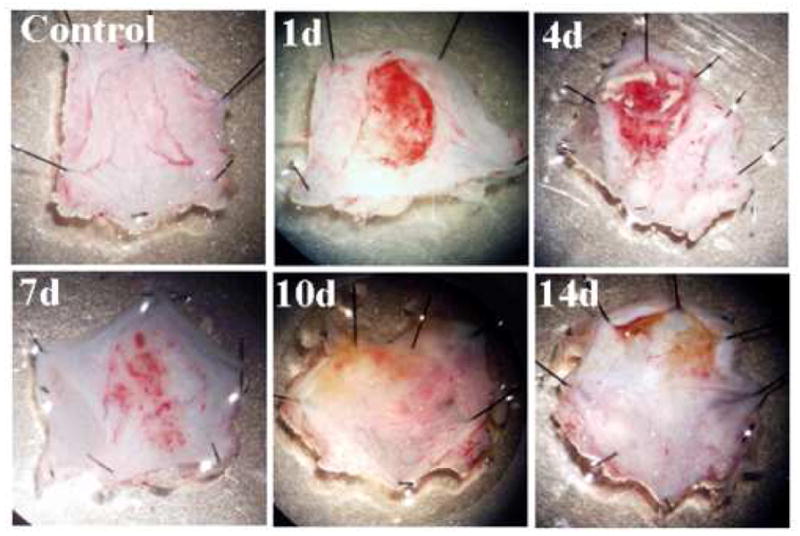
Time-course of the gross pathological changes in rat bladder after HCl-induced chronic irritation. Severe hemorrhagic changes induced by HCl were seen 24 hrs later that seem to persist till 4th day and only partially resolve by the 8th day.
Figure 2.
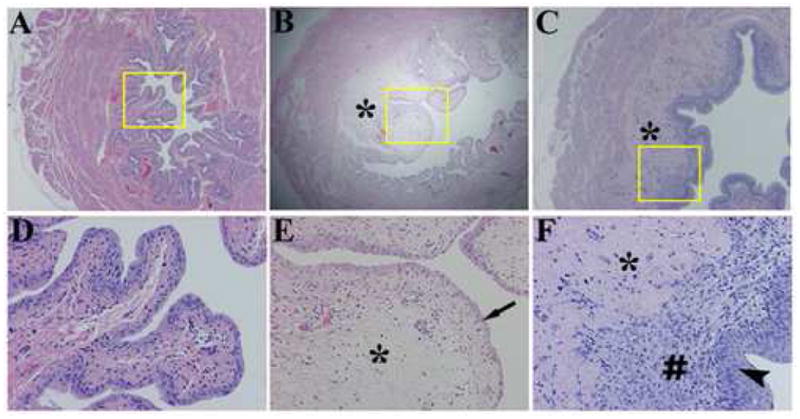
Histological analysis of rat bladder after HCl irritation. (A, D) Sham, (B, E) 1 day, and (C, F) 8 days after treatment. Severe edema in the lamina propria (*) and epithelial denudation (arrow) at day 1, and massive cellular infiltration in the lamina propria (#) and re-epithelization (arrow head) at day 8 after HCl irritation are shown. Hematoxilin and eosin staining. Magnification 20x (A–C), 100x (D–F).
Cytoplasmic extracts did not reveal any visible altered expression comparing the 2D electrophoresis patterns of bladder tissue from normal and chronic (8 days) bladder irritation (data not shown). In contrast, nuclear extracts of irritated rat bladder tissue produced several spots differentially expressed per gel (Fig. 3). There were 48 spots of interest selected by DeCyder software that were digested with trypsin for mass spectrometry. MALDI-TOF and database searching allowed the identification of 33 spots yielding 25 differentially expressed proteins after HCl irritation. Among these, calgranulin A and B showed marked up-regulation, and transgelin (smooth muscle protein 22 alpha) showed marked down-regulation,
Figure 3.
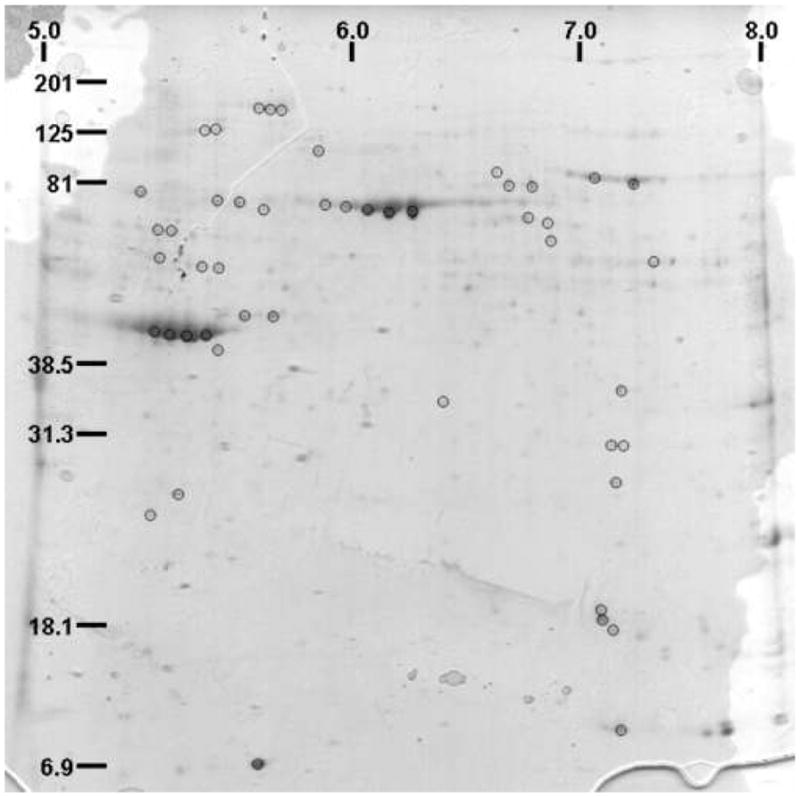
Coomasie-stained 2D-electrophoresis pattern of nuclear extract from rat bladder tissue following chronic bladder irritation. Spots of interest (48 marked) were selected by DeCyder software, isolated from the gels, and submitted to MALDI-TOF analysis, yielding 33 positive identifications from 25 different proteins.
DeCyder software selected 5 protein spots from DIGE images of nuclear extracts from irritated and control rat bladder (Fig. 4). The spots of interest were identified as: Calgranulin A (~10kD; pI 5.68), Calgranulin B (~13kD; pI 7.79), MyT1L (~13kD; pI 4.83) and Fibrinogen gamma chain precursor (~49kD; pI 5.57), which were over-expressed in the bladders of rats with chronic irritation, and transgelin (~22kD; pI 8.88), which was underexpressed in those tissues. Over-expression of Calgranulin A and under-expression of transgelin was most noteworthy (Fig. 4).
Figure 4.
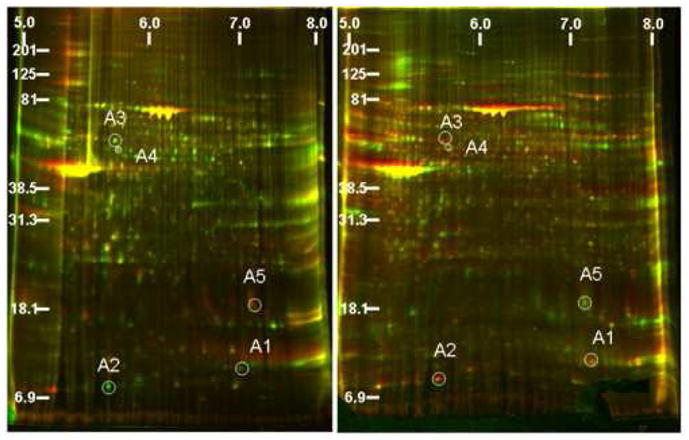
Merged images of DIGE analysis after reciprocal labeling of nuclear extracts from normal and from HCl-irritated rat bladder (day 8). Normal extract was labeled with Cy5 (left) and Cy3 (right) and HCl-treated extract was labeled with Cy3 (left) and Cy5 (right). Computer image analysis selected four spots (A1–A4) as overexpressed and one (A5) as underexpressed in HCl-irritated bladder compared to normal rat bladder. See text for protein ID details.
Time-course expression of Calgranulin A and transgelin in bladder samples after HCl irritation was monitored by Western blotting (Fig. 5). We found that Calgranulin A was over-expressed in the rat bladder shortly after the HCl treatment reaching maximum at day 4. Transgelin, however, showed maximal decrease of expression at day 4 after HCl irritation. Both proteins returned to normal expression at 28 days after the treatment.
Figure 5.
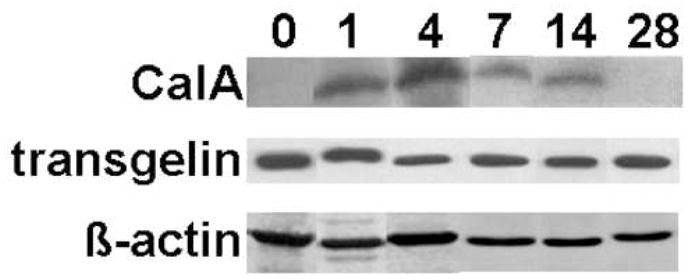
Temporal changes in Calgranulin A and transgelin levels in nuclear extracts of HCl-irritated bladder analyzed by Western blotting. β-actin served as internal loading control.
Histology on bladder sections from these animals showed significantly increased mast cell count by the 8th day following induction and significantly increased eosinophil count by day 4th (Fig. 6). The appearance of inflammatory cells and increase in mast cell count in the bladder do not correlate temporally with differential protein expression in rats.
Figure 6.
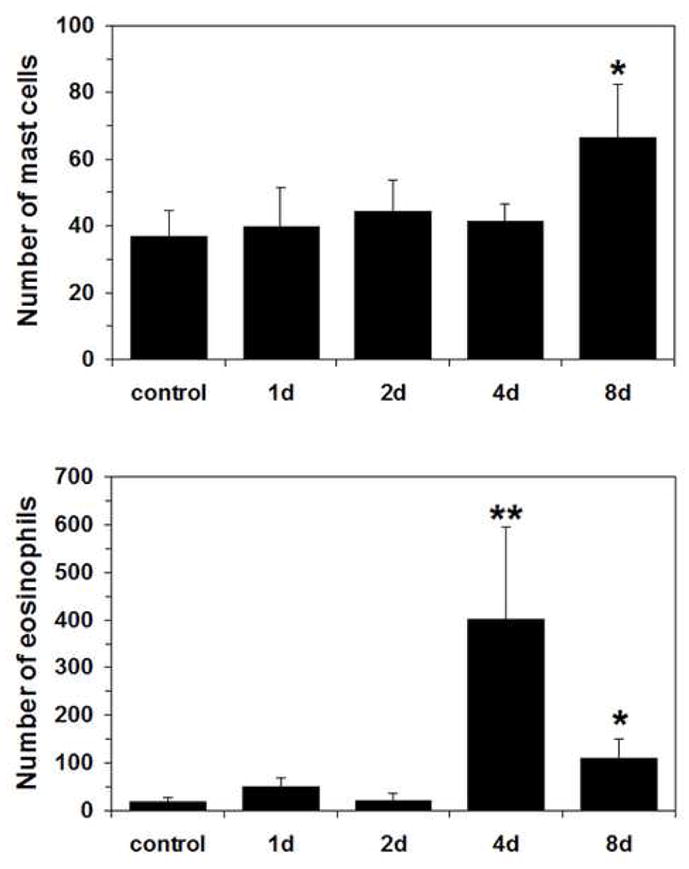
Time-course of the number of mast cell (top) and eosinophil (bottom) infiltration in rat bladder after HCl-induced chronic irritation. *p<0.05,**p<0.01 vs. control group at each point with Wilcoxon test; n=4–9 per group.
Discussion
The study of proteome has recently been introduced as a tool for unmasking difficult-to-diagnose diseases. This approach has been very useful for identifying biomarkers because the information generated reflects the impact of intrinsic genetic effects as well as the environment on the cell. Proteome data combined with histopathological information can provide important, novel clues for understanding disease biology.
The cell/tissue matrix system in a tissue and/or cell is defined as an integrated three-dimensional skeletal network that organizes cellular structure from the peripheral network to the DNA 9. It is apparent from numerous studies that localization of transcription factors on the NM increases the importance of NM in DNA binding and the specific regulation of gene expression11. We hypothesized that changes in nuclear proteins precede the manifestation of IC symptoms in patients and therefore they are suitable for disease specific biomarkers.
We investigated the applicability of proteomics for IC using rats. Many of the structural and functional changes associated with human bladder pathology such as neurogenic inflammation and increased mast cell count can be reproduced in a model of HCl-induced chronic irritation12,13. Damaged bladder epithelial layer in IC patients leads to bladder irritation from toxins which causes pain and decreased bladder capacity 14,15. Similarly, the HCl treatment strips off the upper layers of urothelium and weakens the connections between urothelium and stroma16. Bladder morphology and histologic appearance following the induction of bladder irritation reflected the detrusor mastocytosis and eosinophilia seen in IC patients13 (Figs. 2 and 6).
Investigations on cytoplasmic extract from rat bladder did not reveal any differential changes following irritation but changes in nuclear proteins were observed. We detected over-expression of four nuclear proteins (Calgranulin A, Calgranulin B, MyT1L and Fibrinogen gamma chain precursor), and the under-expression of one protein (transgelin [SM22 alpha]) in our study. Of these, Calgranulin A and transgelin were of particular interest.
Calgranulin A is a Ca2+ and Zn2+ binding protein produced by neutrophils and monocytes and seems to be released during inflammatory processes. A recent study reported that inflammation-associated calcium binding protein S100A8 (MRP-8, calgranulin A) is highly expressed in bladder tumor cells of patients compared to levels expressed in normal urothelium17. Calgranulin A seems to have a possible role as a prognostic marker in inflammatory bladder disorders.
Transgelin is an actin cross-linking/gelling protein found in fibroblasts and smooth muscle and it plays a role in contractility, possibly by affecting actin filament organization18. Decreased transgelin expression was also observed in proteomic investigation of bladder outlet obstruction in rat19. Down-regulation of transgelin may decrease bladder contractility.
In conclusion, chronic bladder irritation in rat by HCl resulted in an increase in the expression of four nuclear proteins and a decrease in the expression of one protein. Of these proteins, Calgranulin A and transgelin are of particular interest.. Bladder irritation alters protein levels in urinary bladder that seem to aggravate the bladder function in this animal study. However, more studies are needed before clinical application of these findings can be established.
Acknowledgments
The work in this study was supported by NIH grant NIDDK DK 066138 and Fishbein Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drabik A, Bierczynska-Krzysik A, Bodzon-Kulakowska A, Suder P, Kotlinska J, Silberring J. Proteomics in neurosciences. Mass Spectrom Rev. 2007 doi: 10.1002/mas.20131. [DOI] [PubMed] [Google Scholar]
- 2.Parsons CL, Greene RA, Chung M, Stanford EJ, Singh G. Abnormal urinary potassium metabolism in patients with interstitial cystitis. J Urol. 2005;173:1182–5. doi: 10.1097/01.ju.0000148361.82074.77. [DOI] [PubMed] [Google Scholar]
- 3.Erickson DR, Tomaszewski JE, Kunselman AR, Bentley CM, Peters KM, Rovner ES, Demers LM, Wheeler MA, Keay SK. Do the National Institute of Diabetes and Digestive and Kidney Diseases cystoscopic criteria associate with other clinical and objective features of interstitial cystitis? J Urol. 2005;173:93–7. doi: 10.1097/01.ju.0000146466.71311.ab. [DOI] [PubMed] [Google Scholar]
- 4.Green M, Filippou A, Sant G, Theoharides TC. Expression of intercellular adhesion molecules in the bladder of patients with interstitial cystitis. Urology. 2004;63:688–93. doi: 10.1016/j.urology.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Slobodov G, Feloney M, Gran C, Kyker KD, Hurst RE, Culkin DJ. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol. 2004;171:1554–8. doi: 10.1097/01.ju.0000118938.09119.a5. [DOI] [PubMed] [Google Scholar]
- 6.Keay SK, Zhang C-O, Shoenfelt J, Erickson DR, Whitmore K, Warren JW, Marvel R, Chai T. Sensitivity and specificity of antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor as urine markers for interstitial cystitis. Urology. 2001;57:9–14. doi: 10.1016/s0090-4295(01)01127-x. [DOI] [PubMed] [Google Scholar]
- 7.Felley-Bosco E, Andre M. Proteomics and chronic inflammatory bowel diseases. Pathol Res Pract. 2004;200:129–33. doi: 10.1016/j.prp.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Pirtskalaishvili G, Getzenberg RH, Konety BR. Use of urine-based markers for detection and monitoring of bladder cancer. Tech Urol. 1999;5:179–84. [PubMed] [Google Scholar]
- 9.Getzenberg RH. Nuclear matrix and the regulation of gene expression: tissue specificity. J Cell Biochem. 1994;55:22–31. doi: 10.1002/jcb.240550105. [DOI] [PubMed] [Google Scholar]
- 10.Unlu M, Morgan ME, Minden JS. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis. 1997;18:2071–7. doi: 10.1002/elps.1150181133. [DOI] [PubMed] [Google Scholar]
- 11.Ellies DL, Krumlauf R. Bone formation: The nuclear matrix reloaded. Cell. 2006;125:840–2. doi: 10.1016/j.cell.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Cayan S, Canpolat B, Cayan F, Yilmaz N, Kartal A, Oguz I, Akbay E. The effect of chronic inflammatory condition of the bladder and estrogen replacement therapy on bladder functions and histology in surgically menopause and chronic cystitis induced rats. Neurourol Urodyn. 2006;25:194–201. doi: 10.1002/nau.20158. [DOI] [PubMed] [Google Scholar]
- 13.Rivas DA, Chancellor MB, Shupp-Byrne S, Shenot PJ, McHugh K, McCue P. Molecular marker for development of interstitial cystitis in rat model: isoactin gene expression. J Urol. 1997;157:1937–40. doi: 10.1016/s0022-5347(01)64905-x. [DOI] [PubMed] [Google Scholar]
- 14.Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol Renal Physiol. 1996;271:F886–894. doi: 10.1152/ajprenal.1996.271.4.F886. [DOI] [PubMed] [Google Scholar]
- 15.Parsons CL. Argument for the use of the potassium sensitivity test in the diagnosis of interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:430–1. doi: 10.1007/s00192-005-1305-6. [DOI] [PubMed] [Google Scholar]
- 16.El Khatib S, Berrahmoune S, Leroux A, Bezdetnaya L, Guillemin F, D’Hallewin MA. A novel orthotopic bladder tumor model with predictable localization of a solitary tumor. Cancer Biol Ther. 2006;5:1327–31. doi: 10.4161/cbt.5.10.3214. [DOI] [PubMed] [Google Scholar]
- 17.Tolson JP, Flad T, Gnau V, Dihazi H, Hennenlotter J, Beck A, Mueller GA, Kuczyk M, Mueller CA. Differential detection of S100A8 in transitional cell carcinoma of the bladder by pair wise tissue proteomic and immunohistochemical analysis. Proteomics. 2006;6:697–708. doi: 10.1002/pmic.200500033. [DOI] [PubMed] [Google Scholar]
- 18.Hoggatt AM, Simon GM, Herring BP. Cell-specific regulatory modules control expression of genes in vascular and visceral smooth muscle tissues. Circ Res. 2002;91:1151–9. doi: 10.1161/01.res.0000047508.30800.4f. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Sohng I, Kim DH, Lee DC, Hwang CH, Park JY, Ryu JW. Investigation of early protein changes in the urinary bladder following partial bladder outlet obstruction by proteomic approach. J Korean Med Sci. 2005;20:1000–5. doi: 10.3346/jkms.2005.20.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]


