Abstract
The purpose of this work – the first of its kind - was to evaluate the impact of chronic selective histone deacetylase (HDAC) inhibitor treatment on brain activity using uptake of the radioligand 18F-fluorodeoxyglucose and positron emission tomography (18FDG-PET). HDAC dysfunction and other epigenetic mechanisms are implicated in diverse CNS disorders and animal research suggests HDAC inhibition may provide a lead toward developing improved treatment. To begin to better understand the role of the class I HDAC subtypes HDAC 1, 2 and 3 in modulating brain activity, we utilized two benzamide inhibitors from the literature, compound 60 (Cpd-60) and CI-994 which selectively inhibit HDAC 1 and 2 or HDACs 1,2 and 3, respectively. One day after the seventh treatment with Cpd-60 (22.5 mg/kg) or CI-994 (5 mg/kg), 18FDG-PET experiments (n = 11–12 rats per treatment group) revealed significant, local changes in brain glucose utilization. These 2–17% changes were represented by increases and decreases in glucose uptake. The pattern of changes was similar but distinct between Cpd-60 and CI-994, supporting that 18FDG-PET is a useful tool to examine the relationship between HDAC subtype activity and brain activity. Further work using additional selective HDAC inhibitors will be needed to clarify these effects as well as to understand how brain activity changes influence behavioral response.
Introduction
HDACs are a family of enzymes catalyzing the removal of acetyl groups from proteins including histones. Subcellular localization and energy requirements divide HDACs into the predominantly nuclear class I (HDAC1, 2, 3 and 8), as well as class II (HDAC4-7, 9-10), class III and class IV enzyme subtypes [7]. Histones – the structural protein core in DNA packaging – are covalently modified, including via lysine acetylation. This alters the binding of transcription factor complexes and thus, class I HDAC activity plays a major part in chromatin-mediated transcriptional regulation.
HDAC activity and transcriptional control are linked to cellular differentiation, growth and apoptosis and have focused the development of HDAC inhibitors as chemotherapeutics and adjuncts in cancer treatment [4, 7, 30]. Beyond cancer, HDAC-mediated mechanisms have been implicated in the underpinnings of diverse psychiatric diseases [1, 9]. Evidence from animal models suggests a role for class I HDAC enzymes in regulating behavioral and molecular effects related to depression, anxiety, deficits in learning and memory, and drug addiction [3, 11, 12, 16, 19, 25]. These reports suggest selective small molecule HDAC inhibitors may be useful leads for clinical CNS disorders treatment. However, outside oncology, development of clinical epigenetic therapies is at a nascent stage as the impact of these compounds on neuroplasticity and neurocircuitry throughout the human brain is not yet well known.
Non-invasive neuroimaging can be utilized to examine effects of drug treatment on brain activity. Analogous imaging experiments can be compared between research animals and humans, providing a powerful translational advantage. Currently, there are no validated tools to directly image the activity of HDAC enzymes in humans. However, remarkable progress was recently made by Yeh in work detailing class II HDAC expression and function in primate brain using a novel, 18F-labeled HDAC substrate [32]. Meanwhile, the impact of HDAC inhibition on brain activity can be investigated using established radioligands and modalities such as PET and magnetic resonance imaging (MRI). Previous results from blood oxygen level-dependent functional MRI indicate that in rats, subchronic administration of the weakly acting, class I HDAC inhibitor, sodium butyrate, enhances the neuronal response to cocaine challenge [10]. However, it is unknown whether basal brain activity is altered by extended HDAC inhibition.
Here, we describe the first evaluation of chronic HDAC inhibitor treatment on brain activity in rats. Part of our overarching question is how class I HDAC enzymes regulate brain activity and, subsequently, how these relate to behavioral response. To begin to address this, we selected two known HDAC inhibitors, Cpd-60 and CI-994 [21, 22]. We used these inhibitors as we have developed an understanding of their effects in biochemical activity assays and in mouse behavioral tests that will be the subject of forthcoming manuscripts. Additionally, these compounds display different selectivity profiles for HDAC1, HDAC2 and HDAC3, and serve as tools to investigate effects of selective HDAC inhibition. We administered compounds once daily for seven days as, using other HDAC inhibitors, this paradigm was sufficient to alter brain biochemistry and behavioral response in mice [3, 25]. We then measured uptake of 18FDG using PET to identify brain regions with significant activity changes compared to vehicle-treated controls.
Our results indicate that chronic HDAC inhibition resulted in focused, 2–17% changes in glucose uptake in limbic nuclei, as well as in subregions of the cortex and cerebellum. We found that changes induced by Cpd-60 or CI-994 were distinct from one another – an expected result given differences in HDAC selectivity and brain pharmacokinetics of these compounds.
We conclude that identifying regional brain activity changes mediated by selective class I HDAC inhibitors provides insight into the role of HDAC subtypes in modulating brain function. By utilizing 18FDG-PET, this work provides a basis for future analogous studies in human with broad implications toward resolving mechanisms of psychiatric disease and effective treatment with chromatin modifying drugs.
Materials and Methods
Additional details are available in online supplement.
Methods Overview
All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Massachusetts General Hospital Institutional Animal Care and Use Facility.
Chemicals
Cpd-60 and CI-994 were synthesized according to published protocols [11, 13, 16], and administered by intraperitoneal (i.p.) injection in vehicle (10% DMSO, 45% PEG-400, 45% saline).
Biochemical Assays
HDAC activity was measured in vitro using recombinant human HDACs 1-9 (BPS Bioscience) using the Caliper EZ reader II system as previously described [8].
Pharmacokinetic Profile Determination
Pharmacokinetics were evaluated in mice treated (i.p.) with Cpd-60 (45 mg/kg) or CI-994 (10 mg/kg) in vehicle and analyzed from brain using LC-MS/MS as described [8].
Western Blotting
Western blotting was performed on dissected brain tissue using standard conditions. Protein extract (~10 μg) was used to assess histone acetylation using antibodies against acetylated histone H4K12 (Millipore #04-119) with normalization to total levels of histone H3 (#07-690).
Image Acquisition
PET and skeletal computed tomography (CT) images were collected on a trimodal scanner and reconstructed using an iterative maximum likelihood expectation maximization.
Post-Acquisition Processing
To compare data across subjects, individual CT and PET scans were transformed into the same coordinate system using standard methodology developed for human imaging experiments, as described [14].
Spatial Preprocessing
Preprocessing was performed as described with minor modifications [14].
Statistical design and analysis in SPM
HDAC inhibitor-treated groups were compared individually against vehicle-treated controls using an unpaired t-test design using statistical parametric mapping as previously described [14].
Results and Discussion
In this study, we utilized two benzamide-based HDAC inhibitors previously published with selectivity for a subset of class I HDAC enzymes [13, 21, 22]. Using an in vitro biochemical assay measuring the deacetylase activity of recombinant human HDACs different from those recently published [2], we clarified that Cpd-60 and CI-994 selectively inhibit HDACs 1-3 in the nanomolar range (Fig. 1b).
Figure 1.
A. Structure of Cpd-60 and CI-994. B. In vitro HDAC inhibition IC50 values following 60 or 180a min incubation of enzyme with substrate and inhibitor indicate selectivity of slow-binding test compounds for a subset of class I HDAC enzymes with no appreciable inhibition of HDAC8 or class II enzymes. C. In vivo pharmacokinetics in mouse brain following i.p. injection of Cpd-60 (45 mg/kg) or CI-994 (10mg/kg) indicate HDAC inhibitors (HDACi) reach peak concentrations within one hour and remain present in brain at >100 nM for at least eight hours. The brain concentration of each inhibitor exceeds the IC50 for targeted HDACs.
The IC50 values for HDAC1-3 we report are following 180 min incubation of the inhibitor with substrate due to the slow binding nature of the compounds. Specifically, Cpd-60 inhibits HDAC1 and 2 with an in vitro IC50 of 1 and 13 nM, respectively. Inhibition of HDAC3 by Cpd-60 revealed an IC50 of 398 nM indicating a >30-fold selectivity for inhibition of HDAC1 and 2 over HDAC3. In contrast, CI-994 inhibited HDAC1, 2 and 3 with a similar potency for each subtype, with an IC50 range of 41–147 nM (Fig. 1b). HDAC8 and class II HDAC enzyme subtypes were not appreciably inhibited by Cpd-60 and CI-994 as IC50 values were >33 μM (Fig. 1b). Brain pharmacokinetics evaluated in mice revealed that treatment with Cpd-60 (45 mg/kg) resulted in a maximum concentration (Cmax) of 0.8 μM; CI-994 treatment (10 mg/kg) resulted in a brain Cmax of 3.2 μM (Fig. 1c). These experiments showed that for both inhibitors, brain concentrations >100 nM were sustained for at least eight hours after injection. Importantly, these concentrations and brain exposures represent the total concentration of each inhibitor – both bound to HDAC targets and free in solution. Our preliminary experiments in mice indicate that these doses of Cpd-60 and CI-994 were sufficient to alter brain histone acetylation (unpublished results). Thus, these findings suggest that at the doses applied in vivo, Cpd-60 predominantly inhibits HDAC 1 and 2, whereas CI-994 inhibits HDAC1, 2 and 3.
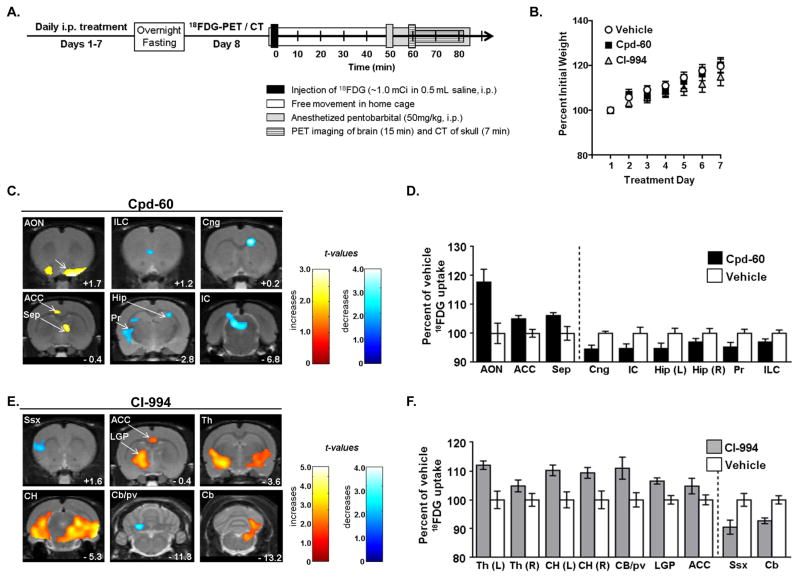
Previous reports showed that treatment for one week with HDAC inhibitors modulate behaviors related to CNS disease including depression, anxiety, and stimulant induced hyperactivity [3, 17, 26]. These studies feature chronic treatment regimens, consistent with the rationale that HDAC inhibitors induce changes in chromatin that over time alter gene expression and neuroplasticity. To examine the effects of chronic selective HDAC inhibition on brain activity using FDG-PET, we treated rats daily with Cpd-60 and CI-994 at doses estimated based on pharmacokinetic study in mice and adjusted for body surface area in rat. Specifically, for seven days, rats were administered Cpd-60 (22.5 mg/kg), CI-994 (5 mg/kg) or an equivalent volume of vehicle as control, summarized in Figure 2a. After the seventh treatment, average weight (Fig 2b) and resting blood glucose levels were not significantly altered by any treatment (Cpd-60, 116 ± 7.0 mg/dL; CI-994, 107.5 ± 4.4 mg/dL; vehicle, 102.9 ± 3.9 mg/dL). Animals were food-restricted overnight to facilitate brain uptake of 18FDG, which, as expected, reduced blood glucose, with no differences between treatment groups (Cpd-60, 84 ± 3.3 mg/dL; CI-994, 84.2 ± 4.6 mg/dL; vehicle, 79.4 ± 3.5 mg/dL).
Figure 2.
A. Chronic treatment schematic with timeline in days (left) and, on day-8, PET/CT imaging schematic with timeline in minutes (right). B. Daily treatment with Cpd-60 (22.5 mg/kg) or CI-994 (5 mg/kg) did not significantly affect rat weight over seven days compared to vehicle-treated controls (n=11–12/group). C. and E. Brain regions with significant changes in glucose uptake induced by chronic treatment with C. Cpd-60, an HDAC1,2 inhibitor or E. CI-994, an HDAC1,2,3 inhibitor. Brain structures abbreviated in upper left corner of each panel comprise significant increases (yellow/red t-value maps) and decreases (blue t-value maps) compared to vehicle treated controls (n=11–12/group). Anterior-Posterior distance from Bregma (mm) is indicated in lower right corner of each panel. D. and F. Histograms showing percent changes in local 18FDG uptake induced by treatment with Cpd-60 (D.) or CI-994 (F.) relative to controls. Changes were evaluated using ROI analysis of regions highlighted in C. and D. and including increases in 18FDG uptake (left of dashed lines) and decreases (right of dashed line).
Next, as further schematized in Figure 2a, 18FDG (~1mCi, i.p.) was administered to awake rats which were returned to their homecage for a 50 min uptake period without anesthesia. Sodium pentobarbital was then used (50 mg/kg, i.p.) to anesthetize rats for imaging. Following data processing (see methods), images from rats treated with Cpd-60 (n=11) or CI-994 (n=11) were compared to vehicle-treated controls (n=12) using SPM. Calculated t-maps were superimposed on a rat MRI template. Representative two-dimensional images from this three-dimensional dataset are shown in Figure 2c,e.
Data were inspected relative to the MRI template and a stereotaxic atlas of the rat brain to assign structural regions [24]. The percent change in 18FDG uptake – both increases and decreases – was determined relative to vehicle-treated controls using a spherical ROI, 12mm in diameter, centered on the voxel of highest regional t-value. Results from voxel-level analysis using SPM and percent change by ROI analysis are summarized in Table 1.
Table 1.
Brain regions showing changes in glucose uptake – Effects of chronic HDAC inhibitor treatment
| Contrast vs Vehicle | Activation | Voxel level
|
Coordinates** (mm)
|
Region | % change | ||||
|---|---|---|---|---|---|---|---|---|---|
| t-value | (Za) | p (uncorr) | x | y | z | ||||
| Cpd-60 | Increases | 2.93 | 2.64 | 0.004 | 18 | 72 | −42 | AON: Anterior offactory nucleus | 17.0 |
| 2.11 | 1.99 | 0.024 | −10 | 36 | 30 | ACC: Anterior cingulate cortex (L) | 5.0 | ||
| 2.36 | 2.19 | 0.014 | 12 | 36 | −6 | Sep: Septum (R) | 6.2 | ||
| Decreases | 3.35 | 2.95 | 0.002 | 20 | 56 | 22 | Cng: Cingulum (R) | 5.5 | |
| 24 | 2.22 | 0.013 | −2 | −30 | 8 | IC: Inferior Colliculus | 5.2 | ||
| 2.06 | 1.94 | 0.026 | −48 | 10 | 18 | Hip: Hippocampus (L) | 5.2 | ||
| 2.03 | 1.92 | 0.028 | 32 | 18 | 26 | Hip: Hippocampus (R) | 2.7 | ||
| 2.85 | 2.59 | 0.005 | −62 | 10 | −10 | Pr: Perirhinal cortex (L) | 4.7 | ||
| 2.09 | 1.97 | 0.024 | −4 | 76 | 4 | ILC: Infralimbic cortex | 3.0 | ||
|
| |||||||||
| CI-994 | Increases | 4.32 | 3.61 | 0 | −38 | 10 | −22 | Th: Thalamic nucleus (ventroposteriomedial, L) | 12.0 |
| 2.4 | 2.23 | 0.013 | 40 | 8 | −24 | Th: Thalamic nucleus (ventroposteriomedial, R) | 4.8 | ||
| 4.15 | 3.5 | 0 | −44 | −36 | 4 | CH: Caudal hippocampus (L) | 10.0 | ||
| 3.6 | 3.14 | 0.001 | 46 | −38 | 8 | CH: Caudal hippocampus (R) | 9.3 | ||
| 2.26 | 2.12 | 0.017 | 34 | −92 | −10 | Cb/pv: Cerebelium (parvocellular nucleus, R) | 11.0 | ||
| 3.3 | 2.93 | 0.002 | −22 | 36 | −14 | LGP: Lateral Globus pallidus (L) | 6.5 | ||
| 2.32 | 2.17 | 0.015 | 4 | 36 | 26 | ACC: Anterior cingulate cortex (R) | 4.8 | ||
| Decreases | 2.31 | 2.16 | 0.016 | −54 | 58 | 2 | Ssx: Somatosensory cortex (L) | 9.5 | |
| 2.88 | 2.62 | 0.004 | −26 | −72 | 0 | Cb: Cerebellum (rostral, L) | 7.3 | ||
The data revealed distinct patterns of activation and deactivation of glucose uptake by Cpd-60 and CI-994 compared to vehicle and that the changes induced by CI-994, particularly activation, were more extensive than by Cpd-60 (Fig 2c–f).
Brain regions with significant HDAC inhibitor-induced changes in glucose uptake included nuclei at midline: infralimbic cortex (ILC), anterior cingulate cortex (ACC), septum (Sep) and inferior colliculus (IC) (Fig. 2c,e). Additional effects were observed bilaterally in anterior olfactory nucleus (AON), hippocampus (Hip), caudal hippocampus (CH), and thalamic nucleus (Th) (Fig. 2c,e). Evidence of unilateral effects was observed in the right cingulum (Cng), left somatosensory cortex (Ssx), left lateral globus pallidus (LGP), left parvocellular nucleus (Cb/pv) and in right cerebellum (Cb) (Fig. 2c,e). We do not consider this evidence of low fidelity as there was no obvious right-left bias to the unilateral changes. Further, published studies have identified unilateral effects in rodent brain, which may indicate hemispheric specialization [6, 28].
We speculate that brain regions with altered glucose uptake may elucidate behaviors important in disease and treatment response. The magnitude of HDAC inhibitor-mediated glucose changes may result in behavioral changes as previous results have shown that rats treated with the antidepressant fluoxetine had brain glucose changes of ~4–14% as well as antidepressant behavioral effects in the forced swim test [15].
The largest 18FDG uptake (17%, Table 1) indicates Cpd-60 increased AON signaling. As reviewed [29], the AON harbors neurons involved in processing olfactory cues facilitating social recognition and other complex behaviors. AON neurons synapse onto the neighboring piriform cortex, a region rich in class I HDAC subtype expression. Therefore, brain activity in these regions may be responsive to HDAC inhibition. That olfactory bulbectomy disrupted citalopram-mediated changes in cerebral glucose in rodents [27] provides new insight to interpret the circuitry underlying demonstrated antidepressant-like effects of HDAC inhibitors [3, 25]. Furthermore, this suggests that drugs altering olfactory signaling, including Cpd-60, may modulate depressive-like behaviors.
CI-994 treatment resulted in increased 18FDG in thalamus (4–12% increase) and hippocampus (9.3–10% increase) in addition to globus pallidus (6.5%) and anterior cingulate cortex (4.8%). Altered signaling in limbic nuclei mediates behaviors related to emotion, reward-seeking and memory. Recent results demonstrate deep brain stimulation in these regions can rescue behavioral deficits in a rodent model of schizophrenia [18]. Additionally, increased hippocampal glucose uptake was shown to enhance spatial memory in rats [20]. This suggests that CI-994 treatment may ameliorate psychiatric-disease related behaviors and improve memory via increased glucose uptake in limbic regions, including the hippocampus.
To provide evidence of HDAC inhibitor target engagement, we used western blotting to profile the acetylation of histone H4 Lys12 (H4K12ac) in the somatosensory cortex, dorsal striatum and cerebellum of treated rats. We examined changes in acetylation, a labile modification, in brain tissue collected one hour after treatment and found that both inhibitors were sufficient to significantly induce H4K12 acetylation in the somatosentory cortex, but only CI-994 treatment resulted in significant increases in the other regions (Supplementary Fig. 1). This evidence confirms that systemic treatment with Cpd-60 (22.5 mg/kg) and CI-994 (5 mg/kg) were sufficient to suppress HDAC activity in brain. These results may indicate a mechanism by which HDAC inhibitors differentially alter regional histone acetylation, and function to modulate gene expression resulting in altered glucose uptake.
18FDG-PET is a robust technique that was recently reported to discriminate patients comprising four dementia subtypes from normal elderly controls with >92% accuracy [23]. This clinical study provides a premier example of utilizing established imaging modalities like 18FDG-PET to identify CNS disease biomarkers. This will be a particular challenge in spectrum disorders such as autism, mood-dysregulation and depression. However refining diagnostic criteria promises to help identify patient subgroups from which to identify patterns of brain activity via neuroimaging that are predictive of disease and effective treatment [5, 31].
Clarifying the role of specific HDAC and other chromatin-modifying enzymes in regulating brain activity and behavioral response is a critical step in refining targets for improved psychiatric disease therapies and in monitoring treatment efficacy in humans. We recognize that the limited number of compounds we have tested in rat prevents a clear understanding of how HDAC subtypes are linked to brain regions whose glucose uptake and therefore neural activity is changed. However, it is tempting to speculate that the regional differences we observed between Cpd-60 and CI-994 may result in part from differences in the degree of HDAC3 inhibition. Addressing this question will require testing a number of compounds with a range of pharmacokinetic properties, including HDAC3-selective inhibitors. Additionally, determining whether compounds like crebinostat, recently shown to enhance learning and memory, mimic the effects of either Cpd-60 or CI-994 may provide insight into regional changes underlying cognitive enhancement [8]. Finally, testing Cpd-60 and CI-994 in rodent models of memory and other behaviors may also help derive the relationship between the neuroimaging signals and behavioral changes.
Supplementary Material
Highlights.
Rats were treated with histone deacetylase (HDAC) inhibitors for seven days
The two inhibitors used were selective for a subset of class I HDAC enzymes.
Treatment resulted in brain glucose uptake changes as measured by PET imaging.
The study is the first to image chronic HDAC inhibitor effects using FDG-PET.
Acknowledgments
Research was supported by the National Institute of Drug Abuse (NIDA) of the National Institutes of Health under grant numbers R01DA030321 (J.M.H.; S.J.H); R01DA028301 (S.J.H.). Additional support was provided by the Alzheimer’s Association and Tau Consortium (S.J.H.). We thank members of the Hooker and Haggarty labs for their critical feedback and Dr. Stephen Sawiak, University of Cambridge, for assistance with SPM software.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akbarian S, Nestler EJ. Epigenetic mechanisms in psychiatry. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:1–2. doi: 10.1038/npp.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, Mazitschek R. Chemical phylogenetics of histone deacetylases. Nature chemical biology. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diyabalanage HV, Granda ML, Hooker JM. Combination therapy: histone deacetylase inhibitors and platinum-based chemotherapeutics for cancer. Cancer letters. 2013;329:1–8. doi: 10.1016/j.canlet.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlop BW, Binder EB, Cubells JF, Goodman MM, Kelley ME, Kinkead B, Kutner M, Nemeroff CB, Newport DJ, Owens MJ, Pace TW, Ritchie JC, Rivera VA, Westen D, Craighead WE, Mayberg HS. Predictors of remission in depression to individual and combined treatments (PReDICT): study protocol for a randomized controlled trial. Trials. 2012;13:106. doi: 10.1186/1745-6215-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endepols H, Sommer S, Backes H, Wiedermann D, Graf R, Hauber W. Effort-based decision making in the rat: an [18F]fluorodeoxyglucose micro positron emission tomography study. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:9708–9714. doi: 10.1523/JNEUROSCI.1202-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fass DM, Kemp MM, Schroeder FA, Wagner FF, Wang Q, Holson EB. Encyclopedia of Molecular Cell Biology and Molecular Medicine. Wiley; 2012. Histone Acetylation and Deacetylation. [Google Scholar]
- 8.Fass DM, Reis SA, Ghosh B, Hennig KM, Joseph NF, Zhao WN, Nieland TJF, Guan JS, Kuhnle CEG, Tang WP, Barker DD, Mazitschek R, Schreiber SL, Tsai LH, Haggarty SJ. Crebinostat: A novel cognitive enhancer that inhibits histone deacetylase activity and modulates chromatin-mediated neuroplasticity. Neuropharmacology. 2013;64:81–96. doi: 10.1016/j.neuropharm.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fass DM, Schroeder FA, Perlis RH, Haggarty SJ. Epigenetic mechanisms in mood disorders: Targeting neuroplasticity. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Febo M, Akbarian S, Schroeder FA, Ferris CF. Cocaine-induced metabolic activation in cortico-limbic circuitry is increased after exposure to the histone deacetylase inhibitor, sodium butyrate. Neuroscience letters. 2009;465:267–271. doi: 10.1016/j.neulet.2009.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, Nieland TJ, Fass DM, Kao PF, Kahn M, Su SC, Samiei A, Joseph N, Haggarty SJ, Delalle I, Tsai LH. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holson EWF, Weïwer M, Zhang YL, Haggarty SH, Tsai LH. WO2012149540. USA: Inhibitors of histone deacetylases. 2012
- 14.Hooker JM, Patel V, Kothari S, Schiffer WK. Metabolic Changes in the Rodent Brain after Acute Administration of Salvinorin A. Mol Imaging Biol. 2009;11:137–143. doi: 10.1007/s11307-008-0192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang DP, Lee SH, Park CW, Lee SY, Kim YB, Cho ZH. Effects of fluoxetine on the rat brain in the forced swimming test: a [F-18]FDG micro-PET imaging study. Neuroscience letters. 2009;451:60–64. doi: 10.1016/j.neulet.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy PJ, Feng J, Robison AJ, Maze I, Badimon A, Mouzon E, Chaudhury D, Damez-Werno DM, Haggarty SJ, Han MH, Bassel-Duby R, Olson EN, Nestler EJ. Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nature neuroscience. 2013;16:434–440. doi: 10.1038/nn.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim WY, Kim S, Kim JH. Chronic microinjection of valproic acid into the nucleus accumbens attenuates amphetamine-induced locomotor activity. Neuroscience letters. 2008;432:54–57. doi: 10.1016/j.neulet.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Klein J, Hadar R, Gotz T, Manner A, Eberhardt C, Baldassarri J, Schmidt TT, Kupsch A, Heinz A, Morgenstern R, Schneider M, Weiner I, Winter C. Mapping brain regions in which deep brain stimulation affects schizophrenia-like behavior in two rat models of schizophrenia. Brain stimulation. 2012 doi: 10.1016/j.brs.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, Rusche JR, Wood MA. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2647–2652. doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiology of learning and memory. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Methot JL, Chakravarty PK, Chenard M, Close J, Cruz JC, Dahlberg WK, Fleming J, Hamblett CL, Hamill JE, Harrington P, Harsch A, Heidebrecht R, Hughes B, Jung J, Kenific CM, Kral AM, Meinke PT, Middleton RE, Ozerova N, Sloman DL, Stanton MG, Szewczak AA, Tyagarajan S, Witter DJ, Secrist JP, Miller TA. Exploration of the internal cavity of histone deacetylase (HDAC) with selective HDAC1/HDAC2 inhibitors (SHI-1:2) Bioorganic & medicinal chemistry letters. 2008;18:973–978. doi: 10.1016/j.bmcl.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Moradei OM, Mallais TC, Frechette S, Paquin I, Tessier PE, Leit SM, Fournel M, Bonfils C, Trachy-Bourget MC, Liu J, Yan TP, Lu AH, Rahil J, Wang J, Lefebvre S, Li Z, Vaisburg AF, Besterman JM. Novel aminophenyl benzamide-type histone deacetylase inhibitors with enhanced potency and selectivity. Journal of medicinal chemistry. 2007;50:5543–5546. doi: 10.1021/jm701079h. [DOI] [PubMed] [Google Scholar]
- 23.Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, Reiman EM, Holthoff V, Kalbe E, Sorbi S, Diehl-Schmid J, Perneczky R, Clerici F, Caselli R, Beuthien-Baumann B, Kurz A, Minoshima S, de Leon MJ. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2008;49:390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paxinos CGW. The Rat Brain in Steretaxic Coordinates. 4. Academic Press, Inc; 1998. [Google Scholar]
- 25.Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biological psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, Tapper AR, Akbarian S. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:2981–2992. doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skelin I, Sato H, Kovacevic T, Diksic M. Chronic therapy with citalopram decreases regional cerebral glucose utilization in OBX, and not sham-operated, rats: an autoradiographic study. Psychopharmacology. 2009;207:315–323. doi: 10.1007/s00213-009-1659-4. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7:131–143. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- 29.Wacker DW, Engelmann M, Tobin VA, Meddle SL, Ludwig M. Vasopressin and social odor processing in the olfactory bulb and anterior olfactory nucleus. Annals of the New York Academy of Sciences. 2011;1220:106–116. doi: 10.1111/j.1749-6632.2010.05885.x. [DOI] [PubMed] [Google Scholar]
- 30.Wagner JM, Hackanson B, Lubbert M, Jung M. Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clinical epigenetics. 2010;1:117–136. doi: 10.1007/s13148-010-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams LM, Rush AJ, Koslow SH, Wisniewski SR, Cooper NJ, Nemeroff CB, Schatzberg AF, Gordon E. International Study to Predict Optimized Treatment for Depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials. 2011;12:4. doi: 10.1186/1745-6215-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh HH, Tian M, Hinz R, Young D, Shavrin A, Mukhapadhyay U, Flores LG, Balatoni J, Soghomonyan S, Jeong HJ, Pal A, Uthamanthil R, Jackson JN, Nishii R, Mizuma H, Onoe H, Kagawa S, Higashi T, Fukumitsu N, Alauddin M, Tong W, Herholz K, Gelovani JG. Imaging epigenetic regulation by histone deacetylases in the brain using PET/MRI with (1)(8)F-FAHA. NeuroImage. 2013;64:630–639. doi: 10.1016/j.neuroimage.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.