Abstract
Orally delivered replicating adenovirus (Ad) vaccines have been used for decades to prevent adenovirus serotype 4 and 7 respiratory illness in military recruits, demonstrating exemplary safety and high efficacy. That experience suggests that oral administration of live recombinant Ads (rAds) holds promise for immunization against other infectious diseases, including those that have been refractory to traditional vaccination methods. Live rAds can express intact antigens from free-standing transgenes during replication in infected cells. Alternatively, antigenic epitopes can be displayed on the rAd capsid itself, allowing presentation of the epitope to the immune system both prior to and during replication of the virus. Such capsid-display rAds offer a novel vaccine approach that could be used either independently of or in combination with transgene expression strategies to provide a new tool in the search for protection from infectious disease.
Keywords: replicating adenovirus vectors, oral administration, capsid-display, hexon, vaccine
Introduction
Oral delivery of immunogens to the gut is regarded as the “Holy Grail” for vaccinologists [1]. The intestine is the largest lymphoid organ and gut-associated immune cells represent up to 90% of immunocompetent cells [2]. Oral immunization offers immunological and logistical advantages including stimulation of mucosal immune responses preferentially at the site of entry for many infectious agents and ability to elicit strong systemic immunity. Oral immunization is cost effective and offers improved patient compliance due to the ease of vaccine administration, freedom from needles and from the requirement for trained medical personnel. All three oral vaccines licensed for use in the US [3] contain live virus. Live-virus vaccines add to the inherent advantages of oral immunization the ability to immunize with small (and hence less expensive) doses, and induction of a breadth of immune responses similar to those induced by natural infection. These characteristics would facilitate routine immunization and response to epidemics or pandemics [4] and make live oral immunization attractive in resource-poor regions, where economy and logistical tractability are critically important.
Licensed oral adenovirus (Ad) serotype 4 and 7 vaccines provide a model for use of live recombinant adenoviruses (rAds) for oral immunization. Since the 1970’s, live oral Ad vaccines have been used by the United States military to prevent acute respiratory disease caused by Ad4 and Ad7 [5]. These vaccines contain lyophilized live, wild type (WT) virus incorporated into enteric tablets that protect the virus against the low pH of the stomach. After oral administration of the tablets, live virus is released into the intestine where asymptomatic replication occurs. In a single dose, the vaccines generate an immune response that was over 95% effective in preventing Ad4- and Ad7-induced respiratory illness in a clinical trial involving more than 40,000 soldiers [6–9]. The historical success of Ad military vaccines suggests great potential for recombinant vaccines using the oral replicating Ad platform.
rAds have been used to deliver vaccine antigens in over 90 pre-clinical and clinical trials [10, 11]. The rationales for use of rAd vaccines include genome stability and ease of manipulation, natural tropism for mucosal inductive sites including the gut and upper respiratory tract and ability to elicit vigorous humoral and cellular immune responses. rAds infect a broad spectrum of cells, including dendritic cells, allowing for efficient antigen presentation and can therefore also prime a robust cell-mediated response [12, 13]. However, most rAd vaccine candidates are replication defective and not intended for oral administration. Here, we review work on replicating rAd vaccines that may provide a route to effective oral immunization.
Replicating rAd Transgene Vectors as Vaccines
Most current rAd vaccine candidates are transgene expression vectors, commonly engineered to express a foreign gene inserted into early region 1 (E1) or, occasionally, early region 4 (E4) of the genome [14]. E1 and E4 are essential for viral replication, and most such rAds are replication-defective [15–17]. Extensive experience with defective recombinants in humans and animal models has shown promise in several cases [18].
Replication-competent transgene vectors can be constructed by careful choice of the site of transgene insertion but relatively few have been extensively investigated. Study of replicating rAd vaccines is complicated by the requirement for a host that supports viral replication if vaccines are to be evaluated under conditions that mimic their intended use in humans. Mice do not support human adenovirus replication. However, golden hamsters, cotton rats, dogs, pigs, monkeys (see below), and chimpanzees all support replication of some human Ads, providing systems that might be exploited to test replicating vaccines [19–24]. Cotton rats and hamsters have found use in characterization of replicating oncolytic adenoviruses [19, 25], and dogs have been used in evaluation of live rAd vaccines [21]. In practice, however, well-developed immunological reagents, perceived similarity of primate and human immune responses, and availability of suitable challenges to assess efficacy have restricted most studies of replication-competent rAds in permissive hosts to primates (chimpanzees or monkeys), or to human volunteers.
In early studies, replication-competent rAd7 and rAd4 expressing the hepatitis B virus surface antigen (HBsAg) were used to immunize (rAd7 HBsAg) and then boost (rAd4 HBsAg) two Ad4, Ad7-seronegative chimpanzees (rAd7/rAd4 HBsAg) by the oral route [23]. After primary vaccinations, both chimpanzees shed vaccine virus for 6–7 weeks and developed Ad7 antibodies, suggesting successful Ad7 replication in the chimpanzee gut. One developed transient seropositivity for HBsAg after the first inoculation; both developed modest titers after the second. A third chimpanzee immunized with WT Ad7 and then rAd4 HBsAg (WTAd7/rAd4 HBsAg) developed no HBsAg antibodies. Both rAd7/rAd4 HBsAg chimpanzees were protected from acute clinical disease but were not protected from infection as evident by development of antibodies against the HBV core protein in response to HBV challenge. The animal that did not seroconvert (WTAd7/rAd4 HBsAg), along with an unimmunized control, became clinically infected with HBV [23]. Three human volunteers in a small phase I vaccine trial immunized with the rAd7 HBsAg vaccine exhibited no adverse effects and shed virus between days 4 and 13 post vaccination with no evidence of person-to-person spread. Although all subjects had a significant increase in Ad7 antibodies, none made antibodies to HBsAg [26]. Protection from disease, if not infection, in chimpanzees, despite lack of seroconversion in humans, suggests potential value in using oral enteric vaccination with rAd to induce humoral immune responses to foreign pathogens.
Most animal studies of replicating rAds have been conducted in macaques. WT Ad2 and Ad5 do not replicate in monkeys, and these experiments therefore require use of an Ad5 host range mutation (hr404), located in the 72k DNA binding protein, that permits replication in monkey cells and macaques [24, 27]. A transgene-type rAd5 hr404 (rAd5hr) virus expressing the env and rev genes from SIV (Ad5hr-SIVenv/rev) was able to replicate in vivo in rhesus macaques [28]. Priming orally and intranasally, followed by intratracheal immunization 12 weeks later with Ad5hr-SIVenv/rev, generated proliferating T cells to Env and strong serum neutralizing anti-Env antibodies. Mucosal secretions also contained Env-specific IgG and IgA antibodies. Although this vaccine did not induce sterilizing immunity, it conferred acute-phase protection following intravaginal challenge with SIV [28]. Partial protection of reboosted and rechallenged transiently viremic macaques was associated with both cellular and humoral immune responses [29]. To broaden rAd-induced immunity to SIV, additional rhesus macaques were immunized simultaneously with replicating constructs expressing SIV env, rev and gag genes through oral and intranasal administration [30]. Specific T-cell responses were generated against all SIV gene products and there was a persistent response to Gag evident for more than 10 weeks post-immunization. Interestingly, immunization primed CD8+ T cells for a persistent and potent response to both dominant and subdominant epitopes [30, 31]. Intrarectal challenge with SIV demonstrated that the vaccine did not induce sterile immunity but acute viral replication was suppressed. Cellular immunity to SIV Gag and Env, along with nasal and vaginal Env-specific IgG antibodies, correlated with a significant reduction of acute phase viremia [32]. Immunized groups exhibited significant protection, with 39% of macaques having either no viremia, cleared viremia or controlled viremia at the threshold of detection 40 weeks post-challenge.
In these studies, only 35% of macaques exhibiting rAd shedding [30], suggesting that the protocol used, bicarbonate neutralization of the stomach prior to virus delivery, might not preserve rAd infectivity. Use of enteric-coated capsules for virus administration resulted in shedding virus in stool samples of 100% of immunized macaques [33] emphasizing the importance of an optimal oral delivery method.
Recently, phase I clinical trial data has been presented for a transgene-type replication-competent rAd4 vaccine (rAd4-H5-Vtn) expressing influenza H5 hemagglutinin (HA) [34]. This virus, which induced protective immune responses in a nonpermissive mouse model [35], contains an insertion of the H5 HA gene in place of part of E3. 166 healthy volunteers received vaccine dosages ranging from 107 to 1011 recombinant virus particles (VP) [34]. Each cohort received three rAd vaccinations orally and an intramuscular boost with inactivated H5N1 vaccine. Administration of the rAd was associated with mild headache, abdominal pain, nasal congestion and diarrhea. There was no confirmed transmission of the rAd4-H5-Vtn virus to household contacts. Pre-existing antibody to Ad4 was associated with a lower immune response to the vaccine, but this effect was overcome in the high-dose cohorts of 1010 and 1011 VP. In mice, this recombinant elicits good humoral Ad4 and HA responses but a low cell-mediated response [35]. In humans, the vaccine induced a significant level of Ad4 seroconversion and HA-specific cellular immune responses in 70% of volunteers receiving 1011 VP [34]. However, HA-specific antibody responses assessed by hemagglutination-inhibition (HAI) were minimal at all doses tested, with seroconversion in 4% to 19% of vaccinated volunteers. Plasma IgA ELISA titers mirrored HAI, although IgG ELISA responses indicated 50% seroconversion in the 1011 VP cohort. The H5 HA antigen is an intrinsically poor immunogen [36], however following boost of the inactivated H5N1 vaccine, 80% to 100% of volunteers seroconverted and 80% to 89% demonstrated antibody titers high enough to be considered protective in the 1010 and 1011 VP cohorts, respectively [34]. This indicates that although the Ad4-H5-Vtn vaccine can induce a cellular response, it is only capable of priming an HA-specific antibody response. The cellular immune response and replication of the vaccine as assessed by Ad4 seroconversion or PCR positive rectal swabs, primarily occurred after the first dose, suggesting that only one oral dose may be necessary to induce a cellular response and prime an antibody response.
The doses of Ad4-H5-Vtn required to induce vector immune responses are 100-fold (or more) greater than that in the Ad4 vaccine (105 – 107 TCID50 [5]). rAd4-H5-Vtn lacks E3, which functions in evading the host immune response [37] and may contribute to the vigor or duration of replication, and thus to the immunogenicity of the, WT vaccines. That possibility has not been experimentally addressed.
Numerous clinical trials of replicating oncolytic rAds have been conducted. In general, these studies do not include analyses of immune responses. Where vector responses have been measured they are efficiently induced [38, 39], but there are no reports of responses to transgene products.
Replicating Capsid Display rAds as Vaccines
Despite the efficacy of the oral Ad4 and Ad7 vaccines and efficient induction of antibodies against the vector, oral rAd vectors induce only modest antibody responses to transgene products in both replicating and non-replicating forms [23, 40] (Berg and Ketner, unpublished). However, a second rAd antigen expression method may offer a more potent approach to induction of humoral immunity. In capsid-display recombinants, segments of foreign antigens are incorporated into one of the capsid proteins such that they are displayed on the surface of the virus particle. Capsid-incorporated antigens are available for binding by surface antibody on B cells and can be processed by the exogenous (MHC class II) pathway. Thus, capsid display recombinants can be immunogenic without intracellular antigen expression, including in systems that do not support virus replication. Replication in a permissive host would further allow persistent antigen presentation via both the exogenous and the endogenous (MHC class I) pathways, with the potential of inducing both humoral and cellular responses. Capsid-display vectors are extremely immunogenic in mice [41–43] and therefore may offer greater efficacy in inducing humoral responses in permissive systems than do transgene rAds.
Several capsid proteins can display foreign epitopes, including hexon, fiber, penton base and pIX (Table 1, Figure 1A, and below). Currently, immunogenicity data is available only in mice, and conclusions therefore have been drawn only in the absence of viral replication.
Table 1.
Capsid Protein Insertion Sites
| Capsid Protein | Insertion Sites | Maximum Foreign Epitope Insertion Sizeb | References |
|---|---|---|---|
|
| |||
| Hexon | HVR1 | 24 | [41, 50–52, 55, 56, 58] |
| HVR2 | 45 | [48, 49, 51, 52, 56, 58, 90, 91] | |
| HVR3 | 6 | [48, 51] | |
| HVR4 | 15 | [51, 56] | |
| HVR5 | 66 | [43, 48–51, 53, 54, 56, 58, 60, 63] | |
| HVR6 | 6 | [48, 51] | |
| HVR7 | 15 | [48, 51, 56] | |
| HVR8 | -- | [51] | |
| HVR9 | -- | [51] | |
| VR1a | 24 | [57] | |
| VR4a | 24 | [57] | |
|
| |||
| Penton | RGD loop | 9 | [60] |
|
| |||
| Fiber | C terminus | 14 | [64, 92–94] |
| CD loop | 14 | [64] | |
| DE loop | 14 | [64] | |
| FG loop | 16 | [64, 95] | |
| HI loop | 22 | [43, 60, 62–68, 95] | |
| IJ loop | 16 | [95] | |
|
| |||
| pIX | C terminus | 393 | [42, 60, 71–75, 96] |
non-human adenovirus serotype with only 5 variable regions
size in amino acids
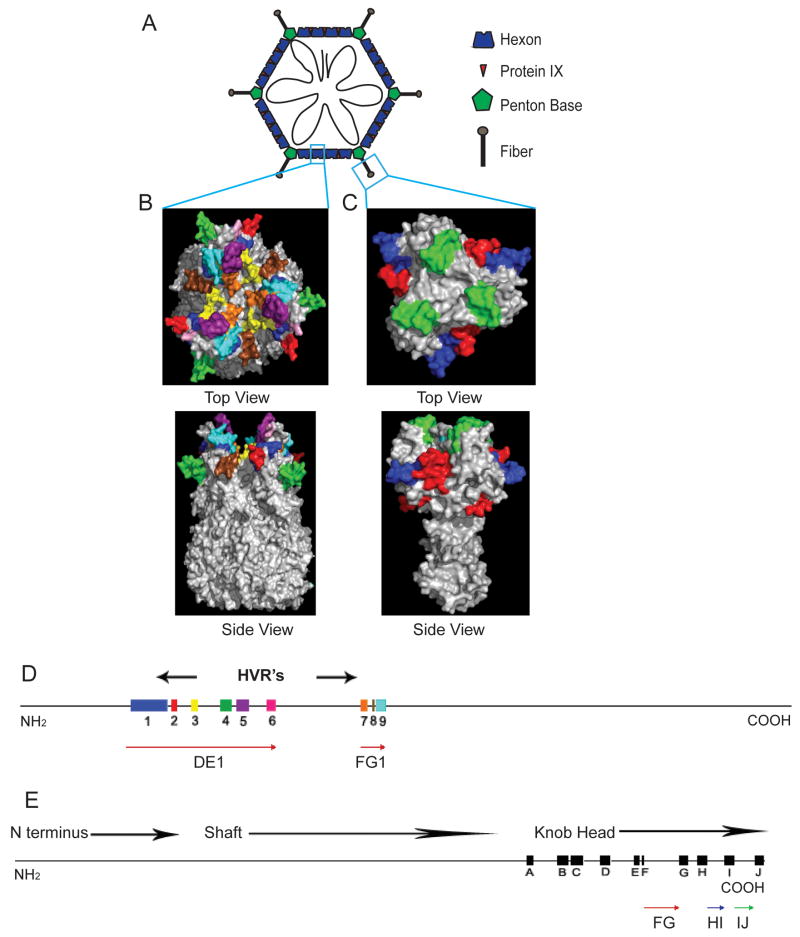
Figure 1.
Adenovirus Capsid Structure A) Cartoon diagram of an adenovirus particle depicting capsid proteins and DNA. B) Surface model of the trimeric Ad5 hexon protein showing the HVR regions. The amino acid location of the region is located in parenthesis. Blue: HVR1 (137–181), red: HVR2 (187–193), yellow: HVR3 (211–218), green: HVR4 (247–260), purple: HVR5 (267–282), brown HVR6 (304–315), orange: HVR7 (418–428), pink: HVR8 (435–436), cyan: HVR9 (440–451). Produced using program PYMOL and PDB 3IYN [97]. C) Surface model of the trimeric Ad2 penton knob and shaft. Red: FG loop (488–515), blue: HI loop (537–549), green: IJ loop (558–572). Produced using program PYMOL and PDB 1QIU [98]. D) Map of the Ad5 hexon protein with labeled HVRs E) Map of the fiber protein with labeled β-strands in black boxes and FG, HI and IJ loops indicated with colored arrows
Hexon (polypeptide II)
The ~960 amino acid Ad hexon protein is the most abundant of the capsid proteins, present in 720 copies per particle [44]. Analysis of hexon amino acid sequences from different serotypes revealed 9 hypervariable regions (HVRs) that diverge in sequence and length among serotypes [45]. Crystal structures of Ad2 and Ad5 hexon show that HVRs reside in two loops that form the surface-exposed portion of hexon. HVR 1–6 are located within the DEI loop and HVR 7–9 lie within the FGI loop (Figure 1 B and D) [45, 46]. These HVRs contain serotype-specific epitopes that are primary targets of neutralizing antibodies (nAb) [47].
X-ray crystallography suggests that HVRs 2, 3, 5, 6 and 7 are unordered and protrude from the capsid surface. Virus containing insertions of His6 peptides with flanking spacers into those HVRs are viable, with normal virion thermostability and infectivity [48]. His6 in HVR2 or 5 is capable of binding tightly to the His6 antibody, suggesting that the peptide is exposed on the virion when incorporated into these regions [48]. Assessed with epitopes of increasing size, HVR5 was found to accommodate a maximum of 65 amino acids, while the maximum length accommodated in HVR2 was 33 amino acids [49]. Modifications in HVR1 or 5 reduced susceptibility to neutralization by preexisting immunity (PEI) to the Ad vector [41, 50]. Substituting all the HVR loops in Ad5 with those derived from Ad43, a serotype with a low prevalence, produced a vector capable of escaping neutralization with anti-Ad5 sera from mouse, rabbit and humans [51] and that was still highly immunogenic in the presence of PEI to the WT virus.
The first capsid display recombinants incorporated 8 amino acids of the poliovirus type 3 VP1 capsid protein into regions now recognized as HVR1/2. Antiserum raised against the rAd recognized the poliovirus capsid itself [52]. Worgall et al. incorporated an immunodominant peptide from the outer membrane protein F (OprF) of Pseudomonas aeruginosa into HVR5 [43, 53]. Immunization with this rAd induced IgG1 and IgG2a antibody subtypes, elicited epitope-specific CD4+ and CD8+ T cell responses, and was capable of protecting 60–80% of mice from a lethal pulmonary challenge with three different P. aeruginosa strains. Efficacy was increased with subsequent boosts [43, 53]. In contrast, a B-cell epitope from Bacillus anthracis protective antigen (PA), a subunit of the lethal toxin, incorporated into HVR5, induced non-neutralizing antibodies and failed to protect against a challenge with lethal toxin [54]. The discordant results from these studies may reflect differential antibody titers or differing properties of the selected epitopes.
Subsequently, Shiratsuchi et al., inserted a B cell epitope from the circumsporozoite protein (CSP) of the murine malaria parasite Plasmodium yoelii into hexon HVR1 or 5 in a recombinant that also expressed CSP as a transgene [41]. The HVR1 recombinant induced high titer antibodies even in mice pre-immunized with WT Ad, suggesting that alteration of HVR1 allowed for evasion of neutralizing Ad antibodies. An rAd incorporating a B-cell epitope from P. falciparum CSP in HVR1 induced high-titer antibodies in mice that recognized parasites expressing the P. falciparum CSP and neutralized sporozoites bearing the P. falciparum CSP gene in vitro [55].
The location of epitopes inserted in hexon is an important determinant of immunological properties [49]. rAds that displayed an epitope from the VP1 capsid protein of Enterovirus 71 in HVRs 1, 2, or 7 were viable and protected neonatal mice from lethal challenge through passive immunization and maternally-acquired antibodies [56]. However, antibody isotype depended on the location of the epitope insertion: insertions into HVR1 induced mostly IgG2a antibodies (Th1) while an HVR7 insertion predominantly produced IgG1 antibodies (Th2), demonstrating that insertion sites on hexon are not immunologically equivalent [56]. Similarly, insertion of the conserved extracellular domain of matrix protein 2 (M2e) of influenza A virus into variable region 1 (VR1) or VR4 of hexon of the chimpanzee-origin adenovirus SAd-V25 (AdC68), only the VR1 recombinant provided partial protection from a lethal influenza challenge [57]. Capsid display recombinants induced more robust responses than a transgene type recombinant expressing an M2e fusion protein, supporting the hypothesis that antibody responses are best induced by antigen displayed in a repetitive and structured fashion to allow for cross-linkage of the B cell receptors.
Recent studies of rAds with modifications in two hexon HVRs have demonstrated the potential for single recombinants to elicit simultaneous antibody responses against two distinct epitopes [58]. ‘Multivalent’ capsid display recombinants offer potential for broadening immune responses or inducing responses to genetically variable pathogens. However, recombinants with different combinations of modified HVRs induced strikingly different responses, indicating that the design of effective multivalent hexon-modified rAds may not be straightforward.
Penton base (polypeptide III)
The penton base and fiber form the penton complex present at the 12 vertices of the capsid (Figure 1A). Each penton base monomer (~570 residues) contains an Arg-Gly-Asp (RGD) integrin-binding motif located within a flexible loop at the capsid surface [59]. An influenza A virus HA epitope inserted into the RGD loop of penton base was accessible to anti-HA antibodies, confirming surface location [60]. However, anti-HA antibodies were not detected in mice immunized with a penton base recombinant containing HA inserted into the RGD loop [60]. The insertion decreased infectivity for DC’s, potentially by interfering with integrin binding, which is involved in virion internalization.
Fiber (polypeptide IV)
Fibers are homotrimers of the fiber protein (polypeptide IV) that protrude from the 12 vertices of the Ad virion and are responsible for attachment to the host cell (Figure 1A). The fiber protein has 3 domains: an N-terminal domain that attaches to the penton base, a central shaft with repeating motifs, and a C-terminal globular knob responsible for virus attachment to the host cell (Figure 1E). Ad5 fiber contains 582 amino acids and is 35–40 nM in length, but fiber length varies among serotypes due to differing numbers of repeats in the fiber shaft.
The crystal structure of the fiber knob reveals that the HI loop (Figure 1C and 1E) does not contribute to intramolecular interactions within the knob, consists mostly of hydrophilic amino acid residues, is exposed on the surface of the knob and is not involved in the formation of cell-binding sites [61]. A FLAG epitope inserted into the HI loop was also accessible to anti-FLAG antibodies, confirming that the HI loop is exposed [62]. Therefore, the HI loop is seen as particularly suitable for manipulation and most modifications initially were made at this location [60, 63]. More recently, a series of rAds with insertions of the P. aeruginosa OprF Epi8 epitope in fiber loops CD, DE, FG, HI and at the C terminus [64] have been examined for effects of insertions on viral growth in vitro and for immunogenicity. Incorporation of Epi8 into the FG and HI loops had little effect on viral growth whereas insertion into the CD and DE loops or at the C terminus strongly reduced infectivity. FG and HI loop insertions also elicited the strongest humoral and cell-mediated immune responses and were partially protective against challenge [64].
Fiber is a target for neutralizing antibodies and substitutions can contribute to evasion of PEI. For example, modification of the HI loop circumvented nAb present in ascites fluid from ovarian cancer patients [65]. Consistent with this, FG and HI loop recombinants were more effective at inducing antibody and protection in the presence of PEI than was a transgene-type recombinant expressing all of OprF [64]. The ability to manipulate fiber at multiple sites to allow for the efficacy in the presence of PEI makes fiber insertions a promising modification for capsid-display vaccines.
Fiber modifications intended to redirect or ablate virus binding to specific cellular receptors have also been explored [43, 66–68]. However, immunogenicity generally is not addressed in those studies.
pIX (polypeptide IX)
pIX (approximately 140 amino acids) is present in about 240 copies per virion. Trimers of pIX contribute to stability of the virus particle [69, 70]. The C-terminus of pIX is exposed on the surface of the virion and has been used as a substrate on which to attach large polypeptides including fluorescent proteins, fully functional enzymes and foreign antigens in viable rAds [71–75]. pIX fusions containing the envelope protein gp70 of the Friend murine leukemia virus (FV) [75] and the Yersinia pestis V and F1 capsular antigens [42] induced high-titer antibodies. The ability of pIX to accommodate very large proteins makes it an attractive site for display of conformational epitopes.
Comparative Immunogenicity
The immunogenicity of influenza A virus HA epitopes inserted into various Ad capsid proteins has been compared [60]. Insertion sites included hexon HVR5, the RGD loop of penton base, the HI loop of the fiber knob and the C terminus of pIX. All HA insertions were located on the virion surface, however, an anti-HA antibody demonstrated strongest binding to HA incorporated into hexon. Infection of A549 cells and DCs showed that HA incorporation into hexon interferes minimally with virus entry in vitro, whereas incorporation into fiber knob, pIX and penton base partially reduced the intracellular Ad genome copy numbers following infection. The humoral immune response was strongest against the hexon insertion when immunizing with the same number of particles but fiber was the most immunogenic when controlling for the number of HA copies per virion [60]. A comparison of an ovalbumin (OVA) epitope inserted into the fiber HI loop or hexon HVR5 indicated that fiber insertions were better detected in native virions and triggered a more dramatic increase in anti-OVA antibody responses upon re-administration [63].
Pre-existing Immunity and Replicating rAds
Antibodies to many Ad serotypes are prevalent in the human population. PEI to the vaccine serotype can interfere with a robust immune response against the foreign antigen even in non-replicating rAds [23, 76], although mucosally administered replication-defective rAd vaccines have elicited transgene-specific antibodies despite the presence of PEI, and homologous serotype boosts can be effective [32, 77, 78]. Importantly, if capable of suppressing the growth of viable rAds, PEI might mitigate the inherent advantage of vaccine vector replication after administration of a low dose [79], and live rAds thus may be more sensitive to PEI than their defective counterparts. PEI can be addressed by use of uncommon human adenovirus serotypes or viruses from other species [80, 81] [82] as vectors. Additionally, as noted above, modifications to both hexon and fiber have been shown to reduce susceptibility to PEI [41, 50, 51] and properly-designed capsid display rAds therefore may be inherently resistant to PEI. Limited experience with replicating rAds has provided some data on the effects of PEI [34], but this topic must be more thoroughly addressed.
Safety of Replicating rAds
Concerns have been expressed over the safety of replicating vaccines due to the possibility of inducing disease in the immunocompromised and to the possibility of unintentional spread to contacts. Systemic adenovirus infections can be fatal in people who are profoundly immunocompromised, for example, in the course of bone marrow transplantation [83, 84]. Further, Ad is commonly present in AIDS-related deaths, although it is not generally believed that it was the cause [85, 86]. Clearly, live rAds cannot be administered to the severely immunodeficient. However, unwitting administration of the military vaccine to a small number of recruits with early HIV infection produced no observed ill effects, nor did the concurrent HIV infection prolong shedding, suggesting a small margin of safety in that population [87]. Transmission of the oral military Ad vaccine did occur, but required intimate contact, as it was not observed among recruits in the barracks [88, 89], and no confirmed transmission of the rAd4-H5-Vtn virus to contacts was observed in its recent clinical trial [34]. This aspect of use of live vaccines, rAd or others, must be carefully investigated.
Conclusion
Replication-competent transgene or capsid display rAds delivered orally to the gut mucosa offer an unconventional immunization approach. Transgene rAds have been shown to induce a robust cellular-mediated immune response, and capsid-display rAds promise to induce strong humoral responses. Critically, transgene and capsid display designs possess complementary immunological characteristics and can be combined in single rAds [41], and such hybrid rAds offer a potential route to greater potency than either approach alone. Multiple-antigen hybrid rAds, in particular, may be capable of increasing the breadth of immune responses to pathogens with a high mutation rate, such as influenza A, or a complex biology, such as malaria. Continued innovation in vaccine research is critical in order to control diseases such as HIV, influenza and malaria that have proven refractory to conventional immunization strategies, and replicating rAds have earned their place among the novel strategies worthy of exploration.
Highlights.
Live oral adenovirus (Ad) vaccines have proven safe and effective.
Live recombinant Ads can express antigens as transgenes or by capsid display.
Ad recombinants are highly immunogenic
Live Ad recombinants induce potent cell-mediated responses in permissive animals
Capsid display recombinants promise good humoral responses.
Acknowledgments
This work was funded by NIH grant 1R01AI079132, the Johns Hopkins Malaria Research Institute, the Marjorie Gilbert Foundation, the Eliasberg Foundation and C.D. was funded through the Johns Hopkins Sommer Scholars. I would also like to thank Dr. Kasey Karen and Laura Gelston for their thoughtful insight and helpful review of this article.
Abbreviations in order of appearance
- Ad
Adenovirus
- rAd
recombinant adenovirus
- WT
wild-type
- E1
early region 1
- E4
early region 4
- HBsAg
hepatitis B virus surface antigen
- hr404
host range mutation in DNA binding protein
- rAd5hr
recombinant Ad 5 with hr404 mutation
- Ad5hrSIVenv/rev
Ad5 with hr404 mutation expressing env and rev genes of SIV
- rAd4-H5-Vtn
recombinant Ad4 expressing influenza H5 hemagglutinin
- HA
influenza hemagglutinin
- VP
virus particles
- MHC class II
major histocompatibility complex II
- MHC class I
major histocompatibility complex I
- HVRs
hypervariable regions
- nAb
neutralizing antibodies
- PEI
preexisting immunity
- OprF
outer membrane protein F of Pseudomonas aeruginosa
- PA
protective antigen of Bacillus anthracis
- CSP
Plasmodium circumsporozoite protein
- M2e
influenza A matrix protein 2 extracellular domain
- VR1
variable region 1
- RGD
Arg-Gly-Asp motif
- FV
Friend murine leukemia virus
- OVA
ovalbumin
Footnotes
Conflict of Interest The authors have no competing financial interests to declare.
Author Contributions C.D. wrote the paper and performed research on the topic. A.P. and G.K. contributed to the conception and revising of the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bourinbaiar AS, Metadilogkul O, Jirathitikal V. Mucosal AIDS vaccines. Viral Immunol. 2003;16(4):427–45. doi: 10.1089/088282403771926274. [DOI] [PubMed] [Google Scholar]
- 2.Silin DS, et al. Oral vaccination: where we are? Expert Opin Drug Deliv. 2007;4(4):323–40. doi: 10.1517/17425247.4.4.323. [DOI] [PubMed] [Google Scholar]
- 3.FDA. Complete List of Vaccines Licensed for Immunization and Distribution in the United States. 2013 [cited 2013 April 26, 2013]; Available from: http://www.fda.gov/BiologicsBloodVaccines/default.htm.
- 4.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 5.Takafuji ET, et al. Simultaneous administration of live, enteric-coated adenovirus types 4, 7 and 21 vaccines: safety and immunogenicity. J Infect Dis. 1979;140(1):48–53. doi: 10.1093/infdis/140.1.48. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz AR, Togo Y, Hornick RB. Clinical evaluation of live, oral types 1, 2, and 5 adenovirus vaccines. Am Rev Respir Dis. 1974;109(2):233–9. doi: 10.1164/arrd.1974.109.2.233. [DOI] [PubMed] [Google Scholar]
- 7.Gutekunst RR, et al. Immunization with live type 4 adenovirus: determination of infectious virus dose and protective effect of enteric infection. Am J Epidemiol. 1967;86(2):341–9. doi: 10.1093/oxfordjournals.aje.a120744. [DOI] [PubMed] [Google Scholar]
- 8.Top FH, Jr, et al. Immunization with live types 7 and 4 adenovirus vaccines. II. Antibody response and protective effect against acute respiratory disease due to adenovirus type 7. J Infect Dis. 1971;124(2):155–60. doi: 10.1093/infdis/124.2.155. [DOI] [PubMed] [Google Scholar]
- 9.Lyons A, et al. A double-blind, placebo-controlled study of the safety and immunogenicity of live, oral type 4 and type 7 adenovirus vaccines in adults. Vaccine. 2008;26(23):2890–8. doi: 10.1016/j.vaccine.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Rollier CS, et al. Viral vectors as vaccine platforms: deployment in sight. Curr Opin Immunol. 2011;23(3):377–82. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 11.US National Institutes of Health . ClinicalTrials.gov. 2013 [Cited May 1, 2013] Available from: www.clinicaltrials.gov/
- 12.Lore K, et al. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179(3):1721–9. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson LJ, et al. Replicating adenovirus-simian immunodeficiency virus (SIV) vectors efficiently prime SIV-specific systemic and mucosal immune responses by targeting myeloid dendritic cells and persisting in rectal macrophages, regardless of immunization route. Clin Vaccine Immunol. 2012;19(5):629–37. doi: 10.1128/CVI.00010-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Small JC, Ertl HC. Viruses - from pathogens to vaccine carriers. Curr Opin Virol. 2011;1(4):241–5. doi: 10.1016/j.coviro.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang MM, Hearing P. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J Virol. 1989;63(6):2605–15. doi: 10.1128/jvi.63.6.2605-2615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberg DH, Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J Virol. 1986;57(3):833–8. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones N, Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978;13(1):181–8. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan NJ, et al. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408(6812):605–9. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 19.Thomas MA, et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66(3):1270–6. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 20.Pacini DL, Dubovi EJ, Clyde WA., Jr A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis. 1984;150(1):92–7. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- 21.Chengalvala M, et al. Evaluation of adenovirus type 4 and type 7 recombinant hepatitis B vaccines in dogs. Vaccine. 1991;9(7):485–90. doi: 10.1016/0264-410x(91)90033-3. [DOI] [PubMed] [Google Scholar]
- 22.Jogler C, et al. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J Virol. 2006;80(7):3549–58. doi: 10.1128/JVI.80.7.3549-3558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubeck MD, et al. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc Natl Acad Sci U S A. 1989;86(17):6763–7. doi: 10.1073/pnas.86.17.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klessig DF, Grodzicker T. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell. 1979;17(4):957–66. doi: 10.1016/0092-8674(79)90335-0. [DOI] [PubMed] [Google Scholar]
- 25.Toth K, et al. Cotton rat tumor model for the evaluation of oncolytic adenoviruses. Hum Gene Ther. 2005;16(1):139–46. doi: 10.1089/hum.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 26.Tacket CO, et al. Initial safety and immunogenicity studies of an oral recombinant adenohepatitis B vaccine. Vaccine. 1992;10(10):673–6. doi: 10.1016/0264-410x(92)90088-2. [DOI] [PubMed] [Google Scholar]
- 27.Kruijer W, van Schaik FM, Sussenbach JS. Structure and organization of the gene coding for the DNA binding protein of adenovirus type 5. Nucleic Acids Res. 1981;9(18):4439–57. doi: 10.1093/nar/9.18.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buge SL, et al. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol. 1997;71(11):8531–41. doi: 10.1128/jvi.71.11.8531-8541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buge SL, et al. Factors associated with slow disease progression in macaques immunized with an adenovirus-simian immunodeficiency virus (SIV) envelope priming-gp120 boosting regimen and challenged vaginally with SIVmac251. J Virol. 1999;73(9):7430–40. doi: 10.1128/jvi.73.9.7430-7440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J, et al. Boosting of SIV-specific immune responses in rhesus macaques by repeated administration of Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinants. Vaccine. 2003;21(25–26):4022–35. doi: 10.1016/s0264-410x(03)00266-4. [DOI] [PubMed] [Google Scholar]
- 31.Malkevitch N, et al. A replication competent adenovirus 5 host range mutant-simian immunodeficiency virus (SIV) recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity to dominant and subdominant epitopes in Mamu-A*01 rhesus macaques. J Immunol. 2003;170(8):4281–9. doi: 10.4049/jimmunol.170.8.4281. [DOI] [PubMed] [Google Scholar]
- 32.Zhao J, et al. Improved protection of rhesus macaques against intrarectal simian immunodeficiency virus SIV(mac251) challenge by a replication-competent Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinant priming/gp120 boosting regimen. J Virol. 2003;77(15):8354–65. doi: 10.1128/JVI.77.15.8354-8365.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Roman VR, et al. Oral delivery of replication-competent adenovirus vectors is well tolerated by SIV- and SHIV-infected rhesus macaques. Vaccine. 2006;24(23):5064–72. doi: 10.1016/j.vaccine.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 34.Gurwith M, et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect Dis. 2013 doi: 10.1016/S1473-3099(12)70345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander J, et al. Pre-clinical evaluation of a replication-competent recombinant adenovirus serotype 4 vaccine expressing influenza H5 hemagglutinin. PLoS One. 2012;7(2):e31177. doi: 10.1371/journal.pone.0031177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talaat KR, et al. An open-label phase I trial of a live attenuated H2N2 influenza virus vaccine in healthy adults. Influenza Other Respi Viruses. 2011;7(1):66–73. doi: 10.1111/j.1750-2659.2012.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wold WS, et al. Immune responses to adenoviruses: viral evasion mechanisms and their implications for the clinic. Curr Opin Immunol. 1999;11(4):380–6. doi: 10.1016/S0952-7915(99)80064-8. [DOI] [PubMed] [Google Scholar]
- 38.Reid T, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62(21):6070–9. [PubMed] [Google Scholar]
- 39.Small EJ, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14(1):107–17. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Lin SW, et al. Intramuscular rather than oral administration of replication-defective adenoviral vaccine vector induces specific CD8+ T cell responses in the gut. Vaccine. 2007;25(12):2187–93. doi: 10.1016/j.vaccine.2006.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiratsuchi T, et al. Replacing adenoviral vector HVR1 with a malaria B cell epitope improves immunogenicity and circumvents preexisting immunity to adenovirus in mice. J Clin Invest. 2010;120(10):3688–701. doi: 10.1172/JCI39812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyer JL, et al. Protective immunity against a lethal respiratory Yersinia pestis challenge induced by V antigen or the F1 capsular antigen incorporated into adenovirus capsid. Hum Gene Ther. 2010;21(7):891–901. doi: 10.1089/hum.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Worgall S, et al. Protective immunity to pseudomonas aeruginosa induced with a capsid-modified adenovirus expressing P. aeruginosa OprF. J Virol. 2007;81(24):13801–8. doi: 10.1128/JVI.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Oostrum J, Burnett RM. Molecular composition of the adenovirus type 2 virion. J Virol. 1985;56(2):439–48. doi: 10.1128/jvi.56.2.439-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rux JJ, Kuser PR, Burnett RM. Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods. J Virol. 2003;77(17):9553–66. doi: 10.1128/JVI.77.17.9553-9566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rux JJ, Burnett RM. Type-specific epitope locations revealed by X-ray crystallographic study of adenovirus type 5 hexon. Mol Ther. 2000;1(1):18–30. doi: 10.1006/mthe.1999.0001. [DOI] [PubMed] [Google Scholar]
- 47.Crawford-Miksza L, Schnurr DP. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J Virol. 1996;70(3):1836–44. doi: 10.1128/jvi.70.3.1836-1844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu H, et al. Identification of sites in adenovirus hexon for foreign peptide incorporation. J Virol. 2005;79(6):3382–90. doi: 10.1128/JVI.79.6.3382-3390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matthews QL, et al. Optimization of capsid-incorporated antigens for a novel adenovirus vaccine approach. Virol J. 2008;5:98. doi: 10.1186/1743-422X-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abe S, et al. Adenovirus type 5 with modified hexons induces robust transgene-specific immune responses in mice with pre-existing immunity against adenovirus type 5. J Gene Med. 2009;11(7):570–9. doi: 10.1002/jgm.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruder JT, et al. Modification of Ad5 hexon hypervariable regions circumvents pre-existing Ad5 neutralizing antibodies and induces protective immune responses. PLoS One. 2012;7(4):e33920. doi: 10.1371/journal.pone.0033920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crompton J, et al. Expression of a foreign epitope on the surface of the adenovirus hexon. J Gen Virol. 1994;75(Pt 1):133–9. doi: 10.1099/0022-1317-75-1-133. [DOI] [PubMed] [Google Scholar]
- 53.Worgall S, et al. Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J Clin Invest. 2005;115(5):1281–9. doi: 10.1172/JCI23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McConnell MJ, Danthinne X, Imperiale MJ. Characterization of a permissive epitope insertion site in adenovirus hexon. J Virol. 2006;80(11):5361–70. doi: 10.1128/JVI.00256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palma C, et al. Adenovirus particles that display the Plasmodium falciparum circumsporozoite protein NANP repeat induce sporozoite-neutralizing antibodies in mice. Vaccine. 2011;29(8):1683–9. doi: 10.1016/j.vaccine.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian X, et al. Protection against enterovirus 71 with neutralizing epitope incorporation within adenovirus type 3 hexon. PLoS One. 2012;7(7):e41381. doi: 10.1371/journal.pone.0041381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou D, et al. Hexon-modified Recombinant E1-deleted Adenovirus Vectors as Dual Specificity Vaccine Carriers for Influenza Virus. Mol Ther. 2012 doi: 10.1038/mt.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu L, et al. Using Multivalent Adenoviral Vectors for HIV Vaccination. PLoS One. 2013;8(3):e60347. doi: 10.1371/journal.pone.0060347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zubieta C, et al. The structure of the human adenovirus 2 penton. Mol Cell. 2005;17(1):121–35. doi: 10.1016/j.molcel.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 60.Krause A, et al. Epitopes expressed in different adenovirus capsid proteins induce different levels of epitope-specific immunity. J Virol. 2006;80(11):5523–30. doi: 10.1128/JVI.02667-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xia D, et al. Structure of the receptor binding domain of adenovirus type 5 fiber protein. Curr Top Microbiol Immunol. 1995;199(Pt 1):39–46. doi: 10.1007/978-3-642-79496-4_3. [DOI] [PubMed] [Google Scholar]
- 62.Krasnykh V, et al. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72(3):1844–52. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lanzi A, et al. Anti-adenovirus humoral responses influence on the efficacy of vaccines based on epitope display on adenovirus capsid. Vaccine. 2010;29(7):1463–71. doi: 10.1016/j.vaccine.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 64.Sharma A, et al. Adenovirus-based vaccine with epitopes incorporated in novel fiber sites to induce protective immunity against Pseudomonas aeruginosa. PLoS One. 2013;8(2):e56996. doi: 10.1371/journal.pone.0056996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blackwell JL, et al. Using a tropism-modified adenoviral vector to circumvent inhibitory factors in ascites fluid. Hum Gene Ther. 2000;11(12):1657–69. doi: 10.1089/10430340050111313. [DOI] [PubMed] [Google Scholar]
- 66.Dmitriev I, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72(12):9706–13. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wickham TJ, et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71(11):8221–9. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Denby L, et al. Development of renal-targeted vectors through combined in vivo phage display and capsid engineering of adenoviral fibers from serotype 19p. Mol Ther. 2007;15(9):1647–54. doi: 10.1038/sj.mt.6300214. [DOI] [PubMed] [Google Scholar]
- 69.McConnell MJ, Imperiale MJ. Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther. 2004;15(11):1022–33. doi: 10.1089/hum.2004.15.1022. [DOI] [PubMed] [Google Scholar]
- 70.Parks RJ. Adenovirus protein IX: a new look at an old protein. Mol Ther. 2005;11(1):19–25. doi: 10.1016/j.ymthe.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 71.Li J, et al. Evaluation of adenovirus capsid labeling versus transgene expression. Virol J. 2010;7:21. doi: 10.1186/1743-422X-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meulenbroek RA, et al. Use of adenovirus protein IX (pIX) to display large polypeptides on the virion--generation of fluorescent virus through the incorporation of pIX-GFP. Mol Ther. 2004;9(4):617–24. doi: 10.1016/j.ymthe.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 73.Kimball KJ, et al. Novel infectivity-enhanced oncolytic adenovirus with a capsid-incorporated dual-imaging moiety for monitoring virotherapy in ovarian cancer. Mol Imaging. 2009;8(5):264–77. [PMC free article] [PubMed] [Google Scholar]
- 74.Matthews QL, et al. Genetic incorporation of a herpes simplex virus type 1 thymidine kinase and firefly luciferase fusion into the adenovirus protein IX for functional display on the virion. Mol Imaging. 2006;5(4):510–9. [PMC free article] [PubMed] [Google Scholar]
- 75.Bayer W, et al. Vaccination with an adenoviral vector that encodes and displays a retroviral antigen induces improved neutralizing antibody and CD4+ T-cell responses and confers enhanced protection. J Virol. 2010;84(4):1967–76. doi: 10.1128/JVI.01840-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. 2007;18(6):546–56. doi: 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiang ZQ, et al. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J Virol. 2003;77(20):10780–9. doi: 10.1128/JVI.77.20.10780-10789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peters W, et al. Oral administration of an adenovirus vector encoding both an avian influenza A hemagglutinin and a TLR3 ligand induces antigen specific granzyme B and IFN-gamma T cell responses in humans. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 79.Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol. 1988;62(7):2321–8. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiang Z, et al. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol. 2002;76(6):2667–75. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dudareva M, et al. Prevalence of serum neutralizing antibodies against chimpanzee adenovirus 63 and human adenovirus 5 in Kenyan children, in the context of vaccine vector efficacy. Vaccine. 2009;27(27):3501–4. doi: 10.1016/j.vaccine.2009.03.080. [DOI] [PubMed] [Google Scholar]
- 82.Kahl CA, et al. Potent immune responses and in vitro pro-inflammatory cytokine suppression by a novel adenovirus vaccine vector based on rare human serotype 28. Vaccine. 2010;28(35):5691–702. doi: 10.1016/j.vaccine.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blanke C, et al. Evolving pathogens in allogeneic bone marrow transplantation: increased fatal adenoviral infections. Am J Med. 1995;99(3):326–8. doi: 10.1016/s0002-9343(99)80169-7. [DOI] [PubMed] [Google Scholar]
- 84.Baldwin A, et al. Outcome and clinical course of 100 patients with adenovirus infection following bone marrow transplantation. Bone Marrow Transplant. 2000;26(12):1333–8. doi: 10.1038/sj.bmt.1702716. [DOI] [PubMed] [Google Scholar]
- 85.Koopmann J, et al. Fatal pneumonia in an AIDS patient coinfected with adenovirus and Pneumocystis carinii. Infection. 2000;28(5):323–5. doi: 10.1007/s150100070028. [DOI] [PubMed] [Google Scholar]
- 86.Khoo SH, et al. Adenovirus Infections in Human Immunodeficiency Virus-Positive Patients: Clinical Features and Molecular Epidemiology. Journal of Infectious Diseases. 1995;172(3):629–637. doi: 10.1093/infdis/172.3.629. [DOI] [PubMed] [Google Scholar]
- 87.Rhoads JL, et al. Safety and immunogenicity of multiple conventional immunizations administered during early HIV infection. J Acquir Immune Defic Syndr. 1991;4(7):724–31. [PubMed] [Google Scholar]
- 88.Mueller RE, Muldoon RL, Jackson GG. Communicability of enteric live adenovirus type 4 vaccine in families. J Infect Dis. 1969;119(1):60–6. doi: 10.1093/infdis/119.1.60. [DOI] [PubMed] [Google Scholar]
- 89.Stanley ED, Jackson GG. Spread of enteric live adenovirus type 4 vaccine in married couples. J Infect Dis. 1969;119(1):51–9. doi: 10.1093/infdis/119.1.51. [DOI] [PubMed] [Google Scholar]
- 90.Matthews QL, et al. HIV antigen incorporation within adenovirus hexon hypervariable 2 for a novel HIV vaccine approach. PLoS One. 2010;5(7):e11815. doi: 10.1371/journal.pone.0011815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flatt JW, et al. CryoEM visualization of an adenovirus capsid-incorporated HIV antigen. PLoS One. 2012;7(11):e49607. doi: 10.1371/journal.pone.0049607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wickham TJ, et al. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol. 1996;14(11):1570–3. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 93.Stevenson SC, et al. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J Virol. 1997;71(6):4782–90. doi: 10.1128/jvi.71.6.4782-4790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krasnykh VN, et al. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70(10):6839–46. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matsui H, et al. Development of fiber-substituted adenovirus vectors containing foreign peptides in the adenovirus serotype 35 fiber knob. Gene Ther. 2009;16(8):1050–7. doi: 10.1038/gt.2009.65. [DOI] [PubMed] [Google Scholar]
- 96.Li J, et al. Genetic incorporation of HSV-1 thymidine kinase into the adenovirus protein IX for functional display on the virion. Virology. 2005;338(2):247–58. doi: 10.1016/j.virol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 97.Liu H, et al. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science. 2010;329(5995):1038–43. doi: 10.1126/science.1187433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Raaij MJ, et al. A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature. 1999;401(6756):935–8. doi: 10.1038/44880. [DOI] [PubMed] [Google Scholar]