To the Editor: Extracellular calcium levels are tightly regulated by parathyroid hormone (PTH). Insufficient production of this hormone, as observed in nonsyndromic isolated hypoparathyroidism, can be caused by mutations in PTH or the genes encoding the parathyroid-specific transcription factor glial cells missing 2 (GCM2) or the calcium-sensing receptor (CaSR). However, most cases of isolated hypoparathyroidism remain genetically undefined.1
We investigated two unrelated white families in which 15 living members had clinical and laboratory findings consistent with autosomal dominant isolated hypoparathyroidism (Fig. 1). After ruling out the presence of mutations in CaSR, PTH, and GCM2 in the index cases (not shown), a genomewide scan revealed linkage to a single chromosomal region for Family A (19p13.3; LOD score, 3.0). Candidate gene sequencing resulted in the identification of a heterozygous nucleotide change in exon 2 of GNA11 (c.178C→T; p.Arg60Cys), the gene encoding the α subunit of the guanine nucleotide-binding protein G11 (Gα11) (Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Whole-exome sequencing of two affected members of Family A (Patients 37 and 44) confirmed this nucleotide transition. Exome sequencing of two members of Family B (Patients 26 and 31) revealed a heterozygous nucleotide transversion in exon 5 of GNA11 (c.632C→G; p.Ser211Trp); no additional variant that affects the same gene in both families was identified. The nucleotide changes were present only in affected family members, and both changes affect amino acid residues that are highly conserved in Gα11 and the closely related Gαq.
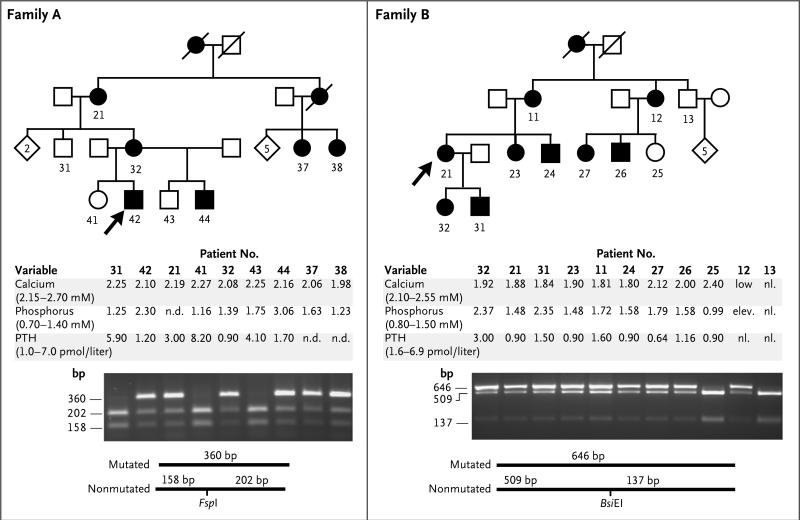
Figure 1. Pedigrees and Laboratory and Genetic Findings for Two Families with Autosomal Dominant Isolated Hypoparathyroidism.
Squares indicate male family members, circles female family members, black symbols affected family members, and white symbols unaffected family members; slashes indicate deceased family members; numbers outside squares and circles indicate family members for whom DNA was available for testing; numbers within white rhombi indicate the number of unaffected siblings. Arrows point to the index cases. Measurements for calcium, phosphorus, and parathyroid hormone (PTH) are shown (with adult normal ranges in parentheses) and reflect values at presentation except in the case of Patients 21 and 32, from whom samples were drawn after treatment was initiated. For affected members, mean (±SE) pretreatment serum levels of calcium were 2.15±0.02 mmol per liter in Family A and 1.91±0.04 mmol per liter in Family B, mean levels of phosphorus were 2.06±0.40 and 1.79±0.13 mmol per liter, respectively, and mean levels of PTH were 1.97±0.42 and 1.33±0.27 pmol per liter, respectively. Values for other variables among patients with autosomal dominant isolated hypoparathyroidism for whom mean pretreatment levels were available were as follows: magnesium, 0.77±0.02 mmol per liter (10 patients; normal range, 0.7 to 1.0); 1,25-dihydroxyvitamin D, 81.8±10.0 pmol per liter (8 patients; normal range, 40 to 150); and urinary calcium:creatinine ratio, normal when measured (8 patients; normal range, <0.6 mmol:mmol). Two novel mutations affecting Gα11 (R60C and S211W) were identified by means of genetic linkage analysis for Family A and exome sequencing of a DNA sample from two affected members of Families A and B (see Fig. 1 in the Supplementary Appendix). Digestion of DNA (amplified by means of polymerase chain reaction) with the endonucleases FspI and BsiEI, respectively, confirmed the two mutations and revealed their segregation with the disease.
Gα11 and Gαq mediate the intracellular signaling that depends on the generation of inositol 1,4,5-trisphosphate and the activation of protein kinase C and occurs downstream of CaSR,2 the main regulator of PTH synthesis and secretion. Homozygous inactivating CaSR mutations cause severe neonatal hyperparathyroidism, as does the combined parathyroid-specific ablation of Gα11 and Gαq in mice.3,4 Conversely, activating CaSR mutations lead to hypocalcemia because of inappropriate PTH secretion.1 The latter findings are similar to those reported for our families with autosomal dominant isolated hypoparathyroidism, thus making it plausible to suggest that the identified Gα11 mutants increase signaling at this receptor.
To evaluate this hypothesis, we analyzed both mutants with the use of molecular modeling (Fig. S2 in the Supplementary Appendix). On the basis of the proposed crystal structure of Gα11, it is probable that the replacement of arginine 60 with cysteine (R60C mutant) in helix α1 of the guanosine triphosphatase (GTPase) domain will disrupt the intramolecular hydrogen bond with asparagine 71, located in αA of the helical domain. This mutant is therefore predicted to destabilize the “closed clamshell” conformation of the helical and GTPase domains, thus allowing either a faster exchange of guanosine diphosphate with GTP or disrupting Gα11 contacts with regulatory proteins. In contrast, the replacement of serine 211 in the switch II region of Gα11 with tryptophan (S211W mutant) is predicted to disrupt the binding of the mutant α subunit to the β subunit, thereby enhancing agonist-dependent signaling.
Activating mutations affecting Gα11 and Gαq cause uveal melanomas5; however, these genetic changes are somatic, as are most disease-causing mutations in other G proteins (Table S1 in the Supplementary Appendix). In fact, only a few activating germline mutations affecting G proteins appear to be compatible with life; these include maternal mutations affecting Gαs that cause gonadotropin-independent male precocious puberty or neonatal diarrhea in combination with pseudohypoparathyroidism type 1a, and three murine germline mutations affecting Gαq or Gα11 that lead to dermal hyperpigmentation. The inherited mutations affecting Gα11 in family members with autosomal dominant isolated hypoparathyroidism are therefore remarkable, particularly since obvious abnormalities affect only the regulation of mineral-ion homeostasis. In summary, genomewide linkage analysis, com bined with whole-exome sequencing, revealed two different heterozygous mutations affecting Gα11 as novel causes of autosomal dominant isolated hypoparathyroidism.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health (K08DK081669-01, R01DK46718-20 and PO1DK11794 [sub-project IV] and X01 HG006062-01) and by the National Heart, Lung, and Blood Institute Exome Sequencing Project (HL102923-26 ad HL103010).
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Michael Mannstadt, Massachusetts General Hospital Boston, MA
Mark Harris, Mater Children's Hospital Brisbane, QLD, Australia
Bert Bravenboer, Catharina Ziekenhuis Eindhoven, the Netherlands
Sridhar Chitturi, Royal Darwin Hospital Tiwi, NT, Australia
Koen M.A. Dreijerink, University Medical Center Utrecht, the Netherlands
David G. Lambright, University of Massachusetts Medical School Worcester, MA
Elaine T. Lim, Broad Institute Cambridge, MA
Mark J. Daly, Massachusetts General Hospital Boston, MA
Stacey Gabriel, Broad Institute Cambridge, MA
Harald Jüppner, Massachusetts General Hospital Boston, MA hjueppner@partners.org
References
- 1.Shoback D. Hypoparathyroidism. N Engl J Med. 2008;359:391–403. doi: 10.1056/NEJMcp0803050. [DOI] [PubMed] [Google Scholar]
- 2.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–8. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 3.Ho C, Conner DA, Pollak M, et al. A mouse model for familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11:389–94. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- 4.Wettschureck N, Lee E, Libutti SK, Offermanns S, Robey PG, Spiegel AM. Parathyroid-specific double knockout of Gq and G11 alpha-subunits leads to a phenotype resembling germline knockout of the extracellular Ca2+-sensing receptor. Mol Endocrinol. 2007;21:274–80. doi: 10.1210/me.2006-0110. [DOI] [PubMed] [Google Scholar]
- 5.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–9. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.