Abstract
DNA methylation and BLC-2 are potential therapeutic targets in acute myeloid leukemia (AML). We investigated pharmacologic interaction between the DNA methyltransferase inhibitor 5-azacytidine (5-AZA) and the BCL-2 inhibitor ABT-737. Increased BCL-2 expression determined by reverse phase protein analysis was associated with poor survival in AML patients with unfavorable cytogenetics (n=195). We found that 5-AZA, which itself has modest apoptotic activity, acts synergistically with ABT-737 to induce apoptosis. The 5-AZA/ABT-737 combination enhanced mitochondrial outer membrane permeabilization, as evidenced by effective conformational activation of BAX and Δψm loss. Although absence of p53 limited apoptotic activities of 5-AZA and ABT-737 as single agents, the combination synergistically induced apoptosis independent of p53 expression. 5-AZA down-regulated MCL-1, known to mediate resistance to ABT-737, in a p53-independent manner. The 5-AZA/ABT-737 combination synergistically induced apoptosis in AML cells from 7 of 8 patients. 5-AZA significantly reduced MCL-1 levels in 2 of 3 samples examined. Our data provide a molecular rationale for this combination strategy in AML therapy.
Keywords: AML, 5-azacytidine, ABT-737, BCL-2, MCL-1, p53
Introduction
Aberrant expression of anti-apoptotic members such as BCL-2 or suppression of pro-apoptotic members such as BAX can lead to tumor formation and promote resistance to therapy in many types of cancer, including acute myeloid leukemia (AML) [1, 2]. ABT-737 is a small molecule BH3 mimetic that binds tightly to a hydrophobic cleft on anti-apoptotic BCL-2 and BCL-XL, and exerts its proapoptotic function by preventing anti-apoptotic BCL-2 family members from sequestering activating BH3 proteins [3]. We have reported that ABT-737 effectively kills AML blasts, progenitor, and stem cells without affecting normal hematopoietic cells [4]. The ABT-737–related clinical compound, ABT-263, is undergoing clinical studies in chronic lymphocytic leukemia (CLL) (NCT00868413; NCT01087151) and diffuse large B-cell lymphoma (NCT01423539), with initial signs of clinical activity. The main side effect is thrombocytopenia resulting from inhibition of BCL-XL [5]. Recent report of the ongoing CLL trial of the 2nd generation BH3 mimetic ABT-199, engineered to selectively inhibit BCL-2 but not BCL-XL, has shown encouraging signs of clinical activity without thrombocytopenia [6]. It remains to be seen, however, whether loss of anti-BCL-XL function will diminish therapeutic efficacy in AML as compared to BCL-2-dependant CLL cells. Hence, elucidation of the combinations of ABT-263 with non-cytotoxic agents desirable to take full advantage of ABT-263’s unique spectrum of biophysical and preclinical activities is warranted.
DNA methyltransferase enzymes add methyl groups to genomic CpG sites. CpG dinucleotides are enriched at certain gene promoter regions and methylation of these sites may contribute to leukemogenesis through silencing of tumor suppressor genes [7]. The cytidine analogue, 5-azacytidine (5-AZA) inhibits DNA methyltransferases, leading to demethylation of DNA in daughter cells with a resultant effect on gene expression and cell differentiation. 5-AZA has been shown to have clinical activity in myelodysplastic syndromes and in AML [8–10].
In this study, we studied pharmacologic interaction between 5-AZA and ABT-737. We found that absence of p53 limits the apoptotic activities of 5-AZA and ABT-737 as single drugs. The combination of 5-AZA and ABT-737 synergistically induces mitochondrial apoptosis irrespective of p53 expression. Importantly, 5-AZA down-regulated MCL-1, a resistance factor for ABT-737, in a p53-independent manner. Strong synergistic apoptosis induction was seen in primary AML cells (7 out of 8 cases studied), supporting future clinical development of this combination strategy in AML patients.
Methods
Reagents
The BCL-2 family small molecule inhibitor, ABT-737 was provided by Abbott Laboratories (Abbott Park, IL). The DNA methylation inhibitor 5-azacytidine (5-AZA) was purchased from Sigma (St Louis, MO). The pan-caspase inhibitor Z-VAD-FMK was purchased from Axxora (San Diego, CA).
Cells lines, primary cells, and cell culture
Human AML cell lines were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS). OCI-AML-3 and MOLM-13 cells have wild-type p53, while U937 and HL-60 do not express p53 [11, 12]. OCI-AML-3 and MOLM-13 cells were stably transduced with retroviruses encoding either p53-specific shRNA or control shRNA as described [13, 14]. Peripheral blood (PB) or bone marrow (BM) samples were obtained from AML patients after informed consent, according to institutional guidelines. Mononuclear cells were purified by Ficoll-Hypaque (Sigma) density-gradient centrifugation, cells resuspended in RPMI 1640 medium supplemented with 10% FCS at a density of 1 × 106 cell/mL. For RPPA analysis, PB and BM specimens were collected from 511 patients with newly diagnosed AML evaluated at MDACC between September 1999 and March 2007. Samples were acquired during routine diagnostic assessments in accordance with the regulations and protocols approved by the investigational review board of MDACC. A total of 387 BM and 283 PB samples were studied; for 140 patients, both BM and PB samples were available. This patient population is typical of the MD Anderson referral pattern: a high percentage of patients with unfavorable cytogenetics (49%) and a very high percentage with an antecedent hematologic disorder (AHD; 40%). The samples were classified by cytogenetic risk groups using the Cancer and Leukemia Group B system, as described previously [15].
Apoptosis analysis
Annexin V binds specifically to phosphatidylserine, a lipid that is normally on the inside of the cell membrane but is exposed on the cell surface early in the apoptotic process. Induction of apoptosis was determined by Annexin V flow cytometry [4]. BAX conformational change was analyzed using an antibody directed against the NH2-terminal region of BAX (YTH-6A7; Trevigen, Gaithersburg, MD), as previously described [13]. Cellular fixation, permeabilization and staining with primary antibody were performed using the Dako IntraStain kit (Dako Cytomation, Carpinteria, CA), according to manufacturer’s instructions. After washing, cells were incubated with Alexa Fluor 488 chicken anti-mouse secondary antibodies (Molecular Probes, Eugene, OR) for 30 min at 4 °C. To measure mitochondrial membrane potential (Δψm), cells were loaded with MitoTracker Red CMXRos (300 nM) and MitoTracker Green (100 M, both from Molecular Probes, Eugene, OR) for 1 hour at 37 °C. The Δψm was then assessed by measuring CMXRos retention (red fluorescence) while simultaneously adjusting for mitochondrial mass (green fluorescence).
Western blot analysis
Western blot analysis was performed as previously described [16], using the Odyssey imaging system (LI-COR Biosciences, Lincoln, NE). The following antibodies were used: mouse monoclonal anti-p53 (Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal anti-MCL-1 (BD Pharmingen, San Diego, CA); mouse monoclonal anti-BCL-2 (Dako Cytomation, Carpinteria, CA); mouse monoclonal anti-BCL-XL (Santa Cruz Biotechnology); rabbit polyclonal anti-BAX (Santa Cruz Biotechnology); rabbit polyclonal anti-BAK (Millipore, Billerica, MA); mouse monoclonal anti-GAPDH (Millipore); and mouse monoclonal anti-β-ACTIN (Sigma).
Animal studies
Animal studies were approved and supervised by the University of Texas M. D. Anderson Cancer Center (MDACC) Institutional Animal Care and Use Committee. Twenty NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were irradiated with 2.5 Gy and injected with cells from a patient with primary refractory AML (1.5 × 106 viable cells per mouse). Seven days after cell injection, mice were treated with vehicle, 5-AZA (intraperitoneally (IP), 4 mg/kg/day for 5 days), ABT-737 (intraperitoneally (IP), 75 mg/kg/day for 10 days), or with the 5-AZA/ABT-737 combination (n = 5 in each group). Nine weeks after AML cell injection, engraftment was determined by immunohistochemical detection of CD45-positive cells in the BM and spleen.
Detection of acute leukemia-associated fusion transcripts and mutations
Leukemia-associated fusion transcripts are tested using nanofluidics-based qualitative multiparametric reverse-transcriptase polymerase chain reaction (PCR) by Molecular Diagnostics Laboratory at M.D. Anderson. The lower limit of detection is approximately 1 in 1,000 to 1 in 10,000. Fluorescently-labeled primers are used to detect the FLT3-ITD and D835 mutations; overnight restriction digestion is done on D835 PCR products. Capillary electrophoresis / GeneScan analysis is used to distinguish the wild type. Activating point mutations in exon 17 of the KIT, mutations in codons 12 and 13 as well as codon 61 of NRAS and KRAS2, and TP53 mutations in exons 4 to 9 were detected by PCR-based DNA sequencing. Mutations in exon 12 of the NPM1 were detected PCR-based amplification followed by amplicon sizing using Genescan. The sensitivity of detection is approximately 2.5% for FLT3 and NPM1 mutations, and approximately 20% for KIT, RAS and TP53 mutations.
Reverse phase protein analysis (RPPA)
Samples were enriched for leukemic cells, and a whole-cell lysate was prepared [17]. The methodology and validation of the proteomic profiling technique have been described previously [17–19]. Slides were probed with a strictly validated mouse anti–human monoclonal primary antibody against BCL-2 (clone 124, Dako), a secondary antibody to amplify the signal, and finally a stable dye was precipitated [20]. The stained slides were analyzed using Microvigene Version 2.9 software (Vigene Tech, Carlisle, MA).
Statistical analysis
The statistical analysis was performed using the two-tailed Student t test. Statistical significance was considered when P < .01. Average values were expressed as mean ± SD. The extent of apoptosis was quantified as percentage of Annexin V-positive cells, and the extent of drug-specific apoptosis was assessed by the formula: % specific apoptosis = (test − control) × 100 / (100 − control). In the formula, the numerator is the actual amount of killing that occurred and the denominator is the potential amount of killing that could occur. The combination index (CI), a numerical description of combination effects, was calculated using the more stringent statistical assumption of mutually non-exclusive modes of action. The averaged CI values were calculated from the values for ED50, ED75 and ED90. When CI = 1, this equation represents the conservation isobologram and indicates additive effects. CI values less than 1.0 indicate a more than expected additive effect (synergism), while CI values greater than 1.0 indicate antagonism between the 2 drugs. For RPPA, supercurve algorithms were used to generate a single value from the 5 serial dilutions [21]. Loading control [22] and topographical normalization procedures accounted for protein concentration and background staining variations. Expression was compared with that observed in normal CD34+ samples, and the patients were divided into 3 cohorts (expression above, below, or within the range of the normal CD34+ samples) based on the 95% confidence intervals. The Kaplan-Meier method was used to generate the survival curves.
Results
Increased BCL-2 expression predicts for poor survival in AML patients with unfavorable cytogenetics
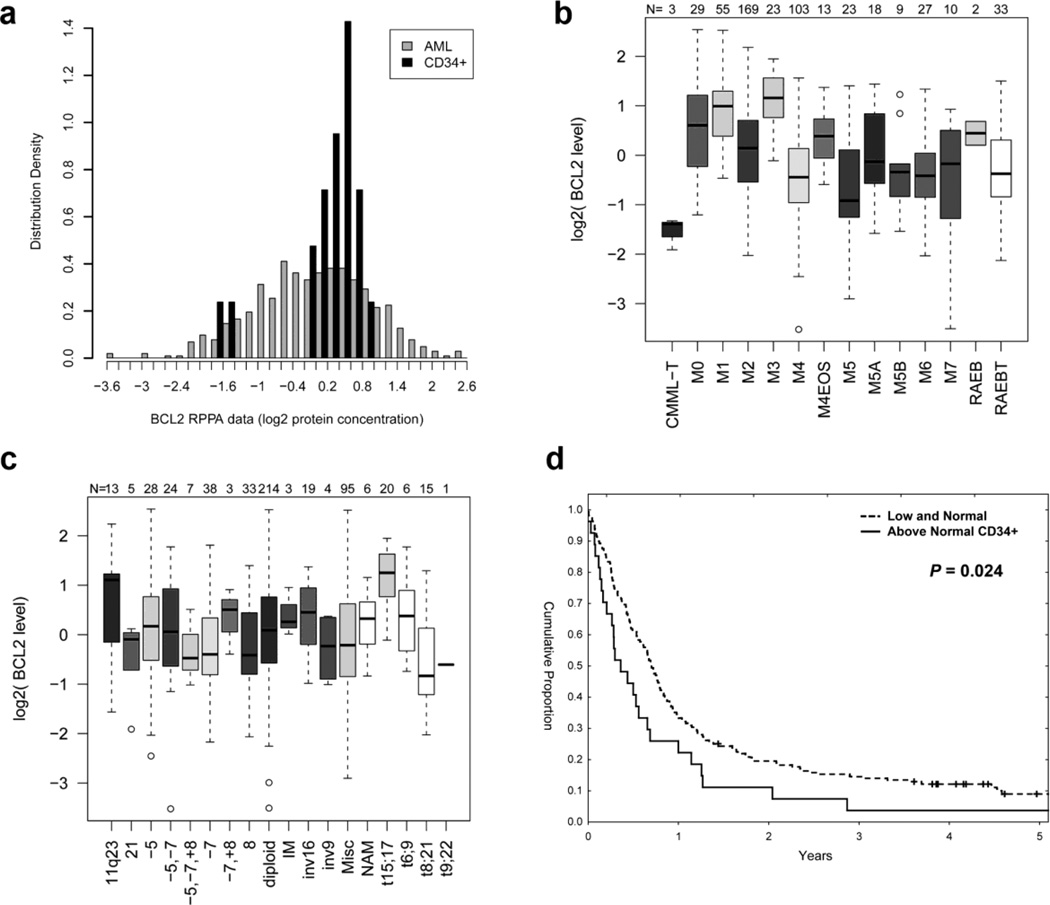
BCL-2 protein expression was analyzed in 511 newly diagnosed AML and 21 newly diagnosed APL patients by RPPA. BCL-2 expression in 140 same-day paired PB and BM samples was compared and found to be statistically similar (P = 0.90), demonstrating that the sample source did not affect the protein levels (not shown). BCL-2 levels did not significantly differ at the time of relapse compared with levels at diagnosis in the 47 paired samples (P = 0.25; not shown). Compared with expression in normal CD34+ cells, BCL-2 levels were above normal CD34+ cells in 15.2% and below normal in 38% of AML patients. Fig. 1a shows the expression of BCL-2 levels in the 511 newly diagnosed AML patients relative to normal CD34+ cells.
Figure 1. Increased BCL-2 expression predicts for poor survival in AML patients with unfavorable cytogenetics.
(a) Histogram of BCL-2 expression in all AML patients relative to normal CD34+ cells. (b) BCL-2 expression levels in patients with AML classified according to FAB classification. (c) BCL-2 expression levels in patients with AML classified according to cytogenetic abnormalities. (d) Kaplan Meier curves for overall survival in patients with unfavorable cytogenetics.
BCL-2 expression levels were not strongly correlated with French-American-British classification, although levels were higher in AML-M1 and APL (Fig. 1b). BCL-2 levels were elevated in patients with 11q23 and t(15;17) (APL) cytogenetic abnormalities (Fig. 1c). BCL-2 levels did not vary based on FLT3, RAS, or TP53 mutation status (not shown). BCL-2 levels were not associated with the attainment of complete remission. No difference in overall survival was seen based on different BCL-2 levels among patients with intermediate prognosis cytogenetics (median 77 vs 64 weeks; P = 0.32, not shown). In turn, among patients with unfavorable cytogenetics those with high BCL-2 (n=27) had inferior survival compared with those who had low-normal expression of BCL-2 (n=168), withmedian survival of 18 vs 36 weeks, P = 0.024, Fig. 1d). There was no significant difference in remission duration (P = 0.53, not shown). These findings indicate that high BCL-2 levels in patients with unfavorable cytogenetics may contribute to their refractoriness to standard chemotherapeutic agents, and validate BCL-2 as a potential therapeutic target.
5-AZA and ABT-737 synergistically induce apoptosis in AML cell lines
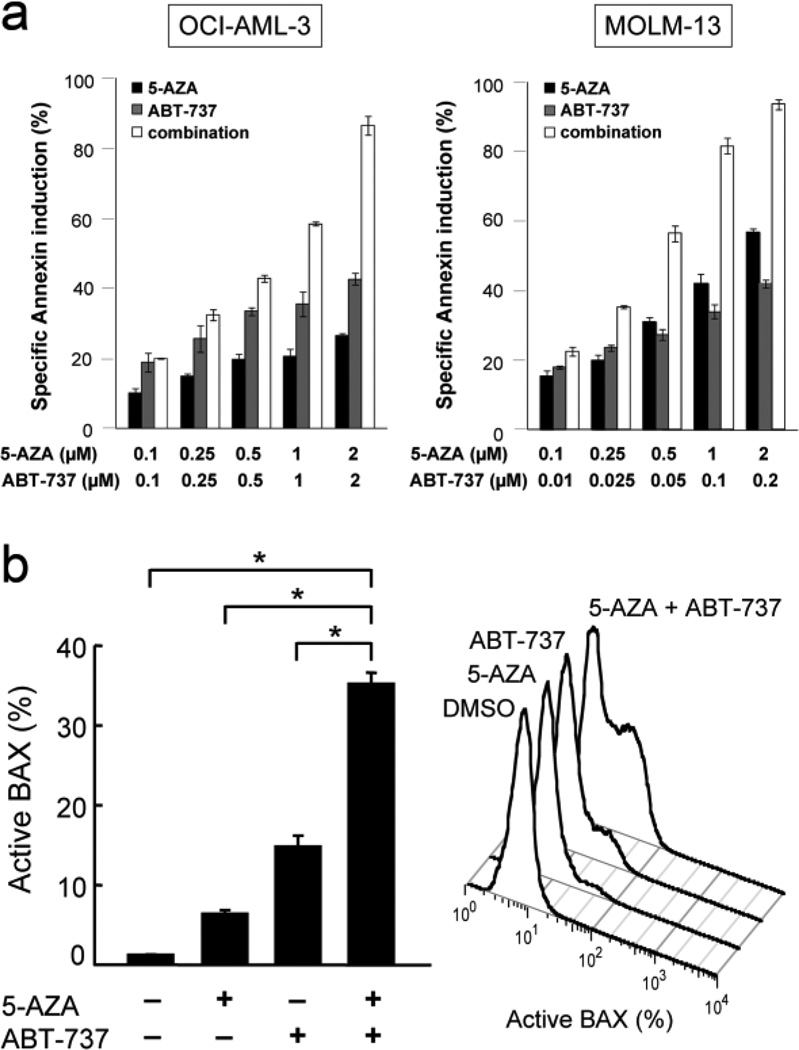
We examined the anti-proliferative and apoptotic effect of 5-AZA and ABT-737 in cultured AML cell lines. OCI-AML-3 and MOLM-13 cells have wild-type p53, while U937 and HL-60 do not express p53 [11,12]. Cells were treated for 72 hours with 100, 250, 500, 1000 or 2000 nM 5-AZA and ABT-737, either as individual agents or in combination. The concentration ratio of ABT-737 to 5-AZA was set at 1:1 in OCI-AML-3 and U937, and 1:10 in MOLM-13 and HL-60 cells, based on their different sensitivities to ABT-737. As shown in Fig. 2a and Table 1, the 5-AZA/ABT-737 combination showed highly synergistic anti-proliferative and apoptotic effects in OCI-AML-3 and MOLM-13 cells. The calculated CI values for phosphatidylserine externalization were 0.19 for ED50, 0.08 for ED75 and 0.04 for ED90 in OCI-AML-3 cells, and were 0.28, 0.17 and 0.11 in MOLM-13 cells, exerting strong synergy over a wide concentration range. A CI value of < 1 indicates synergy (i.e., greater than the expected additive effect when two agents are combined), the CI value of < 0.3 indicates strong synergy [23]. Synergistic effect was less prominent in U937 and HL-60 cells (Table 1).
Figure 2. 5-AZA and ABT-737 synergistically induce apoptosis in AML cells.
(a) OCI-AML-3 and MOLM-13 cells were treated with a range of concentrations of 5-AZA and ABT-737 for 72 hours, either as individual agents or in combination, and the Annexin V-positive fractions were measured by flow cytometry. Results are expressed as mean ± SD. A minority of untreated cells (6.5 ± 0.9% in OCI-AML-3, 5.3 ± 0.6% in MOLM-13 and 4.8 ± 2.0% in HL-60 cells) was positive for Annexin V. (b) OCI-AML-3 cells were treated for 24 hours with 2 µM 5-AZA and 6 hours with 2 µM ABT-737, and BAX conformational change was determined. To block the caspase activation-mediated conformational change of BAX, cells were preincubated for 1 hour with 50 M Z-VAD-FMK. Results are expressed as mean SD of triplicate measurements. Asterisk (*) indicates significance at P < 0.01.
Table 1.
Averaged combination index values for anti-proliferative and apoptotic effects of 5-AZA and ABT-737
| anti-proliferation | apoptosis induction | |
|---|---|---|
| OCI-AML-3 | 0.26 | 0.10 |
| MOLM-13 | 0.57 | 0.19 |
| U937 | 0.85 | 0.66 |
| HL-60 | 1.16 | 0.79 |
5-AZA and ABT-737 synergistically activate BAX in AML cells
The BH3 mimetic ABT-737 neutralizes anti-apoptotic BCL-2 and BCL-XL, resulting in BAX activation and apoptosis in AML cells. Mitochondrial dysfunction mediated by BAX activation is a critical step in mammalian cell apoptosis. To investigate if 5-AZA enhances ABT-737-induced BAX activation, BAX conformational change was analyzed in OCI-AML-3 and MOLM-13 cells by means of an antibody directed against the NH2-terminal region of BAX (clone YTH-6A7), which reacts only with BAX in its active conformation [13]. Considering the different pharmacokinetic profiles between 5-AZA and ABT-737, 5-AZA (2 µM) and ABT-737 (2 µM) were added into the medium 24 and 6 hours before the analysis, respectively. As shown in Fig. 2b, 5-AZA and ABT-737 only modestly induced BAX conformational change in OCI-AML-3 cells. Importantly, the combination synergistically induced BAX conformational change, suggesting that 5-AZA and ABT-737 synergistically activate BAX and the intrinsic apoptotic pathway. The synergistic activation of BAX by 5-AZA and ABT-737 was also found in MOLM-13 cells, in which the percentages of cells undergoing specific BAX conformational change after exposure to 2 µM 5-AZA, 0.2 µM ABT-737 and their combination were 10.4 ± 1.7%, 31.9 ± 3.3% and 48.9 ± 2.1%, respectively. Total BAX levels were not affected by 5-AZA, ABT-737 or the 5-AZA/ABT-737 combination in either OCI-AML-3 (Supplemental Fig. S1) or MOLM-13 cells (figure not shown). 5-AZA, ABT-737 and their combination did not affect levels of other BCL-2 family proteins BAK, BCL-XL and BCL-2, or p53. These results suggest that 5-AZA and ABT-737 synergize to activate BAX without affecting the levels of BAX, BAK, BCL-2, BCL-XL or p53.
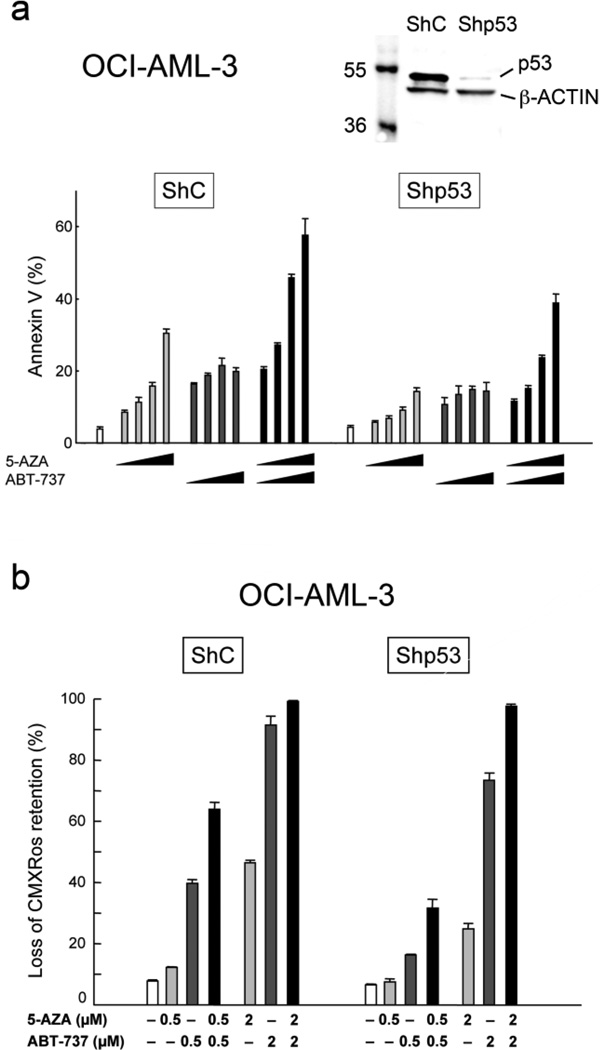
p53 knockdown leads to decreased apoptosis induction by 5-AZA and ABT-737 but does not impair their synergistic apoptotic effect
It has been reported that 5-AZA can activate p53-mediated apoptotic signaling [24]. We have recently demonstrated that loss of p53 limits the apoptotic activity of ABT-737 [14]. To elucidate if p53 expression determines AML cell sensitivity to 5-AZA, ABT-737 or their combination, we investigated the effect of 5-AZA and ABT-737 in OCI-AML-3 and MOLM-13 cells that were infected with lentivirus encoding either negative control shRNA or p53-specific shRNA [13, 14]. p53-specific shRNA reduced p53 basal levels by more than 90% in both OCI-AML-3 and MOLM-13 cells (Fig. 3a and Supplemental Fig. S2). p53 knockdown has rendered these cells resistant to p53-mediated apoptosis. Cells were incubated with the indicated concentrations of 5-AZA or ABT-737 for 48 hours, and the Annexin V–positive fractions were measured by flow cytometry. p53-knockdown OCI-AML-3 cells were less susceptible to 5-AZA- and ABT-737-induced apoptosis than cells expressing negative control shRNA, suggesting that p53 expression may be required for full induction of apoptosis by 5-AZA or ABT-737 (Fig. 3a). Interestingly, however, the synergistic combination effect was maintained in p53-knockdown OCI-AML-3 cells. The calculated CI values for phosphatidylserine externalization were 0.19 for ED50, 0.18 for ED75 and 0.18 for ED90 in p53-knockdown OCI-AML-3 cells, which were lower than those in OCI-AML-3 cells expressing negative control shRNA (0.23 for ED50, 0.25 for ED75 and 0.26 for ED90). p53-dependent cell sensitivity to 5-AZA and ABT-737 and p53-independent synergistic apoptotic induction by the combination were also observed in MOLM-13 cells (Supplemental Fig. S2). The averaged CI values were 0.61 for MOLM-13 cells expressing negative control shRNA and were 0.10 for p53-knockdown cells. Our data suggest that p53 knockdown affects AML cell sensitivity to 5-AZA and ABT-737 as single agents and that 5-AZA and ABT-737 synergistically induce apoptosis irrespective of p53 expression.
Figure 3. 5-AZA and ABT-737 synergistically induce mitochondrial apoptosis in AML cells irrespective of p53 expression.
(a) OCI-AML-3 cells transduced with retroviruses encoding either scrambled shRNA (shC) or p53-specific shRNA (shp53), were treated for 72 hours with 250, 500, 1000 or 2000 nM 5-AZA and ABT-737, either as individual agents or in combination. The concentration ratio of ABT-737 to 5-AZA was 1:1. Annexin V–positive fractions were measured by flow cytometry. Results are expressed as mean ± SD. (b) OCI-AML-3 cells expressing scrambled shRNA (shC) or p53-specific shRNA (shp53), were treated for 18 hours with indicated concentrations of 5-AZA and ABT-737, either as individual agents or in combination. Δψm loss was determined. Results are expressed as mean ± SD.
5-AZA and ABT-737 synergistically induce mitochondrial apoptosis
We next investigated if mitochondrial apoptotic signaling is involved in the synergistic apoptotic effect between 5-AZA and ABT-737. OCI-AML-3 expressing shp53 and its negative control cells were treated with 5-AZA and ABT-737, and Δψm was determined. As shown in Fig. 3b, p53-knockdown OCI-AML-3 cells were less sensitive to 5-AZA- and ABT-737-induced Δψm loss than those expressing negative control shRNA. Importantly, 5-AZA synergized with ABT-737 to induce Δψm loss independent of p53 expression. The profound effect of the 5-AZA/ABT-737 combination on Δψm loss would support the idea that 5-AZA and ABT-737 synergistically induce apoptosis in AML cells mainly through a mitochondrial apoptotic pathway.
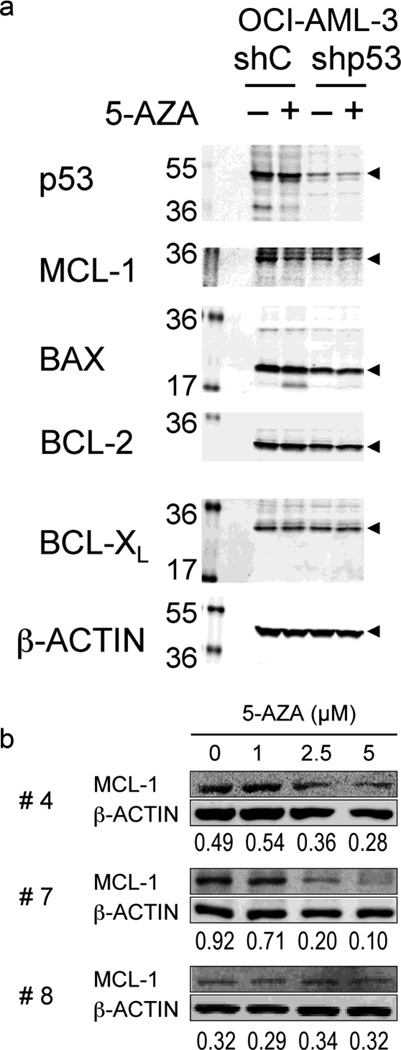
5-AZA reduces MCL-1 expression independent of p53 expression
It has been reported that ABT-737 neutralizes BCL-2, BCL-XL, and BCL-W but not MCL-1 and that ABT-737 efficiently induces mitochondrial apoptosis if MCL-1 is neutralized [4, 25, 26]. To see if 5-AZA affects MCL-1 expression independent of p53, OCI-AML-3 cells expressing p53-specific shRNA and those expressing negative control shRNA were treated with 1 µM 5-AZA for 48 hours. As shown in Fig. 4a, 5-AZA treatment did not induce p53 levels, and led to reduced expression of MCL-1 but not BAX, BCL-2 or BCL-XL in both, p53-wt and p53-KD cells. Steady-state BAX levels were low in p53-knockdown cells compared to control cells, reflecting low transcriptional activity of p53 [14].
Figure 4. 5-AZA reduces MCL-1 levels in a p53-independent manner.
(a) OCI-AML-3 cells transduced with retroviruses encoding either scrambled shRNA (shC) or p53-specific shRNA (shp53) and were treated for 48 hours with 1 µM 5-AZA. (b) Primary AML cells from 3 patients were incubated for 72 hours with the indicated concentrations of 5-AZA.
5-AZA and ABT-737 synergistically induce apoptosis in primary AML blasts
We cultured primary cells from 8 AML patients (Supplemental Table S1) with 5-AZA at 0, 1, 2.5 and 5 µM and ABT-737 at 0, 10, 25 and 50 nM either as individual agents or in combination, and evaluated apoptosis after 72 hours. The results indicated highly synergistic interaction of 5-AZA and ABT-737 in induction of apoptosis in 7 of 8 samples (Table 3). The combination effect was antagonistic in one case (case #3), in which the averaged CI value was 2.61. Chromosome analysis revealed a normal karyotype, and molecular studies did not detect fusion transcripts of BCR/ABL, AML1/ETO, PML/RAR α, CBFβ /MYH11 or MLL/AF4 or FLT3, RAS, C-KIT or NPM1 mutations. The possible molecular aberrations that affected the combination effects in the case #3 were not determined. DNMT3A, TET2 or IDH1/2 mutations were not analyzed in our series. Data suggest that 5-AZA enhances ABT-737-induced apoptosis in a majority of primary AML cells. In accordance with the data in AML cell lines, 72-hour exposure to 2.5 µM 5-AZA and/or 25 nM ABT-737 did not affect p53 levels in 2 primary AML samples (not shown). To investigate if 5-AZA reduces MCL-1 levels in patient AML cells, MCL-1 expression levels were determined in three AML samples (#4, 7 and 8) after 72-hour treatment of 5-AZA. As shown in Fig. 4b, 5-AZA treatment significantly reduced MCL-1 levels in 2 of the 3 samples.
5-AZA and ABT-737 decrease leukemia infiltration of murine bone marrow in mice injected with primary human AML cells
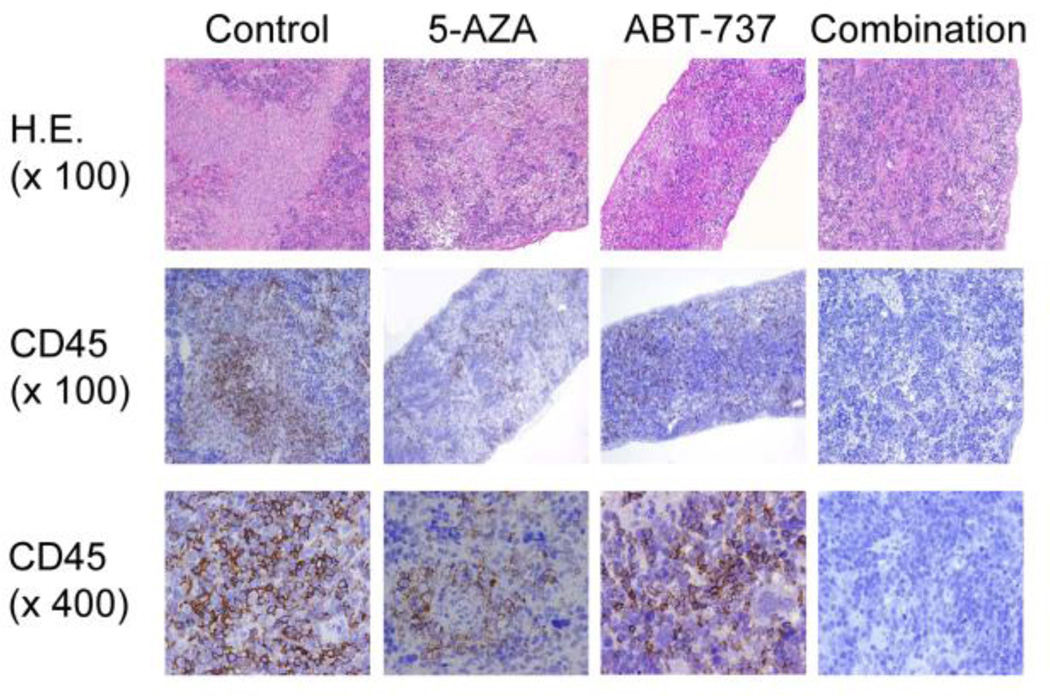
The combined effects of 5-AZA and ABT-737 were further investigated in NSG mice injected with BM blasts from a patient with primary refractory AML. The sample had 93% BM blasts. Seven days after leukemia cell injection, mice were treated with vehicle (group 1), 5-AZA (group 2), ABT-737 (group 3) or with the combination (group 4). Leukemia progression was monitored by enumeration of CD45(+) human leukemic cells in murine PB by flow cytometry. At six weeks of injection, circulating leukemic cells were detectable in PB from two out of three vehicle-treated mice (Supplemental Table S2), all three ABT-737-treated mice and only in one of the three 5-AZA-treated animals. Both 5-AZA and ABT-737 exerted an anti-leukemia effect, as evidenced by significant reduction in leukemia cells (Fig. 5, Supplemental Table S2). Engraftment was investigated in 2 mice from the group 4; no CD45+ AML cells were detected in organs of mice co-treated with 5-AZA and ABT-737. This was confirmed by immunohistochemical assessment of spleen tissues from selected mice sacrificed at nine weeks post injection.
Figure 5. 5-AZA and ABT-737 decrease the infiltrated CD45+ human leukemic cells in spleen.
Mouse spleen sections were stained with hematoxylin and eosin (H.E.) and anti-human CD45. The frequency of CD45+ cells enumerated by pathologist was as following: control, 30%; 5-AZA, 3%; ABT-737, 10%; 5-AZA+ABT-737, 0%.
Discussion
Since many signaling components are frequently affected in AML, synergistic targeted therapies that inhibit multiple targets are required. 5-AZA has been associated with a relatively low response rate (~20%) in AML patients. More importantly, although mutations in epigenetic regulator genes DNMT3A, TET2 or IDH1/2 may predict favorable response to 5-AZA, the treatment response has not correlated with overall survival [27, 28]. ABT-263, the clinical derivative of ABT-737, is a promising agent being tested in clinical trials for solid tumors and lymphoid malignancies. One of the major limitations of ABT-737/ABT-263 reported in preclinical studies is that high levels of MCL-1 confer resistance to ABT-737/ABT-263 [4, 25, 26]. Our results suggest that 5-AZA strongly enhances ABT-737-induced mitochondrial apoptosis in AML cell lines and patient AML cells. The strikingly low averaged combination index values in patient samples (median 0.28; < 0.4 in 6 out of 8 samples) suggest that a combination strategy aimed at inhibiting DNA methyltransferase and anti-apoptotic BCL-2 proteins is potentially effective in AML. Because of the limited number of samples, however, further study is needed to confirm the efficacy of combination therapy.
Our data indicate that loss of expression of p53 may lead to decreased apoptosis induction by 5-AZA or ABT-737 when they are administered as single agents. 5-AZA induces cell death in AML cells via multiple mechanisms, leading to mitochondrial apoptosis [29]. ABT-737 directly binds to anti-apoptotic BCL-2 family proteins and induces mitochondrial apoptosis [3]. We have recently reported that loss of p53 can lead to reduced expression of pro-apoptotic BCL-2 proteins BAX, PUMA and NOXA, stabilizing mitochondrial membrane potential and that p53 loss was associated with decreased apoptosis induction by ABT-737 in AML and CLL cells [14]. Therefore, mitochondrial membrane stabilization associated with loss of p53 might contribute to low susceptibility to 5-AZA in p53-knockdown AML cells. In contrast to single agent treatment, the synergistic apoptotic effect between 5-AZA and ABT-737 did not necessarily require p53. Importantly, 5-AZA reduced expression of MCL-1 in a p53-independent manner. Since MCL-1 is a known resistance factor for ABT-737, 5-AZA enhances ABT-737-induced mitochondrial apoptosis at least partially by reducing MCL-1 expression. The presence of p53-independent apoptotic activity in the 5-AZA/ABT-737 combination, if operational in vivo, could delay or prevent the selection of p53 mutant subclones during therapy.
Recently, Bogenberger et al. have utilized high-throughput RNA-interference screening, to identify targets for rational combination therapies with 5-AZA in AML [30]. They found that silencing of anti-apoptotic BCL-2 family potently synergizes with 5-AZA to reduce cell viability in AML cell lines TF-1, HEL, THP-1 and ML-2. They have also reported that 5-AZA synergizes with ABT-737 to induce cell death in primary AML samples. Considering that their model cell lines have mutant p53, their data may support the hypothesis that p53 is not necessarily required for the synergism between 5-AZA and BCL-2 inhibition. This is particularly important in view of recent findings of high frequency of p53 alterations in AML patients with complex karyotype which is associated with dismal outcome in this patient population [31]. Although further detailed studies using samples from this cohort of patients are required (in our series, only pt#4 exhibited complex karyotype), it is plausible to imagine potential utility of this drug combination in these patients. Further, since the frequency of genomic complexity is increasing with age, utilization of non-myelosuppressive agent such as 5-AZA has further advantages in elderly patients with poor prognosis cytogenetics. Our high-throughput proteomic analysis highlighted higher levels of BCL-2 in a cohort of patients with unfavorable cytogenetics, further supporting the premise of utilizing this combination in this poor-prognosis patient population. Finally, combination with 5-AZA offers major benefit due to limited effect of this agent on platelet production and hence should be tolerable in combination with ABT-263 in patients with moderately high platelet counts.
In summary, 5-AZA and ABT-737 synergistically induce apoptosis in AML cells in a p53-independent manner. The mechanisms of this pharmacologic interaction involve synergistic mitochondrial outer membrane permeabilization by the 5-AZA/ABT-737 combination, as evidenced by effective conformational activation of BAX and Δψm loss. These findings provide strong rationale for testing this combination in rationally designed clinical trials in elderly AML patients unsuitable for intensive chemotherapy options.
Supplementary Material
Table 2.
Combination effect of 5-AZA and ABT-737 on apoptosis induction
| ED50 | ED75 | ED90 | Averaged CI | |
|---|---|---|---|---|
| #1 | 0.90 | 0.74 | 0.60 | 0.75 |
| #2 | 0.03 | 0.03 | 0.05 | 0.04 |
| #3 | 1.98 | 2.51 | 3.35 | 2.61 |
| #4 | 0.18 | 0.27 | 0.39 | 0.28 |
| #5 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| #6 | 0.30 | 0.37 | 0.46 | 0.38 |
| #7 | 0.19 | 0.27 | 0.39 | 0.28 |
| #8 | 0.28 | 0.24 | 0.21 | 0.24 |
Acknowledgments
Grant Support
National Institutes of Health Lymphoma SPORE (CA136411), P01 “The Therapy of AML” (CA55164), Leukemia SPORE (CA100632) and the Paul and Mary Haas Chair in Genetics (M. Andreeff).
Footnotes
STATEMENT OF AUTHORSHIP
T.T., Y.S., H.L., S.K., A.A., V.R., S.K., Y-H.Q and K.R.C. performed the research and analyzed the data; M.A. contributed to discussion; K.K. designed and performed the research, analyzed the data, and wrote the paper; M.K. designed the research, analyzed the data, and wrote and edited the paper.
Disclosure of Potential Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 2.Kornblau SM, Vu HT, Ruvolo P, et al. BAX and PKC modulate the prognostic impact of BCL2 expression in acute myelogenous leukemia. Clin Cancer Res. 2000;6:1401–1409. [PubMed] [Google Scholar]
- 3.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of BCL-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 4.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steven WElmore. ABT-199: A potent and selective inhibitor of Bcl-2. Proc Am Assoc Cancer Res AACR. 2012;239 [Google Scholar]
- 7.Rice KL, Hormaeche I, Licht JD. Epigenetic regulation of normal and malignant hematopoiesis. Oncogene. 2007;26:6697–6714. doi: 10.1038/sj.onc.1210755. [DOI] [PubMed] [Google Scholar]
- 8.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 9.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 10.Soriano AO, Yang H, Faderl S, et al. Safety and clinical activity of the combination of 5-AZAytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110:2302–2308. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 11.Kojima K, Konopleva M, Samudio IJ, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–3159. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petitjean A, Mathe E, Kato S, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 13.Kojima K, Shimanuki M, Shikami M, et al. The dual PI3 kinase/mTOR inhibitor PI-103 prevents p53 induction by Mdm2 inhibition but enhances p53-mediated mitochondrial apoptosis in p53 wild-type AML. Leukemia. 2008;22:1728–1736. doi: 10.1038/leu.2008.158. [DOI] [PubMed] [Google Scholar]
- 14.Kojima K, Duvvuri S, Ruvolo V, Samaniego F, Younes A, Andreeff M. Decreased sensitivity of 17p-deleted chronic lymphocytic leukemia cells to a small molecule BCL-2 antagonist ABT-737. Cancer. 2012;118:1023–1031. doi: 10.1002/cncr.26360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimwade D. Impact of cytogenetics on clinical outcome in AML. In: Karp JE, editor. Acute Myeloid Leukemia. Totowa, NJ: Humana Press; 2007. pp. 177–192. [Google Scholar]
- 16.Kojima K, McQueen T, Chen Y, et al. p53 activation of mesenchymal stromal cells partially abrogates microenvironment-mediated resistance to FLT3 inhibition in AML through HIF-1α-mediated down-regulation of CXCL12. Blood. 2011;118:4431–4439. doi: 10.1182/blood-2011-02-334136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornblau SM, Qiu YH, Zhang N, et al. Abnormal expression of FLI1 protein is an adverse prognostic factor in acute myeloid leukemia. Blood. 2011;118:5604–5612. doi: 10.1182/blood-2011-04-348052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibes R, Qiu YH, Lu Y, et al. Reverse phase protein array (RPPA): validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoetic stem cells (HSC) Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 19.Kornblau SM, Tibes R, Qiu YH, et al. Functional proteomic profiling of AML predicts response and survival. Blood. 2009;113:154–164. doi: 10.1182/blood-2007-10-119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunyady B, Krempels K, Harta G, Mezey E. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem. 1996;44:1353–1362. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- 21.Hu J, He X, Baggerly KA, et al. Non-parametric quantification of protein lysate arrays. Bioinformatics. 2007;23:1986–1994. doi: 10.1093/bioinformatics/btm283. [DOI] [PubMed] [Google Scholar]
- 22.Neeley ES, Kornblau SM, Coombes KR, Baggerly KA. Variable slope normalization of reverse phase protein arrays. Bioinformatics. 2009;25:1384–1389. doi: 10.1093/bioinformatics/btp174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 24.Kiziltepe T, Hideshima T, Catley L, et al. 5-Azacytidine, a DNA methyltransferase inhibitor, induces ATR-mediated DNA double-strand break responses, apoptosis, and synergistic cytotoxicity with doxorubicin and bortezomib against multiple myeloma cells. Mol Cancer Ther. 2007;6:1718–1727. doi: 10.1158/1535-7163.MCT-07-0010. [DOI] [PubMed] [Google Scholar]
- 25.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 27.Traina F, Jankowska AM, Visconte V, et al. Impact of molecular mutations on treatment response to hypomethylating agents in MDS [abstract 461] Blood. 2011;118 [Google Scholar]
- 28.Walker AR, Metzeler KH, Geyer S. Impact of DNMT3A mutations on clinical response to the hypomethylating agent decitabine in older patients (pts) with acute myeloid leukemia (AML) [abstract 944] Blood. 2011;118 [Google Scholar]
- 29.Khan R, Schmidt-Mende J, Karimi M, et al. Hypomethylation and apoptosis in 5-azacytidine-treated myeloid cells. Exp Hematol. 2008;36:149–157. doi: 10.1016/j.exphem.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Bogenberger JM, Shi C-X, Gonzales G, et al. RNAi screening identifies BCL-XL as an erythroid lineage-specific 5-Azacytidine sensitizer while the BCL-2/BCL-XL/BCL-W inhibitor ABT-737 results in more universal sensitization in leukemia cells [abstract 3513] Blood. 2011;118 [Google Scholar]
- 31.Rücker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119:2114–2121. doi: 10.1182/blood-2011-08-375758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.