Abstract
Background
Awareness of detail in ambiguous complaints may help GPs suspect cancer when a malignancy is present.
Aim
To study the contribution of symptoms and patient characteristics to GPs’ suspicions of cancer being present, and to what degree these suspicions were confirmed.
Design and setting
Prospective cohort study of patients in 283 rural and urban general practices throughout Norway.
Method
During patient consultations (over a period of 10 days) GPs registered whether there was a suspicion of cancer when a patient presented with at least one of seven focal symptoms and three general symptoms commonly considered to be warning signs of cancer. Follow-up questionnaires were sent to GPs 6–7 months later, requesting information on any subsequent diagnosis of cancer in these patients.
Results
Out of 51 073 patients, 6321 presented with warning signs of cancer; of these, 106 had a subsequent cancer diagnosis. Of the patients presenting with warning signs, 1515 (24%) patients were suspected of having cancer; this was correct for 3.8% of suspected cases. Of the 106 patients diagnosed with cancer who presented with warning signs, cancer was suspected in 58 (54.7%). GPs’ correct cancer suspicions were six times more frequent than their erroneous lack of suspicion. Multiple symptoms, previous cancer, comorbidity, and multiple consultations increased the probability of cancer, but only multiple symptoms and previous cancer increased suspicion. Suspicion led to an increase in the number of diagnostic procedures undertaken. The proportion of cancer cases where GPs recorded a lack of suspicion was relatively small, but important.
Conclusion
Selected symptoms appropriately resulted in GPs suspecting cancer. Comorbidity and multiple consultations were underestimated by GPs as factors associated with cancer. Cancer suspicion should rely on symptoms in combination with other relevant information.
Keywords: early detection of cancer, early diagnosis, family practice, general practice, neoplasms
INTRODUCTION
The majority of patients with cancer begin their diagnostic process in general practice.1,2 To improve the cancer diagnostic process, it is important to understand how the thought of cancer arises in a GP’s mind.3,4 Diagnostic strategies vary, but initial hypotheses often occur early and may be triggered by symptom presentation.5 A ‘critical style’ with awareness of detail in ambiguous complaints may help a GP to arrive at a correct conclusion when a malignancy is present.6
This study investigated the relationship between symptoms presented in general practice and cancer, including the GP’s suspicion of cancer being present. In a previous article the frequency of ‘alarm symptoms’ or warning signs of cancer and the relationship between symptoms and cancer were reported.7 Historically, different symptoms have been promoted by cancer organisations and charities to encourage early consultations and, thus, early diagnoses; the symptoms studied are commonly associated with cancer in many countries. In this study, the frequency of warning signs of cancer were present 12.4% of patients who consulted their GP and in 40% of patients who were subsequently diagnosed as having cancer. The warning signs of cancer were valid in relation to a diagnosis of cancer but had a low positive predictive value as single symptoms.
Few studies deal directly with cancer suspicion. The qualitative study by Johansen et al4 showed that practising basic knowledge based on explicit rules and skills (such as alarm symptoms and clinical know-how) is only one important factor among others that makes the thought of cancer arise in a GP consultation. This article describes:
how GPs assess warning signs of cancer and other factors as possible signs of cancer;
what actions were taken by GPs; and
to what extent a cancer suspicion proved to be correct.
METHOD
The methods of this study will be outlined here in brief but have been described in more detail elsewhere.7
Study design and setting
A simple and well-established audit-type questionnaire (Appendix 1; available from the authors)7 was sent to all the registered GPs in Norway in April 2006 (n = 3910). A total of 396 (10.1%) GPs participated in the study; after exclusions, 51 073 patients were included in the study population. Some 6–7 months later a new questionnaire was sent to the GPs; 283 (71.5%) completed this, of whom 156 reported cancer diagnosis in a total of 261 patients.
Questionnaires
The initial questionnaire was sent to GPs, together with two pages of instructions and examples of how to complete the form. GPs were instructed to register all patients who presented to them over a period of 10 working days. Birth date and sex were requested for every consultation and, if the patients had one or more warning signs of cancer, the GPs were to complete the remaining questions, including choosing between ‘cancer possible, follow-up needed’ or ‘cancer not likely’. In the instructions, this was specified as a choice between two options: cancer was a possibility that should be pursued, or cancer seemed unlikely based on the GP’s evaluation.
How this fits in
Few studies explore what makes a GP suspect cancer during a consultation. This study confirmed the importance of selected symptoms, and of multiple symptoms. GPs may have confidence in their ability to suspect cancer and should explore cues given by symptoms and patient characteristics. Increased awareness of comorbidity and multiple consultations could increase the proportion of correct cancer suspicion.
The warning signs of cancer were divided into seven focal symptoms and three general symptoms; these are listed in Table 1. The remaining questions dealt with:
previous cancer,
important comorbidity,
whether this was a first or subsequent consultation,
duration of symptoms, and
actions taken: supplementary laboratory tests, imaging, and/or referrals.
Table 1.
GPs’ suspicion of cancer when a patient presents with warning signs of cancer, by cancer diagnosis and non-diagnosis (n = 6321)
| Symptom | All warning signs of cancer | Cancer possible, needs follow-up | Cancer not likely | Missing registration of suspicion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| n, all | n, cancera | PPV | %, all cancers | n, all (%) | n, cancera (%) | n, all (%) | n, cancera (%) | n, all (%) | n, cancera (%) | |
| Focal symptom | ||||||||||
| Non-healing skin lesion | 309 | 5 | 1.6 | 4.7 | 105 (34.0) | 3 (60.0) | 149 (48.2) | 1 (20.0) | 55 (17.8) | 1 (20.0) |
| Lump/nodule | 918 | 12 | 1.3 | 11.3 | 202 (22.0) | 4 (33.3) | 611 (66.6) | 4 (33.3) | 105 (11.4) | 4 (33.3) |
| Unusual bleeding | 509 | 17 | 3.3 | 16.0 | 197 (38.7) | 12 (70.6) | 255 (50.1) | 3 (17.6) | 57 (11.2) | 2 (11.8) |
| Pigmented skin lesion (mole) | 518 | 4 | 0.8 | 3.8 | 101 (19.5) | 3 (75.0) | 356 (68.7) | 1 (25.0) | 61 (11.8) | 0 (0.0) |
| Persistent digestive problem | 1042 | 28 | 2.7 | 26.4 | 321 (30.8) | 19 (67.9) | 586 (56.2) | 4 (14.3) | 135 (13.0) | 5 (17.9) |
| Cough/hoarseness of uncertain origin | 774 | 15 | 1.9 | 14.2 | 158 (20.4) | 7 (46.7) | 497 (64.2) | 5 (33.3) | 119 (15.4) | 3 (20.0) |
| Other symptom suspicious of cancer | 879 | 22 | 2.5 | 20.84 | 343 (39.0) | 15 (68.2) | 321 (36.5) | 1 (4.5) | 215 (24.5) | 6 (27.3) |
| One or more of the focal symptoms | 4726 | 92 | 1.9 | 86.8 | 1307 (27.7) | 55 (59.8) | 2704 (57.2) | 17 (18.5) | 715 (15.1) | 20 (21.7) |
|
| ||||||||||
| General symptom | ||||||||||
| Unintentional weight loss | 339 | 13 | 3.8 | 12.3 | 162 (47.8) | 9 (69.2) | 114 (33.6) | 2 (15.4) | 63 (18.6) | 2 (15.4) |
| Unusual fatigue | 1152 | 21 | 1.8 | 19.8 | 254 (22.0) | 13 (61.9) | 715 (62.1) | 3 (14.3) | 183 (15.9) | 5 (23.84) |
| Unusual pain | 1264 | 16 | 1.3 | 15.1 | 271 (21.4) | 6 (37.5) | 835 (66.1) | 6 (37.5) | 158 (12.5) | 4 (25.0) |
| One or more of the general symptoms | 2497 | 40 | 1.6 | 37.7 | 585 (23.4) | 22 (55.0) | 1561 (62.5) | 10 (25.0) | 351 (14.1) | 8 (20.0) |
|
| ||||||||||
| All symptoms | ||||||||||
| One or more of all warning signs | 6321 | 106 | 1.7 | 100 | 1515 (24.0) | 58 (54.7) | 3854 (61.0) | 24 (22.6) | 952 (15.1) | 24 (22.6) |
|
| ||||||||||
| Multiple symptoms | ||||||||||
| 2 symptoms | 955 | 21 | 2.2 | 19.8 | 359 (37.6) | 13 (61.9) | 477 (49.9) | 6 (28.6) | 119 (12.5) | 2 (9.5) |
| ≥3 symptoms | 193 | 12 | 6.3 | 11.3 | 109 (56.8) | 10 (83.3) | 51 (26.6) | 0 (0.0) | 33 (17.1) | 2 (16.7) |
Number of patients with subsequent cancer. PPV = positive predictive value of symptom in relation to cancer.
The follow-up questionnaire was sent to GPs 6–7 months later. The GP was asked to:
find, in the electronic database, all patients with a cancer that had been diagnosed after the initial registration, and
complete an anonymous five-page questionnaire about diagnosis and treatment for each patient diagnosed with cancer.
This questionnaire was a modified version of a cancer care questionnaire used in a previous study after testing in several countries and being reliability tested in Norway.2
Variables used in this article were found in the initial questionnaire; the confirmed cancer diagnosis is the only information from the follow-up questionnaire used here.
Analysis of data
The data were analysed using SPSS (version 19). The association between the symptoms and the GP’s suspicion of cancer was examined using the χ2 test and a logistic regression model, thereby comparing each symptom with all other symptoms. Possible interaction effects were tested by including product terms for each pair of symptoms in the logistic regression model.
The analysis revealed significant two-way interaction effects between several of the symptoms. A symptom would change a suspicion of cancer by varying degrees when combined with other symptoms. In order to avoid this effect of interaction on the odds ratios (ORs), calculations for single symptoms were limited to patients for whom only one symptom had been recorded (82% of patients). ORs for each symptom were adjusted for age and sex only, and not for other symptoms.
As not all GPs recorded a suspicion of cancer, a sensitivity analysis was performed for the two extreme cases: that all missing cases had been recorded as either ‘cancer possible, follow-up needed’ or as ‘cancer not likely’. Combinations that occurred frequently were examined separately in the group of patients with the same number of recordings, that is two symptom recordings for twin symptoms and three for ‘triplets’. A χ2 test was also used to relate cancer suspicion and subsequent cancer diagnosis to prior cancer, comorbidity, first or not-first consultation, duration of symptoms, and subsequent action by the GP.
Differences between means were compared using the Mann-Whitney U-test. The level of statistical significance was P<0.05. Positive predictive values (PPVs) for symptoms in relation to cancer were calculated using the following formula:8
RESULTS
Cancer suspicion based on warning signs of cancer
Among the 51 073 patients studied, one or more warning signs of cancer were registered in 6321 (12.4%) patients. The average age of patients was 54 years; 2386 were male and 3935 were female. In total, 261 patients had cancer at the time of follow-up, of whom 106 (40%) had one or more warning signs of cancer. The GPs assessed the possibility of cancer in 85% of the patients who presented with warning signs; 1515 (24.0%) were assessed as ‘cancer possible, follow-up needed’, while 3854 (61.0%) were assessed as ‘cancer not likely’ (Table 1). Overall, 3.8% of the positive suspicions proved correct and 0.6% of patients were diagnosed as having cancer in spite of a ‘cancer not likely’ recording; as such, a cancer suspicion that proved to be correct was six times more likely to occur than an erroneous lack of suspicion.
The patients who were diagnosed with cancer and had presented to their GP with warning signs had a mean age of 70 years (range 51–91 years). PPV for being diagnosed with cancer after having presented with one or more warning signs was 1.7% (Table 1).
On average, patients with warning signs of cancer had 1.4 warning signs each when there was a subsequent diagnosis of cancer and 1.2 warning signs each when there was no such subsequent diagnosis (P<0.001). Of the 106 patients diagnosed with cancer who presented with warning signs, cancer was suspected in 58 (54.7%) and not suspected in 24 (22.6%); on average, 1.6 and 1.3 warning signs of cancer were evident per patient, respectively. In the remaining 24 patients the GPs did not record whether or not they suspected cancer.
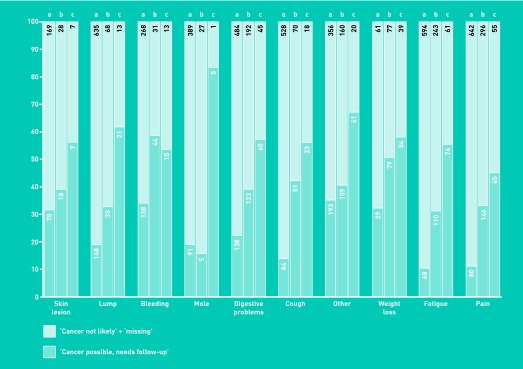
The suspicion rate was higher for males than females (27% versus 22% [P<0.001]) and increased with age (Table 2). Suspicion rates varied with different symptoms. Digestive problem was the most common focal symptom, no matter whether cancer was or was not diagnosed; this symptom caused GPs to suspect the presence of cancer in 31% of the patients who presented with it (Table 1). In patients with cancer, GPs suspected cancer in around 70% of the patients who initially presented with bleeding, digestive problem, and weight loss, and also in the few cancer patients who had a recording of a mole. They suspected cancer in around 60% of patients with a recording of fatigue or skin lesion. Cancer was suspected less frequently in patients presenting with lump, cough, and pain (Table 1).
Table 2.
Logistic regression model showing the association between warning signs of cancer and GPs’ suspicion of cancer by single symptoms and the most common symptom combinations, each adjusted for age and sex
| n | OR for suspicion | 95% CI | P-value |
Sensitivity analysis
|
||
|---|---|---|---|---|---|---|
| Missing = possible | Missing = not likely | |||||
| Age (increasing) | 1.0 | 1.03 to 1.04 | <0.001 | |||
|
| ||||||
| Sex (females =1, males = 2) | 1.3 | 1.1 to 1.5 | <0.001 | |||
|
| ||||||
| Skin lesion | 247 | 1.6 | 1.2 to 2.2 | 0.003 | 1.5 | 1.5 |
|
| ||||||
| Lump | 783 | 1.0 | 0.8 to −1.2 | n.s. | 0.9 | 1.1 |
|
| ||||||
| Bleeding | 406 | 2.4 | 1.9 to 3.0 | <0.001 | 1.7 | 2.4 |
|
| ||||||
| Mole | 480 | 1.1 | 0.8 to 1.4 | n.s. | 1.0 | 1.1 |
|
| ||||||
| Digestive problem | 622 | 1.1 | 0.9 to 1.3 | n.s. | 1.0 | 1.1 |
|
| ||||||
| Cough | 612 | 0.5 | 0.4 to 0.7 | <0.001 | 0.7 | 0.5 |
|
| ||||||
| Weight loss | 90 | 1.7 | 1.0 to 2.7 | 0.040 | 1.5 | 1.5 |
|
| ||||||
| Fatigue | 662 | 0.4 | 0.3 to 0.5 | <0.001 | 0.7 | 0.4 |
|
| ||||||
| Pain | 712 | 0.4 | 0.3 to 0.5 | <0.001 | 0.5 | 0.4 |
|
| ||||||
| Two warning signs of cancer | ||||||
| Lump and fatigue | 24 | 0.8 | 0.3 to −2.4 | n.s. | 1.0 | 0.6 |
| Lump and pain | 36 | 0.6 | 0.3 to 1.3 | n.s. | 0.6 | 0.7 |
| Bleeding and digestive problem | 21 | 2.0 | 0.8 to 5.2 | n.s. | 1.6 | 2.3 |
| Bleeding and fatigue | 18 | 6.5 | 1.7 to 24.7 | 0.006 | 6.0 | 2.8 |
| Bleeding and pain | 19 | 1.9 | 07 to 5.2 | n.s. | 1.6 | 1.7 |
| Digestive problem and weight loss | 71 | 1.6 | 0.9 to 2.7 | n.s. | 1.3 | 1.7 |
| Digestive problem and fatigue | 57 | 0.8 | 0.4 to 1.4 | n.s. | 0.7 | 0.9 |
| Digestive problem and pain | 147 | 0.7 | 0.5 to 1.1 | n.s. | 0.7 | 0.8 |
| Cough and weight loss | 12 | 2.8 | 0.6 to 13.4 | n.s. | 2.6 | 2.0 |
| Cough and fatigue | 67 | 0.8 | 0.4 to 1.3 | n.s. | 0.8 | 0.9 |
| Cough and pain | 18 | 1.8 | 0.9 to 5.0 | n.s. | 1.5 | 2.1 |
| Weight loss and fatigue | 34 | 1.2 | 0.5 to 2.7 | n.s. | 1.5 | 0.8 |
| Fatigue and pain | 68 | 0.4 | 0.2 to 0.7 | 0.003 | 0.4 | 0.4 |
|
| ||||||
| Three warning signs of cancer | ||||||
| Digestive problem, weight loss, and fatigue | 29 | 2.8 | 0.8 to 10.0 | n.s. | 3.2 | 1.0 |
| Digestive problem, fatigue, and pain | 22 | 0.3 | 0.1 to 0.8 | 0.016 | 0.6 | 0.4 |
6321 patients with warning signs of cancer (one recorded symptom, n = 5173; two symptoms, n = 955; three symptoms, n = 161; ≥4 symptoms, n = 32). OR = odds ratio. n.s. = non-significant.
When symptoms were compared one by one with all other symptoms, skin lesion (OR = 1.6), bleeding (OR = 2.4), and weight loss (OR = 1.7) showed significant association with cancer suspicion, while cough (OR = 0.5), fatigue (OR = 0.4), and pain (OR = 0.4) showed a negative association with suspicion (Table 2). In the sensitivity analysis with the elimination of missing cases, the ORs showed minor variation up or down, but did not have an effect on the conclusions drawn (Table 2).
Multiple symptoms
Multiple symptoms were reported in 1148 (18%) of the patients who presented with warning signs of cancer, and 193 of these had three or more symptoms. Cancer suspicions rose with an increasing number of warning signs (Table 1, Figure 1). In addition, cancer incidence increased. When the GP had a cancer suspicion, 13 of 359 (3.6%) patients with two registered symptoms and 10 of 109 (9.2%) with three symptoms had the suspicion confirmed. Where cancer was not suspected, six of 477 (1.3%) patients with two symptoms and no patients with three symptoms turned out to have cancer (Table 1).
Figure 1.
Cancer suspicion by number of GP-recorded warning signs of cancer (WSC).
Bleeding and fatigue (OR = 6.5) showed a positive association with cancer suspicion compared with all other double combinations. Fatigue and pain (OR = 0.4) and digestive problem, fatigue, and pain (OR = 0.3) similarly showed a negative association (Table 2).
The number of cancer cases encountered for each combination of symptoms was low (n = 0–2 in most cases), but for 71 patients with digestive problem and weight loss (OR = 1.6) there were six cases of cancer and five of these were suspected by the GP.
Digestive problem was the most common focal symptom; it occurred in 420 patients with multiple symptoms (36.6% of all combinations, most often with one or more general symptoms). Weight loss was unique in that it was the only warning sign that occurred more often in combinations than as a single symptom, and all cancer cases where the patient had presented with weight loss had multiple warning signs of cancer.7 In spite of this, weight loss as a single symptom resulted in a suspicion of cancer three times as often as the two other general symptoms.
Cancer suspicion based on single symptoms occurred in 10–35% of warning signs of cancer registrations, while suspicion rates based on two symptoms, or on three or more symptoms, ranged from 16% to 59% and from 45% to 83%, respectively (Figure 1).
Other factors influencing cancer suspicion
Data discussed here are presented in Table 3. Previous cancer was registered in 610 patients with warning signs of cancer; in 35.4% of these patients cancer was suspected, while in 20.8% of them it was not. Cancer occurred in 38 (6.2%) of these people, and 26 of them had a relapse. A previous cancer diagnosis was the only non-symptom variable for which the percentage of a positive cancer suspicion was higher than that for the non-suspicion of cancer. Of the patients with cancer who had previously had cancer, 50.0% were registered as ‘cancer possible, follow-up needed’ while for 7.9% the GP did not suspect that the symptoms were caused by cancer.
Table 3.
Factors related to cancer being diagnosed or not, or suspected or not (n = 6321)a
|
All patients with warning sign(s) of cancer
|
Cancer possible, needs follow-up
|
Cancer not likely
|
Missing registration
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | n, cancerb | PPV | % of all cancers | n (%) | n, cancerb (%) | n (%) | n, cancerb (%) | n (%) | n, cancerb (%) | |
| Previous cancer | 610 | 38 | 6.2 | 35.8 | 216 (35.4) | 19 (50.0) | 127 (20.8) | 3 (7.9) | 267 (43.8) | 16 (42.1) |
|
| ||||||||||
| Comorbidity | 651 | 21 | 3.2 | 19.8 | 170 (26.1) | 10 (47.6) | 346 (53.1) | 6 (28.6) | 135 (20.7) | 5 (23.8) |
|
| ||||||||||
| First consultation | 2847 | 36 | 1.3 | 34.0 | 747 (26.2) | 22 (61.1) | 1840 (64.6) | 11 (30.6) | 260 (9.1) | 3 (8.3) |
|
| ||||||||||
| Not first consultation | 2553 | 60 | 2.4 | 56.6 | 640 (25.1) | 33 (55.0) | 1458 (57.7) | 12 (20.0) | 455 (17.8) | 15 (25.0) |
|
| ||||||||||
| Duration of symptoms, weeks | ||||||||||
| 0–1 | 535 | 8 | 1.5 | 7.5 | 113 (21.1) | 4 (50.0) | 366 (68.4) | 3 (37.5) | 55 (10.3) | 1 (12.5) |
| 2–7 | 1283 | 19 | 1.5 | 17.9 | 359 (28.0) | 16 (84.2) | 795 (62.0) | 3 (15.8) | 129 (10.1) | 0 (0) |
| 8–23 | 1320 | 25 | 1.9 | 23.6 | 426 (32.3) | 18 (72.0) | 775 (58.7) | 4 (16.0) | 116 (8.8) | 3 (12.0) |
| 24–51 | 491 | 10 | 2.0 | 9.4 | 140 (28.5) | 6 (60.0) | 288 (58.7) | 2 (20.0) | 61 (12.4) | 2 (20.0) |
| ≥52 | 785 | 13 | 1.7 | 12.3 | 148 (18.9) | 5 (38.5) | 524 (66.8) | 2 (15.4) | 107 (13.6) | 6 (46.2) |
|
| ||||||||||
| Supplementary actions | ||||||||||
| Laboratory tests | 2719 | 52 | 1.9 | 49.1 | 838 (30.8) | 33 (63.5) | 1569 (57.7) | 12 (23.1) | 312 (11.5) | 7 (13.5) |
| Imaging | 1140 | 33 | 2.9 | 31.1 | 461 (40.4) | 20 (60.6) | 561 (49.2) | 7 (21.2) | 118 (10.4) | 6 (18.2) |
| Referrals | 1342 | 41 | 3.1 | 38.7 | 555 (41.4) | 30 (73.2) | 614 (45.8) | 4 (9.8) | 173 (12.9) | 7 (17.1) |
| 0 action | 2370 | 24 | 1.0 | 22.6 | 249 (10.5) | 5 (20.8) | 1644 (69.4) | 8 (33.3) | 466 (19.7) | 11 (45.8) |
| 1 action | 2847 | 42 | 1.5 | 39.6 | 758 (26.6) | 25 (59.5) | 1729 (60.7) | 10 (23.8) | 353 (12.4) | 7 (16.7) |
| 2 actions | 958 | 36 | 3.8 | 34.0 | 428 (44.7) | 26 (72.2) | 428 (44.7) | 5 (13.9) | 97 (10.1) | 5 (13.9) |
| 3 actions | 146 | 4 | 2.7 | 3.84 | 80 (54.8) | 2 (50.0) | 53 (36.3) | 1 (25.0) | 12 (8.2) | 1 (25.0) |
Patients presenting with warning signs of cancer, of whom 106 got cancer.
Number of patients with subsequent cancer. PPV = positive predictive value in relation to cancer.
Important comorbidity did not seem to increase the GPs’ suspicion of cancer, noted for 26% of all patients with comorbidity. Of 651 patients with comorbidity, 3.2% were later diagnosed with cancer; 19.8% of the patients with cancer and warning signs of cancer had important comorbidity.
Compared with the first consultation, the proportion of cancer cases almost doubled with two or more consultations; this did not appear to influence the GPs’ judgement, with almost the same proportion of patients suspected of having cancer in the two groups.
The duration of warning signs of cancer was registered for 4414 or around (70%) patients. The same percentage applied for the patients with cancer. Median duration before presenting to the GP was 10 weeks. There was no sex difference. A duration of 2–23 weeks was noted for 59% of all patients for whom duration of symptoms was recorded, whether or not they were later diagnosed with cancer. Suspicion did not vary greatly between duration intervals.
GPs’ actions
The GPs’ actions markedly increased when cancer was suspected. In 54.8% of the cases when a GP suspected cancer, they initiated laboratory testing, as well as referrals to diagnostic imaging and referrals to a hospital and/or specialist; in 10.5% of cases, none of these were requested. In comparison, for 69.4% of the group in whom cancer was not suspected, none of these actions were requested; for 36.3% all three kinds of actions were undertaken. When cancer was suspected, the GP tended to request imaging or referral relatively more frequently than laboratory tests (Table 3).
DISCUSSION
Summary
Warning signs of cancer vary in their tendency to make GPs suspect cancer. All warning signs of cancer are valid symptoms of the disease,7 but symptoms such as cough and pain may be more easily connected with benign diagnoses in a GP’s daily practice. When the number of symptoms increased in a patient, cancer suspicion also increased. One-quarter of all patients with warning signs of cancer and more than half of those diagnosed with cancer who presented to the GP with warning signs were suspected of having cancer. Not all cancer suspicions proved correct, but cancer was six times more likely to be diagnosed when it was suspected than when it was not.
For a single symptom like weight loss, which, in itself, raised a high degree of suspicion, there was no corresponding cancer case. Only when weight loss occurred in combination with other symptoms were some of the suspicions confirmed. Cough seemed to be underestimated as a symptom that aroused suspicion. The combination of digestive problem and weight loss warning signs had an OR of 1.6, which was not significant in relation to cancer suspicion, but GPs recorded cancer suspicion in five of six cancer cases. Overall, the GPs seemed to have a reason both for their recordings of suspicion and for their lack of suspicion; much higher ORs, both for single warning signs of cancer and for combinations, should not be expected.
One in three patients diagnosed with cancer who had presented with a warning sign had had a previous cancer; GPs seemed to be aware of the importance of this. Comorbidity did not trigger the GPs’ suspicion to the same extent, even though the cancer incidence almost doubled when the patient had presented both warning signs of cancer and important comorbidity. Both previous cancer and important comorbidity should increase a GP’s awareness of cancer; for patients with a history of cancer, this is especially so with regard to the possibility of recurrence of the same cancer type.
Cancer cases also doubled when the patient came for a second or subsequent consultation. This may signal an important message to GPs because the degree of cancer suspicion was the same for first and for later consultations.
The duration of symptoms did not seem to greatly influence GPs’ suspicion of cancer or the reality of it being present.
As expected, the number of actions taken by GPs increased in line with cancer suspicion however, in spite of this, more than 10% of GPs carried out no laboratory tests, diagnostic imaging, or referrals even though they suspected cancer. In another Norwegian study most patients where there was a suspicion of cancer were given new appointments in general practice.9
Strengths and limitations
The prospective recording of cancer eliminated knowledge about the diagnosis at the time of symptom recording. The GP’s judgement about the likelihood of cancer was missing in 15% of patients with one or more warning signs of cancer, 24 (23%) of whom had cancer. This may have introduced some bias in the balance between ‘cancer possible’ and ‘cancer not likely’; however, because the missing data are distributed between all warning signs of cancer and GPs deal with uncertainty in different ways, it is not thought that the bias is systematic. The results did not change greatly in the sensitivity analyses where values were assigned to the missing data.
To avoid interaction effects on the estimated ORs, analysis for each single symptom was limited to 82% of the patient material. OR estimates for multiple symptoms have wide confidence intervals because of fewer patients. Any study asking GPs to consider the possibility of cancer during a consultation may tend to raise their consciousness of the disease, possibly increasing the proportion of ‘cancer possible’ answers; however, it may also make GPs more critical in their assessments.
The interval between initial recordings and the registration of cancer was chosen to increase the possibility that a symptom would have relevance for any cancer that was not yet diagnosed. In some cases, the consultation may have taken place before cancer could have been suspected; the fact that 55% of the 106 patients who presented with warning signs of cancer and then developed cancer were suspected of having cancer reflects only a cross-sectional view at a certain point in time before diagnosis. Some of the cancer that had not been suspected may have had recordings of non-cancer symptoms only.
Some other considerations concerning GP participation and double recordings of patients are discussed in Ingebrigtsen et al;7 however, these are not considered relevant to the results presented here. In order to limit the workload for the GP, they were not asked to record suspicions of cancer if there were no warning signs. As a result of this, there is no such data for the 156 patients who were diagnosed with cancer but showed no warning signs.
Comparison with existing literature
Symptoms and the other factors studied are not the only things to influence GPs’ suspicions of cancer. Cognitive factors have been shown to be important,10,11 as is interpersonal awareness; that is, being alert to changes in the patients’ appearance or behaviour, and to cues they give,4 however, this was not intended to be part of the study.
There is current interest in the physician’s gut feeling12 based on the hypothesis that analytical and non-analytical processes interact during the doctor’s diagnostic work.13 However, gut feeling sometimes may be based on clinical observations rather than vague impressions, that is, general appearance, breathing pattern, and weight loss.14 It is an open question whether first impressions inspire measurements and closer clinical examination, thereby leading to more comprehensive referrals by GPs.
The Cancer Prediction in Exeter (CAPER) studies have provided evidence that alarm symptoms are important, but also that, for cancers like colorectal and lung cancer, most patients will experience lower-risk symptoms rather than the more obvious ‘red flags’ represented by rectal bleeding and haemoptysis.15 Most GPs are aware of this, and it is rational not to suspect cancer every time a patient presents with a warning sign of cancer. The role of symptoms has also been confirmed in a recent British study investigating the journey of the patient with cancer.16 This, together with the finding that patients (especially older people) are commonly diagnosed through emergency presentation17 may support the finding that comorbidity confounds the GP’s thinking and leads to an underestimation of the role of symptoms in older patients. Consistent with Nylenna18 this current study found that tests had been taken from about half of patients in whom cancer was suspected, and the referral rate was similar.
Because the PPV for cancer will always be low for single symptoms, further information gained through the medical history, clinical examination, and supplementary tests is important for diagnostic thinking.7 Kostopoulou et al19 found that physicians tended to distort incoming medical information to support an emerging diagnosis — and more so when the physician was less experienced. If comorbidity is present, one condition may mask another so that a second morbidity is not suspected.20 Jiwa et al21 found that coexisting multiple pathologies could increase diagnostic delay.
In this study, more than half of the patients with cancer had paid more than one visit to the GP. Lyratzopoulos et al22,23 found that many patients with cancer have several primary care visits before getting a diagnosis of cancer, but that 82% were referred after the first or second consultation; this varied for different types of cancer. Hansen et al24 found that 3 weeks was the median patient delay. Symptoms in this current study had a median duration of 10 weeks but, for half of the patients, this was not a first consultation, which contributes to a longer symptom interval.
Implications for research and practice
The GP succeeded in suspecting cancer in more than half of the patients with cancer and correct suspicions were six times more likely to occur than erroneous lack of suspicion. In view of the cross-sectional design, in which most patients had a single consultation where suspicion could arise, the study believes this shows that GPs are attentive to the possibility of cancer.
Some asymptomatic cancers are discovered through screening or otherwise, or with an acute presentation, meaning that GPs can never suspect 100% of cases. Multiple symptoms, previous cancer, and advancing age increased both cancer suspicion and cancer incidence. This study shows that comorbidity and more than one consultation for the same symptom should also cause a GP to suspect cancer. Symptoms that remain unclear at the end of a GP consultation may, at least, merit a follow-up appointment; perhaps this is particularly true for cough, fatigue or pain.
Overall, the quantification of relevant symptoms and other risk factors should help GPs to balance the awareness and suspicion of cancer and enhance the early diagnostic process. More studies connecting symptoms with specific forms of cancer are needed, and ideally, they should have a prospective design. Research exploring the concept of gut feeling may prove useful, both for emergency situations and in routine consultations where the low prevalence of serious disease is a challenge.25 In recent years, qualitative studies have added to the understanding of delay in diagnosing cancer, the factors in the patient–doctor relationship that are important for cancer awareness during a consultation, and how it affects diagnostic reasoning. Further studies should be encouraged.
Acknowledgments
Thanks to Tonje Braaten for statistical assistance, Per Baadnes for technical assistance, and to Robin Holtedahl for language assistance.
Funding
This work was supported by the Northern Norway Regional Health Authority (grant number: SAT 395-05), and by the General Practice Research Unit at the University of Tromsø.
Ethical approval
The survey protocol was accepted by the Norwegian Data Inspectorate and ethical approval for the project was given by the Regional Committee for Medical and Health Research Ethics of Northern Norway (P Rek Nord 44/2005). No patients were contacted and personal data that were recorded were limited to sex, year of birth, and type of cancer. Only the individual GP knew the identity of any single patient.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Allgar VL, Neal RD. General practitioners’ management of cancer in England: secondary analysis of data from the National Survey of NHS Patients — Cancer. Eur J Cancer Care (Engl) 2005;14(5):409–416. doi: 10.1111/j.1365-2354.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 2.Demagny L, Holtedahl K, Bachimont J, et al. General practitioners’ role in cancer care: a French-Norwegian study. BMC Res Notes. 2009;2(1):200. doi: 10.1186/1756-0500-2-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton W. Cancer diagnosis in primary care. Br J Gen Pract. 2010 doi: 10.3399/bjgp10X483175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen M-L, Holtedahl KA, Rudebeck CE. How does the thought of cancer arise in a general practice consultation? Interviews with GPs. Scand J Prim Health Care. 2012;30(3):135–140. doi: 10.3109/02813432.2012.688701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heneghan C, Glasziou P, Thompson M, et al. Diagnostic strategies used in primary care. BMJ. 2009;338:b946. doi: 10.1136/bmj.b946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuyvenhoven MM, Spreeuwenberg C, Touw-Otten FW. Diagnostic styles of general practitioners confronted with ambiguous symptoms. An exploratory study. Scand J Prim Health Care. 1989;7(1):43–48. doi: 10.3109/02813438909103670. [DOI] [PubMed] [Google Scholar]
- 7.Ingebrigtsen SG, Scheel BI, Hart B, et al. Frequency of ‘warning signs of cancer’ in Norwegian general practice, with prospective recording of subsequent cancer. Fam Pract. 2013;30(2):153–160. doi: 10.1093/fampra/cms065. [DOI] [PubMed] [Google Scholar]
- 8.Sackett Dl, Haynes RB, Guyatt GH. Clinical epidemiology. A basic science for clinical medicine. 2nd edn. Boston: Little, Brown and Company; 1991. PT. [Google Scholar]
- 9.Nylenna M. Diagnosing cancer in general practice: when is cancer suspected? BMJ. 1986;293(6541):245–248. doi: 10.1136/bmj.293.6541.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493–1499. doi: 10.1001/archinte.165.13.1493. [DOI] [PubMed] [Google Scholar]
- 11.Evans J, Ziebland S, McPherson A. Minimizing delays in ovarian cancer diagnosis: an expansion of Andersen’s model of ‘total patient delay’. Fam Pract. 2007;24(1):48–55. doi: 10.1093/fampra/cml063. [DOI] [PubMed] [Google Scholar]
- 12.Stolper E, Wiel M, Royen P, et al. Gut feelings as a third track in general practitioners’ diagnostic reasoning. J Gen Int Med. 2011;26(2):197–203. doi: 10.1007/s11606-010-1524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelaccia T, Tardif J, Triby E, Charlin B. An analysis of clinical reasoning through a recent and comprehensive approach: the dual-process theory. Med Educ Online. 2011 doi: 10.3402/meo.v16i0.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruel AVd, Thompson M, Buntinx F, Mant D. Clinicians’ gut feeling about serious infections in children: observational study. BMJ. 2012;345:e6144. doi: 10.1136/bmj.e6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton W. The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer. 2009;101(Suppl 2):S80–86. doi: 10.1038/sj.bjc.6605396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliss-Brookes L, McPhail S, Ives A, et al. Routes to diagnosis for cancer — determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107(8):1220–1226. doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kmietowicz Z. One in three cases of cancer in patients over 70 are diagnosed at emergency admission. BMJ. 2012;345:e6402. doi: 10.1136/bmj.e6402. [DOI] [PubMed] [Google Scholar]
- 18.Nylenna M. Diagnosing cancer in general practice: from suspicion to certainty. BMJ. 1986;293(6542):314–317. doi: 10.1136/bmj.293.6542.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kostopoulou O, Russo JE, Keenan G, et al. Information Distortion in Physicians’ Diagnostic Judgments. Med Decis Making. 2012;32(6):831–839. doi: 10.1177/0272989X12447241. [DOI] [PubMed] [Google Scholar]
- 20.Kostopoulou O, Delaney BC, Munro CW. Diagnostic difficulty and error in primary care — a systematic review. Fam Pract. 2008;25(6):400–413. doi: 10.1093/fampra/cmn071. [DOI] [PubMed] [Google Scholar]
- 21.Jiwa M, Reid J, Handley C, et al. Less haste more speed: factors that prolong the interval from presentation to diagnosis in some cancers. Fam Pract. 2004;21(3):299–303. doi: 10.1093/fampra/cmh314. [DOI] [PubMed] [Google Scholar]
- 22.Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012;13(4):353–365. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 23.Lyratzopoulos G, Abel GA, McPhail S, et al. Measures of promptness of cancer diagnosis in primary care: secondary analysis of national audit data on patients with 18 common and rarer cancers. Br J Cancer. 2013;108(3):686–690. doi: 10.1038/bjc.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen RP, Vedsted P, Sokolowski I, et al. Time intervals from first symptom to treatment of cancer: a cohort study of 2212 newly diagnosed cancer patients. BMC Health Serv Res. 2011;11:284. doi: 10.1186/1472-6963-11-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buntinx F, Mant D, Van den Bruel A, et al. Dealing with low-incidence serious diseases in general practice. Br J Gen Pract. 2011 doi: 10.3399/bjgp11X548974. [DOI] [PMC free article] [PubMed] [Google Scholar]


