Abstract
Background
Uterine cancer is the fourth most common cancer in women in the UK, with approximately 7700 new diagnoses and 1700 deaths annually.
Aim
To identify and quantify features of uterine cancer in primary care.
Design and setting
Case–control study using electronic primary care records in primary care in the UK.
Method
Putative features of uterine cancer were identified in the year before diagnosis, and odds ratios (ORs) calculated using conditional logistic regression. Positive predictive values (PPVs) were calculated for women who consulted.
Results
A total of 2732 women aged ≥40 years with uterine cancer between 2000 and 2009, and 9537 age-, sex- and practice-matched controls were selected from the General Practice Research Database. The median age at diagnosis was 67 years. Nine features were significantly associated with uterine cancer: postmenopausal bleeding (OR = 160; 95% confidence interval [CI] = 100 to 240), excessive vaginal bleeding (OR = 22; 95% CI = 12 to 42), irregular menstruation (OR = 42; 95% CI = 27 to −63), vaginal discharge (OR = 14; 95% CI = 10 to 21), haematuria (OR = 8.7; 95% CI = 5.0 to 15), abdominal pain (OR = 2.0; 95% CI = 1.4 to 2.8), low haemoglobin (OR = 2.1; 95% CI = 1.5 to 2.9), raised platelets (OR = 1.5; 95% CI = 1.0 to 2.3), and raised glucose (OR = 1.4; 95% CI = 1.1 to 1.8); all P<0.01, other than raised platelets, P = 0.05 and raised glucose, P = 0.02. In the year before diagnosis, 1725 (63%) cases had a record of abnormal vaginal bleeding compared to 135 (1%) controls. The PPV of uterine cancer with postmenopausal bleeding was 4%, and was higher in women with multiple or repeated symptoms.
Conclusion
This study confirms the importance of several features, particularly postmenopausal bleeding, for uterine cancer. Haematuria is an important risk marker. The results of this study may inform GPs in the selection of women for investigation and should assist the NICE in their update of GP referral guidance.
Keywords: diagnosis, general practice, primary health care, uterine neoplasms
INTRODUCTION
Uterine cancer is the fourth most common cancer in women in the UK, with approximately 7700 new diagnoses and 1700 deaths annually.1 The incidence is slowly increasing, particularly in postmenopausal women. The cancer generally originates in the endometrium, although tumours of the uterine muscle and trophoblastic cancers also occur. Uterine cancer has different risk factors to cervical cancer, and may also have different symptoms, despite the cervix being anatomically part of the uterus. Five-year survival rates for uterine cancer have improved to more than 75%.2 However, survival in the UK lags behind that in other European countries, with an estimated 100 additional uterine cancer deaths annually when compared to the European average, or 240 when compared to the highest European survival.3 Other European countries have reported delay in diagnosis of uterine cancer.4,5
There is no screening available for uterine cancer (in contrast to cervical cancer), so recognition of the cancer depends on the woman having symptoms. In the UK, and in many other high-income countries, women with symptoms first report them to their family doctor or GP. However, at the level of an individual GP, uterine cancer is rare, so GPs build up little personal experience of diagnosing the condition. The full range of symptoms of uterine cancer has not been researched in primary care, although secondary care studies have highlighted the importance of postmenopausal bleeding.6 One primary care study has examined this symptom, with 1.7% of women who described it developing a relevant cancer in the next 2 years.7 Only two features of possible uterine cancer are reported in the influential National Institute for Health and Clinical Excellence (NICE — now the National Institute for Health and Care Excellence) Referral guidelines for suspected cancer (2005) — postmenopausal bleeding, and pelvic masses.8 All these gynaecological cancer recommendations were based on grade C evidence or below. The proportion of women referred with such symptoms who transpire to have uterine cancer is below 10%, and improved guidance has been called for.9 Furthermore, 34% of women with uterine cancer do not have one of these alarm symptoms, and suffer delays in diagnosis as a result.5 Thus, both the sensitivity and the specificity of the advice in NICE guidance are relatively poor, with the low sensitivity perhaps contributing to the UK’s poor record in uterine cancer mortality. The aim of this study was to identify the features of uterine cancer in primary care (where the clinical problem of selection of women for investigation exists) and to quantify the risk of cancer for these features.
METHOD
Data sources
This was a case–control study using data from the General Practice Research Database (GPRD; now the Clinical Practice Research Datalink) in the UK. The GPRD maintains an anonymised copy of participating practices’ medical records: these contain full information of the patient, including all consultations, recorded symptoms, investigations, and diagnoses. There are stringent checks on validation and data quality.10,11 Similar methods have been used previously for several cancer diagnostic studies.12,13
How this fits in
Only postmenopausal bleeding and pelvic masses are recommended for urgent investigation in current National Institute for Health and Care Excellence (NICE) referral guidelines. Less than 10% of women referred with such symptoms transpire to have uterine cancer, and improved guidance has been called for. This study confirms the importance of several features, including haematuria as a significant risk marker. The results may help GPs in selection of women for investigation and should assist NICE in their update of GP referral guidance.
Identification of cases and controls
A list of 30 uterine tumour diagnostic codes (available from the authors) was collated from the GPRD master code library, all mapping to Read Codes within the B43..00 tree for uterine cancer. Codes relating to cervical cancer were omitted. All women aged ≥40 years with one of these codes, diagnosed between 1 January 2000 and 31 December 2009, were identified. Cases with <1 year of data meeting GPRD quality standards before the first diagnostic code were excluded. For each case, five controls, matched to the case by year of birth, sex, and practice, were randomly selected, using a computer-generated sequence. These stages were performed by GPRD staff. The date of the first cancer code in the records was taken to be the date of diagnosis; this was labelled the index date. The matched controls were assigned the index date of their case. The following exclusion criteria were applied: leiomyosarcoma (the GPRD had ascribed all leiomyosarcomas to uterine origin: as there are several possible sites for leiomyosarcomas, all were excluded, indeed some were in men); diagnosis before 1 January 2000; controls diagnosed with uterine cancer before the index date; metastatic cancer from a non-uterine primary cancer; women with a recorded hysterectomy before the index date; and women with no consultations in the year before the index date.
Women with a hysterectomy recorded >3 months before their first uterine cancer code were also excluded, as the date of diagnosis was unreliable; if the discrepancy was <3 months, the index date was taken to be the date of the hysterectomy.
Selection of possible features of uterine cancer
All symptoms, signs, or abnormal investigations previously recorded in the uterine cancer literature and cancer charity websites (full list available from authors) were studied. For simplicity, these are called ‘features’ from now on. The GPRD stores clinical information in just over 100 000 medcodes, each describing a facet of primary care, such as a symptom. There are several codes for each symptom, differing usually in a qualifier such as duration or severity, so generally containing more information than a specific Read Code. All the codes pertaining to individual symptoms were collated into single symptom libraries. All codes for fractures were also identified, as a test for any recording bias between cases and controls (making the assumption that the fracture rate would be approximately equal). Occurrences of symptoms in the year before the index date were identified. Features were only retained in the study if they occurred in ≥2% of the cases or controls (this was invariably cases). A list of plausible laboratory abnormalities was assembled a priori, using the literature and the authors’ clinical knowledge. All abnormal laboratory results in the year before the index date were also identified, using the local laboratory’s normal range, which is supplied with the data. Women without a test were considered to be equivalent to those with a normal result. Some abnormal tests were grouped: abnormal liver function was defined as the presence of any liver enzyme above the normal range. The variable ‘raised inflammatory markers’ was defined as a raised erythrocyte sedimentation rate, C-reactive protein, or plasma viscosity. These simplifications were necessary, as different localities in the UK contributing to the GPRD have different tests available.
Analysis
Analysis followed the methods used in several previous studies.14 The main analytical method was conditional logistic regression. Analysis was carried out in three stages. First, univariable analysis was performed: any feature with P<0.1 was retained for the multivariate analysis.
Multivariate conditional regression was then performed in stages, collecting similar features together, such as those indicating abnormal bleeding. This stage used a threshold value of P<0.05 for retention. The final model was derived from those features that survived the earlier staged regressions and used a threshold value of P<0.05. All rejected variables were checked again to establish whether they added information to the final model. As all 2732 cases analysed had consulted their GP in primary care but only 90% of controls had consulted during the study period, the posterior odds were divided by 0.90 to give predictive values for the consulting population. One subgroup analysis examined women aged ≥55 years, as a proxy for being postmenopausal, and for this analysis all abnormal menstrual bleeding variables were categorised as postmenopausal bleeding. Analyses were performed using Stata (version 11). Lastly, four clinically plausible interactions were tested.
Calculation of positive predictive values
Positive predictive values (PPVs) were estimated for features shown to be independently associated with uterine cancer in the multivariable analysis, using Bayes’ theorem, whereby the posterior odds = the prior odds × the likelihood ratio.15 The prior odds were calculated from the age-specific national incidence rate of uterine cancer for 2008.16 This was repeated for pairs of symptoms and for second attendances with the same symptom.
As the number of cases in the GPRD was fixed, a power calculation was performed, rather than a sample size calculation. Three thousand cases and 10 000 controls (the initial estimates from the GPRD) provided 97% power (5% two-sided alpha) to detect a change in a rare variable from 2% in cases to 1% of controls. For a more common variable, the study had >95% power to detect a change in prevalence of 20% in cases to 17% in controls.
RESULTS
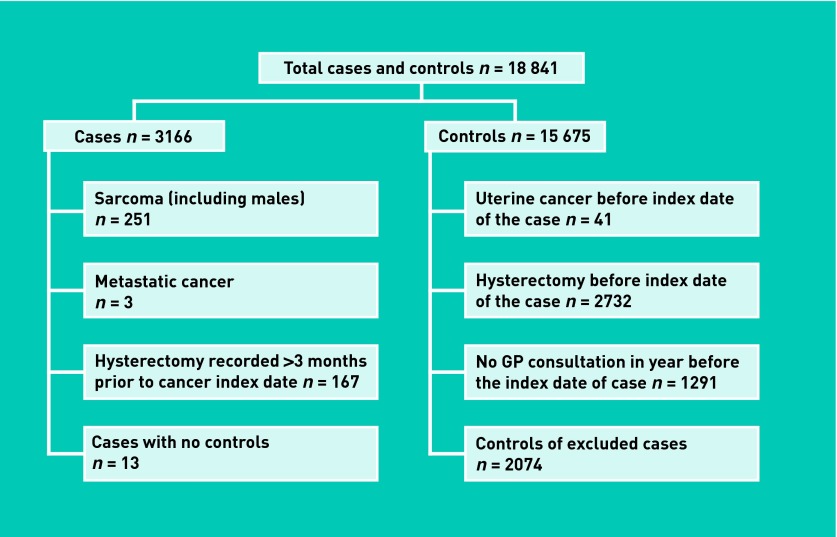
The GPRD provided a total of 18 841 women (3166 cases and 15 675 controls). Application of the exclusion criteria (Figure 1) resulted in 12 269 eligible women (2732 cases and 9537 controls). The median age at diagnosis was 67 years (interquartile range = 59–75 years). The frequency of consultations is presented in Table 1.
Figure 1.
Application of exclusion criteria for cases and matched controls.
Table 1.
Consultations in the year before diagnosis
| Consultations | Cases, median number (IQR) | Controls, median number (IQR) | Rank sum test P-value |
|---|---|---|---|
| In the year before the index date | 14 (9 to 21), (n = 2732) | 8 (4 to 14), (n = 9537) | <0.001 |
| In the 6 months before the index date | 9 (6 to 14), (n = 2732) | 4 (2 to 8), (n = 8614) | <0.001 |
IQR = interquartile range.
Clinical features
Twenty-five symptoms and 22 abnormal test results were studied initially. The frequency, univariable likelihood ratio, and multivariable odds ratio (OR) for features associated with uterine cancer are shown in Table 2. Fractures were similar in cases (n = 39, 1.4%) and controls (n = 179, 1.9%), P = 0.16; 2072 (76%) cases had at least one of the nine features from Table 2 recorded in their notes, compared to 1627 (17%) controls; and 1725 (63%) cases had a record of abnormal vaginal bleeding of any type compared to just 135 (1%) controls. An antagonistic interaction was identified only in the dataset of women aged ≥55 years; this interaction was between vaginal discharge and postmenopausal bleeding (interaction OR = 0.06, P = 0.002).
Table 2.
Clinical features of uterine cancer (all ages)
| Feature | Cases, n (%), n = 2732 | Controls, n (%), n = 9537 | LR (95% CI) | OR in multivariate analysis (95% CI) |
|---|---|---|---|---|
| Symptoms | ||||
| Postmenopausal bleeding, one visit to GPa | 926 (33.9) | 53 (0.6) | 61.0 (46.4 to 80.2) | 154.5 (100.4 to 237.8) |
| Postmenopausal bleeding, two or more visits to GPa | 349 (12.8) | 7 (0.1) | 174.0 (82.5 to 367.3) | 578.9 (187.0 to 1792.1) |
| Excessive bleeding | 109 (4.0) | 35 (0.4) | 10.9 (7.5 to 15.9) | 22.28 (11.86 to 41.84) |
| Irregular menstruation, one visit to GPa | 426 (15.6) | 43 (0.5) | 34.6 (25.4 to 47.2) | 41.50 (27.31 to 63.05) |
| Irregular menstruation, two visits to GPa | 154 (5.6) | 11 (0.1) | 48.9 (26.6 to 90.0) | 69.04 (31.62 to 150.72) |
| Vaginal discharge | 204 (7.5) | 65 (0.7) | 11.0 (8.31 to 14.4) | 13.75 (9.98 to 21.04) |
| Haematuria | 120 (4.4) | 51 (0.5) | 8.21 (5.94 to 11.37) | 8.71 (5.03 to 15.06) |
| Abdominal pain, one visit to GPa | 138 (5.1) | 306 (3.2) | 1.57 (1.29 to 1.92) | 1.97 (1.40 to 2.76) |
| Abdominal pain, two visits to GPa | 64 (2.3) | 95 (1.0) | 2.35 (1.72 to 3.22) | 3.41 (1.99 to 5.83) |
|
| ||||
| Investigations | ||||
| Low haemoglobin | 202 (7.4) | 398 (4.2) | 1.77 (1.50 to 2.09) | 2.12 (1.53 to 2.94) |
| Raised platelets | 110 (4.0) | 207 (2.2) | 1.86 (1.48 to 2.33) | 1.50 (1.00 to 2.25) |
| Raised glucose | 316 (11.6) | 673 (7.1) | 1.64 (1.44 to 1.86) | 1.37 (1.06 to 1.77) |
LR = likelihood ratio. OR = odds ratio.
The ORs presented for the first or second time a symptom is reported to the GP compares to a patient who has not reported the presence of a symptom to their GP. All associations in the model have P<0.001, other than raised platelets, P = 0.049 and raised glucose, P = 0.016.
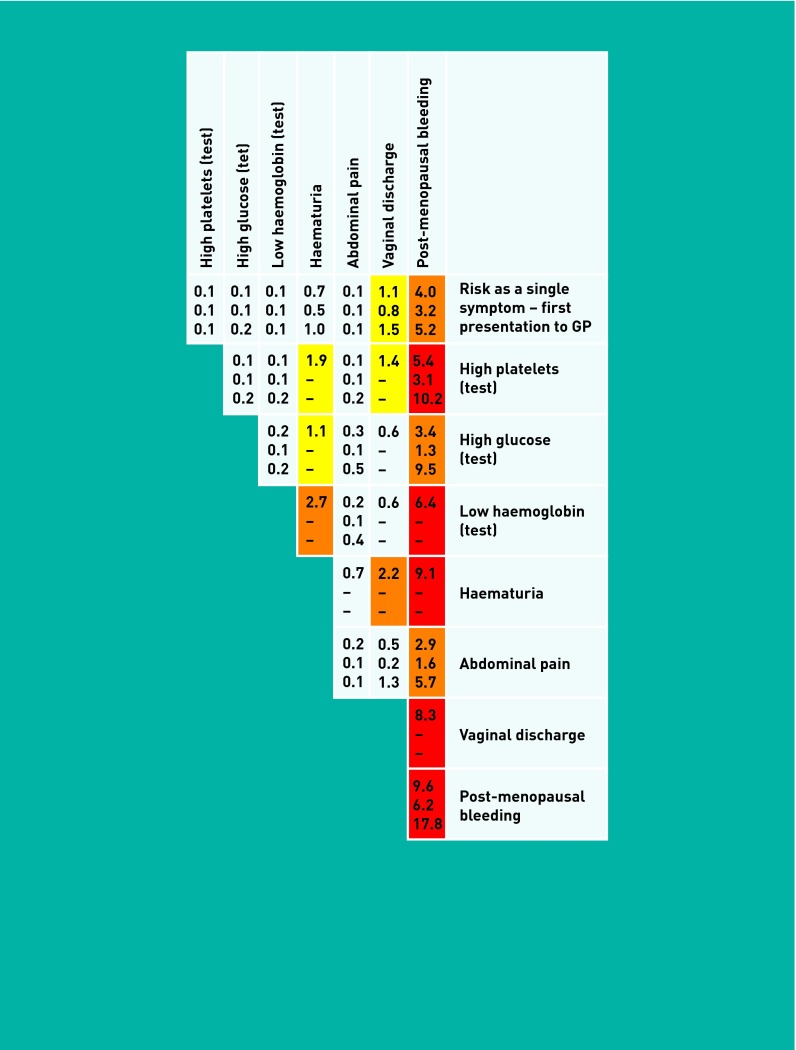
Figure 2 shows the PPVs for uterine cancer for women aged ≥55 years reporting single, paired, or repeated symptoms to their GP.
Figure 1.
Positive predictive value for uterine cancer for women reporting single or paired features of the cancer to primary care (aged ≥55 years).
DISCUSSION
Summary
This is the first study of the clinical features of uterine cancer in primary care. As expected, most of the symptoms reported from secondary care studies were also strongly associated with uterine cancer in primary care. However, the risk of uterine cancer with these features, other than postmenopausal bleeding, was relatively low, reflecting the rarity of uterine tumours and that many of the symptoms are common in benign conditions. The risk of an underlying uterine cancer was higher in women with multiple symptoms.
Strengths and limitations
This study is large, and uses primary care data. This is crucial: selection of women for investigation is performed by primary care, so primary care data must be used to study the selection process. The GPRD is the largest and most established of the longitudinal patient databases from primary care. It has been used in nearly 1000 research articles and its validity has been well documented.10,11,17 The patient population in the database is also broadly representative of the UK population. Additionally, laboratory results are transmitted directly to the database, allowing the local normal range to be used to identify abnormal results, and avoiding transcription errors.
It was not possible to check the accuracy of diagnosis in the cases by histology, or to determine the staging. However, most cases had multiple records of a uterine neoplasm. It is unlikely that such a serious disease would be recorded incorrectly with any frequency. However, the most significant limitation is that the study had to rely upon accurate recording of symptoms by GPs. It was possible to identify all symptoms in the main field of the records, but some may be recorded in an inaccessible part of the GPRD; the so-called ‘free-text’ area. Encouragingly, a recent study of ovarian cancer identified relatively little hidden data in these fields.18 However, when calculating likelihood ratios and PPVs, under-recording is only important if the proportion of under-recording is differentially higher in either cases or controls.
Comparison with existing literature
Women with uterine cancer attended their doctors roughly twice as often as controls; this excess is less extreme than seen in many other cancers, and mirrors data from the National Cancer Patient Experience Survey, where only 17.6% of women with uterine cancer reported consulting their GP at least three times before diagnosis.19 This may be because the possibility of gynaecological cancer is considered early on in a woman with abnormal vaginal bleeding, whereas this is not the case for some other cancers, such as ovarian cancer, with less characteristic symptoms.20
Recent studies of secondary care clinics in the UK have reported that 5–6% of women with postmenopausal bleeding are diagnosed with endometrial carcinoma.9,21,22 The risk for postmenopausal bleeding reported from primary care in this study is 4%. The similarity between these figures suggests that the importance of postmenopausal bleeding has been recognised by GPs, and that most, but not all, patients are being referred for investigation. This fits with a recent article suggesting that two-thirds of women with postmenopausal bleeding were referred immediately, although it does leave the question of why the other one-third were not.23 Only 63% of women in the present study with uterine cancer had any record of abnormal vaginal bleeding, similar to the 66% reported in a large Danish secondary care study.5 An association between diabetes and uterine cancer has been reported before and with a similar strength to the association with a raised glucose found in the present study.24 Finally, a raised platelet count has been reported in primary care studies of lung, and pancreatic cancer,25,26 as well as secondary care studies of ovarian cancer.27
Implications for practice and research
This study identified other significant symptoms for uterine cancer. Arguably, the main value of the results is these other symptoms, which may allow earlier identification of women with uterine cancer, particularly when the symptoms are multiple. Two combinations were notable: haematuria together with anaemia or vaginal discharge. The PPV for both of these is >2% in women aged ≥55 years. It was not possible to tell in this study whether the recorded haematuria was a true symptom, or misattributed vaginal bleeding. However, this is an important clue for GPs: women describing haematuria may not be at risk of having a urological cancer, it may be gynaecological.
A further point is that publishing the risks of uterine cancer in this form does not mandate GPs referring all women with one of the features in Figure 2. GPs can and do use such clinical decision support to reassure when the risk is low, as well as to investigate when the risk is higher. This was demonstrated in the authors’ study of similar colorectal and lung tools.28
Other European countries have better outcomes from uterine cancer than the UK.3 Some of this may reflect earlier investigation of symptomatic women. The data presented in this study may guide GPs in the selection of women for urgent investigation. Although other methods of definitive diagnosis, such as biomarkers, may emerge in this field, these will still require selection of patients for testing and should probably use these results. This study provides primary care evidence for the forthcoming revision of the UK NICE referral guidance.
Funding
The Policy Research Unit in Cancer Awareness, Screening and Early Diagnosis receives funding for a research programme from the Department of Health Policy Research Programme. It is a collaboration between researchers from seven institutions (Queen Mary University of London, UCL, King’s College London, London School of Hygiene and Tropical Medicine, Hull York Medical School, Durham University, and Peninsula Medical School). The views expressed are those of the authors and not necessarily those of the NHS, or the Department of Health.
Ethical approval
Ethical approval was granted by the Independent Scientific Advisory Committee in November 2009, reference number 09_111.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
William Hamilton is the clinical lead for the upcoming NICE revision of cancer referral guidelines. The other authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Evans T, Sany O, Pearmain P, et al. Differential trends in the rising incidence of endometrial cancer by type: data from a UK population-based registry from 1994 to 2006. Br J Cancer. 2011;104(9):1505–1510. doi: 10.1038/bjc.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office for National Statistics Mortality Statistics: deaths registered in England and Wales (Series DR), 2010. http://www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-230730 (accessed 17 Jul 2013).
- 3.Abdel-Rahman M, Stockton D, Rachet B, et al. What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable? Br J Cancer. 2009;101(suppl 2):S115–S224. doi: 10.1038/sj.bjc.6605401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson KM, Ottesen B, Christensen KB, Krasnik A. Diagnostic delay experienced among gynecological cancer patients: a nationwide survey in Denmark. Acta Obstet Gynecol Scand. 2009;88(6):685–692. doi: 10.1080/00016340902971482. [DOI] [PubMed] [Google Scholar]
- 5.Vandborg M. Reasons for diagnostic delay in gynecological malignancies. Int J Gynecol Cancer. 2011;21(6):967–974. doi: 10.1097/IGC.0b013e31821d2770. [DOI] [PubMed] [Google Scholar]
- 6.Brand A. The woman with postmenopausal bleeding. Aust Fam Physician. 2007;36(3):116–120. [PubMed] [Google Scholar]
- 7.Parker C, Hippisley-Cox J, Coupland C, Vinogradova Y. Rectal and postmenopausal bleeding: consultation and referral of patients with and without severe mental health problems. Br J Gen Pract. 2007;57(538):371–376. [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health and Clinical Excellence . Referral guidelines for suspected cancer. London: National Instiitute for Health and Clinical Excellence; 2005. [Google Scholar]
- 9.Burbos N. Predictive value of urgent referrals for women with suspected gynecologic malignancies. Gynecol Oncol. 2010;116(3 suppl 1):S53. [Google Scholar]
- 10.Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract. 2010 doi: 10.3399/bjgp10X483562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrett E, Thomas SL, Schoonen WM, et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton W, Kernick D. Clinical features of primary brain tumours: a case-control study using electronic primary care records. Br J Gen Pract. 2007;57(542):695–699. [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton W, Lancashire R, Sharp D, et al. The importance of anaemia in diagnosing colorectal cancer: a case-control study using electronic primary care records. Br J Cancer. 2008;98(2):323–327. doi: 10.1038/sj.bjc.6604165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton W. The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer. 2009;101(suppl 2):S80–S86. doi: 10.1038/sj.bjc.6605396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knottnerus JA. The evidence base of clinical diagnosis. London: BMJ Books; 2002. [Google Scholar]
- 16.Cancer Research UK Cancer incidence statistics. http://info.cancerresearchuk.org/cancerstats/incidence/ (accessed 17 Jul 2013).
- 17.Fombonne E, Heavey L, Smeeth L, et al. Validation of the diagnosis of autism in general practitioner records. BMC Public Health. 2004;4:5. doi: 10.1186/1471-2458-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tate AR, Martin AGR, Ali A, Cassell JA. Using free text information to explore how and when GPs code a diagnosis of ovarian cancer: an observational study using primary care records of patients with ovarian cancer. BMJ Open. 2011;1(1):e000025. doi: 10.1136/bmjopen-2010-000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012;13(4):353–365. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton W, Peters TJ, Bankhead C, Sharp D. Risk of ovarian cancer in women with symptoms in primary care: population based case-control study. BMJ. 2009;339:b2998. doi: 10.1136/bmj.b2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atiomo WR, Shrestha AD, Falconer W. Evaluation of a one-stop clinic for the rapid assessment of post-menopausal bleeding. J Obstet Gynaecol. 1998;18(2):148–150. doi: 10.1080/01443619867902. [DOI] [PubMed] [Google Scholar]
- 22.Burbos N, Musonda P, Giarenis I, et al. Predicting the risk of endometrial cancer in postmenopausal women presenting with vaginal bleeding: the Norwich DEFAB risk assessment tool. Br J Cancer. 2010;102(8):1201–1206. doi: 10.1038/sj.bjc.6605620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBride D, Hardoon S, Walters K, et al. Explaining variation in referral from primary to secondary care: cohort study. BMJ. 2010;341:c6267. doi: 10.1136/bmj.c6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saltzman BS, Doherty JA, Hill DA, et al. Diabetes and endometrial cancer: an evaluation of the modifying effects of other known risk factors. Am J Epidemiol. 2008;167(5):607–614. doi: 10.1093/aje/kwm333. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton W, Peters TJ, Round A, Sharp D. What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax. 2005;60(12):1059–1065. doi: 10.1136/thx.2005.045880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stapley S, Peters TJ, Neal RD, et al. The risk of pancreatic cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2012;106(12):1940–1944. doi: 10.1038/bjc.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton W, Green T, Macleod U, et al. Evaluation of risk assessment tools for suspected cancer in general practice: a cohort study. Br J Gen Pract. 2013 doi: 10.3399/bjgp13X660751. [DOI] [PMC free article] [PubMed] [Google Scholar]