Abstract
Individuals with Mirror-Touch Synaesthesia (MTS) experience touch on their own bodies when observing another person being touched. Whilst somatosensory processing in MTS has been extensively investigated, the extent to which the remapping of observed touch on the synaesthete’s body can also lead to changes in the mental representation of the self remains unknown. We adapted the experimental paradigm of the ‘Enfacement Illusion’ to quantify the changes in self-face recognition as a result of synaesthetic touch. MTS and control participants observed the face of an unfamiliar person being touched or not, without delivering touch on the participant’s face. Changes in self-representation were quantified with a self-face recognition task, using ‘morphed’ images containing varying proportions of the participant’s face and the face of the unfamiliar other. This task was administered before and after the exposure to the other face. While self-recognition performance for both groups was similar during pre-test, MTS individuals showed a significant change in self-recognition performance following the observation of touch delivered to the other face. Specifically, the images that participants had initially perceived as containing equal quantities of self and other became more likely to be recognised as the self after viewing the other being touched. These results suggest that observing touch on others not only elicits a conscious experience of touch in MTS, but also elicits a change in the mental representation of the self, blurring self-other boundaries. This is consistent with a multisensory account of the self, whereby integrated multisensory experiences maintain or update self-representations.
Keywords: mirror-touch synaesthesia, multisensory integration, self, face recognition, body representation
1. Introduction
Mirroring properties in neurons in the primate brain have been well documented since the discovery of the mirror neurons in macaque monkeys (Rizzolatti, Fadiga, Gallese, & Fogassi, 1996). There is now strong evidence supporting the presence of a similar Mirror Neuron System (MNS) in humans, which is thought to provide a neural basis for the interpersonal sharing of motor representations (e.g. Buccino et al., 2001; Mukamel, Ekstrom, Kaplan, Iacoboni, & Fried, 2010). Furthermore, evidence has suggested that the MNS is not restricted to the motor cortex in humans, but that we also possess a ‘somatosensory mirror system’ that is activated both when we perceive touch to others, and when we experience touch to the self (e.g. Blakemore, Bristow, Bird, Frith, & Ward, 2005; Ebisch et al., 2008; Keysers et al., 2004; Keysers, Kaas, & Gazzola, 2010; Rossetti, Miniussi, Maravita, & Bolognini, 2012). This vicarious activation of the somatosensory cortex may form a neural basis for the understanding of others’ sensory experiences, and may play an important role in empathy (e.g. Schaefer, Heinze, & Rotte, 2012).
Interestingly, this vicarious somatosensory activity to observed touch has measurable behavioural effects. Perception of touch to our own bodies, when delivered near the perceptual threshold, can be modulated by the observation of touch to others (e.g. Cardini et al., 2011; Serino, Giovagnoli, & Làdavas, 2009; Serino, Pizzoferrato, & Làdavas, 2008). For example, viewing someone being touched on the cheek can enhance detection of a tactile stimulus being applied to our own cheek in a congruent location, an effect known as Visual Remapping of Touch (VRT: Serino et al., 2008). The effect of observed touch on tactile perception has been shown to be extinguished when somatosensory activity is disrupted using TMS (Fiorio & Haggard, 2005), which suggests that this effect is reliant on vicarious activation of the ‘somatosensory mirror system’.
Although the observation of touch can enhance perception of tactile stimulation delivered to the body, it very rarely elicits a conscious experience of touch in the absence of actual tactile stimulation. However, a type of synaesthesia has been identified which provides an interesting exception to this case. For individuals with Mirror Touch Synaesthesia (MTS), observing others being touched consistently produces a marked conscious experience of touch on their own body. This experience is thought to occur in approximately 1.6% of people (Banissy, Cohen Kadosh, Maus, Walsh & Ward, 2009) and to be a consequence of increased cortical activity within the somatosensory mirror system (Blakemore et al., 2005). Consistent with the purported role of this system in social cognition, MTS individuals show enhanced emotion recognition (Banissy et al., 2011) and score more highly on empathy measures (Banissy & Ward, 2007) than non-synaesthetes. Although not yet explicitly tested, it has been suggested that the increased activity within the somatosensory mirror system in MTS is mediated by mechanisms involved in self-other discrimination. Moreover, several authors have suggested that MTS may be linked to a blurring of self-other boundaries when perceiving touch to another person (Banissy et al., 2009; Banissy, Walsh & Muggleton, 2011; Amiola-Davis & White, 2012), leading to a disinhibition of normal somatosensory mirror mechanisms (Fitzgibbon et al., 2012).
Intriguingly, in non-synaesthetes, we can experimentally induce a blurring of self-other boundaries by employing synchronous visuotactile stimulation (Tsakiris, 2010). This type of stimulation can evoke bodily illusions, induce misattributions of viewed tactile sensations to the self, and eventually change the perceptual boundaries between self and other. For example, in the Rubber Hand Illusion (RHI), tactile stimulation delivered in synchrony to the participant’s own unseen hand and a visible fake rubber hand can induce illusory ownership over the rubber hand, and induces the participant to attribute the tactile sensations on their own hand to the touch they can see on the rubber hand (Botvinick & Cohen, 1998).
In a facial analogue of the RHI, touch is delivered to a participant’s face whilst they view a video in which another person is being touched on a specularly-congruent location in synchrony with the participant’s felt touch (Sforza, Bufalari, Haggard & Aglioti, 2010; Tsakiris, 2008). This procedure, known as the ‘Enfacement Illusion’, elicits a situation akin to looking at oneself in a mirror, yet seeing an unfamiliar person’s face in place of your own reflection. During enfacement, participants report a change in the experience of the source of sensation from their own face to the other’s, and a subjective increase in perceived similarity between the other and themselves (Tajadura-Jimenez, Grehl & Tsakiris, 2012).
This subjective increase in self-other similarity is accompanied by a measurable behavioural change in the way participants represent their own facial appearance (the ‘self-face representation’). Tajadura-Jimenez et al. (2012) presented participants with morphed faces containing varying percentages of their own face, and they decided whether each face looked more like themselves, or an unfamiliar other. After experiencing the enfacement illusion with the other’s face, the images that participants had initially perceived as containing equal quantities of self and other became more likely to be recognised as the self. The direction of this change was elucidated by Tajadura-Jimenez et al. in an additional experiment, in which they demonstrated that the enfacement illusion independently affected recognition of the self-face, while recognition of the other’s face remained unchanged. Specifically, they showed that when participants watched a video of another’s face gradually morphing into their own face after a period of enfacement, they accepted faces with a higher percentage of ‘other’ as ‘self’. Importantly, however, this change did not occur when they watched a video showing the other direction of morphing, from self to other. This suggests that the synchronous shared touch of the enfacement illusion induced participants to incorporate features of the other’s face into their own face representation, resulting in the participants representing the other’s face as more similar to their own.
This supports a multisensory account of the self, whereby our stored representations of our physical appearance (our ‘body representations’) are not solely derived from stable representations, but instead is maintained and updated by integrated multisensory experiences (Tsakiris, 2008). We may recognize and form a mental representation of our own face because our mirror reflection moves when we move, and we see it being touched when we feel touch ourselves. In both the RHI and enfacement illusion, an individual experiences a touch that they see on another body, resulting in measurable changes in their body representations. This sharing of another’s tactile experience bears similarities to MTS. This raises the intriguing possibility that when MTS individuals view touch on others, it not only elicits a shared tactile experience, but actually alters their body representation, in the same way that bodily illusions such as enfacement and RHI induce change in non-synaesthetes.
We aimed to investigate changes in body representation in MTS, by inducing the enfacement illusion in MTS individuals without delivering physical touch to their faces, and measuring the effect of pure tactile observation on their stored self-face representation. Aimola-Davies and White (2012) recently demonstrated that RHI can be induced in MTS participants without delivering physical touch to their own hand, by allowing them merely to observe touch on the rubber hand. The synaesthetic touch that they experienced induced a subjective incorporation of the rubber hand into their body representation. However, it remains to be answered whether synaesthetic touch can change stored mental representations of a key feature of one’s self-identity, such as one’s own face.
This study consisted of two experiments. In the first experiment, a group of MTS participants and a group of non-synaesthetic controls viewed another’s face being touched, but were not physically touched themselves. For MTS individuals, we hypothesised that the synaesthetic experience of seeing touch on another’s face could change their self-face representation, in the same way that physical experience of touch seen on another’s face changes the self-face representations of non-synaesthetic individuals. To test this, we measured self face-recognition before and after the enfacement session to investigate whether there were any changes in self-face representation induced by the observation of touch. We also measured the participants’ subjective experiences of ownership, self-other similarity, and self-attribution of touch. A control condition was employed, in which the face that the participants viewed was untouched. This controlled for effects of mere exposure to the other’s face, and thus ensured that any effect we did find was specific to experienced touch, rather than mere visual exposure to the face of another individual. In a second control experiment, we investigated the similarity of this effect to the standard Enfacement Illusion in participants without synaesthesia. Another non-MTS control group observed touch on another’s face whilst physical touch was delivered on their own face, following the standard procedure of the enfacement illusion. Subsequent changes in self-face recognition were compared to those elicited by the mere observation of touch in MTS individuals.
2. Material and Methods
2.1. Participants
Potential MTS participants were first selected through self-report via a web-based questionnaire investigating different types of synaesthesia. Those (n=25) who answered ‘agree’ or ‘strongly agree’ to the statement ‘I sometimes feel touch when I see other people being touched’ on a 5-point Likert scale were subsequently contacted to complete a further web screening devised by Holle and colleagues (Holle, Banissy, Wright, Bowling & Ward, 2011). Participants saw a series of videos of people and objects being either touched or approached by a finger. Participants were asked to report on their experiences of touch, if any, for each video. Six participants (all female1, MAGE = 19.0 years) who gave reports of experienced touch in two or more of the videos in which people were touched, reflecting 4.3% of the total questionnaire respondents, were selected to participate in Experiment 1.
Twenty non-MTS participants (all female) were also recruited for the study. Non-MTS status was confirmed by self-report on the web-based questionnaire, to which all participants answered “strongly disagree” to the statement ‘I sometimes feel touch when I see other people being touched’. Ten of these non-MTS participants (MAGE = 19.9 years), referred to as the Control-1 group, participated in Experiment 1and performed exactly the same task as the MTS individuals. The remaining ten non-MTS participants (MAGE = 20.7 years), referred to as the Control-2 group, participated in Experiment 2 which followed the standard enfacement procedure, allowing us to compare the effect of touch observation in MTS with the effect of standard enfacement in non-MTS participants.
2.2. Procedure
The procedure employed to generate the experimental stimuli was identical for both Experiments 1 and 2. Two female individuals were selected to model as the face of the ‘other’. A photo and two videos were recorded with each model looking straight into the camera with a neutral expression. For the ‘TOUCH’ video, their right cheek was stroked with a cotton bud every three seconds, whereas for the ‘NO-TOUCH’ video, no tactile stimulation was delivered. Before the experiment began, we took a photo of each participant’s face, which we subsequently mirror-reversed to most closely match their stored facial representation. From this photo, morphed face stimuli were generated by morphing the participant’s face with the two models’ faces. This produced two sets of 100 images for each participant, which contained increasing amounts of the participant’s face ranging from 0% (100% model) to 100% (0% model). These images were used as stimuli in both experiments.
2.2.1. Procedure for Experiment 1
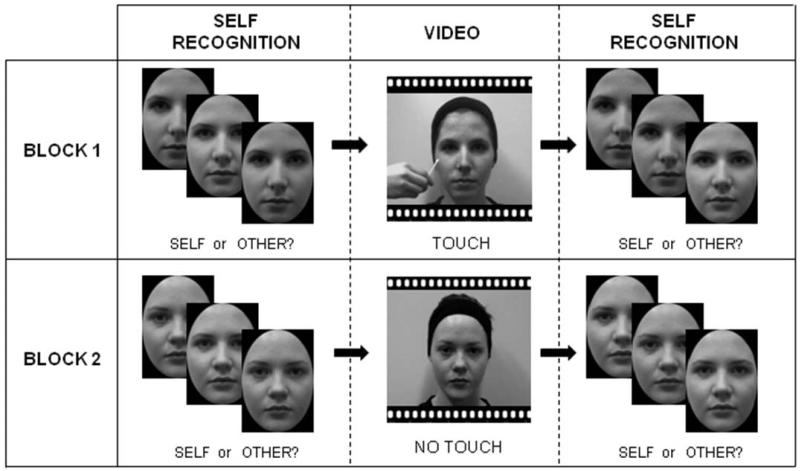
The MTS group and the Control-1 group participated in Experiment 1. The experiment comprised two experimental blocks; one for the TOUCH condition and one for the NO-TOUCH condition (see Figure 1). Each block began with a self-recognition task, in which each trial displayed a morphed image, and the participant had to decide whether the image looked more like the self, or the other. The first set of trials were presented in an interleaved double-staircase procedure, following that of Tajadura-Jimenez et al. (2012), in order to ascertain the participant’s ‘point of subjective equality’; the image at which they responded ‘self’ and ‘other’ at chance levels. This was taken as the baseline image. For the final 60 trials of the self-recognition task, the participant was presented with images containing subjectively more other than self (‘Other’ trials: 8% less self than baseline, and 4% less self than baseline), the baseline image, and images containing subjectively more self than other (‘Self’ trials: 4% more self than baseline, and 8% more self than baseline). The choice of these images was based on the results of previous studies investigating the effect of enfacement on self-recognition. The enfacement effect has been shown to only occur when self-other discrimination is difficult, and thus only to images close to Baseline (the PSE). Previous studies have shown a change in self-recognition around the Baseline of between 3% and 6% (e.g. Sforza et al., 2010; Tajadura-Jimenez et al., 2012; Tsakiris, 2008). Thus, we tested self-recognition at Baseline, as well as to images with up to 8% more Other (Other images), and up to 8% more Self (Self images) in order to ensure that we had a high change of detecting any likely changes to self recognition in the MTS group. There were 20 ‘Other’ trials, 20 baseline trials, and 20 ‘Self’ trials presented in total, and order of trials was randomised.
Figure 1.
The design of Experiment 1. Each participant completed two experimental blocks, comprising TOUCH and NO-TOUCH conditions. A self-recognition task, performed both before and after viewing a video, required participants to decide whether morphed images looked more like their own face, or that of another.
The participant was then shown a 2-minute video of the model (either the TOUCH or NO-TOUCH video), during which the participant was instructed to keep as still as possible whilst viewing the video, and to keep their eyes on the model’s face at all times. The participant then completed another self-recognition task, with the same range of −8%, −4%, baseline, +4% and +8% images as in the pre-video stage. Finally, the participant reported their subjective experiences by completing an Illusion Questionnaire, in which they were presented with a set of statements (9 for the TOUCH condition, 8 for the NO-TOUCH condition, adapted from Tajadura-Jimenez et al., 2012) on a computer screen in a random order, and rated their agreement with each one using a 7-point Likert scale.
Each participant completed one TOUCH block and one NO-TOUCH block. The model featuring as the ‘other’ face was consistent within, but differed between, experimental blocks. A different model was used for each block to avoid the experience with a model in one condition ‘carrying over’ to affect subsequent self-recognition responses in the following condition. The order of conditions and the model assigned to each condition was counterbalanced between participants.
2.2.2. Procedure for Experiment 2
The Control-2 group participated in Experiment 2, which contained two experimental conditions. For the ENFACEMENT condition, they performed self-recognition before and after observing a TOUCH video, during which they received concurrent touch to their left cheek in synchrony with the touch they observed on the model’s cheek, as per the standard enfacement illusion procedure. For the NO-TOUCH condition, they performed self-recognition before and after the NO-TOUCH video, in which they merely viewed the model in the absence of touch delivered to either the participant’s or the model’s face. The procedure for this condition was identical to that of the NO-TOUCH condition in Experiment 1. All other aspects of the task and procedure (e.g. videos, face-recognition task, Illusion Questionnaire) were identical to Experiment 1.
3. Results
First, we analysed the data from Experiment 1 to investigate the effects of touch observation on self-face recognition in MTS and non-MTS individuals. We then analysed the data from Experiment 2 and conducted statistical comparisons with key results from Experiment 1, to allow us to assess the similarities between the effect of touch observation in the MTS group to the effect of the Enfacement Illusion in the non-MTS (Control-2) group.
3.1. Results of Experiment 1
For each participant, the proportion of ‘self’ responses given to each type of image was calculated for both the pre- and post-video self-recognition tasks. The −8% and −4% images (containing a lower percentage of self than the baseline image) were categorized as the ‘Other’ images, and the +4% and +8% images (containing a higher percentage of self than baseline) were categorized as the ‘Self’ images.
Given the small sample sizes, our data were most suited to non-parametric analysis. However, due to the limited applicability of non-parametric methods to mixed factorial designs, we first performed an initial ANOVA in order to identify any interactions between factors, before proceeding to investigate and verify these interactions using non-parametric analyses. To begin, the proportion of ‘self’ responses were entered into a 2(time: Pre vs. Post-video) × 3(image: Other vs. Baseline vs. Self) × 2(condition: TOUCH vs. NO-TOUCH) × 2(group: MTS vs. Control-1) repeated measures ANOVA. Residuals were subjected to Shapiro-Wilk tests for normality and none deviated significantly from a normal distribution, all p-values > .05. Although Mauchly’s Test of Sphericity was not violated, W = 0.876, p = .422, we proceeded to use Greenhouse-Geisser corrections to ensure the most conservative tests for our small samples. There was no significant main effect of group on proportion of self-responses, F(1,14) = 0.75, p = .400. There was however an expected main effect of image, F(1.23,14.00) = 53.96, p < .001, with Self images eliciting the highest proportion of self-responses, M = .72, followed by Baseline images, M = .54, followed by Other images, M = .33. Importantly, this main effect was modulated by a four-way interaction between time, image, condition and group, F(1.78,24.91) = 3.74, p = .042. No other main effects or interactions were significant.
To investigate the four-way interaction, we first ensured that there were no significant differences between the two groups on pre-video self-recognition performance. Pre-video scores were entered into a mixed repeated-measures ANOVA with image (Other vs. Baseline vs. Self), condition (TOUCH vs. NO-TOUCH) and group (MTS vs. Control-1) as factors, which revealed an expected main effect of image, F(1.47,20.60) = 49.08, p < .001, but no main effect of group, F(1,14) = 0.68, p = .425, nor any interactions (p > .05). We then calculated change scores by subtracting pre- from post-video self-responses, and investigated the effect of condition and image type on self-recognition change for each group separately.
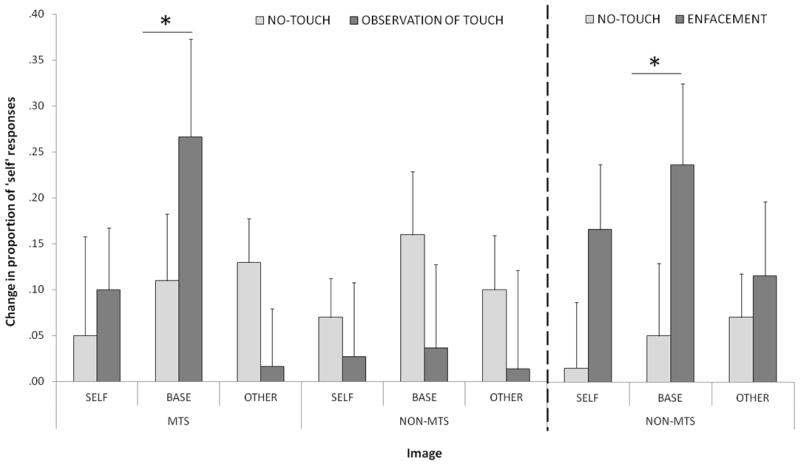
In the Control-1 group, a 3(image: Other vs. Baseline vs. Self) × 2(condition: TOUCH vs. NO-TOUCH) ANOVA did not reveal any significant main effect of condition, F(1,9) = 0.66, p = .803, nor a Condition × Image interaction, F(1.98,17.77) = 0.57, p = .571. In the MTS group, however, the ANOVA yielded an interaction between image and condition on change in self-responses, F(1.24,6.21) = 16.56, p = .010. Visual inspection of the means suggested that only self-recognition change to the baseline image had been affected by condition (see Figure 2). This was confirmed using non-parametric pairwise comparisons. Wilcoxon signed rank tests were used to compare self-recognition change scores between TOUCH and NO-TOUCH conditions for Self, Baseline and Other image types individually. For the MTS group, change scores to Baseline images were significantly higher in the TOUCH condition, M = .267 (SD = .260) than the NO-TOUCH condition, M = .067 (SD = .178), z = −2.06, p = .039. A one-sample Wilcoxon Signed Rank test on change scores for NO-TOUCH showed no significant difference from zero, p = .496, suggesting that the NO-TOUCH condition did not yield any significant changes in self-recognition. There were no significant differences between conditions for Self images, z = −0.11, p = .916, or for Other images, z = −1.47, p = .141. For the Control-1 group, there were no significant differences between conditions for any of the three image types, all p>.05, thus confirming the general pattern of interaction illustrated in the initial ANOVA.
Figure 2.
Left Panel: Change in proportion of ‘self’ responses given by MTS and non-MTS groups after viewing TOUCH and NO-TOUCH videos, for Self, Baseline, and Other images. Starred contrast indicates significance at two-tailed level. Positive change in ‘self’ responses signifies an increase in the proportion of ‘self’ responses after viewing the video. Right Panel: Change in proportion of ‘self’ responses given by non-MTS group after experiencing ENFACEMENT, in which synchronous tactile stimulation is delivered to the face during observation of TOUCH video (Experiment 2). Again, starred contrast indicates significance at two-tailed level. Error bars indicate S.E.M.
Responses to the Illusion Questionnaire were then analysed. Independent t-tests were employed to compare mean responses across all questions between groups, for each condition. For the TOUCH condition, the MTS group gave a significantly higher mean rating to the Illusion Questionnaire (across all questions) than did the Control-1 group, t(13.93) = −3.57, p = .003. For the NO-TOUCH condition, the ratings given by the two groups did not significantly differ, t(14) = −1.01, p = .332. Group differences in median responses for each individual question were analysed using Mann-Whitney U tests, the results of which can be found in Table 1.
Table 1.
Table showing median Likert responses given by MTS and non-MTS (Control-1) groups to each Illusion question ranging from −3 (strongly disagree) to +3 (strongly agree), for TOUCH and NO-TOUCH conditions. P-values for individual questions indicate the results of Mann-Whitney U tests comparing the responses to each questionnaire item between MTS and non-MTS groups. P-values for ‘Total Mean Response’ indicate the results of independent t-tests comparing the mean response across all items of the questionnaire between groups. Asterisks indicate significance at α=.05.
| Illusion Question | TOUCH M(SD) | NO-TOUCH M(SD) | ||||
|---|---|---|---|---|---|---|
| MTS | NON-MTS | p-value | MTS | NON-MTS | p-value | |
| “I felt like the other’s face was my face” | 1.00 (1.37) | −2.50 (1.37) | .008* | 0.50 (1.47) | −2.00 (1.95) | .122 |
| “It seemed like the other’s face belonged to me” | 0.00 (2.17) | −2.00 (0.67) | .126 | −1.50 (1.83) | −2.00 (1.94) | .781 |
| “It seemed like I was looking at my own mirror reflection” | 0.50 (1.54) | −2.00 (1.94) | .263 | −0.50 (2.07) | −2.00 (1.51) | .342 |
| “It seemed like the other’s face began to resemble my own face” | 0.50 (2.06) | −1.50 (1.51) | .098 | 0.00 (1.47) | 0.00 (2.12) | .657 |
| “It seemed like my own face began to resemble the other person’s face” | 1.00 (1.03) | −2.50 (1.64) | .005* | 0.50 (1.94) | −1.00 (2.01) | .473 |
| “It seemed like my own face was out of my control” | −0.50 (1.63) | −2.00 (1.84) | .200 | 0.00 (1.63) | −2.00 (1.34) | .095 |
| “It seemed like the experience of my face was less vivid than normal” | 1.50 (1.26) | 0.00 (2.32) | .203 | 0.50 (1.47) | 0.00 (1.96) | .378 |
| “I felt that I was imitating the other person” | 1.00 (1.03) | −0.50 (1.94) | .305 | 1.00 (1.52) | 1.00 (1.99) | .197 |
| “I felt a touch on my face when I saw the cotton bud touching the other’s face” | 1.00 (1.83) | −2.50 (1.33) | .032* | - | - | - |
| Total Mean Response | 0.30 (0.70) | −1.49 (1.30) | .003* | 0.10 (1.07) | −0.86 (1.50) | .332 |
3.2. Results of Experiment 2
The results from the Control-2 group in Experiment 2 allowed us to investigate the similarity of the reported effect in MTS to the standard Enfacement Illusion in non-MTS participants. First, we confirmed and replicated the standard enfacement effect on self-recognition (see Sforza et al., 2010; Tajadura-Jimenez et al., 2012; Tsakiris, 2008). Change scores for Control-2 were calculated by subtracting pre- from post-video self-responses, as in the previous analysis of MTS results. The change scores to Baseline images were compared within-subjects between ENFACEMENT and NO-TOUCH conditions with a Wilcoxon Signed Rank test. As predicted, there was a significant difference in the proportion of self-responses in the ENFACEMENT condition and the NO-TOUCH condition, z = −1.94, p = .052, with the ENFACEMENT condition yielding a significantly larger increase in self-recognition than the NO-TOUCH condition, MENFACEMENT = .235 (SD = .277), MNO-TOUCH = .050 (SD = .247).
We were then interested in comparing our Enfacement effect with the effect of viewing touch, both for individuals with MTS and for non-synaesthetes. For our three groups of participants (MTS, Control-1, Control-2), we calculated difference scores by subtracting change scores in the experimental condition (for MTS and Control-1, this was the TOUCH condition, and for Control-2, this was the ENFACEMENT condition) from change scores in the NO-TOUCH condition (which did not significantly differ between groups, Kruskal-Wallis test p = .208). These difference scores reflected the differential effect of our experimental manipulation (viewing touch for MTS and Control-1, or Enfacement for Control-2) relative to that of merely viewing an untouched face. The calculation of these scores allowed us to compare the differential effects of our experimental conditions on self-recognition between groups, using non-parametric methods, without losing vital information regarding the relative changes between experimental and control conditions.
We first compared difference scores between TOUCH in non-MTS participants (Control-1) and ENFACEMENT in non-MTS participants (Control-2). This revealed a significant difference, U = 19.5, p = .021, whereby for non-MTS participants, ENFACEMENT yielded a significantly larger difference score, Mdn = 0.25, than did TOUCH, Mdn, −.05. This suggests that, as expected, ENFACEMENT had a significantly larger effect than TOUCH for non-MTS participants on self-recognition change, relative to the NO-TOUCH control condition. We then compared the differential effects of TOUCH for MTS participants to the differential effects of ENFACEMENT for Control-2 participants, using a Mann-Whitney U test on difference scores. This revealed no significant difference, U = 28.0, p = .827, which suggests that the behavioural effect of TOUCH on self-recognition change for the MTS group was equivalent to that of ENFACEMENT for non-MTS individuals, relative to the NO-TOUCH control condition.
Finally, the non-MTS group’s responses to the Illusion Questionnaire after the ENFACEMENT condition were compared to the MTS group’s responses after the TOUCH condition, to investigate whether their subjective experiences during the videos were also equivalent. A Mann-Whitney U test revealed no significant difference in average strength of illusory experience (comprising the mean response to all nine illusion questions) between the groups, U = 25.0, z = −0.543, p = .587, suggesting that the TOUCH condition for MTS individuals elicited an illusory experience of a similar strength to that elicited by the ENFACEMENT condition in non-synaesthetes. We then compared scores given on individual items between groups; there were no significant group differences on any questionnaire item, p > .05.
4. Discussion
We investigated the malleability of self-other boundaries with a self-face recognition task in a group of individuals with Mirror-Touch Synaesthesia (MTS). While somatosensory processing in MTS has been extensively investigated, the extent to which synaesthetic touch can also lead to changes in self-other boundaries remains unknown. To investigate this, MTS and control individuals took part in an adapted version of the ‘enfacement illusion’ paradigm, in which they observed the face of an unfamiliar person being touched or not, without being touched themselves. To quantify the changes in self-other boundaries as a result of synaesthetic experience, we administered a self-face recognition task before and after the exposure to the other face. While self-recognition performance for both groups was similar during pre-test, the MTS participants showed a specific and significant change in self-recognition performance following the observation of touch delivered to the other face. During the standard enfacement illusion paradigm, tactile stimulation is delivered to participants’ faces whilst they observe another person’s face being touched in synchrony. This experience of synchronous ‘shared touch’ elicits a measurable change in participants’ stored mental representation of their own face, to incorporate elements of the other’s face (Tajadura-Jimenez et al., 2012). The results of the present study suggest that, in a way that is analogous to the classic enfacement illusion, for MTS the observation of touch on others not only elicits a conscious experience of touch, but also a change in the mental representation of the self-face, analogous to the change induced in non-synaesthetes when exposed to the enfacement illusion.
In the MTS group, but not the non-MTS group, self-face recognition was significantly altered after viewing touch on another’s face. Specifically, the images that MTS participants had initially perceived as containing equal quantities of self and other became more likely to be recognised as the self after viewing the other being touched. A ‘no-touch’ control condition did not yield any changes in self-recognition, demonstrating that the effect was specific to the experience of touch rather than any general effect of visual familiarity. Our results suggested that the MTS participants’ mental representations of their facial appearance had been updated to incorporate features of the other’s face, enhancing perceived self-other similarity. This behavioural effect was accompanied by subjective reports of increased self-other resemblance, ownership and illusory touch whilst watching the other being touched.
In a second experiment, we compared the effect of touch observation in MTS individuals to the effect of the standard Enfacement Illusion in non-synaesthetes. We demonstrated that the effect of viewing touch on self-recognition in MTS was equivalent to the change in self-recognition elicited by enfacement in a group of non-MTS participants. Furthermore, analysis of questionnaire responses showed that the observation of touch elicited a phenomenology in MTS participants that was of equivalent intensity and subjective quality to the phenomenological experience elicited by the Enfacement Illusion in non-synaesthetes.
Imaging studies have identified a network of brain areas involved in representing and distinguishing self from other, comprising the inferior parietal lobule and inferior frontal gyrus, the temporoparietal junction, and the right insula (see Northoff, Qin & Feinberg, 2011, for review). Banissy et al. (2009) highlight this network as likely to be atypical in MTS, leading to a remapping of observed sensations onto the self. In particular, the right insular lobe has been shown to be involved in key domains of self-processing, such as body-ownership (Tsakiris, Hesse, Boy, Haggard & Fink, 2007), empathy (Singer et al., 2004) and self-face recognition (Devue et al., 2007; Morita et al., 2008). Intriguingly, Blakemore et al. (2005) found that the anterior insula was the only area to be activated solely in an individual with MTS, and not in control participants, during the observation of touch. The anterior insula is anatomically connected to the secondary somatosensory cortex (Mesulam & Mufson, 1985), which might act as a neural pathway whereby self-related processing and tactile awareness interact. Further work is needed to elucidate the causal connections between these areas, both in MTS and non-MTS individuals.
The self-face recognition task employed in the current study does not give information about the directionality of the change in face recognition. However, we believe it likely that our effect observed in the MTS group reflects a specific change in the representation of the self-face, rather than the other-face, for two reasons. First, the results of a video-morphing task used by Tajadura-Jimenez et al. (2012) found that enfacement in non-synaesthetic participants elicited a significant change in self-face recognition, whilst leaving other-face recognition unchanged. Given that our study has revealed notable behavioural and phenomenological similarities between the enfacement illusion in non-MTS individuals, and the observation of touch in MTS individuals, it is likely that the direction of the effect in the MTS group is the same as that reported by Tajadura-Jimenez et al. Second, this prediction is also consistent with the overall phenomenology of MTS, as a type of synaesthesia characterised by an interjection of the other into the self, rather than a projection of the self into others. For example, MTS individuals incorporate the touch of others onto their own bodies, but do not project their own touch experience onto other’s bodies. To be consistent with this general phenomenology of MTS, we would expect the features of others to be incorporated into the self-face representation, rather than the features of one’s own face being projected onto the other’s face. Concordantly, the MTS group in the current study gave significantly higher agreement ratings to the statement “It seemed like my own face began to resemble the other person’s face” during the TOUCH video than they did to the statement “It seemed like the other’s face began to resemble my own face”, relative to the non-MTS control group, which again suggests that the effect seen in this group reflects a specific, directional change in the representation of the self-face, rather than the other-face.
It is possible that the effect of viewing touch on self-face representation in MTS individuals may not be due to the experience of illusory touch on their own face, but a more general effect of increased attention or tactile imagery. However, previous work has shown that attentional factors are unlikely to explain MTS, as individuals with MTS experience touch when they see touch on faces, but not when they see touch on objects (Holle et al., 2011) nor when a light flash merely cues attention to a specific area of an observed face (Banissy et al., 2009). In addition, it has been shown that imagery alone is not enough to induce MTS in such individuals, as they experience touch only when they see touch on another face, but not when they see a hand merely approaching the face (Holle et al., 2011). Our MTS participants were recruited following a screening protocol which involved videos of hands delivering touch to, or merely approaching, a variety of stimuli (objects, dummy body-parts, and humans). Our MTS participants reported synesthetic touch solely during observation of touch to humans, and not during observation of touch to objects or dummies. Furthermore, in line with previously verified individuals with MTS, they did not experience synesthetic touch when viewing hands merely approaching faces. Therefore, it is unlikely that changes in self-face representation in the MTS group were due merely to higher tactile imagery abilities, or increased attention to tactile events.
In conclusion, we have demonstrated that the observation of touch can induce a measurable change in the stored self-face representations of MTS individuals. After viewing another’s face being touched, MTS participants incorporated features of the other’s face into their own face representation. This lends further support to the multisensory account of the self, which argues that our representations of our own body, including the representation of our face, are continually updated by integrated multisensory experiences. Importantly, this study has shown that, for MTS individuals, the presence of physical touch is not necessary to update body representations. In this case, the integrated experience of observed touch and synaesthetic touch is sufficient to cause a significant change in self-face representation and self-other boundaries. This effect was shown to be quantitatively and qualitatively similar to the change in self-recognition seen after the Enfacement Illusion in non-synaesthetes. Whilst other studies have investigated the remapping of observed touch to the self in MTS, ours is the first to demonstrate that this remapping of touch significantly changes the way synaesthetes represent their own bodies. Given the documented engagement of the insula and secondary somatosensory cortex in MTS (Blakemore et al., 2005) as well as in body-awareness in non-MTS individuals (Tsakiris et al., 2007), the behavioural results of the present study advance our understanding of the multisensory basis of the self and its involvement in key social cognition processes such as the self-other distinction.
Acknowledgements
This study was funded by ESRC First Grant RES-061-25-0233, European Research Council (ERC-2010-StG-262853) to MT. MJB is supported by the British Academy.
Footnotes
The fact that all MTS participants were female may be partly due to the high proportion of females initially responding to the web-based questionnaire (76%). Because of this gender bias in selection, we make no empirical claims about the gender ratio of mirror-touch synaesthetes in the general population. Only females were chosen for subsequent control groups in order to match the MTS group for gender.
References
- Aimola-Davies AM, White RC. A sensational illusion: Vision-touch synaesthesia and the rubber hand paradigm. Cortex. 2012 doi: 10.1016/j.cortex.2012.01.007. IN PRESS. [DOI] [PubMed] [Google Scholar]
- Banissy MJ, Cohen Kadosh R, Maus GW, Walsh V, Ward J. Prevalence, characteristics and a neurocognitive model of mirror-touch synaesthesia. Experimental Brain Research. 2009;198(2-3):261–272. doi: 10.1007/s00221-009-1810-9. [DOI] [PubMed] [Google Scholar]
- Banissy MJ, Garrido L, Kusnir F, Duchaine B, Walsh V, Ward J. Superior facial expression, but not identity recognition, in mirror-touch synesthesia. The Journal of Neuroscience. 2011;31(5):1820–4. doi: 10.1523/JNEUROSCI.5759-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banissy MJ, Walsh VZ, Muggleton NG. Mirror-touch synaesthesia: A case of faulty self-modelling and insula abnormality. Cognitive Neuroscience. 2011;2:114–115. doi: 10.1080/17588928.2011.585232. [DOI] [PubMed] [Google Scholar]
- Banissy MJ, Ward J. Mirror-touch synesthesia is linked with empathy. Nature Neuroscience. 2007;10(7):815–6. doi: 10.1038/nn1926. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, Bristow D, Bird G, Frith C, Ward J. Somatosensory activations during the observation of touch and a case of vision-touch synaesthesia. Brain. 2005;128(7):1571–83. doi: 10.1093/brain/awh500. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands “feel” touch that eyes see. Nature. 1998;391(6669):756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. European Journal of Neuroscience. 2001;13(2):400–404. [PubMed] [Google Scholar]
- Cardini F, Costantini M, Galati G, Romani GL, Làdavas E, Serino A. Viewing one’s own face being touched modulates tactile perception: an fMRI study. Journal of Cognitive Neuroscience. 2011;23(3):503–13. doi: 10.1162/jocn.2010.21484. [DOI] [PubMed] [Google Scholar]
- Devue C, Collette F, Balteau E, Degueldre C, Luxen A, Maquet P, Brédart S. Here I am: The cortical correlates of visual self-recognition. Brain Research. 2007;1143:169–82. doi: 10.1016/j.brainres.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Ebisch SJH, Perrucci MG, Ferretti A, Del Gratta C, Romani GL, Gallese V. The sense of touch: embodied simulation in a visuotactile mirroring mechanism for observed animate or inanimate touch. Journal of Cognitive Neuroscience. 2008;20(9):1611–23. doi: 10.1162/jocn.2008.20111. [DOI] [PubMed] [Google Scholar]
- Fiorio M, Haggard P. Viewing the body prepares the brain for touch: effects of TMS over somatosensory cortex. The European Journal of Neuroscience. 2005;22(3):773–7. doi: 10.1111/j.1460-9568.2005.04267.x. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon BM, Enticott PG, Rich AN, Giummarra MJ, Georgiou-Karistianis N, Bradshaw JL. Mirror-sensory synaesthesia: exploring “shared” sensory experiences as synaesthesia. Neuroscience and Biobehavioral Reviews. 2012;36(1):645–657. doi: 10.1016/j.neubiorev.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Holle H, Banissy MJ, Wright T, Bowling N, Ward J. “That’s not a real body”: Identifying stimulus qualities that modulate synaesthetic experiences of touch. Consciousness & Cognition. 2011;20:720–726. doi: 10.1016/j.concog.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Current opinion in neurobiology. 2009;19(6):666–671. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nature Reviews Neuroscience. 2010;11(6):417–28. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton J-L, Fogassi L, Gallese V. A Touching Sight: SII/PV Activation during the Observation and Experience of Touch. Neuron. 2004;42(2):335–346. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Mufson EJ. The insula of Reil in man and monkey. In: Peters A, Jones EG, editors. Cerebral Cortex. Plenum; New York: 1985. pp. 179–226. [Google Scholar]
- Morita T, Itakura S, Saito DN, Nakashita S, Harada T, Kochiyama T, Sadato N. The role of the right prefrontal cortex in self-evaluation of the face: A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20(2):342–355. doi: 10.1162/jocn.2008.20024. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Current Biology. 2010;20(8):750–6. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Qin P, Feinberg TE. Brain imaging of the self - Conceptual, anatomical and methodological issues. Consciousness and Cognition. 2011;20(1):52–63. doi: 10.1016/j.concog.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3(2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rossetti A, Miniussi C, Maravita A, Bolognini N. Visual perception of bodily interactions in the primary somatosensory cortex. The European Journal of Neuroscience. 2012;36(3):2317–23. doi: 10.1111/j.1460-9568.2012.08137.x. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Heinze H-J, Rotte M. Embodied empathy for tactile events: Interindividual differences and vicarious somatosensory responses during touch observation. NeuroImage. 2012;60(2):952–7. doi: 10.1016/j.neuroimage.2012.01.112. [DOI] [PubMed] [Google Scholar]
- Serino A, Giovagnoli G, Làdavas E. I feel what you feel if you are similar to me. PloS one. 2009;4(3):e4930. doi: 10.1371/journal.pone.0004930. (P. F. Ferrari, Ed.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino A, Pizzoferrato F, Làdavas E. Viewing a face (especially one’s own face) being touched enhances tactile perception on the face. Psychological Science. 2008;19(5):434–438. doi: 10.1111/j.1467-9280.2008.02105.x. [DOI] [PubMed] [Google Scholar]
- Sforza A, Bufalari I, Haggard P, Aglioti SM. My face in yours: Visuo-tactile facial stimulation influences sense of identity. Social Neuroscience. 2010;5(2):148–162. doi: 10.1080/17470910903205503. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Tajadura-Jimenez A, Grehl S, Tsakiris M. The other in me: Interpersonal multisensory stimulation changes the mental representation of the self. PloS one. 2012;7(7):e40682. doi: 10.1371/journal.pone.0040682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M. Looking for myself: current multisensory input alters self-face recognition. PloS one. 2008;3(12):e4040. doi: 10.1371/journal.pone.0004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia. 2010;48(3):703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: A sensory network for bodily self-consciousness. Cerebral Cortex. 2007;17(10):2235–44. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]