Abstract
BACKGROUND:
Pityriasis versicolor (PV) is a cutaneous pigmentation disorder caused by a lipophilic yeast of the genus Malassezia sp. It is a superficial mycosis characterized by well-defined, slightly scaly skin lesions of variable color. In Brazil, the number of reported cases is small, and there are few epidemiological studies.
OBJECTIVES:
to assess incidence, characteristics of the lesions, effectiveness of the Zileri's Sign procedure, and the epidemiological profile of PV in the urban area of Buerarema - Bahia.
METHODS:
Biological samples were collected on pre-established days at Basic Health Care Units from July to September 2010. Sample collection was followed by laboratory diagnosis using Porto's Method.
RESULTS:
Of the 158 patients with suspected PV participating in the study, 105 (66.5%) were positive; 72 (68.6%) were female and 33 (31.4%) were male. Sex and location of lesions showed statistically significant differences (p<0.05). The region with the highest rate of cases of PV was found to be the center of the city, with 40.9% of diagnosed cases. The most affected age group was between 10 and 19 years. There was a significant association between the results produced through Zileri's Sign and Porto's Method in relation to positive and negative results (p<0.05).
CONCLUSIONS:
The results showed a higher prevalence of PV among individuals at puberty. The Zileri's Sign method proved to be counterproductive, because it showed low efficacy as a method for clinical diagnosis, yielding negative results for 36 (34.3%) patients who had been diagnosed with PV through laboratory examination.
Keywords: Diagnosis, Epidemiology, Pityriasis, Tinea versicolor
Abstract
FUNDAMENTOS:
A pitiríase versicolor é um distúrbio de pigmentação cutânea causada pela levedura lipofílica do gênero Malassezia sp. É uma micose superficial caracterizada por produzir lesões delimitadas, com descamação fina e de cor variável. No Brasil, o relato do número de casos e de estudos é restrito.
OBJETIVOS:
Analisar a incidência, as características das lesões, a eficácia do Sinal de Zileri e o perfil epidemiológico da pitiríase versicolor na área urbana do município de Buerarema-BA.
MÉTODOS:
Foram coletadas amostras biológicas no período de julho a setembro de 2010, em dias pré-establecidos, nas Unidades Básicas de Saúde. O Método de Porto foi utilizado como diagnóstico laboratorial.
RESULTADOS:
Dos 158 pacientes cadastrados com suspeita de pitiríase versicolor, 105 (66,5%) mostraram-se positivos, sendo 72 (68,6%) do sexo feminino e 33 (31,4%) do sexo oposto. Ocorreu diferença estatisticamente significante entre sexo e localização das lesões (p<0,05). A região com maior índice de pitiríase versicolor foi detectada no centro do município com 40,9% dos casos diagnosticados. A faixa etária mais acometida foi entre 10 e 19 anos. Houve associação significante entre o resultado do Sinal de Zileri e do Método de Porto em relação aos resultados positivos e negativos (p<0,05).
CONCLUSÕES:
Frente aos resultados, observou-se maior prevalência da pitiríase versicolor em jovens no período da puberdade. O Sinal de Zileri mostrou-se contraproducente, pois não demonstrou eficácia como método de diagnóstico clínico ao gerar resultados negativos em 36 (34,3%) pacientes, cujas amostras ostentaram positividade no exame laboratorial.
INTRODUCTION
Pityriasis versicolor (PV) is a cutaneous pigmentation disorder caused by a lipophilic yeast of the genus Malassezia sp. It is a superficial mycosis that occurs worldwide, especially in tropical and subtropical regions. In temperate regions, a higher incidence of PV occurs during summer and autumn.1,2
PV was first recognized as a fungal disease in 1846 by Eichstedtand. Over a period of 160 years of scientific advances, several fungal species have been discovered as disease-causing microbes through morpho-physiological criteria.3,4 However, the terminology "Malassezia yeast species" has been retained for these lipophilic fungi that are part of the skin microbes.5
Seven species of the gender Malassezia are routinely known: M. furfur, M. pachydermatis, M. sympodialis, M. globosa, M. obtusa, M. restricta, and M. slooffiae. All but one of these species require external lipids for their development. M. pachydermatis is the only one that grows on routine mycological media without lipid supplementation. With application of molecular techniques, six new species have been identified: M. dermatis, M. japonica, M. yamatoensis, M. nana, M. caprae, and M. equine. 6-10
M. furfur was considered the main etiologic agent of PV for a long time. However, this changed during the last decade as research to further investigate the distribution of this fungus in affected humans revealed a predominance of other species such as M. globosa and M. sympodialis. 11-15
Fungal infection occurs in both sexes and in all races and can affect patients from infancy to old age. It is, however, more frequent in young, post-puberty adults, probably due to physiological changes in skin surface lipids during puberty.5,16 PV is also known as the "Beach Ringworm", because sun-exposed individuals reveal preexisting spots more clearly.17 The fungus affects the keratinized outer layers of the skin and scalp and is easily diagnosed and treated.1,2
PV-caused skin lesions are characterized by well-defined macules, with slight desquamation and color ranging from white to brownish and brown. Such lesions may spread and coalesce to cover large areas, thus compromising trunk, shoulders, upper limbs, neck, face, and flexural folds.5,18
Hyperpigmented skin patches occur due to an excessive increase in melanosome size and to changes in their distribution in the epidermis, giving the affected region a darker-than-normal skin color.19 Hypopigmented lesions, in turn, may result from inhibition of the enzyme dopa-tyrosinase by lipid fractions, because the fungus produces azelaic acid at the infected injury site, which inhibits tyrosinase, interfering with melanogenesis. 2,5
Clinical diagnosis of PV is based on the manifestation of scaly patches with limited or generalized distribution, which can be evidenced by stretching the skin - Zileri's sign (ZS). There is slight collapse of the keratin in this region, which facilitates observing fine desquamation.5,20
Pityriasis capitis (PC), in turn, is the most common clinical manifestation of lipophilic mycosis. It is generically known as dandruff and is often associated with colonization by Malassezia species. Some authors believe that PC is a smooth and non-inflammatory form of seborrheic dermatitis. Its clinical manifestations are achromic or hypopigmented, scaly macules of small size located in the region of the scalp.3,4,21,22
In Brazil, there are no official governmental figures on the prevalence of PV. Its notification to the Unified Health System is not compulsory, and control measures do not apply.23 However, scientific studies have been carried out in this line of research with the aim of helping to establish a distribution profile of PV in Brazil.
This is a pioneering study on the clinical and laboratory diagnosis of PV in public and private health care units in the municipality of Buerarema-BA. This study was conducted in order to describe the local epidemiological profile of the disease, analyze the characteristics of the lesions, investigate the relationship between pathology and use of cosmetics, and evaluate the effectiveness of ZS as a method for the clinical diagnosis of PV.
METHODS
Place of study
This work was performed in the municipality of Buerarema from July to September 2010. In addition to its center, the city has the following neighboring settlements: Cosme e Damião, Santo Antônio, São Bento, Santa Helena, and São Sebastião. Buerarema is located in the state of Bahia. It is about 450 km south of Salvador and has an urban population of about 17,000 inhabitants.24
Procedure
We decided to carry out a descriptive case series. A feature of the descriptive model is the use of standardized techniques for collecting data, such as questionnaires and systematic observation of groups.25 Samples were selected through non-probability judgmental sampling, according to the criteria outlined below.
The studied population was comprised of patients with suspected PV, approached by researchers and agents from basic health care units located in each neighborhood. Criteria for patient inclusion in the project were the following: individuals at any age, of any gender and race with dyschromic macules (hypo or hyperpigmented) and positive or negative results on the ZS test who were voluntarily participating in the project. Exclusion criteria were the following: patients with extensive desquamation, inflammation, wounds or abscesses associated with skin lesions, and characteristics that did not match PV.
The health care community agents were trained by researchers in courses of medical mycology aimed at enabling them to diagnose the first signs of the disease in patients from the community. Skin samples were collected at health care units or at strategic places (public schools). Patients considered positive for PV had samples of scales from their lesions collected by researchers on pre-established days.
Sample collection was performed in triplicate as recommended by Porto's Method. Samples were stained with methylene blue and then evaluated by optical microscopy (10 and 40 X) at the Laboratory of Clinical Research. 26
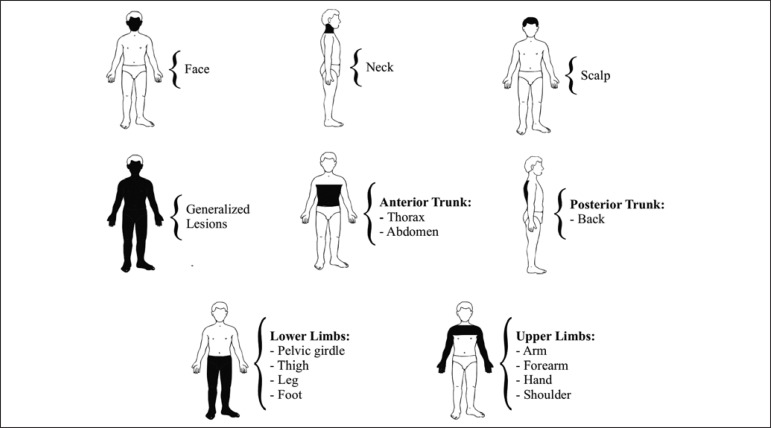
The human body was divided into eight anatomical regions to facilitate understanding the results related to location of the injuries caused by PV (Figure 1).
FIGURE 1.
Distribution of lesions according to anatomic location
Statistical analysis
Results were evaluated and plotted with the help of the statistical tool Microsoft Excel 2010(r), which was also used for constructing the graphs. We applied the Chi square test (χ2 test), where p < 0.05 was considered statistically significant. Vector illustrations were created by CorelDraw Graphics Suite X.
Benefit for the community
To ensure a return to the community of Buerarema, our analytical results, i.e., data of the direct mycological examination, were made available for free to the participating patients. The report included suggestions for further medical monitoring and treatment.
Ethical considerations
The study protocol and the Free and Informed Consent were approved by the Ethics and Research Committee of Maria Milza College - CEP/FAMAM. Protocol number 1409/10; approval received on 21/05/2010.
RESULTS
The project in the city and suburbs of Buerarema had 158 participating patients with suspected PV who met the inclusion criteria. Among these, 105 patients had a confirmed laboratory diagnosis of PV. The city center had the greatest incidence of PV, with 40.9% of diagnosed cases (Table 1).
TABLE 1.
Prevalence of PV in the six areas of the municipality of Buerarema-BA, 2010
| Locality | No. | % |
| TOTAL | 105 | 100 |
| Center | 43 | 40.9 |
| São Bento | 26 | 24.8 |
| Santa Helena | 25 | 23.8 |
| São Sebastião | 6 | 5.7 |
| Cosme e Damião | 3 | 2.9 |
| Santo Antônio | 2 | 1.9 |
Regarding sex, there was a predominance of female volunteers, with a gender distribution of 72 (68.6%) females and 33 (31.4%) males infected with PV. The most affected age group was that between 10 and 19 years, totaling 29.5% of cases (Table 2).
TABLE 2.
Distribution of cases according to sex and age
| Sex | No. | % |
| TOTAL | 105 | 100 |
| Male | 33 | 31.4 |
| Female | 72 | 68.6 |
| TOTAL | 105 | 100 |
| Age (years) | ||
| 0 – 9 | 9 | 8.6 |
| 10 – 19 | 31 | 29.5 |
| 20 – 29 | 14 | 13.3 |
| 30 – 39 | 14 | 13.3 |
| 40 – 49 | 17 | 16.2 |
| ≥ 50 | 20 | 19.1 |
According to physical examination, 93.3% of patients had hypochromic lesions, followed by hyper-pigmented lesions: brownish lesions with a frequency of 5.7% and pink lesions with 0.95% of cases. The anatomical region with the highest prevalence of fungus-caused injuries was the upper limbs, with 23 (21.9%) cases, followed by the anterior trunk, with 19 (18.0%) patients (Table 3).
TABLE 3.
Distribution of patients according to location of lesions in anatomical regions
| Location of lesions | No. | % |
| Upper Limbs | 23 | 21.90 |
| Anterior Trunk | 19 | 18.10 |
| Posterior Trunk | 17 | 16.19 |
| Lower Limbs | 17 | 16.19 |
| Generalized Lesions | 17 | 16.19 |
| Scalp | 6 | 5.71 |
| Face | 5 | 4.76 |
| Neck | 1 | 0.95 |
In the age group between 0 and 9 years, the injuries were most frequent on the face and were found in 5 (55.5%) children. In other 3 (33.3%) children, the lesions were found on the scalp. There was a statistically significant difference between sex and body location of the lesions (p < 0.05). While 22.2% of the female patients had a predominance of PV on the lower limbs, the opposite sex presented lesions on the upper limbs and anterior trunk, both locations with a prevalence of 24.2%.
The frequency of PC was higher in males, with 9.1% of cases, while the opposite sex had a frequency of 4.2%. Analyzing the age group of patients with PC, fungal infections were more frequent in children aged 0 to 9 years and in young adults between 20 and 29 years old.
An analysis of the use of cosmetics (moisturizer) revealed that 49.5% of patients did not make daily use of aesthetic products and that 50.5% reported using them.
Studying the condition of the patients' skin types, 47 (44.8%) volunteers reported having dehydrated skin, 27 (25.7%) reported having a tendency to the normal type, 20 (19.0%) indicated having the mixed type, and 11 (10.5%) patients reported seborrheic skin. It is noteworthy that out of the patients who presented dehydrated skin, 28 (59.6%) reported not using any kind of daily moisturizer. A statistical analysis of the data showed no significant difference between sex and skin type (p > 0.05).
There was a statistically significant association between the outcomes of clinical examinations (ZS) and laboratory tests (Porto's Method) in relation to positive or negative results (p < 0.05). It was noted that the ZS test showed to be effective in only 69 (65.7%) out of 105 positive patients.
Pruritus was observed in 27 (25.7%) patients, out of whom 24 (88.9%) had hypochromic lesions, and 03 (11.1%) had brownish lesions. Observing the relationship between pruritus and effectiveness of the ZS test, the clinical diagnosis used was effective in 19 (70.4%) patients. Making a correlation between pruritus and skin type, pruritus was more frequent in patients with dehydrated epidermis, being reported by 14 patients.
Of the 105 patients diagnosed with PV in the study, 47 (44.8%) volunteers mentioned that they had never had previous episodes of PV. On the other hand, 58 (55.2%) reported previous events (Table 4).
TABLE 4.
Distribution of patients according to previous episodes
| Preceding episodes | No. | % |
| Total | 58 | 100 |
| None | 47 | 44.8 |
| At least one | 58 | 55.2 |
| Total | 105 | 100 |
| One | 10 | 17.2 |
| Two | 28 | 48.3 |
| Three | 13 | 22.4 |
| 4 or more | 07 | 12.1 |
There was no statistically significant difference between patients with PV preceding the research and the use of cosmetics for the skin (p >0.05). Still analyzing the relationship between patients who had at least one previous episode and skin types, dehydrated epidermis corresponded to 37.5% (21) of the patients, followed by normal texture (25.0%), mixed skin (21.4%), and seborrheic skin (16.1%).
DISCUSSION
An analysis of the patients' slides on a microscope revealed presence of clusters of round or oval cells associated with short hyphae of bizarre forms, as described by Lacaz et al. based on the same diagnostic method (Porto's Method).26According to an analysis of the literature, the structures found while examining the slides are fungi of the genus Malassezia, which cause PV and PC. 4,5,26-28
As table 1 shows, the greatest number of cases of PV in the municipality of Buerarema is concentrated in the city center. It is noteworthy that the central area is geographically larger than the other areas investigated and has the highest number of patients diagnosed with PV. On the other hand, the lowest index was found in the neighborhood of Santo Antônio, because it has been created recently and has a few residents. The neighborhoods of São Sebastião and Cosme e Damião also had low rates of PV because they are small locations. The former has only three streets, and the second, despite having several thoroughfares, has been recently founded.
PV does not show sex predilection. The greater frequency of PV among women can be explained by the fact that this group seeks medical care more frequently than men and has the habit of using oily cosmetics for the skin. This could predispose the onset of fungus infections and the appearance of skin lesions.17
The occurrence of PV in young people going through puberty corroborates the results of a study conducted in Bangkok, where 178 (43.4%) patients aged between 12 and 21 years were diagnosed with the condition, as well as the results of another work conducted in southern Brazil.29,30 This fact can be explained by the increased activity of sebaceous glands during puberty, which is an important endogenous factor.17
The prevalence of hypochromic lesions found in our study is in agreement with other studies conducted in Brazil, Bangkok, and Spain.11,12,29 The different shades of injuries are related to an interference in the production of melanin caused by a fungus of the genus Malassezia. When the distribution of melanin in the skin becomes irregular, the spots appear.1
Prevalence of macules on the upper limbs of the human body corroborates the data presented in a study conducted in João Pessoa - PB, where 29.5% of patients had lesions in this anatomic region. According to the authors, it may be explained by the higher concentration of sebaceous glands in these places.17 On the other hand, these results disagree with those obtained in Venezuela, where the highest prevalence of PV was found on the anterior trunk of 49 patients, out of a total of 175 cases diagnosed with the condition.31
Considering the relationship between PV and PC, there was only a 38-year-old patient with a previous history of PV. In the study accomplished at the School of Medicine of the University of Zulia (Venezuela), 3 students with PC were diagnosed in a sample of 56 PV-affected students aged between 16 and 25 years.32
Considering daily use of cosmetics, some authors report that these products induce occlusion of the skin, thus favoring the development of lesions. It occurs because these products result in increased concentration of carbon dioxide in the epidermis, leading to changes in the microflora, lower pH, and the appearance of fungus.2,4,5,21,33
Prevalence of dehydrated skin (44.8%) among patients may be related to the climatic conditions at the time of collection of biological material (July to September). According to Magnoli et al., this is the time period in the southern hemisphere when the climate is characterized by cold temperatures, leading to dry air, which contributes to a dry epidermis.34
Comparing the diagnostic methods used, the ZS method showed low efficacy as a sole method for the clinical diagnosis of PV, yielding negative results in 36 (34.3%) patients whose biological samples tested positive in laboratory tests. According to Sidrim et al., the ZS should be used in association with a Wood's lamp for better results, and it should always be complemented by laboratory diagnosis.2
Pruritus showed higher frequency in patients with dehydrated skin, according to dermatological analyses conducted by researchers. Itching is most evident when the patient's lesions are exposed to high temperatures.35,36
The patients' history of previous episodes of PV may not only correspond to relapses, but also to treatment protocols that did not confirm PV through clinical and laboratorial diagnosis. Regardless of the therapeutic method employed, recurrence will be highly probable, for fungi of the genus Malassezia are part of the human microbiota.2
CONCLUSIONS
Although PV does not show gender preference, there was a higher frequency among women (68.3%). It is prevalent in young people at puberty. Most lesions were hypochromic (93.3%), with predominance in the region of the upper body (21.9%). It was noted that the ZS showed efficacy in only 69 (65.7%) of 105 positive patients.
For lack of detailed studies on PV in a municipality, there is need for continuing research to delineate the current profile of the dermatological problem in a community, since its notification to the Unified Health System is not compulsory and control measures do not apply.
Acknowledgments
We thank the nurses and community agents of the basic health care units of the city of Buerarema - BA.
Footnotes
* Work conducted at the Laboratory of Clinical Research - School of Pharmacy, Faculdade do Sul - Unime, Itabuna - Lomanto (BA), Brazil.
Financial support: none.
Conflict of interests: none.
REFERENCES
- 1.Murray PR, Rosenthal KS, Pfaller MA. Microbiologia médica. 5. ed. Rio de Janeiro: Elsevier; 2006. 979 p [Google Scholar]
- 2.Sidrim JJC, Moreira JLB. Fundamentos clínicos e laboratoriais da micologia médica. Rio de Janeiro: Guanabara Koogan; 1999. 287 p [Google Scholar]
- 3.Ashbee RA, Evans EGV. Immunology of Diseases Associated with Malassezia Species. Clin Microbiol Rev. 2002;15:21–57. doi: 10.1128/CMR.15.1.21-57.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desgarennes M del CP. Pitiriasis Versicolor. Dermatología Rev Mex. 2005;49:157–167. [Google Scholar]
- 5.Zaitz C, Ruiz LRB, Souza VM. Dermatoses associadas às leveduras do gênero malassezia. An Bras Dermatol. 2000;75:129–142. [Google Scholar]
- 6.Cabañes J, Theelen B, Castellá G, Boekhout T. Two new lipid dependent Malassezia species from domestic animals. FEMS Yeast Res. 2007;7:1064–1076. doi: 10.1111/j.1567-1364.2007.00217.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirai A, Jano R, Makimura K, Duarte ER, Hamdan JS, Lachance MA, et al. A Malassezia nana sp. nov., a novel lipid-dependent yeast species isolated from animals. Int J Syst Evol Microbiol. 2004;54:623–627. doi: 10.1099/ijs.0.02776-0. [DOI] [PubMed] [Google Scholar]
- 8.Sugita T, Takashima M, Shinoda M, Suto T, Unno H, Tsuboi R, et al. New yeast species, Malassezia dermatis, isolated from patients with atopic dermatitis. J Clin Microbiol. 2002;40:1363–1367. doi: 10.1128/JCM.40.4.1363-1367.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugita T, Takashima M, Kodama M, Tsuboi R, Nishikawa A. Description of a new yeast species, Malassezia japonica, and its detection in patients with atopic dermatitis and healthy subjects. J Clin Microbiol. 2003;41:4695–4699. doi: 10.1128/JCM.41.10.4695-4699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugita T, Tajima M, Takashima M, Amaya M, Saito M, Tsuboi R, et al. A new yeast, Malassezia yamatoensis, isolated from a patient with seborrheic dermatitis, and its distribution in patients and healthy subjects. Microbiol Immunol. 2004;48:579–583. doi: 10.1111/j.1348-0421.2004.tb03554.x. [DOI] [PubMed] [Google Scholar]
- 11.Aspiroz C, Ara M, Varea M, Rezusta A, Rubio C. Isolation of Malassezia globosa and M. sympodialis from patients with pityriasis versicolor in Spain. Mycopathologia. 2001;154:111–117. doi: 10.1023/a:1016020209891. [DOI] [PubMed] [Google Scholar]
- 12.Franil VM de S, Melhem MSC, Szeszs MW, Corneta EC, Zaitz C. Pitiríaseversicolor: isolamento e identificação das principais espécies de Malassezia. An Bras Dermatol. 2010;85:111–114. doi: 10.1590/s0365-05962010000100021. [DOI] [PubMed] [Google Scholar]
- 13.Gaitanis G, Velegraki A, Alexopoulos EC, Chasapi V, Tsigonia A, Katasambas A. Distribution of Malassezia species in pityriasis versicolor and seborrhoeic dermatitis in Greece. Typing of the major pityriasisversicolor isolate M. globosa. Br J Dermatol. 2006;154:854–859. doi: 10.1111/j.1365-2133.2005.07114.x. [DOI] [PubMed] [Google Scholar]
- 14.Giusiano G, Sosa M de los A, Rojas F, Vanacore ST, Mangiaterr M. Prevalence of Malassezia species in pityriasis versicolor lesions in northeast Argentina. Rev Iberoam Micol. 2010;27:71–74. doi: 10.1016/j.riam.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Prohic A, Ozagovic L. Malassezia species isolated from lesional and non-lesional skin in patients with pityriasis versicolor. Mycoses. 2007;50:58–63. doi: 10.1111/j.1439-0507.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 16.Miranda LG do A. Identificação de espécies de Malassezia em pacientes com pitiríase versicolor atendidos no ambulatório de dermatologia Came-Primavera em João Pessoa-PB. Recife, (PE): Universidade Federal de Pernambuco; 2004. 92f [Google Scholar]
- 17.Trabulsi LR, Alterthum F. Microbiologia. 5. ed. São Paulo: Atheneu; 2008. 760p [Google Scholar]
- 18.Lima EO, Belém LF, Cechinel FV, Corrêa R, Nunes RJ, Andricopulo A, et al. Avaliação da Sensibilidade de Cepas de Malasseziafurfur a Imidas Cíclicas. Rev Bras Cienc Farm. 2002;38:443–450. [Google Scholar]
- 19.Zaitz C. Compêndio de micologia médica. 2. ed. Rio de Janeiro: Guanabara Koogan; 2010. [Google Scholar]
- 20.Oliveira JR, Mazocco VT, Steiner D. Pitiríase Versicolor. An Bras Dermatol. 2002;77:611–618. [Google Scholar]
- 21.Danby FW, Maddin WS, Margesson LJ, Rosenthal AD. A randomized, double-blind, placebo-controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2.5% shampoo in the treatment of moderate to severe dandruff. J Am Acad Dermatol. 1993;29:1008–1012. doi: 10.1016/0190-9622(93)70282-x. [DOI] [PubMed] [Google Scholar]
- 22.Cunha AR, Silva RS, Chorilli M. Desenvolvimento e avaliação da estabilidade física de formulações de xampu anticaspa acrescidas ou não de extratos aquosos de hipérico, funcho e gengibre. Rev Bras Farm. 2009;90:190–195. [Google Scholar]
- 23.BRASIL. Ministério da Saúde. Secretaria de Políticas de Saúde. Departamento de Atenção Básica . Dermatologia na atenção básica de saúde. Brasília: Ministério da Saúde; 2002. 142p. (Série Cadernos de Atenção Básica). n. 09. Série A. Normas e Manuais Técnicos; n. 174. [Google Scholar]
- 24.Governo do Estado da Bahia. Secretaria do Planejamento. Superintendência de Estudos Econômicos e Sociais da Bahia . Estatísticas dos Municípios Baianos. II. Salvador: SEI; 2009. [Google Scholar]
- 25.Pereira MG. Epidemiologia teoria e prática. Rio de Janeiro: Guanabara Koogan; 2008. [Google Scholar]
- 26.Lacaz CS, Porto E, Martins JEC. Tratado de micologia médica. 9. São Paulo: Sarvier; 2002. 1104p [Google Scholar]
- 27.Ghahfarokhi MS, Abyaneh MR. Rapid Identification of Malassezia furfur from other Malassezia Species: A Major Causative Agent of PityriasisVersicolor. Iran J Med Sci. 2004;29:36–39. [Google Scholar]
- 28.Moura R A, Wada CS, Purchio A, Almeida TV. Técnicas de laboratório. 3 ed. São Paulo: Atheneu; 2008. [Google Scholar]
- 29.Imwidthaya P, Thianprasit M, Srimuang S. A studyofpityriasis versicolor in Bangkok (Thailand) Mycopathologia. 1989;105:157–161. doi: 10.1007/BF00437248. [DOI] [PubMed] [Google Scholar]
- 30.Petty V, Tanhausen F, Weiss L, Milan T, Mezzari A, Weber MB. Identification of Malassezia yeast species isolated from patients with pityriasisversicolor. An Bras Dermatol. 2011;86:803–806. doi: 10.1590/s0365-05962011000400032. [DOI] [PubMed] [Google Scholar]
- 31.Borelli D. Pitiriasis versicolor por Malasseziaovalis. Mycopathologia. 1985;89:147–153. doi: 10.1007/BF00447023. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Valero S, Mesa LM, Gonzalez-Morán E, Delmonte ML, Robertiz S, Valero A. Phenotypic characterization of species of Malassezia in healthy skin of a university student population. Invest Clin. 2005;46:329–335. [PubMed] [Google Scholar]
- 33.Padilla C, Acar MR, Castillo DM, Zambrano SCG, Espada LM, Garibay AR. Pitiriasis versicolor: Presentación de tres casos. Rev Cent Dermatol Pascua. 2004;13:49–55. [Google Scholar]
- 34.Magnoli D, Araújo R. A nova geografia: Estudos de geografia geral. 2. São Paulo: Editora Moderna; 1995. [Google Scholar]
- 35.Moore M. Cultivation of Malassezia furfur, Etiological Agent of Pityriasis (Tinea) Versicolor. Mycopathologia. 1938;1:53–61. [Google Scholar]
- 36.Rai MK, Wankhade S. Tinea versicolor: Anepidemiology. J Microbial BiochemTechnol. 2009;1:51–56. [Google Scholar]