Abstract
Kaposi's sarcoma is the most common neoplasia diagnosed in AIDS patients and the expression of the human herpesvirus-8 (HHV-8) latent nuclear antigen-1 has been useful for its histological diagnosis. The aim of this study is to confirm that immunohistochemistry is a valuable tool for differentiating KS from its simulators in skin biopsies of HIV patients. Immunohistochemical and histological analyses were performed in 49 Kaposi's sarcoma skin biopsies and 60 of its histological simulators. Positivity was present in the 49 Kaposi's sarcoma skin biopsies and no staining was observed in the 60 simulators analyzed, resulting in sensibility and specificity of 100%. HHV-8 immunohistochemical detection is an effective tool for diagnosing Kaposi's sarcoma, especially in early lesions in which neoplastic features are not evident. It also contributes to its histological differential diagnosis.
Keywords: Herpesvirus-8, human; Immunohistochemistry; Sarcoma, Kaposi
Abstract
O sarcoma de Kaposi é a neoplasia mais diagnosticada em pacientes com SIDA e a expressão do antígeno nuclear latente-1 do herpesvírus humano tipo-8 (HHV-8) tem se mostrado útil no seu diagnóstico histológico. O objetivo deste estudo é confirmar que o método imuno-histoquímico é uma ferramenta útil para diferenciar o sarcoma de Kaposi cutâneo de seus simuladores histológicos em pacientes HIV positivos. Análise histológica e imuno-histoquímica foram realizadas em 49 casos de sarcoma de Kaposi cutâneo e 60 casos de seus simuladores histológicos. Positividade à imuno-histoquímica para o antígeno nuclear latente 1 do HHV-8 foi observada nos 49 casos de sarcoma de Kaposi e nenhuma reação foi detectada nos 60 simuladores analisados, resultando em 100% de sensibilidade e especificidade. A detecção do HHV-8 por imuno-histoquímica é uma ferramenta útil para o diagnóstico de sarcoma de Kaposi, especialmente na lesão inicial cujo caráter neoplásico não é evidente, e contribui para seu diagnóstico diferencial histológico.
INTRODUCTION
Kaposi's sarcoma (KS) is an endothelial neoplasia that is found typically in cutaneous lesions whose development stages entail macules, plaques and nodules, and may also involve lymph nodes and viscera. It has a more aggressive form when associated to AIDS and despite the reduction of its occurrence with the introduction of Highly Active Antiretroviral Therapy (HAART), it remains the most common neoplasia associated to AIDS. It is the only malignancy included as a defining element of AIDS by the Centre for Disease Control (CDC).1,2
Its viral etiology was confirmed with the identification of the Kaposi Sarcoma associated herpesvirus (KSHV) or Human Herpes Virus Type-8 (HHV-8). This virus has been found in KS cells in all forms of the disease, regardless of the clinical stage of the lesions.3-6
The histopathological spectrum for Cutaneous KS varies with the clinical stage of the lesions, and for this reason the differential diagnosis includes the range of diseases that histologically simulate it, leading to difficulty in diagnosis. These lesions range from inflammatory conditions to malignant tumors.1,7-14
The immunohistochemistry technique using the antibody anti-latent nuclear antigen -1 (LNA-1) of the HHV-8 has been found to be a reliable and cost-effective method to detect the presence of the virus in the tumor cells and to differentiate KS from its histological simulators, when compared to molecular biology methods.15 On the other hand, the literature shows conflicting results, probably related to the different steps of IHC reactions. In our study, we apply IHC to demonstrate the sensibility of this technique in the diagnosis of cutaneous KS in our cohort and to differentiate it from its histological simulators.
METHODS
Design and outline of the study
This is a descriptive study involving analyzing skin biopsies diagnosed as KS. The biopsy samples were taken from HIV infected patients followed in IPEC from 2003 to 2010. The skin biopsies corresponded to 44 male patients and 5 females with ages from 25 to 46 years old. The study was approved by the IPEC Research Ethics Committee under procedure No. 0072.0.009.000-09.
Selection of the sample
Study materials
49 cases diagnosed as with cutaneous Kaposi's sarcoma were included, as well as 60 cases of lesions that simulate it microscopically. Among the lesions that simulate Kaposi's sarcoma described in literature we found the following in our records: 12 dermatofibromas, 17 hemangiomas, 8 pyogenic granulomas and 23 cases of stasis dermatitis.
Histopathological analysis
All the cases identified and included in this study were submitted to histological review by two pathologists for diagnosis confirmation. The classical criteria as found in the literature were applied to recognize this neoplasia.16
Immunohistochemical method
The histological cuts were placed in an autoclave at 56ºC for 20 minutes. After that we conducted a deparaffinization in xylol, in two 20-minute stages with later re-hydration in alcohol solutions and water. The activity of the endogenous peroxidase was blocked with a solution of 55ml methanol and 45ml perhydrol (hydrogen peroxide) at 13%. Antigenic recovery was obtained with a citrate 10mM, pH6 tampon, in a pressure cooker for 3.5 minutes.
The non-specific bonds were blocked with Blocking Protein (Novocastra, Leica Biosystem New Castle) for 30 minutes. After that, the slides were incubated, adding the lyophilized monoclonal human herpesvirus (type 8) (latent nuclear antigen) - NCLHHV8-LNA (Novocastra, Newcastle-upon-Tyne, UK) in the 1/200 dilution for 16 hours overnight at 4ºC. The Post Primary Block (Novocastra, Leica Biosystem New Castle) was then added for 30 minutes and the Polymer (Novolink Polymer Ref 7112) was incubated for an additional 30 minutes.
The slides were developed with Diaminobenzidine - DAB (DakoCytomation Ref S3000, Carpinteria, CA, USA) and counter-stained with Harris's hematoxylin. The brown color, diffused or dotted, was considered positive, as found in the nuclei of the lesion's cells.
In between incubations the slides were washed in TBS - Tris Buffered Solution (Wash Buffer 10x, DakoCytomation Ref S3006, Carpinteria, CA, USA) tampon for 5 minutes.
A case of lymph node KS was used as positive control.
RESULTS
Review of the histological aspects of the 49 fragments of skin with the diagnosis of Kaposi's sarcoma showed correlation with the clinical stage of disease.
Early lesions (maculae) displayed increased interstitial cellularity and vascular proliferation consisting of crevasse-like spaces between the collagen fibers and/or around the adnexes; the more advanced lesions, with a nodular (nodule) histological architecture displayed all the alterations, in exuberance, associated to the higher proliferation of fusiform cells with occasional mitosis figures. The plaques displayed alterations of intermediate intensity, between macule and nodule. Other histological changes included: vessels surrounding the adnexes and in between collagen fibers, inflammatory infiltration consisting mainly of lymphocytes and plasma cells, old hemorrhage, presence of promontory and hyaline globules.16
Similar to the KS, the 12 dermatofibroma cases had a nodular conformation resulting from the proliferation of fusiform cells with atypical trend. The 17 hemangiomas studied had vascular channels, sometimes anastomosing, lined by round or elongated endothelium cells. The pyogenic granulomas were nodular proliferations of capillaries with an ulcerated surface, followed by an accentuated mixed inflammatory infiltration. The 23 cases of stasis dermatitis displayed vascular proliferation on the dermis, with varying degrees of fibrosis, inflammatory infiltration consisting of lymphocytes, plasma cells and histiocytes, red cell extravasation and the presence of free hemosiderin or within histiocytes.
Positive nuclear reaction to HHV-8 was detected in the lesional areas in all cases of KS (Figures 1 and 2). The endothelium cells of the normal vessels were negative. Negative reaction was observed in all of its histological simulators analyzed. Immuno-histochemical positive nuclear reaction was observed in all lymph node KS slides used as controls.
FIGURE 1.
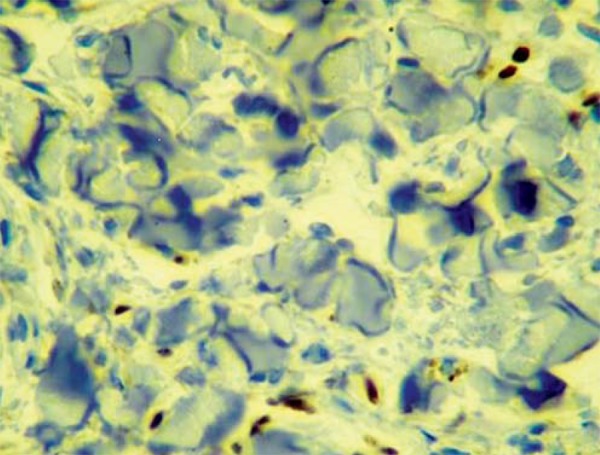
Immunohistochemistry - Inicial KS lesion. Positivity for the HHV-8-LNA-1 antigen as observed in endothelium cells is initial KS lesion. (IHQ, 40x)
FIGURE 2.
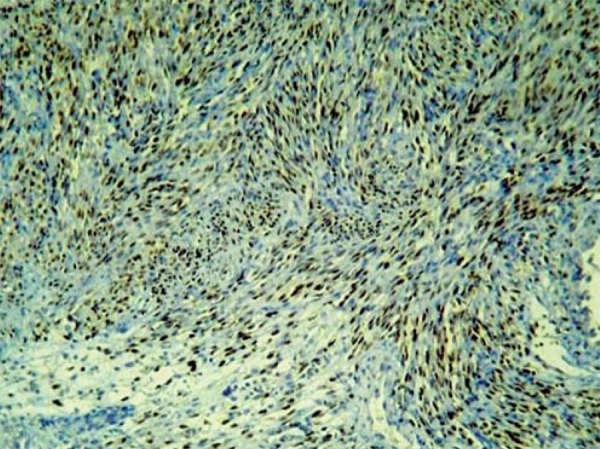
Immuno histochemistry - Advanced KS lesion. Positivity for the HHV-8-LNA-1 antigen as observed in fusiform and endothelium cells in advanced KS lesion. (IHQ, 40x)
DISCUSSION
The distinction between KS and a series of benign and malignant tumors, as well as inflammatory conditions, can be difficult due to the superimposing of the histological changes, generating diagnostic doubt. The discovery of the HHV-8 in all the forms of KS allowed the use of viral detection to diagnose this tumor. The use of the monoclonal anti-HHV8-LNA-1 antibody to identify the HHV-8 in fixed tissue is an efficient and low-cost way to differentiate KS from its histological simulators when compared to the molecular methods traditionally used. 6,17,18
We found that the detection of the HHV-8 was particularly useful in the diagnosis of the initial KS lesions in which the neoplastic alterations are not immediately revealed. In these cases, inflammatory processes are the main differential diagnosis to be considered, stasis dermatitis being the most important. Other histopathological alterations such as ulceration, lymphedema and secondary infections can change the histopathology that is characteristic of KS, rendering the viral detection crucial for the diagnosis.
Some histopathological variants of KS offer additional diagnostic difficulty. Pyogenic granuloma-like KS is marked by the proliferation of well-formed capillaries organized in lobules with an epidermal collarette, with no solid aggregate of fusiform cells, although the positivity of the HHV-8 provides the diagnosis in cases where doubt may exist.19 The value of IHC is also seen in superficial biopsies of ulcerated KS, which display an inflamed granulation tissue with the imprisoning of positive fusiform HHV-8 cells. Angiomatous areas of KS lesions may be confused with hemangiomas in superficial biopsies and the anaplastic KS can be confused with angiosarcoma for its capability to invade the subcutaneous tissue. Solid aggregates of fusiform cells may mask the vascular nature of KS, simulating benign and malignant neoplasias such as dermatofibroma and leiomyosarcoma respectively.1
The technique applied to KS histological simulators resulted in negative reaction in all cases. The study done by Ryan et al (2002) describes the detection of HHV-8 in two cases of pyogenic granuloma although this result was not reproduced in later studies, including ours, in which the eight cases of pyogenic granuloma were negative for this antigen.20
The results of our study are aligned with most of the studies related to the use of IHC technique in the detection of the HHV-8 to diagnosis Kaposi's sarcoma in doubtful cases. The use of the monoclonal anti-HHV8-LNA-1 antibody produced 100% sensitivity and specificity when compared to hematoxilin and eosin alone, confirming that the use of this antibody defines the diagnosis of cutaneous KS in all stages of the disease. Therefore, the use of IHC technique to detect the viral antigen has allowed pathologists to diagnose Kaposi's sarcoma with a greater degree of confidence.
Our results confirm that the detection of HHV8 is a valuable tool for diagnosing cutaneous lesions of KS and to differentiate it from its simulators.
Footnotes
* Study conducted at Evandro Chagas Clinical Research Institute - Oswaldo Cruz Foundation (IPEC-FIOCRUZ) - Manguinhos (RJ), Brazil.
Financial support: Oswaldo Cruz Foundation
Conflict of interests: Study approved by the IPEC Research Ethics Committee (No. 0072.0.009.000-09).
REFERENCES
- 1.Ramdial PK. Dermatopathological challenges in the human immunodeficiency virus and acquired immunodeficiency syndrome era. Histopathology. 2010;56:39–56. doi: 10.1111/j.1365-2559.2009.03456.x. [DOI] [PubMed] [Google Scholar]
- 2.Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027–1038. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein WB, Little RF, Wilson WH, Yarchoan R. Acquired immunodeficiency syndrome-related malignancies in the era of highly active antiretroviral therapy. Int J Hematol. 2006;84:3–11. doi: 10.1532/IJH97.06088. [DOI] [PubMed] [Google Scholar]
- 4.Leão JC, Hinrichsen SL, Freitas BL, Porter SR. Herpes virus humano-8 and Kaposi's sarcoma. Rev Ass Med Brasil. 1999;45:55–62. doi: 10.1590/s0104-42301999000100011. [DOI] [PubMed] [Google Scholar]
- 5.Mesri EA, Cesarman E, Boshoff C. Kaposi's sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456–460. doi: 10.1038/modpathol.3800061. [DOI] [PubMed] [Google Scholar]
- 7.Kempf W, Cathomas G, Burg G, Trueb RM. Micronodular Kaposi's sarcoma - the new variant of classic-sporadic Kaposi's sarcoma. Dermatology. 2004;208:255–258. doi: 10.1159/000077313. [DOI] [PubMed] [Google Scholar]
- 8.Nickoloff BJ, Griffiths CE. The spindle-shaped cells in cutaneous Kaposi's sarcoma. Histologic simulators include factor XIIIa dermal dendrocytes. Am J Pathol. 1989;135:793–800. [PMC free article] [PubMed] [Google Scholar]
- 9.The'Donnell PJ, Pantanowitz L, Grayson W. Unique histologic variants of cutaneous Kaposi sarcoma. Am J Dermatopathol. 2010;32:244–250. doi: 10.1097/DAD.0b013e3181b7f6a7. [DOI] [PubMed] [Google Scholar]
- 10.Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190. doi: 10.1186/1471-2407-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantanowitz L, Mullen J, Dezube BJ. Primary Kaposi sarcoma of the subcutaneous tissue. World J Surg Oncol. 2008;6:94. doi: 10.1186/1477-7819-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouhani P, Fletcher CD, Devesa SS, Toro JR. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: An analysis of 12,114 cases. Cancer. 2008;113:616–627. doi: 10.1002/cncr.23571. [DOI] [PubMed] [Google Scholar]
- 13.Weedon D, Strutton G, Rubin AI. Weedon D. Weedon´s Skin Pathology. Philadelphia: Churchill Livingstone Elsevier; 2010. Vascular Tumors; pp. 913–918. [Google Scholar]
- 14.Yu Y, Demierre MF, Mahalingam M. Anaplastic Kaposi's sarcoma: an uncommon histologic phenotype with an aggressive clinical course. J Cutan Pathol. 2009;37:1088–1091. doi: 10.1111/j.1600-0560.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-da-Silva S, Elgui-de-Oliveira D, Borges L, Bacchi CE. Kaposi's sarcoma-associated herpesvirus infection and Kaposi's sarcoma in Brazil. Braz J Med Biol Res. 2006;39:573–580. doi: 10.1590/s0100-879x2006000500002. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher CD, Unni KK, Mertens F, editors. World Health Organization. Classification of Tumours. Lyon: IARC; 2002. [Google Scholar]
- 17.Robin YM, Guillou L, Michels JJ, Coindre JM. Human herpesvirus 8 immunostaining: the sensitive and specific method for diagnosing Kaposi sarcoma in paraffin-embedded sections. Am J Clin Pathol. 2004;121:330–334. doi: 10.1309/96U1-6LRR-AN5H-WWVE. [DOI] [PubMed] [Google Scholar]
- 18.Horenstein MG, Moontasri NJ, Cesarman E. The pathobiology of Kaposi's sarcoma: advances since the onset of the AIDS epidemic: advances since the onset of the AIDS epidemic. J Cutan Pathol. 2008;35(Suppl 2):40–44. doi: 10.1111/j.1600-0560.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 19.Pantanowitz L, Otis CN, Dezube BJ. Immunohistochemistry in Kaposi's sarcoma. Clin Exp Dermatol. 2009;35:68–72. doi: 10.1111/j.1365-2230.2009.03707.x. [DOI] [PubMed] [Google Scholar]
- 20.Ryan P, Aarons S, Murray D, Markham T, O'Sullivan S, Lyons F, et al. Human herpesvirus 8 (HHV-8) detected in two patients with Kaposi's sarcoma-like pyogenic granuloma. J Clin Pathol. 2002;55:619–622. doi: 10.1136/jcp.55.8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]


