Abstract
CDDO-Me, an oleanane synthetic triterpenoid has shown strong antitumorigeic activity towards diverse cancer cell types including colorectal cancer cells. In the present study, we investigated the role of free radicals in the growth inhibitory and apoptosis-inducing activity of CDDO-Me in colorectal cancer cells lines. Results demonstrated that CDDO-Me potently inhibited the growth of colorectal cancer cells and pretreatment of cancer cells with small-molecule antioxidant N-acetylcysteine (NAC) completely blocked the growth inhibitory activity of CDDO-Me. CDDO-Me caused the generation of reactive oxygen species, which was inhibited by NAC and mitochondrial chain 1 complex inhibitors DPI and rotenone. CDDO-Me induced apoptosis as demonstrated by the cleavage of PARP-1, activation of procaspases -3, -8, and -9 and mitochondrial depolarization and NAC blocked the activation of these apoptosis related processes. Furthermore, induction of apoptosis by CDDO-Me was associated with the inhibition of antiapoptotic/prosurvival Akt, mTOR and NF-κB signaling proteins and the inhibition of these signaling molecules was blocked by NAC. Together these studies provided evidence that CDDO-Me is a potent anticancer agent, which imparts growth inhibition and apoptosis in colorectal cancer cells through the generation of free radicals.
Keywords: CDDO-Me, colorectal cancer, ROS, apoptosis, signaling proteins
INTRODUCTION
Colorectal cancer is the fourth most common cancer in men and women in the United States. It is more common in people over 50, and the risk increases with age. High-fat diet, family history of colorectal cancer and inactivation of adenomatous polyposis coli (apc) gene, a tumor suppressor gene, are some of the risk factors for colon cancer (1–3). The mutation or deletion apc gene results in the development of adenomatous polyps, which are considered precursor lesions of colonic carcinomas (3–5). Current therapies (surgery, chemotherapy and radiotherapy) often fail to control more advanced metastatic colorectal cancer. Since the incidence of colon cancer increases with advancing age, this malignancy is expected to become an increasingly greater health problem as life expectancy improves. Thus, novel strategies are needed to prevent and treat colorectal cancer.
Aberration of apoptosis has been implicated in malignant transformations and resistance of tumors to conventional cancer therapies (6). In deed, apoptosis has been implicated in preventing the development and progression of premalignant colonic epithelial cells to colon tumors (7,8). Thus, promotion of apoptosis in colon cancer cells with novel agents could lead to tumor regression and improved prognosis.
Synthetic triterpenoids derived from oleanolic acid (CDDO, CDDO-Im and CDDO-Me) have shown potent antiproliferative and antitumorigenic activity against diverse types of tumor cell lines, including leukemia, multiple myeloma, osteosarcoma, breast, brain, prostate and lung cancer cells in culture (9–13). CDDOs have also shown strong chemopreventive activity in animal models of liver, breast and lung cancer (14,15). Although the mechanisms of the anticancer effects of CDDOs are not fully understood, cancer cell differentiation, induction of apoptosis and modulation of MAPK (Erk1/2), NF-κB, TGF-β/Smad and PPARγ signaling pathways contribute to the antitumor activity of CDDOs (16–19). In a previous study, we demonstrated the strong growth inhibitory and apoptosis inducing activity of CDDO-Me in several human colon cancer cell lines through the inhibition of prosurvival Akt, NF-κB and mammalian target of rapamycin (mTOR) signaling proteins (20). Since most anticancer agents act, at least in part, by inducing reactive oxygen species (ROS) (21–23), we investigated whether ROS generation plays a role in the antitumorigenic effects of CDDO-Me in colon cancer cells. Our results demonstrate that CDDO-Me induces intracellular ROS production in colorectal cancer cells and inhibition of ROS generation reverses growth inhibition, apoptosis and prevent down-regulation of prosurvival Akt, NF-κB and mTOR signaling proteins.
MATERIALS AND METHODS
Reagents
CDDO-Me was obtained from the National Cancer Institute, Bethesda, MD, through the Rapid Access to Intervention Development Program. Antibodies against p-Akt (ser473), NF-κB (p65), p-mTOR (Ser2448), procaspase3,-8,-9 and PARP-1 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). H2DCF-DA oxidative fluorescent probe was from Molecular Probes (Eugene, OR). Annexin V-FITC apoptosis detection kit II was obtained from BD Pharmingen (San Diego, CA). Rotenone and diphenylene iodonium (DPI) were purchased from Sigma Chemical Inc. (St. Louis. MO). 96 AQueous One Solution Proliferation Assay System was purchased from Promega (Madison, WI).
A 100 mM solution of CDDOs was prepared in DMSO and all test concentrations were prepared by diluting the stock solution in tissue culture medium.
Cell lines and cell culture
HCT 8, HCT-15, HT-29 and Colo 205 human colorectal cancer cell lines were obtained from the American Type Culture Collection (ATCC), Rockville, MD. HT-29 cells were grown in modified McCoy 5α culture medium (ATCC Cat #30-2007), whereas HCT8, HCT-15 and Colo 205 cells were grown in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Hyclone, Logan, UT), 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate. All cell lines were cultured at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air, and maintained by subculturing cells twice a week.
Measurement of cell viability (MTS assay)
Cells (1 ×104) were seeded into each well of a 96-well plate in 100 μl of tissue culture medium. After 24 h incubation to allow cells to adhere, cells were treated with CDDO-Me at concentrations shown in individual experiments for 72 h. Cell viability was then determined by the colorimetric MTS assay using CellTiter 96 AQueous One Solution Proliferation Assay System from Promega (Madison, WI) following instructions provided by the vendor.
Annexin V-FITC binding
Induction of apoptosis was assessed by the binding of annexin V-FITC to phosphotidylserine, which is externalized to the outer leaflet of the plasma membrane early during induction of apoptosis. Briefly, cells treated with CDDO-Me for 24 h were resuspended in the binding buffer provided in the annexin V-FITC apoptosis detection kit II (BD Biosciences, Pharmingen). Cells were reacted with 5 μl of annexin V-FITC reagent and 5 μl of propidium iodide (PI) for 30 min at room temperature in the dark. Stained cells were analyzed by flow cytometry.
Measurement of ROS
H2DCF-DA fluorescent probe was used to measure the generation of intracellular hydrogen peroxide (H2O2). Briefly, 1 ×106 tumor cells were plated in 6-well plates and allowed to attach overnight. Cells were treated or not with CDDO-Me for 24 h and then reacted with 5 μM of H2DCF-DA for 30 min at 37°C. Cells were collected by trypsinization and DCF-DA fluorescence was analyzed by flow cytometry.
Western blotting
Total cellular proteins were isolated by detergent lysis (1% Triton-X 100 (v/v), 10 mM Tris-HCl (pH 7.5), 5 mM EDTA, 150 mM NaCl, 10% glycerol, 2 mM sodium vanadate, 5 μg/mL leupeptin, 1 μg/mL aprotinin, 1 μg/mL pepstatinin, and 10 μg/mL 4-2-aminoethyl-benzenesulfinyl fluoride). Lysates were clarified by centrifugation at 14,000 ×g for 10 min at 4°C, and protein concentrations were determined by Bradford assay. Samples (50 μg) were boiled in an equal volume of sample buffer (20% glycerol, 4% SDS, 0.2% Bromophenol Blue, 125 mM Tris-HCl (ph 7.5), and 640 mM 2-mercaptoethanol) and separated on 10–14% SDS-polyacrilamide gels. Proteins resolved on the gels were transferred to nitrocellulose membranes. Membranes were blocked with 5% milk in 10 mM Tris-HCl (pH 8.0), 150 mM NaCl with 0.05% Tween 20 (TPBS) and probed with protein specific antibodies to PARP-1, procaspases 3, 8 or 9, p-Akt, p-mTOR, NF-κB or β-actin. The protein bands were visualized by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
Mitochondrial depolarization assay
Loss of mitochondrial potential by CDDO-Me was determined using mitochondrial potential sensor JC-1 (Molecular Probes, Invitrogen, San Diego, CA). 1 ×106 control (untreated) and CDDO-Me treated cells in 1 ml culture medium were loaded with mitochondrial sensor JC-1 dye (10 μg/ml) for 10 minutes at 22°C and analyzed by flow cytometry. In normal cells, dye is aggregated in mitochondria, fluoresces red, and is detected in the FL2 channel. In cells with altered mitochondrial potential, the dye fails to accumulate in the mitochondria, remains as monomers in the cytoplasm, fluoresces green, and is detected in the FL1 channel.
RESULTS
NAC blocks the growth inhibitory activity of CDDO-Me
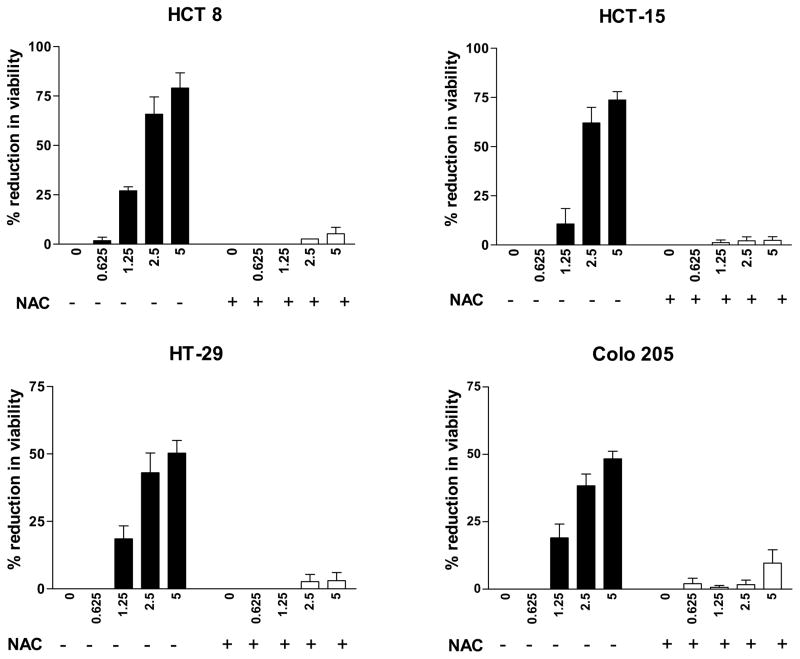
To evaluate the role of ROS in the growth inhibitory activity of CDDO-Me in colon cancer cells, we investigated whether N-acetylcysteine (NAC), a small-molecule antioxidant, will block the growth inhibitory activity of CDDO-Me on colorectal cancer cells. For this purpose, HCT-15, HCT 8, HT-29 or Colo 205 cells were plated in 96-well microtiter plate and pre-treated or not with NAC for 2 h before treating with CDDO-Me (0.625 to 5 μM) for 72 h. The viability of cell cultures was determined by MTS assay. As shown in Figure 1, CDDO-Me inhibited the growth of each of the cell line in a dose-dependent manner. Significant growth inhibition was observed at concentrations of 2.5–5 μM (50% to 78% inhibition). Measurable growth inhibition also occurred at 1.25 μM CDDO-Me. Overall, HCT-15 and HCT-8 cells were more sensitive to CDDO-Me than HT-29 and Colo 205 cells. In contrast, pre-treatment with NAC completely protected each of the cell lines from the growth inhibitory effect of CDDO-Me. The prevention of growth inhibition by NAC indicated that ROS generation plays an essential role in the growth inhibitory activity of CDDO-Me.
Figure 1. NAC blocks the growth inhibitory effect of CDDO-Me on colorectal cancer cells.
1 × 104 colon cancer cells (HCT 8, HCT-15, HT-29 or Colo 205) were seeded in each well of a microtiter plate in 0.1 ml of culture medium. After allowing cells to adhere for 24 h, they were treated or not with NAC (5 mM) for 2 h before treating with CDDO-Me (0 to 5 μM) in triplicates for 72 h. Cell viability was measured by MTS assay using CellTiter AQueous assay system from Promega. Similar results were obtained in 3 independent experiments.
CDDO-Me induces ROS generation in colorectal cancer cells
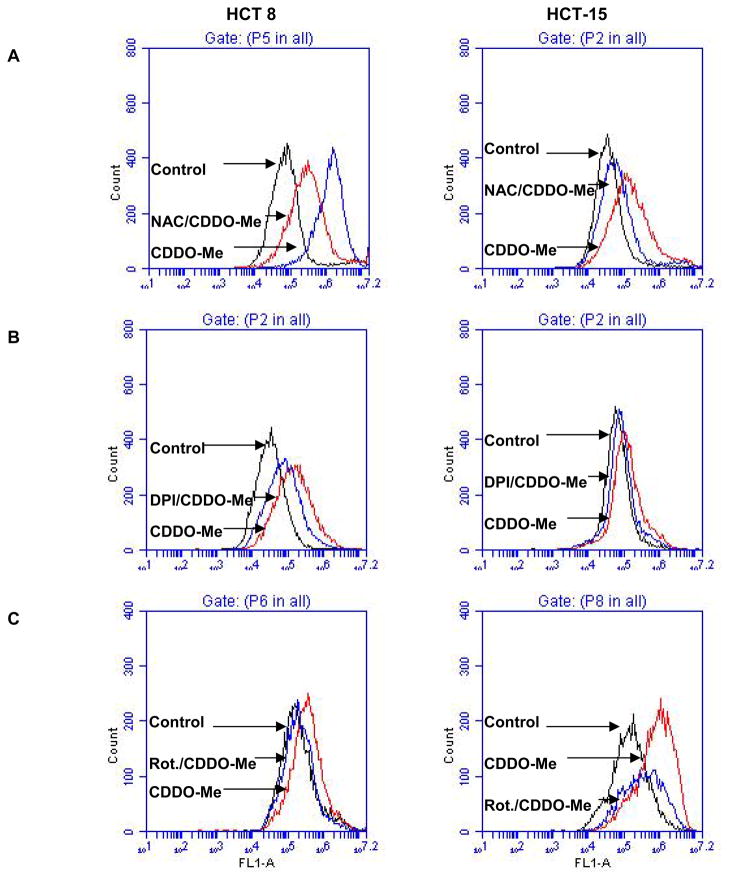
To further investigate whether generation of intracellular ROS is part of the mechanism by which CDDO-Me inhibits growth and induce apoptosis in colon cancer cells, we first measured the generation of free radicals in cancer cells treated with CDDO-Me. The generation of ROS by CDDO-Me was assessed by using fluorescent probe H2DCFDA that detects intracellular H2O2. HCT8 and HCT-15 cells were treated with CDDO-Me in the presence or absence of antioxidant NAC and reacted with H2DCFDA. Treatment with CDDO-Me resulted in 3.6-fold (HCT8) and 5.5-fold (HCT-15) increase in DCF fluorescence intensity compared to vehicle-treated control cells (Fig. 2A). On the other hand, pretreatment of cells with antioxidant NAC significantly reduced the CDDO-Me induced increase in DCF fluorescence in both cell lines (HCT-8 = 1.4-fold; HCT-15 = 2.8-fold).
Figure 2. CDDO-Me induces ROS generation in colorectal cells which is blocked by NAC, DPI and rotenone.
Subconfluent HCT 8 and HCT-15 cells were pretreated or not with 5 mM NAC (A) or 10 μM DPI (B) or 200 nM rotenone (C) for 2 h before treating with CDDO-Me (2.5 μM) for 24 h. Cells were then reacted with 5 μM H2DCFDA for 30 min at 37°C. Cells were collected and DCF fluorescence was measured by flow cytometry. Flow cytographs show an increase in the mean fluorescence intensity in both cell lines treated with CDDO-Me, which was blocked by NAC, DPI and rotenone.
Next, we investigated whether mitochondrial ROS are also generated in colon cancer cells treated with CDDO-Me. For this purpose, HCT8 and HCT-15 cells were treated with CDDO-Me in the presence or absence of DPI, a NADPH oxidase inhibitor or rotenone, a specific inhibitor of the respiratory chain complex 1 and ROS generation was measured by oxidation of H2DCFDA by flow cytometry (24). As shown in Figure 2B, DPI significantly inhibited the CDDO-Me-mediated oxidation of H2DCFDA in both cell lines (HCT8, from 5.7-fold to 2.8-fold; HCT-15, from 2.3-fold to 1.5-fold). Treatment with rotenone also partially blocked the CDDO-Me induced oxidation of H2DCFDA (fig. 2C). Together, these data showing the inhibition of CDDO-Me-induced ROS generation by NAC, DPI and rotenone indicated that both mitochondrial and non-mitochondrial oxidative pathways are involved in ROS generation by CDDO-Me.
NAC blocks CDDO-Me induced apoptosis in colorectal cancer cells
Whether ROS plays a role in induction of apoptosis by CDDO-Me in colorectal cancer cells, we investigated the effect of NAC on CDDO-Me-induced binding of annexin V-FITC and cleavage of PARP-1 and procaspases.
Effect of NAC on annexin V-FITC-binding
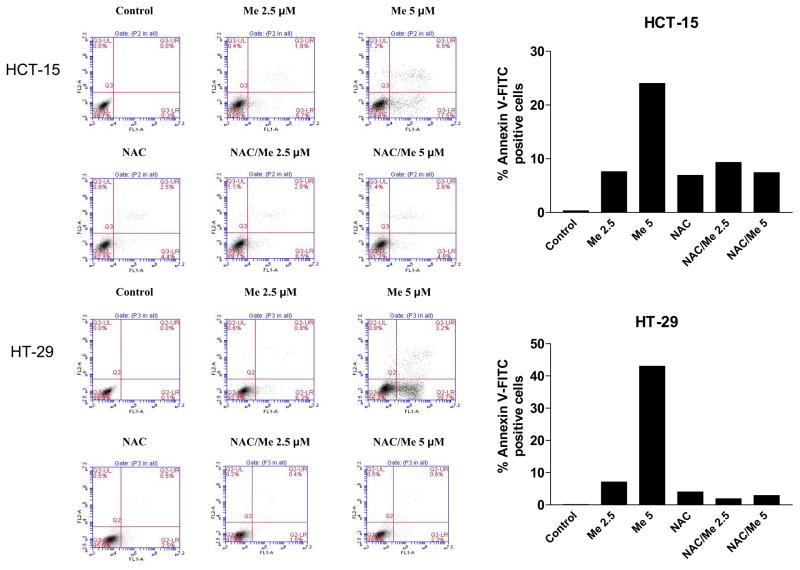
HCT-15 and HT-29 cells were pretreated with NAC for 2 h before applying CDDO-Me for 24 h. Cells were stained with annexin V-FITC and PI and analyzed by flow cytometry. As shown in Figure 3A, 8% to 24% percent of HCT-15 cells and 7% to 43% of HT-29 cells bound annexin V-FITC after treatment with CDDO-Me at 2.5 and 5 μM, respectively. In contrast, pretreatment with NAC blocked the CDDO-Me-induced binding of annexin V-FITC.
Figure 3. NAC prevents induction of apoptosis in colorectal cells.
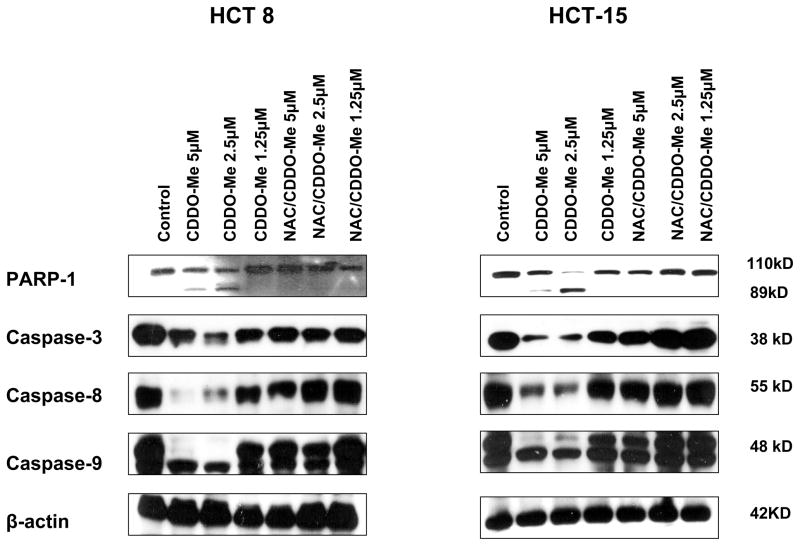
A. Binding of annexin V-FITC. HCT-15 and HT-29 cells were treated with NAC for 2 h prior to treating with CDDO-Me at concentrations of 2.5 and 5 μM for 24 h. Cells were then reacted with 5 μl of annexin V-FITC + PI for 30 min at room temperature. The percentage of annexin V-FITC positive tumor cells was determined by flow cytometry. B. Cleavage of PARP-1 and procaspases. HCT 8 and HCT-15 cells were treated as described above and cleavage of PARP-1 and procaspases -3, -8 and -9 was analyzed by immunoblotting. Each experiment was repeated twice.
Effect of NAC on cleavage of PARP-1 and procaspases
Next, we determined the effect NAC has on the cleavage of PARP-1 and procaspases by CDDO-Me in HCT8 and HCT-15 cells. Figure 3B clearly shows the cleavage of PARP-1 by CDDO-Me in both cell lines as demonstrated by the presence of 89 kDa cleaved PARP-1 fragment at concentrations of 1.25 to 5 μM. In contrast, pretreatment with NAC blocked the cleavage of PARP-1 by CDDO-Me. Similarly, cells treated with CDDO-Me showed processing, i.e., significant reduction in the band density of native procaspases-3, -8 and -9 at 2.5 and 5 μM CDDO-Me in both cell lines (Fig. 3B). On the other hand, pretreatment with NAC partially to completely prevented the cleavage of these procaspases by CDDO-Me.
Taken together, the blocking of CDDO-mediated annexin V-FITC-binding and cleavage of PARP-1 and procaspases-3,-8,-9 by NAC indicated that ROS plays a significant role in induction of apoptosis by CDDO-Me in colorectal cancer cells.
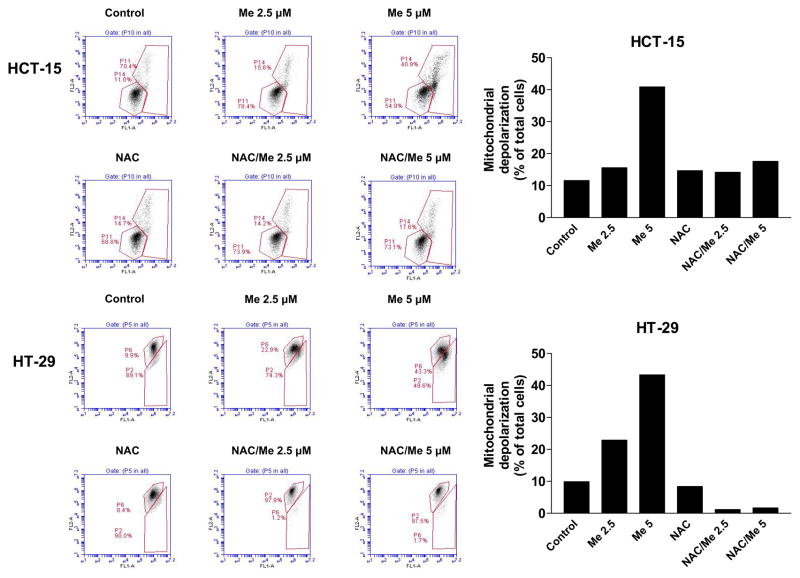
CDDO-Me induces mitochondrial depolarization and NAC inhibits it
To measure the involvement of mitochondria and the role of ROS in mitochondrial depolarization by CDDO-Me, HCT-15 and HT-29 cells were pretreated or not with NAC for 2 h before treating with CDDO-Me for 24 h. Cells were loaded with mitochondrial potential sensor dye JC-1 and analyzed by flow cytometry. There was a significant change in the mitochondrial membrane potential in both cell lines after treatment with CDDO-Me for 24 h. The percentage of HCT-15 cells with green fluorescence changed from 11% (untreated) to 16% and 41% and from 10% (untreated) to 23% and 45% in the case of HT-29 cells after treatment at CDDO-Me at 2.5 and 5 μM (Fig. 4). In both cell lines, pretreatment with NAC effectively blocked the mitochondrial depolarization caused by CDDO-Me, indicating the involvement of ROS in CDDO-Me-induced mitochondrial depolarization.
Figure 4. NAC prevents CDDO-Me-induced mitochondrial depolarization.
A. HCT-15 and HT-29 cells were treated with 5 mM NAC prior to treating with CDDO-Me for 24 h. 1×106 cells were resuspended in 1ml of culture medium and loaded with mitochondrial potential sensor JC-1 (10 μg/ml) for 10 minutes at 22°C. Cells were analyzed by flow cytometry for fluorescence emission. Data are shown as flow cytographs of cells fluorescing red (FL2 channel) or green (FL1 channel). B. Histograms showing percentage of cells with loss of mitochondrial potential difference. Similar results were obtained in two separate experiments.
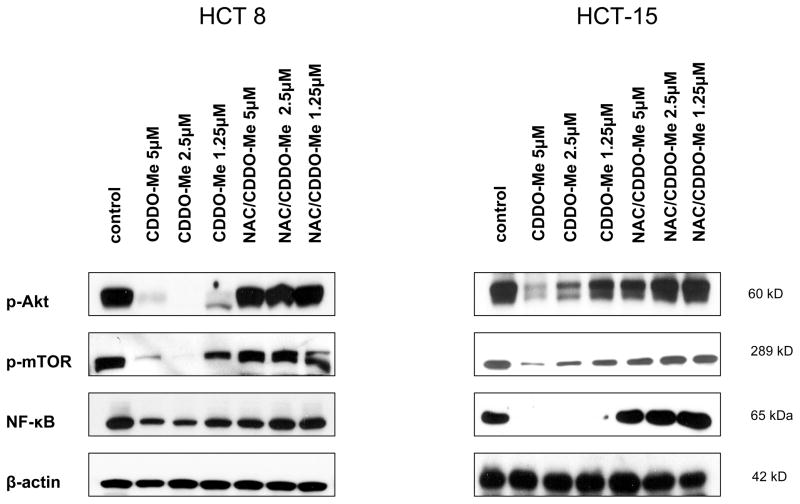
NAC prevents the inhibition of p-Akt, p-mTOR and NF-κB by CDDO-Me
Akt/mTOR and Akt/NF-κB are major antiapoptotic pathways that confer survival advantage and resistance of cancer cells to various forms of anticancer therapies. We have previously reported that CDDO-Me inhibits Akt, NF-κB, and mTOR in colorectal cells (20). Here we investigated whether ROS is involved in the inhibition of Akt, NF-κB and mTOR by CDDO-Me. HCT-8 and HCT-15 cells showed significant to complete inhibition of p-Akt, p-mTOR and NF-κB after treatment with CDDO-Me at 1.25 to 5 μM for 24 h (Fig. 5). Pre-treatment with NAC prevented the inhibition of these proteins by CDDO-Me. This result suggested that ROS may have a role in inhibition of these cell survival signaling proteins by CDDO-Me.
Figure 5. NAC blocks inhibition of p-Akt, p-mTOR and NF-κB by CDDO-Me.
HCT 8 and HCT-15 cells were pretreated or not with 5 mM NAC for 2 h before treating with CDDO-Me for 24 h. After treatment, cell lysates were prepared and analyzed by Western blotting using anti-p-Akt, anti-p-mTOR, anti-NF-κB (p65) or anti-β-actin antibody (loading control). Similar result was obtained in two independent experiments.
DISCUSSION
We have previously reported that CDDO-Me is a potent inhibitor cell growth of several human colorectal cancer cell lines (20). The growth inhibition of colorectal cancer cells by CDDO-Me was attributed to the induction of apoptosis as demonstrated by an increase in annexin-V binding, cleavage of PARP-1 and activation of caspases. In addition, these studies also identified progrowth/prosurvival signaling proteins such as Akt, mTOR and NF-κB as potential molecular targets of CDDO-Me (20).
Since generation of ROS is part of the mechanism by which most chemotherapeutic agents and ionizing radiation kill tumor cells (21–23), the aim of the present study was to determine whether ROS are also involved in growth inhibition and apoptosis induction by CDDO-Me in colorectal cancer cells. ROS are generated intracellularly as byproducts of normal aerobic metabolism or as second messengers in various signal transduction pathways or in response to environmental stress. Depending upon the concentration, ROS elicit a wide spectrum of biological responses ranging from mitogenic to proliferative effects at low concentration to macromolecular damage and cell death at high concentrations (25). Our present studies demonstrated that antioxidant NAC protected colorectal cancer cells from the growth inhibitory activity of CDDO-Me, suggesting that ROS might be involved in killing of cancer cells by CDDO-Me. The generation of ROS by CDDO-Me was confirmed by the oxidation of H2DCFDA in cells treated with CDDO-Me. This was further supported by the finding that pretreatment with NAC blocked the oxidation of H2DCFDA by CDDO-Me. In addition, the inhibition of H2DCFDA oxidation by mitochondrial chain 1 complex inhibitors DPI and rotenone suggested the involvement of mitochondrial respiratory chain reaction in production of ROS. These findings are consistent with the results of previous studies showing participation of ROS in killing of leukemia and pancreatic cancer cells by CDDO-Me (13,26).
We also investigated the role of ROS in CDDO-Me-induced apoptosis in colorectal cancer cells. Induction of apoptosis by CDDO-Me was confirmed by an increase in binding of annexin V-FITC, PARP-1 cleavage and activation of procaspases-3, -8 and -9. Activation of the most apical initiator procaspase-8, effector procaspase-3 and activation of caspase-9 and loss of mitochondrial potential indicated that both the death receptor (extrinsic) and mitochondrial (intrinsic) pathways of apoptosis participate in the destruction of colorectal cancer cells by CDDO-Me. The activation of both pathways of apoptosis by CDDO-Me in colorectal cancer cells is consistent with previous reports showing activation of caspase-dependent and caspase-independent pathways of apoptosis by CDDOs in leukemic and solid tumor cell lines (26). The inhibition of each of these apoptosis-related processes by NAC indicated the involvement of ROS in CDDO-Me-induced apoptosis.
Akt, mTOR and NF-κB are major signaling proteins that control cellular processes such as cell growth, proliferation, survival, motility, angiogenesis and apoptosis (27–29) and are frequently hyperactivated in most cancers (30,31). Our results demonstrated that p-Akt, p-mTOR and NF-κB were inhibited by CDDO-Me, indicating that inhibition of these antiapoptotic proteins may be necessary for induction of apoptosis by CDDO-Me. The inhibition of these signaling proteins by CDDO-Me was prevented by NAC. The latter implies that ROS may have a role in inhibition of these signaling molecules by CDDO-Me. Free radicals have been shown to activate pro-survival pathways including Akt/mTOR/NF-κB signaling pathway (32); however, the exact role of ROS in inhibition of Akt, mTOR and NF-κB by CDDO-Me is unclear. Obviously, more work is needed to precisely define the role of ROS in inhibition of the antiapoptotic and pro-survival Akt, mTOR and NF-κB signaling proteins in cancer cells by CDDO-Me.
CONCLUSION
Oleanane-derived synthetic triterpenoids have shown stronger anti-inflammatory and antitumorigenic activity in several experimental models. The present study demonstrated the generation of oxygen free radicals by CDDO-Me in colorectal cancer cells and their role in the growth inhibitory and apoptosis inducing activity of CDDO-Me. Data also implicated ROS in the inhibition of antiapoptotic/prosurvival Akt, mTOR and NF-κB signaling proteins by CDDO-Me. Thus, better understanding of the mechanism of the anticancer activity of CDDO-Me could potentially improve strategies to treat and/or prevent colorectal cancer.
Acknowledgments
This work was supported by NIH grants 1R01 CA130948 (S.C.G.).
References
- 1.Giovannucci E, Willett WC. Dietary factors and risk of colon cancer. Ann Med. 1994;26:443–452. doi: 10.3109/07853899409148367. [DOI] [PubMed] [Google Scholar]
- 2.Rao CV, Hirose Y, Indranie C, Reddy BS. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Res. 2001;61:1927–1933. [PubMed] [Google Scholar]
- 3.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 4.Kinzler KW, Vogelstein B. Cancer-susceptibility genes: gate- keepers and caretakers. Nature. 1997;386:761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 5.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 6.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 8.Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCM in mice. Cancer Res. 2006;66:7370–7377. doi: 10.1158/0008-5472.CAN-05-4619. [DOI] [PubMed] [Google Scholar]
- 9.Gao X, Deeb D, Jiang H, Liu Y, Dulchavsky S, Gautam S. Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF-κB and Notch1 signaling. J Neurooncol. 2007;84:147–157. doi: 10.1007/s11060-007-9364-9. [DOI] [PubMed] [Google Scholar]
- 10.Deeb D, Gao X, Dulchavsky SA, Gautam SC. CDDO-Me induces apoptosis and inhibits Akt, mTOR and NF-κB in prostate cancer cells. Anticancer Res. 2007;27:3035–3044. [PubMed] [Google Scholar]
- 11.Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estrov Z, Leysath CE, Zhao S, Harris D, Chang S, Jackson CE, Munsell M, Suh N, Gribble G, Honda T, May WS, Sporn MB, Andreeff M. Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood. 2002;99:326–335. doi: 10.1182/blood.v99.1.326. [DOI] [PubMed] [Google Scholar]
- 12.Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB. A synthetic triterpenoid, CDDO-Me, inhibits Ikappa Balpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res. 2006;12:1828–1838. doi: 10.1158/1078-0432.CCR-05-2044. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda T, Sporn M, Honda T, Gribble GW, Kufe D. The novel triterpenoid CDDO and its derivatives induce apoptosis by disruption of intracellular redox balance. Cancer Res. 2003;63:5551–5558. [PubMed] [Google Scholar]
- 14.Xiaoyang L, Konopleva M, Zeng Z, Ruvolo V, Stephens LC, Schober W, McQueen T, Dietrich M, Madden TL, Andreeff M. The novel triterpenoid C-28 methyl ester of 2-cyano-3, 12-dioxoolen-1, 9-dien-28-oic acid inhibits metastatic murine breast tumor growth through inactivation of STAT3 signaling. Cancer Res. 2007;67:4210–4218. doi: 10.1158/0008-5472.CAN-06-3629. [DOI] [PubMed] [Google Scholar]
- 15.Liby K, Royce DB, Williams CR, Risingsong R, Yore MM, Honda T, Gribble GW, Dmitrovsky E, Sporn TA, Sporan MB. The synthetic triterpenoid CDDO-methyl ester and CDDO-amide prevent lung cancer induced by vinyl carbomate in A/J mice. Cancer Res. 2007;67:2414–2419. doi: 10.1158/0008-5472.CAN-06-4534. [DOI] [PubMed] [Google Scholar]
- 16.Konopleva M, Tsao T, Estrov Z, Lee RM, Wang RY, Jackson CE, McQueen T, Monaco G, Munsell M, Belmont J, Kantarjian H, Sporn MB, Andreeff M. The synthetic triterpenoid 2-cyano-3, 12-dioxooleana-1, 9-dien-28-oic acid induces caspase-dependent and -independent apoptosis in acute myelogenous leukemia. Cancer Res. 2004;64:7927–7935. doi: 10.1158/0008-5472.CAN-03-2402. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Pandey P, Sporn MB, Datta R, Kharbanda S, Kufe D. The novel triterpenoid CDDO induces apoptosis and differentiation of human osteosarcoma cells by a caspase-8 dependent mechanism. Mol Pharmacol. 2001;59:1094–1099. doi: 10.1124/mol.59.5.1094. [DOI] [PubMed] [Google Scholar]
- 18.Konopleva M, Contractor R, Kurinna SM, Chen W, Andreeff M, Ruvolo PP. The novel triterpenoid CDDO-Me suppresses MAPK pathways and promotes p38 activation in acute myeloid leukemia cells. Leukemia. 2005;19:1350–1354. doi: 10.1038/sj.leu.2403828. [DOI] [PubMed] [Google Scholar]
- 19.Suh N, Roberts AB, Birkey Reffey S, Miyazono K, Itoh S, ten Dijke P, Heiss EH, Place AE, Risingsong R, Williams CR, Honda T, Gribble GW, Sporn MB. Synthetic triterpenoids enhance transforming growth factor beta/Smad signaling. Cancer Res. 2003;63:1371–1376. [PubMed] [Google Scholar]
- 20.Gao X, Deeb D, Jiang H, Liu Y, Dulchavsky SA, Gautam SC. Synthetic triterpenoids inhibit growth, induce apoptosis, and suppress pro-survival Akt, mTOR, and NF-κB signaling proteins in colorectal cancer cells. Anticancer Res. 2010;30:785–792. [PMC free article] [PubMed] [Google Scholar]
- 21.Gao J, Liu X, Rigas B. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc Natl Acad Sci USA. 2005;102:17207–12. doi: 10.1073/pnas.0506893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramanathan B, Jan KY, Chen CH, Hour TC, Yu HJ, Pu YS. Resistance to paclitaxel is proportional to cellular total antioxidant capacity. Cancer Res. 2005;65:8455–60. doi: 10.1158/0008-5472.CAN-05-1162. [DOI] [PubMed] [Google Scholar]
- 23.Yu Sun, Rigas B. The thioredoxin system mediates redox-induced cell death in human colon cancer cells: Implications for the mechanism of action of anticancer agents. Cancer Res. 2008;68:8269–77. doi: 10.1158/0008-5472.CAN-08-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Antosiewicz AH. Sulphoraphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–24. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 25.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: Signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 26.Samudio I, Konopleva M, Hail N, Jr, Shi Y-X, McQueen T, Hsu T, Evans R, Honda T, Gribble GW, Sporn M, Gilbert HF, Safe S, Andreeff M. 2-cyano-3, 12-dioxooleana-1, 9-dien-28-imidazolide (CDDO-Im) directly targets mitochondrial glutathione to induce apoptosis in pancreatic cancer. J Biol Chem. 2005;280:36273–82. doi: 10.1074/jbc.M507518200. [DOI] [PubMed] [Google Scholar]
- 27.Marte BM, Downward J. PKB/Akt: connecting phosphoinositide3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 28.Mayo MW, Baldwin AS. The transcription factor NF-κB: control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta. 2000;1470:M55–M62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 29.Hidalgo M, Rowinsky EK. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 2000;19:6680–6686. doi: 10.1038/sj.onc.1204091. [DOI] [PubMed] [Google Scholar]
- 30.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 31.Altomare DA, Testa JR. Perturbations of the Akt signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 32.Lee SJ, Seo KW, Yun MR, Bae SS, Lee WS, Hong KW, Kim CD. 4-Hydroxynonenal enhances MMP-2 production in vascular smooth muscle cells via mitochondrial ROS-mediated activation of the Akt/NF-kappaB signaling pathways. Free Radic Biol Med. 2008;45:1487–92. doi: 10.1016/j.freeradbiomed.2008.08.022. [DOI] [PubMed] [Google Scholar]