Abstract
Context
A previous study reported a gene × environment interaction in which a haplotype in the corticotropin-releasing hormone receptor 1 gene (CRHR1) was associated with protection against adult depressive symptoms in individuals who were maltreated as children (as assessed by the Childhood Trauma Questionnaire [CTQ]).
Objective
To replicate the interaction between childhood maltreatment and a TAT haplotype formed by rs7209436, rs110402, and rs242924 in CRHR1, predicting adult depression.
Design
Two prospective longitudinal cohort studies.
Setting
England and New Zealand.
Participants
Participants in the first sample were women in the E-Risk Study (N= 1116), followed up to age 40 years with 96% retention. Participants in the second sample were men and women in the Dunedin Study (N= 1037), followed up to age 32 years with 96% retention.
Main Outcome Measure
Research diagnoses of pastyear and recurrent major depressive disorder.
Results
In the E-Risk Study, the TAT haplotype was associated with a significant protective effect. In this effect, women who reported childhood maltreatment on the CTQ were protected against depression. In the Dunedin Study, which used a different type of measure of maltreatment, this finding was not replicated.
Conclusions
A haplotype in CRHR1 has been suggested to exert a protective effect against adult depression among research participants who reported maltreatment on the CTQ, a measure that elicits emotional memories. This suggests the hypothesis that CRHR1’s protective effect may relate to its function in the consolidation of memories of emotionally arousing experiences.
Major depression is one of the most common and disabling medical disorders and is associated with excess of morbidity, mortality, and costs to society.1 Research documents the contribution of both genetic and environmental risk factors,2 particularly maltreatment occurring early in life,3,4 to the development of depression.
Molecular genetic studies have evaluated the association between a wide range of candidate polymorphisms in different genes and major depressive disorder (MDD).5 Although positive associations have been detected, replications are scarce.5 One possible reason for the lack of consistency across genetic association studies of depression is that these studies focus on genetic main effects on disease rather than on genetic effects on vulnerability to environmental causes of disease.6 Early-life stress has been substantially associated with neurobiological modifications7 and increased risk of depression.8 However, there are substantial differences in the way that individuals respond to the same stressful event, and such response variability may be under genetic influence.9 Several studies have reported that specific polymorphisms exert genetic control of sensitivity to stressful experiences in early life,10–12 and it is likely that there are more polymorphisms involved.
The concept that genes may moderate the causal effect of environmental stressors on depression has direct implications for the selection of candidate genes for the disorder. Genes associated with the physiological response to environmental stressors,13 particularly in the hypothalamicpituitary-adrenal (HPA) axis,14 would be natural candidates. Accordingly, Bradley et al15 investigated the interaction between child abuse and 10 singlenucleotide polymorphisms (SNPs) in the corticotropin-releasing hormone receptor 1 gene (CRHR1), and haplotypes formed by selected SNPs, in relation to depressive symptoms. The CRHR1 is a G protein–coupled receptor localized in frontal cortical areas, forebrain, brainstem, amygdala, cerebellum, and the anterior pituitary.16 This receptor plays a key role in the regulation of the HPA axis in response to stressful events, mediating the action of corticotropin-releasing hormone (CRH) on the pituitary to release adrenocorticotropic hormone that stimulates the production of cortisol in the adrenal cortex.9,17 Moreover, CRH acts as a neurotransmitter via CRHR1 eliciting anxiety-related behavior and influencing arousal, attention, executive functions, the conscious experience of emotions, and learning and memory consolidation related to these emotions.16,18 Bradley et al15 reported that a TAT haplotype formed by the 3 most significant CRHR1 SNPs investigated (rs7209436, rs110402, and rs242924) was associated with a protective effect in terms of reduced depression symptoms among individuals exposed to moderate to severe child abuse, as assessed by the Childhood Trauma Questionnaire (CTQ).15 The effect was also detected for a TCA haplotype formed by rs7209436, rs4792887, and rs110402.15
Since replication is a sine qua non for accepting a genetic hypothesis,19 we aimed to test the gene × environment interaction (G × E) between childhood maltreatment and CRHR1 haplotypes in the development of depression. In this study, we extend the original finding by offering 4 complementary methodological features: (1) We evaluated 2 representative community cohorts to complement the samples selected in hospital and clinical settings in the original report. (2) We assessed exposure to childhood maltreatment using 2 different measures, the CTQ, as used by Bradley et al,15 and a combined prospectiveretrospective measure, to complement the exclusive retrospective assessment originally used. (3) We report DSM diagnoses of depression assessed through standardized interviews to complement the self-completed symptom scales originally used. (4) We assessed individuals longitudinally to evaluate the effect of the investigated interaction on recurrent episodes of MDD to complement the cross-sectional assessment of depressive symptoms originally performed.
METHODS
SAMPLE
Participants in the first sample were the mothers of the children in the Environmental Risk (E-Risk) Longitudinal Twin Study. The base sample of the E-Risk Study was formed in 1999–2000, when 1116 mothers with 5-year-old twins (93% of eligible families) participated in home-visit assessments. Details about the sample are reported elsewhere.20 Participants represent the full range of socioeconomic status in the general population of England and more than 90% are white. Four assessments were undertaken: women were on average 33 years of age at the initial assessment and 35, 38, and 40 years (range, 26–55 years; SD= 5.8) on the subsequent assessments. In the most recent assessment, undertaken in 2007–2008, we evaluated 96% of the women. Women gave written informed consent before participating. The Maudsley Hospital Ethics Committee approved each phase of the study.
Participants in the second sample were members of the Dunedin Multidisciplinary Health and Development Study.21 Of infants born in Dunedin, New Zealand, between April 1972 and March 1973, 1037 children (91% of eligible births; 52% male) participated in the first follow-up at age 3 years, constituting the base sample for the longitudinal study. Participants represent the full range of socioeconomic status in the general population of New Zealand’s South Island and more than 90% are white. Assessments were undertaken at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, and 26 years and most recently at age 32 years when we assessed 96% of the 1015 study members still alive in 2004–2005. Study members gave written informed consent before participating. The Otago Ethics Committee approved each phase of the study.
MEASURES
Childhood Maltreatment
In the E-Risk Study, women completed the CTQ22 when they were on average 40 years of age. This is the same measure used in the 2 samples studied by Bradley et al.15 The CTQ inquires about the history of 5 categories of childhood maltreatment: emotional, physical, and sexual abuse and emotional and physical neglect. The validity of the instrument has been previously demonstrated in clinical and community samples.22 Bradley et al used an abbreviated 3-scale version of the CTQ; the E-Risk Study used all 5 scales. We used the score classification evaluated and recommended by the CTQ manual22 and considered a specific category of maltreatment present if the woman had a moderate to severe score. Subsequently, we derived a cumulative exposure index for each woman by counting the number of maltreatment categories present: 75.5% of women experienced no maltreatment, 16.8% experienced some maltreatment (1–2 categories), and 7.7% experienced severe maltreatment (≥3 categories).
In the Dunedin Study, we used the maltreatment measure described in our previous publications.23–25 Briefly, maltreatment between ages 3 to 11 years was evaluated. Evidence of childhood maltreatment was ascertained from 5 sources. Three sources were prospective: (1) behavioral observations by researchers of rejecting mother-child interactions at age 3 years; (2) parental reports of harsh disciplinary practices at ages 7 and 9 years; and (3) multiple changes in the person occupying the role of the child’s primary caregiver during the first decade of life. Two sources were retrospective: study members’ reports in adulthood of (4) physical and (5) sexual abuse. We derived a cumulative exposure index by counting the number of maltreatment experiences during the first decade of life: 64% of the cohort experienced no maltreatment, 27% experienced some maltreatment (1 indicator), and 9% experienced severe maltreatment (≥2 indicators).
Major Depressive Disorder
In the E-Risk Study, MDD was evaluated with the Diagnostic Interview Schedule,26 and diagnoses followed DSM-IV27 criteria at the 4 waves of assessment. In the Dunedin Study, MDD was also evaluated with the Diagnostic Interview Schedule.26,28 At ages 18 and 21 years, diagnoses followed the thencurrent DSM-III-R,29 and at ages 26 and 32 years, diagnoses followed DSM-IV.27 In both studies, (1) diagnostic interviews were conducted by mental health trainees or professionals, (2) functional impairment was required for the MDD diagnosis, and (3) diagnosis at each assessment covered the period of the past year.
Past-year MDD was defined as the presence of MDD in the most recent wave of assessment, ie, at mean age of 40 years for the participants of the E-Risk Study and at age 32 years for the participants of the Dunedin Study. Recurrent MDD was defined as the presence of past-year MDD at 2 or more waves of assessment. In the E-Risk Study, recurrent MDD refers to 2 or more diagnoses during the 8-year period from mean age 33 to 40 years. In the Dunedin Study, recurrent MDD refers to 2 or more diagnoses during the 15-year period from age 18 to 32 years.
DNA EXTRACTION AND GENOTYPING
In the E-Risk Study, DNA was obtained via buccal swabs from 94% of women. In the Dunedin Study, DNA was obtained from 97% of participants (93% via blood and 7% via buccal swabs). To avoid potential problems of population stratification, we studied white individuals from both samples. Genotyping for both samples was carried out in the same laboratory, by the same technician, who was blind to data on maltreatment and depression and who genotyped cases in birthdate order (ie, not cases vs controls).
Following the findings from Bradley et al,15 we genotyped the following 4 SNPs located at CRHR1 (GenBank NM_004382): dbSNP accession numbers rs7209436, rs110402, rs242924, and rs4792887. Single-nucleotide polymorphisms were genotyped on an Applied Biosystems 7900HT TaqMan genotyping platform (Applied Biosystems, Foster City, California) in Allelic Discrimination mode. Five-microliter reactions contained 1 × QPCR ROX universal genotyping mastermix (Abgene, Epsom, England), 1 × Applied Biosystems Assays-on-Demand primer/probe mix (Applied Biosystems), and 20 ng of genomic DNA. Fluorescence data files from each plate were analyzed by using automated software SDS 2.1 (Applied Biosystems). The rate of successful genotyping for each SNP individually was 97% to 99% in both samples.
Genotype frequencies are presented in Table 1. All SNPs in both samples were in Hardy-Weinberg equilibrium (HWE).
Table 1.
Genotype Frequencies of the CRHR1 Polymorphisms Evaluated in the E-Risk and Dunedin Studies
| Frequency, % |
||
|---|---|---|
| SNP Accession No. | E-Risk Study | Dunedin Study |
| rs7209436 | ||
| TC | 46 | 50.3 |
| CC | 35.4 | 31.9 |
| TT | 18.6 | 17.8 |
| rs110402 | ||
| AG | 46.9 | 49.9 |
| GG | 33.7 | 30.9 |
| AA | 19.4 | 19.2 |
| rs242924 | ||
| TG | 47.4 | 49.9 |
| GG | 33 | 30.8 |
| TT | 19.6 | 19.2 |
| rs4792887 | ||
| CC | 81.5 | 80.6 |
| TC | 17.2 | 18.3 |
| TT | 1.3 | 1.1 |
Abbreviation: SNP, single-nucleotide polymorphism.
Herein, we report the interaction between childhood maltreatment and haplotypes formed by rs7209436, rs110402, and rs242924. The TAT haplotype, derived from these 3 SNPs, exerted the most significant protective effect in the original study by Bradley et al.15 We also derived the haplotypes from rs7209436, rs4792887, and rs110402, as the original study detected a significant protective effect of a TCA haplotype. However, as a consequence of the strong correlations among the 4 SNPs, fewer than 1% of individuals from the E-Risk and Dunedin studies were estimated to have a different number of TAT and TCA haplotype copies. Since the information provided by each haplotype individually was redundant, we decided to focus on the original, most significant haplotype to avoid unnecessary multiple testing.
Haplotypes were estimated using the haplo.glm method, described in the “Statistical Analyses” subsection. In the E-Risk Study, 2 common haplotypes were derived from rs7209436, rs110402, and rs242924 (r2 between the 3 SNPs was 0.90,0.87, and 0.97). The common haplotypes were CGG, with an estimated frequency of 56%, and TAT, with an estimated frequency of 42%. All remaining haplotypes together had a frequency of 2%. In the Dunedin Study, the same 2 common haplotypes were derived from rs7209436, rs110402, and rs242924 (r2 between the 3 SNPs was 0.91, 0.91, and 0.99). CGG had an estimated frequency of 55%, and TAT had an estimated frequency of 43%. All remaining haplotypes together had a frequency of 2%.
STATISTICAL ANALYSES
We tested the haplotype × environment interaction in a logistic regression framework with the haplo.glm method.30 The haplo.glm method iteratively and simultaneously estimates both regression parameters and haplotype frequencies by maximizing the prospective likelihood, even when the phase of the genetic data are not known. Haplotypes with estimated frequencies of 10% or less are by default collapsed into a single category, “rare” haplotypes.
Using inferred haplotype frequencies to study G × E relies on an assumption of HWE in the pooled data. Although there was no indication of Hardy-Weinberg disequilibrium in any of the individual SNP genotypes in our data, this assumption is difficult to verify. Haplotype inference using the prospective likelihood is generally relatively robust to departures from HWE31 and simulations suggest that the haplo.glm method is not affected by departures from HWE.30
An interaction model including the haplotypes (with the most common haplotype CGG as the reference category), childhood maltreatment (treated as a continuous variable), and the interactions between haplotypes and childhood maltreatment was fitted to data from the E-Risk and Dunedin studies, under an additive genetic model assumption. Analyses for the Dunedin Study included sex as a covariate.
RESULTS
Table 2 provides information about the distribution of demographic characteristics, maltreatment exposure, and depression phenotypes, according to the estimated number of CRHR1 TAT haplotype copies, in each cohort.
Table 2.
Main Characteristics of Participants According to the Number of TAT Copies in the E-Risk and Dunedin Studies
| No. (%) of Participants |
||||
|---|---|---|---|---|
| Characteristic | 0 TAT Copies (n=356) |
1 TAT Copy (n=458) |
2 TAT Copies (n=185) |
Statistic; P Value |
| E-Risk Study (n=999) | ||||
| Women | 356 (100) | 458 (100) | 185 (100) | …a |
| Age, y, mean (SD) | 39.6 (5.9) | 40.1 (5.9) | 40.3 (5.7) | F2=1.05; .34 |
| Socioeconomic status | ; .10 | |||
| Low | 125 (35.1) | 154 (33.6) | 48 (25.9) | |
| Intermediate | 123 (34.6) | 140 (30.6) | 68 (36.8) | |
| High | 108 (30.3) | 164 (35.8) | 69 (37.3) | |
| Exposure to severe maltreatment in childhood | 36 (10.1) | 30 (6.6) | 10 (5.4) | ; .07 |
| Diagnosis of past-year MDD | 58 (16.3) | 57 (12.4) | 24 (13.0) | ; .26 |
| Diagnosis of recurrent MDDb | 47 (13.2) | 60 (13.0) | 20 (10.8) | ; .69 |
|
No. (%) of Participants |
||||
| Characteristic |
0 TAT Copies (n=292) |
1 TAT Copy (n=448) |
2 TAT Copies (n=159) |
Statistic; P Value |
| Dunedin Study (n=899) | ||||
| Women | 156 (53.4) | 217 (48.4) | 69 (43.4) | ; .11 |
| Age, y, mean | 32 | 32 | 32 | …c |
| Socioeconomic status | ; .93 | |||
| Low | 90 (30.8) | 141 (31.5) | 49 (31) | |
| Intermediate | 155 (53.1) | 228 (50.9) | 79 (50) | |
| High | 47 (16.1) | 79 (17.6) | 30 (19) | |
| Exposure to severe maltreatment in childhood | 28 (9.6) | 35 (7.8) | 14 (8.8) | ; .69 |
| Diagnosis of past-year MDD | 53 (18.2) | 70 (15.6) | 25 (15.7) | ; .63 |
| Diagnosis of recurrent MDDb | 54 (18.1) | 70 (15.4) | 31 (19.0) | ; .45 |
Abbreviation: MDD, major depressive disorder.
Because the entire sample was composed of women, we did not compare the 3 groups on the distribution of sex.
Individuals included in a minimum of 2 assessment periods were evaluated for recurrent MDD and included in the analysis for this outcome. E-Risk Study, n=1000; Dunedin Study, n=918.
Because the entire sample was composed of individuals of the same age, we did not compare the 3 groups on the mean age.
E-RISK STUDY
At mean age 40 years, 13.9% of women presented with past-year MDD. Between ages 33 and 40 years, 12.7% of the women had recurrent MDD. Of those with recurrent MDD, 66% were depressed at the most recent assessment at age 40 years.
Childhood maltreatment was significantly associated with adult depression. As the severity of reported maltreatment increased, so did rates of past-year (; P< .001) and recurrent (; P< .001) MDD. Among women with no exposure to childhood maltreatment, the rate of past-year MDD was 9.6%; among women with some maltreatment, the rate of past-year MDD was 19.8%; and among women with severe maltreatment, the rate of past-year MDD was 43.4%. The comparable rates of recurrent MDD were 8.1%, 19.0%, and 43.4%. In contrast, there was no significant association between number of TAT haplotype copies and either the rate of past-year (; P=.26) or recurrent (; P=.69) MDD (Table 2).
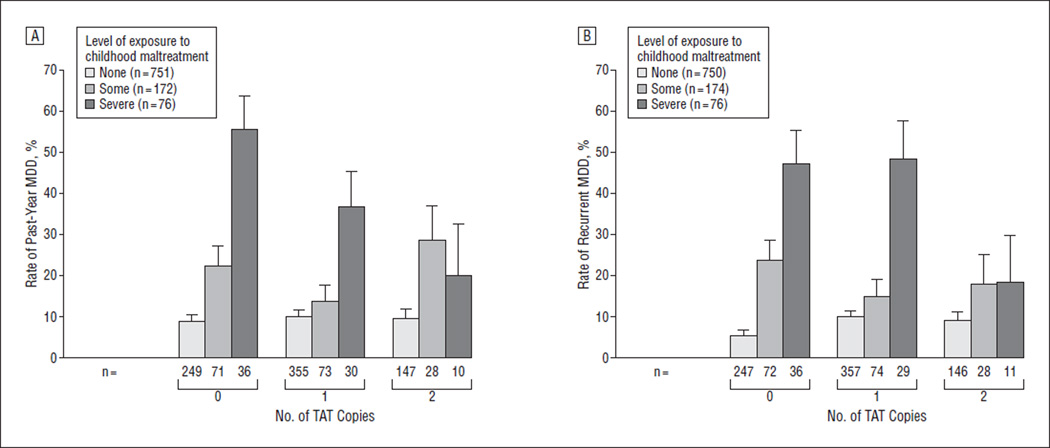
Logistic regression analysis demonstrated a significant interaction between maltreatment and number of CRHR1 TAT haplotype copies in predicting past-year MDD (P=.04) (Table 3). The odds ratio of past-year MDD in women exposed to severe maltreatment in comparison with those exposed to no or some maltreatment was 9.27 (95% confidence interval [CI], 4.42–19.43) for those with 0 copies, 4.80 (95% CI, 2.15–10.73) for those with 1 copy, and 1.73 (95% CI, 0.34–8.72) for those with 2 copies of the TAT haplotype (Figure 1A).
Table 3.
Effect of Childhood Maltreatment CRHR1 Haplotypes, and Their Interaction on the Development of Past-Year and Recurrent MDD in the E-Risk and Dunedin Studies
| Past-Year MDD |
Recurrent MDD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Coef | SE | t | P Value | Coef | SE | t | P Value | |
| E-Risk Study | ||||||||
| Childhood maltreatment | 1.22 | 0.19 | 6.55 | <.001 | 1.42 | 0.20 | 7.15 | <.001 |
| CRHR1 rare haplotypes | 0.06 | 0.55 | 0.10 | .92 | 0.39 | 0.53 | 0.74 | .46 |
| CRHR1 TAT haplotype | 0.09 | 0.17 | 0.52 | .60 | 0.27 | 0.18 | 1.51 | .13 |
| Childhood maltreatment by CRHR1 rare haplotypes | −0.46 | 0.54 | −0.86 | .39 | −0.53 | 0.51 | −1.05 | .29 |
| Childhood maltreatment by CRHR1 TAT haplotype | −0.37 | 0.18 | −2.03 | .04 | −0.43 | 0.19 | −2.31 | .03 |
| Dunedin Study | ||||||||
| Sex | −0.62 | 0.19 | −3.30 | <.001 | −0.58 | 0.18 | −3.14 | .002 |
| Childhood maltreatment | 0.38 | 0.21 | 1.85 | .07 | 0.52 | 0.20 | 2.54 | .01 |
| CRHR1 rare haplotypes | −1.07 | 0.83 | −1.29 | .20 | 0.28 | 0.54 | 0.51 | .61 |
| CRHR1 TAT haplotype | −0.05 | 0.17 | −0.30 | .77 | 0.09 | 0.17 | 0.52 | .61 |
| Childhood maltreatment by CRHR1 rare haplotypes | 1.48 | 0.81 | 1.82 | .07 | 0.20 | 0.56 | 0.36 | .71 |
| Childhood maltreatment by CRHR1 TAT haplotype | 0.01 | 0.19 | 0.08 | .94 | −0.02 | 0.18 | −0.09 | .93 |
Abbreviations: Coef, coefficient; MDD, major depressive disorder.
Figure 1.
Major depressive disorder (MDD) according to the number of TAT copies and the level of exposure to childhood maltreatment in the E-Risk Study. A, Past-year MMD. B, Recurrent MDD.
Logistic regression analysis also demonstrated a significant interaction between maltreatment and number of CRHR1 TAT haplotype copies in predicting recurrent MDD (P=.03) (Table 3). The odds ratio of recurrent MDD in women exposed to severe maltreatment in comparison with those exposed to no or some maltreatment was 8.61 (95% CI, 4.05–18.33) for those with 0 copies, 7.81 (95% CI, 3.54–17.21) for those with 1 copy, and 1.92(95% CI, 0.38–9.61) for those with 2 copies of the TAT haplotype (Figure 1B).
DUNEDIN STUDY
At age 32 years, 16.5% of the participants presented with past-year MDD. Between ages 18 and 32 years, 16.9% of the participants had recurrent MDD. Of those with recurrent MDD, 60% were depressed at the most recent assessment at age 32 years.
As the severity of reported childhood maltreatment increased, so did rates of past-year (; P< .001) and recurrent (; P<.001) MDD. Among individuals with no exposure to maltreatment, the rate of past-year MDD was 14.1%; among individuals with some maltreatment, the rate of past-year MDD was 16.6%; and among individuals with exposure to severe maltreatment, the rate of past-year MDD was 33.8%. The comparable rates of recurrent MDD were 13.5%, 20.3%, and 31.3%. There was no significant association between number of TAT haplotype copies and either rate of past-year (; P = .63) or recurrent (; P = .45) MDD (Table 2).
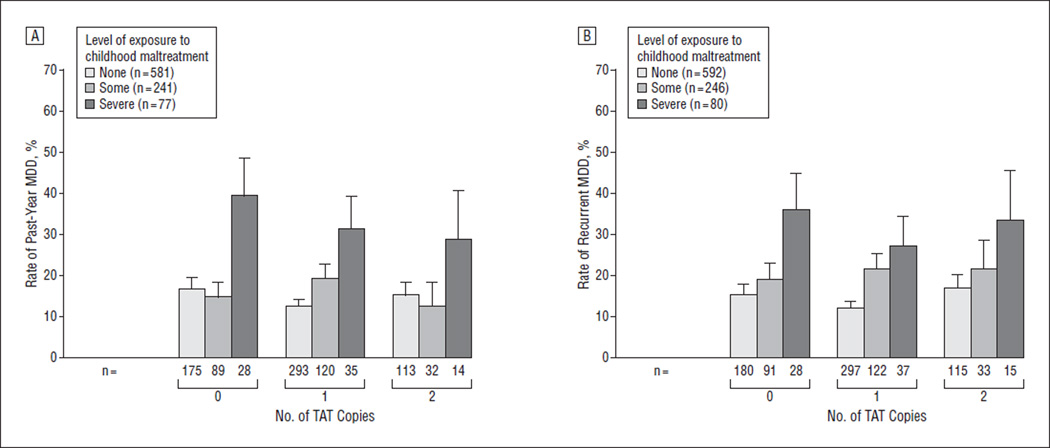
In contrast to findings from the E-Risk Study, the interaction between childhood maltreatment and number of CRHR1 TAT haplotype copies in predicting past-year MDD was not significant (P=.94) (Table 3). The odds ratio of MDD in individuals exposed to severe maltreatment in comparison with those exposed to no or some maltreatment was 3.42 (95% CI, 1.49–7.82) for those with 0 copies, 2.75 (95% CI, 1.27–5.90) for those with 1 copy, and 2.36 (95% CI, 0.67–8.23) for those with 2 copies (Figure 2A). Similarly, there was no significant G × E predicting recurrent MDD (P = .93) (Table 3). The odds ratio of recurrent MDD was 2.86 (95% CI, 1.24–6.62) for those with 0 copies, 2.21 (95% CI, 1.02–4.81) for those with 1 copy, and 2.34 (95% CI, 0.73–7.43) for those with 2 copies of the TAT haplotype (Figure 2B).
Figure 2.
Major depressive disorder (MDD) according to the number of TAT copies and the level of exposure to childhood maltreatment in the Dunedin Study. A, Past-year MMD. B, Recurrent MDD.
We considered the possibility that the interaction between maltreatment and CRHR1 may be female specific. In the original report,15 61% of sample 1 were women and 100% of sample 2 and 100% of participants in the positive replication in the E-Risk Study. However, when we restricted the Dunedin analysis to women, the pattern of results did not differ from that in the full cohort. Among women with severe maltreatment, the rates of pastyear MDD were 47.1%, 25%, and 37.5% for those with 0, 1, and 2 copies of TAT haplotype, respectively, and the interaction term was not significant (P=.46). The comparable rates of recurrent MDD were 47.1%, 28.6%, and 50%, with a nonsignificant interaction term (P=.96). This excluded the possibility that the Dunedin Study’s negative findings resulted from its mixed sex composition.
We also considered the possibility that the interaction between maltreatment and CRHR1 may depend on retrospective assessment of childhood maltreatment. In the original report15 and in the E-Risk Study, maltreatment was ascertained via retrospective reports (on the CTQ). In Dunedin, maltreatment was ascertained using a combination of prospective and retrospective indicators. To test this hypothesis, we disaggregated the original composite measure used in the Dunedin Study23–25 and repeated our analyses separately for the prospective and retrospective indicators of maltreatment. The rates of pastyear MDD in individuals exposed to prospectively measured maltreatment were 20.9%, 19.6%, and 15.6% for those with 0, 1, and 2 copies of TAT haplotype, respectively, with a nonsignificant interaction term (P=.81). The comparable rates of recurrent MDD were 21.6%, 18.1%, and 23.5%, with a nonsignificant interaction term (P=.98). The rates of past-year MDD in individuals exposed to retrospectively measured maltreatment were 29.5%, 30.2%, and 20% for those with 0, 1, and 2 copies of TAT haplotype, respectively, with a nonsignificant interaction term (P=.55). The comparable rates of recurrent MDD were 31.8%, 31.3%, and 23.8%, with a nonsignificant interaction term (P=.43). This excluded the possibility that the occurrence of the G × E is a function of retrospective data collection.
COMMENT
We undertook a replication test of the G × E between childhood maltreatment and a haplotype formed by rs7209436, rs110402, and rs242924 in CRHR1 in predicting the development of adult depression. In the E-Risk Study, the TAT haplotype was associated with significant protection of women exposed to severe maltreatment against developing past-year and recurrent MDD. In the Dunedin Study, we did not replicate this G × E finding.
The significant G × E in the E-Risk Study corroborates the original findings15 and extends them, considering that (1) a community sample (vs a clinical sample) was evaluated, (2) depression was diagnosed according to DSM-IV criteria (vs the symptom ratings previously used), (3) depression was operationalized not only as past-year MDD, but also as recurrent MDD (during a multiyear follow-up period), and (4) an ethnically homogeneous European sample was studied (vs the original American samples).
Unexpectedly, results of the Dunedin Study did not corroborate the G × E finding originally reported in the 2 samples studied by Bradley et al15 and replicated here in the E-Risk Study. If this G × E is valid, what could explain the negative results from the Dunedin Study? The discrepant results between the E-Risk and Dunedin studies are unlikely to arise from sampling differences; the 2 samples were equally powered to detect the same effect sizes, both samples were of European ancestry and similar age, and analyses excluded the possibility that findings differed because the Dunedin cohort contained males. The discrepancy is unlikely to arise from phenotyping differences because the 2 cohorts shared the same DSM-based diagnostic interview for MDD. The discrepancy is unlikely to arise from genotyping differences because our 2 cohorts shared the same laboratory and technical procedures. It is unlikely that population stratification affected the results, since the linkage disequilibrium structure was very similar in the UK-European and New Zealand–European samples, and both samples differed from the original African American sample.15 In fact, the positive findings in the samples studied by Bradley et al15 and in the E-Risk Study, which presented different haplotype frequencies and different linkage disequilibrium structures, provide important transpopulational replication. This may suggest that the TAT haplotype has functional relevance, or tags functional variant(s) across populations of different geographic origins. Finally, the discrepancy is unlikely to arise from retrospective vs prospective measurement of maltreatment because, although the G × E was observed in studies using the retrospective CTQ, it was not observed when retrospective reports of maltreatment were analyzed in the Dunedin Study.
A remaining explanation for the discrepant findings concerns differences in the nature of the measures of childhood maltreatment. The CTQ taps depression-relevant emotional memories, whereas arguably the measure used in the Dunedin Study does not. The CTQ evaluates memories that elicit intense emotions in individuals with a maltreatment history through items like “I thought my parents wished I had never been born,” “Someone threatened to hurt me or tell lies about me unless I did something sexual with them,” or “I felt that someone in my family hated me.” In direct contrast, the composite measure of maltreatment in the Dunedin Study relied in part on staff observations and parental reports of childhood events that adult study members may not recall. Moreover, the Dunedin Study’s retrospective self-reported assessment of childhood maltreatment was deliberately designed to minimize respondent distress by avoiding emotional content. Items were matter-of-fact questions asked in the context of interviews on violence victimization and reproductive health. For example, computer-administered questions included “Before you turned 11, did someone touch your genitals?” and “Before you turned 11, did someone get you to touch their genitals?” Some, but not all, adults who respond positively to such factual questions also sustain emotionally laden memories about their experience. Finally, the Dunedin measure referred to the first 10 years of life, whereas the CTQ refers to both childhood and adolescence. The adolescent period may be remembered more vividly.
Two lessons may be learned about stress exposure assessment. The first lesson relates to the importance of how stress exposures are measured. In the 3 studies replicating the G × E (the original 2 samples evaluated by Bradley et al15 and the E-Risk Study), childhood maltreatment was assessed using the same measure, the CTQ. To our knowledge, this is the first instance in the history of psychiatric G × E research that stress has been ascertained by more than 1 study using the same instrument. Although comparable characterization of genotypes and diagnostic phenotypes is now routinely attainable across studies, heterogeneity in the measurement of environmental stressors across studies has been and continues to be a major obstacle to replication in G × E research. For replication, it is important that similar measures of stress are used as much as possible. Failure to take differences in exposure measurement seriously may produce seemingly inconsistent findings. Moreover, studies with different environmental measures may not be comparable and, therefore, suitable for meta-analysis. We recommend that once enough studies have accumulated in the literature, meta-regression methods can be used to compare findings from studies that use different types of stress-exposure measurement. In this way, the effect of methodological factors on the heterogeneity of G × E results can be evaluated more rigorously, paving the road to meta-analytical strategies.
The second lesson relates to the important question of how adverse experiences in childhood can result in depression that only emerges years later, during adulthood. What mediating cognitive and biological processes ensue during the decades-long span separating cause from effect? A hypothesis suggests itself: the CTQ successfully identifies individuals who have sustained memories with emotional content since childhood, such emotional memories may be necessary to precipitate MDD, and such memories may be influenced by CRHR1.
A consistent body of evidence has implicated the stress hormones, including CRH, in mediating the effects of emotional arousal on memory consolidation.16,18 The basolateral complex of the amygdala is a key area in modulating the effects of CRH on memory32 and the consolidation of memories of emotionally arousing experiences18 through projections to several brain regions. The basolateral amygdala contains a high density of CRHR1 receptors,33 and experimental evidence shows their selective blockade after stressful training impairs the consolidation of fear memory,34,35 which demonstrates the importance of this receptor in emotional memory consolidation. Although the role of the studied haplotype in the receptor functioning remains elusive, our results point in the same direction as experimental studies18 and might indicate that individuals with 2 copies of the TAT haplotype have impaired activation of fear memory consolidation processes, resulting in relatively unemotional cognitive processing of memories of childhood experiences. Such a cognitive mechanism could protect them from depression. If the hypothesis were correct, it could explain why the pattern of G × E was not found in the Dunedin Study.
The importance of negative and ruminative memories to the development of depression has long been recognized.36,37 Emotional arousal and memory consolidation, 2 processes intrinsically linked, are influenced by projections from a number of different systems, such as noradrenergic and HPA systems, that are integrated by the amygdala.16,32 The amygdala has been shown to modulate memories congruent with sad mood in individuals with a history of depression.38 Recently, amygdala activity during an emotionally loaded memory task was assessed with neuroimaging techniques.39 During the recall of negative memories, depressed individuals showed increased activity in the right amygdala, which was correlated with activity in caudate-putamen and hippocampus, demonstrating the importance of this area in the negative memories of individuals with depression and the activation of neural systems that encode affective material.39 Taken together, these findings suggest the hypothesis that the role of CRHR1 in the consolidation of aversive memories may be involved in the mediation of the pathway from childhood maltreatment to adulthood depression. This should be investigated using genetic neuroimaging designs. Moreover, the results presented herein indicate that retrospective measures can be of great value (contrary to what is commonly argued) when the aim is to measure not only the objective occurrence of events, but also the emotional memories associated with events, which may be important in the developmental etiology of depression. Unfortunately, because we do not have an objective measure of maltreatment and the CTQ in the same sample, we were unable to test this hypothesis.
Stressful experiences, especially those occurring during childhood, are linked to depression and other mental disorders. Better understanding of the processes by which adverse experiences produce distinctive patterns of response between individuals, from severe psychopathology to adaptive and even improved functioning, is fundamental to understanding and reducing mental illness. The identification of genes involved in these processes is likely to improve understanding, and to eventually improve treatment and prevention.40 Progress will be hastened by integrating the fields of epidemiology, genetics, neuroscience, and psychiatry.40–42
Acknowledgments
Funding/Support: This research received support from Medical Research Council grants G9806489, G0100527, and G0601483; National Institute of Mental Health grants MH077874, MH45070, and MH49414; the William T. Grant Foundation; and a NARSAD Young Investigator Award (Dr Polanczyk). Dr Caspi is a Royal Society–Wolfson Merit Award holder. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council.
Footnotes
Author Contributions: All authors had full access to the data. Dr Polanczyk takes responsibility for the integrity and accuracy of the data.
Financial Disclosure: None reported.
Additional Information: The study protocols were approved by the institutional review boards of the participating universities. All study members gave written informed consent before participating.
Additional Contributions: We thank the members of the E-Risk and Dunedin studies.
REFERENCES
- 1.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 3.McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EB. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997;277(17):1362–1368. [PubMed] [Google Scholar]
- 4.Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am J Psychiatry. 2006;163(6):993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- 5.Lo´pez-Leo´n S, Janssens AC, Gonzalez-Zuloeta Ladd AM, Del-Favero J, Claes SJ, Oostra BA, van Duijn CM. Meta-analyses of genetic studies on major depressive disorder. Mol Psychiatry. 2008;13(8):772–785. doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- 6.Rutter M. Genetic Effects on Environmental Vulnerability to Disease. Chichester, England: John Wiley & Sons; 2008. [Google Scholar]
- 7.Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29(4):641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC, Magee WJ. Childhood adversities and adult depression: basic patterns of association in a US national survey. Psychol Med. 1993;23(3):679–690. doi: 10.1017/s0033291700025460. [DOI] [PubMed] [Google Scholar]
- 9.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 10.Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Dev Psychopathol. 2007;19(4):1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101(49):17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widom CS, Brzustowicz LM. MAOA and the “cycle of violence”: childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60(7):684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62(5):473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 14.Mello AF, Juruena MF, Pariante CM, Tyrka AR, Price LH, Carpenter LL, Del Porto JA. Depression and stress: is there an endophenotype? [in Portuguese] Rev Bras Psiquiatr. 2007;29(suppl 1):S13–S18. doi: 10.1590/s1516-44462007000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steckler T, Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry. 1999;46(11):1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 17.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropinreleasing factor. Pharmacol Rev. 1991;43(4):425–473. [PubMed] [Google Scholar]
- 18.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 19.Merikangas KR, Risch N. Will the genomics revolution revolutionize psychiatry? Am J Psychiatry. 2003;160(4):625–635. doi: 10.1176/appi.ajp.160.4.625. [DOI] [PubMed] [Google Scholar]
- 20.Moffitt TE E-Risk Study Team. Teen-aged mothers in contemporary Britain. J Child Psychol Psychiatry. 2002;43(6):727–742. doi: 10.1111/1469-7610.00082. [DOI] [PubMed] [Google Scholar]
- 21.Moffitt TE, Caspi A, Rutter M, Silva PA. Sex Differences in Antisocial Behaviour: Conduct Disorder, Delinquency, and Violence in the Dunedin Longitudinal Study. Cambridge, England: Cambridge University Press; 2008. [Google Scholar]
- 22.Bernstein D, Fink L. Childhood Trauma Questionnaire Manual. San Antonio, TX: The Psychological Corp; 1998. [Google Scholar]
- 23.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 24.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65(4):409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 26.Robins LN, Cottler L, Bucholz KK, Compton W. Diagnostic Interview Schedule for DSM-IV. St Louis, Mo: Washington University School of Medicine; 1995. [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 28.Robins LN, Helzer JE, Cottler L, Goldring E. Diagnostic Interview Schedule, Version III-R. St Louis, MO: Washington University School of Medicine; 1989. [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1987. revised. [Google Scholar]
- 30.Lake SL, Lyon H, Tantisira K, Silverman EK, Weiss ST, Laird NM, Schaid DJ. Estimation and tests of haplotype-environment interaction when linkage phase is ambiguous. Hum Hered. 2003;55(1):56–65. doi: 10.1159/000071811. [DOI] [PubMed] [Google Scholar]
- 31.Satten GA, Epstein MP. Comparison of prospective and retrospective methods for haplotype inference in case-control studies. Genet Epidemiol. 2004;27(3):192–201. doi: 10.1002/gepi.20020. [DOI] [PubMed] [Google Scholar]
- 32.McGaugh JL. Memory—a century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like im-munoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420(3):305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150(4):818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci U S A. 2002;99(21):13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradley BP, Mogg K, Williams R. Implicit and explicit memory for emotioncongruent information in clinical depression and anxiety. Behav Res Ther. 1995;33(7):755–770. doi: 10.1016/0005-7967(95)00029-w. [DOI] [PubMed] [Google Scholar]
- 37.Watkins PC, Mathews A, Williamson DA, Fuller RD. Mood-congruent memory in depression: emotional priming or elaboration? J Abnorm Psychol. 1992;101(3):581–586. doi: 10.1037//0021-843x.101.3.581. [DOI] [PubMed] [Google Scholar]
- 38.Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol Psychiatry. 2007;61(2):231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63(12):1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet. 2008;9(7):527–540. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- 41.An unnecessary battle. Nature. 2008;454(7201):137–138. doi: 10.1038/454137b. [DOI] [PubMed] [Google Scholar]
- 42.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7(7):583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]