Abstract
In the present study, we demonstrated the utility of the nonmammalian model system Galleria mellonella for studying the pathogenesis of Staphylococcus aureus infection. By use of clinical and laboratory strains that had been exposed to vancomycin, we showed that both agr functional status and vancomycin minimum inhibitory concentration are determinants associated with the virulence of S. aureus in G. mellonella. These results show that G. mellonella can be effectively used to facilitate the in vivo study of S. aureus virulence and, more specifically, the relationship between antibiotic drug resistance and the pathogenesis of infection.
The emergence of methicillin-resistant Staphylococcus aureus (MRSA) in both the hospital and community setting is concerning and has led to necessary changes in the empirical prescribing of antibiotics in health care institutions worldwide. In most institutions, vancomycin remains the first-line antibiotic for MRSA infection. Not surprisingly, staphylococcal strains with reduced susceptibility to vancomycin are increasingly being reported. To date, frank resistance to van comycin is rare; however, true intermediate resistance (MIC ≥ 4 μg/mL; vancomycin-intermediate S. aureus [VISA]) and vancomycin heteroresistance (heteroresistant VISA [hVISA]) are encountered [1–4].
Serious infections caused by hVISA/VISA strains are associated with poor clinical outcomes [1, 5]. However, these outcomes are confounded by the inherent nature of hVISA/VISA infections, which are often associated with prosthetic material, deep-seated abscesses, and endocarditis [1,5]. Surprisingly, such patients can have very prolonged periods of bacteremia (up to 87 days has been reported) [5] without acute clinical instability. Moreover, patients with staphylococcal bacteremia have been shown to be significantly less likely to suffer shock as the vancomycin MIC increases from 1 to 2 μg/mL [6]. Thus, an interrelationship between staphylococcal susceptibility to vancomycin and pathogenicity is plausible. In support of that hypothesis, dysfunction of an important global virulence regulator in S. aureus, the accessory gene regulator (agr), has been associated with the emergence of the hVISA/VISA phenotype [4, 7], thus highlighting the potential interaction between virulence and susceptibility to vancomycin.
In the present study, we used the nonmammalian model system Galleria mellonella to identify the pathogenic consequences of reduced vancomycin susceptibility in S. aureus infection. G. mellonella is a wax moth that has been used to study hostpathogen interactions for a range of organisms, including Pseudomonas aeruginosa, Burkholderia cepacia, Proteus mirabilis, Bacillus cereus, Francisella tularensis, and several pathogenic fungi [8–10]. Importantly, a correlation has been established between the virulence of an organism in G. mellonella and that in mammalian models [9, 10]. Finally, the G. mellonella model is well suited to the study of human pathogens, because it can be maintained at temperatures of 37°C. Thus, we studied the utility of the G. mellonella model for assessing the virulence of S. aureus and, in particular, the pathogenic consequences of reduced susceptibility to vancomycin.
Methods
The bacterial strains used in this study are shown in table 1. Susceptibility to vancomycin was determined previously by agar dilution, according to Clinical and Laboratory Standards Institute guidelines. All isolates within each series were indistinguishable by pulsed-field gel electrophoresis [4]. RN6607 is a methicillin-susceptible, agr group II prototype S. aureus strain, and RN9120 is its isogenic agr knockout mutant [11]. The in vitro passage of strains on vancomycin, described elsewhere [7], gave rise to RN6607V, RN9120V, and RN9120-VISA (table 1). The agr function was determined by δ-hemolysin activity, as reported elsewhere [4]. For growth kinetic experiments, an overnight culture was diluted 1:1000 in 200 μL of brain-heart infusion broth in the wells of a 96-well plate and incubated at 37°C. Eight replicate wells were used for each strain, and optical density at 600 nm (OD600) was recorded for 24 h. Growth kinetic experiments were performed twice.
Table 1.
Bacterial strains used in this study with their respective clinical sites of isolation, vancomycin MICs, and accessory gene regulator (agr) function.
| Strain | Site of isolation | Vancomycin MIC, μg/mL | agr functiona |
|---|---|---|---|
| Clinical pairs | |||
| Pair 1 | |||
| A8090b (MRSA) | Blood | 1 | – |
| A8094 | 8 | – | |
| Pair 2 | |||
| A9635 (MSSA) | Blood | 1 | – |
| A9639 | 4 | – | |
| Pair 3 | |||
| A5937 (MRSA) | Blood and porcine aortic valve | 1 | + |
| A5940 | 2 | – | |
| Pair 3 | |||
| A6224 | Blood | 2 | – |
| A6226 | 3 | – | |
| Laboratory strains | |||
| agr+ pair | |||
| RN6607 (agr+) | … | 1 | + |
| RN6607V | 2 | + | |
| agr− series | |||
| RN9120 (agr−) | … | 1 | – |
| RN9120V | 2 | – | |
| RN9120-VISA | 8 | – | |
| ATCC strain 29213 | … | 1 | … |
For the G. mellonella killing assays, caterpillars in the final instar larval stage (Vanderhorst) were stored in the dark and used within 7 days after shipment. Caterpillars 250–350 mg in weight were used in all assays. Sixteen randomly chosen caterpillars were used for each group in an experiment. Before inoculation into G. mellonella, bacterial cells were washed with PBS and then diluted to an appropriate cell density, as determined by OD600. A 10-μL Hamilton syringe was used to inject 10-μL aliquots of the inoculum into the hemocoel of each caterpillar via the last left proleg (figure 1) [9]. Bacterial colony counts on Luria-Bertani agar were used to confirm all inocula. After injection, caterpillars were incubated in plastic containers, and the number of dead caterpillars was scored daily. Caterpillars were considered dead when they displayed no movement in response to touch.
Figure 1.

Inoculation of Galleria mellonella caterpillars with Staphylococcus aureus.
For all experiments, 2 control groups were used. The first group included caterpillars that were inoculated with PBS to monitor for killing due to physical trauma, and the second group included caterpillars that received no injection. Experiments that had >2 dead caterpillars in either control group were discarded and repeated. For simplicity, the control groups are not included in the figures. Survival curves were plotted using the Kaplan-Meier method, and differences in survival were calculated using the log-rank test (Stata software; version 6). Differences were considered statistically significant at P < .05. Atleast 3 independent experiments were performed for all G. mellonella killing experiments. Representative experiments are presented.
Results
As shown in figure 2A, inoculation of G. mellonella with a reference strain of S. aureus (ATCC 29213) resulted in rapid killing of the caterpillars. Killing was significantly dependent on the number of S. aureus cells injected (figure 2A) as well as the incubation temperature after inoculation. The rate of killing was slower at 30°C (figure 2B) than at 37°C (figure 2A). Thus, for further experiments we used inocula of 1 × 105−1 × 106 cfu/larva and incubated the infected G. mellonella at 37°C To evaluate whether G. mellonella killing was caused by a noninfectious reaction, such as that due to staphylococcal cell wall components, we determined the survival of caterpillars after injection of heat-killed S. aureus. No killing was observed in this group (data not shown). Melanization is also part of the infection process in insects [8], and during the course of S. aureus infection caterpillars demonstrated obvious signs of melanization (figure 2C and figure 2D).
Figure 2.
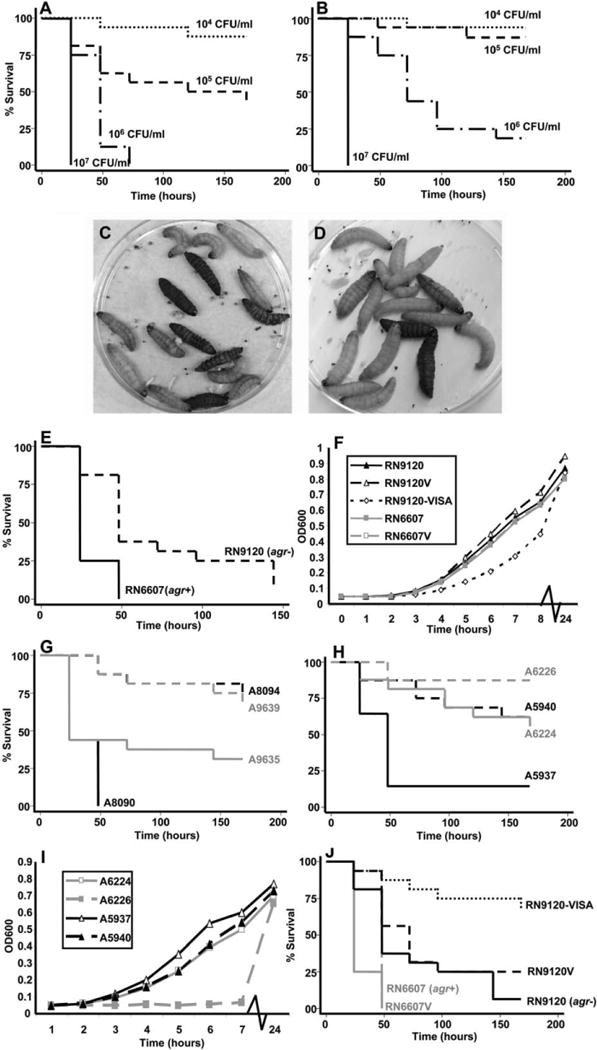
Effects of reduced susceptibility to vancomycin on the pathogenesis of Staphylococcus aureus infection in Galleria mellonella. The killing of G. mellonella was dependent on the injected S. aureus inoculum and the temperature of incubation, with greater killing observed at 37°C (A) than at 30°C (B). Progressive melanization of G. mellonella was evident after S. aureus infection (C and D) and was more profound in a vancomycin-susceptible strain (A8090) (C) than in its vancomycin-nonsusceptible derivative (A8094) (D) at the same time point (18 h). A S. aureus accessory gene regulator (agr) knockout mutant (RN9120) caused significantly less killing than its isogenic parent strain (RN6607) (P< .001) (E). Growth kinetics of the laboratory strains RN6607 and RN9120 and their vancomycin-exposed derivatives were measured by optical density at 600 nm (OD600) (F). The killing of G. mellonella was significantly attenuated after infection with a vancomycin-nonsusceptible S. aureus strain compared with an isogenic, vancomycin-susceptible progenitor strain (for A8094 vs. A8090, P < .001; for A9639 vs. A9635, P < .05; for A5940 vs. A5937, P < .01; for A6226 vs. A6224, P < .05) (G and H). The growth kinetics of clinical pairs A6224/A6226 and A5937/A5940 were measured by OD600(I). Infection of G. mellonella with laboratory strains of S. aureus that were exposed to vancomycin showed that derivatives of the agr− mutant (RN9120V and RN9120-VISA) were significantly attenuated in their killing compared with the derivative of the agr+ strain (RN6607V) (P < .01) (J). In an agr− background (RN9120), attenuation of virulence was correlated with the vancomycin MIC.
To confirm the utility of G. mellonella as a model to study the virulence of S. aureus, we assessed the effects of dysfunction in the global virulence regulator agr on killing by staphylococci in G. mellonella. This virulence regulator has been shown to be important in a range of hosts, including mammals [12]. As shown in figure 2E, infection with an agr knockout mutant (RN9120) caused significantly less killing than its agr+ parent strain (RN6607) (P < .01), thus highlighting the importance of this virulence regulator in the pathogenesis of S. aureus infection in G. mellonella. The growth kinetics of these isogenic strains were comparable (figure 2F).
To assess the pathogenic consequences of reduced susceptibility to vancomycin in S. aureus, we used a carefully selected sample of isogenic clinical pairs of strains from patients who had been exposed to vancomycin (table 1). All pairs demonstrated the same pattern, whereby the strain with the elevated vancomycin MIC was significantly less virulent than its vancomycin-susceptible progenitor when injected at a similar inoculum into G. mellonella (figure 2G. and figure 2H). Interestingly, the degree of difference in killing was proportional to the difference in the vancomycin MIC. For example, the pair A8090/A8094 (1 and 8 μg/mL, respectively) showed the greatest difference in G. mellonella killing between the susceptible and resistant isolates (figure 2G). The attenuation in G. mellonella killing by the less susceptible of each clinical pair was similar whether the initial progenitor strain was methicillin susceptible (A9635) or methicillin resistant (all others). The functional agr activity for each strain is shown in table 1.
Given the known association between slower growth kinetics and the hVISA/VISA phenotype [13], we assessed the growth rates of all included strains. At 24 h, all pairs reached a similar OD600 value (figures 2I and 3). Interestingly, the pair with the greatest difference in growth at earlier time points (A6224/A6226) (figure 2I) had the smallest difference in killing between the susceptible and nonsusceptible strains (figure 2H). Moreover, a pair that had similar growth over 24 h (A5937/A5940) (figure 2I) exhibited a larger difference in killing (figure 2H). Moreover, in contrast to A5937 (susceptible progenitor), A5940 had evidence of agr dysfunction, providing further support that agr dysfunction significantly attenuates virulence without necessarily affecting the growth of S. aureus in vitro.
Figure 3.

Growth kinetics of clinical Staphylococcus aureus pairs A8090/A8094 and A9635/A9639.
To confirm our observations using paired clinical isolates, we assessed the effects of laboratory derived hVISA/VISA strains in the G. mellonella infection model. This allowed control of both antibiotic exposure (vancomycin only) and agr function. As has been shown previously, the vancomycin MIC increases significantly more with an agr knockout mutant (RN9120, RN9120V, and RN9120-VISA) than with its parent strain (RN6607 and RN6607V) during in vitro vancomycin exposure (table 1) [7]. When inoculated into G. mellonella, the attenuation in virulence was significantly more pronounced in the derivatives of the agr knockout strain (RN9120V and RN9120-VISA) than in its vancomycin-exposed agr+ parent (RN6607V) (figure 2J). Similar to what we observed using the clinical strains, as the vancomycin MIC increased (RN9120 to RN9120-VISA), the degree of G. mellonella killing significantly decreased (P < .001) (figure 2J). Thus, these experiments support a role for both agr functional status and for vancomycin MIC as correlates of S. aureus virulence in G. mellonella. At 24 h, all vancomycin-exposed laboratory strains had a similar OD600 value (figure 2F).
Discussion
This report describes, for the first time, the utility of the greater wax moth, G. mellonella, as amodelto studythe virulence of S. aureus. Using both clinical and laboratory strains, we have demonstrated that with the evolution of reduced susceptibility to vancomycin, the virulence of S. aureus becomes attenuated. The degree to which virulence is attenuated appears to be proportional to the vancomycin MIC.
The mechanism of this attenuation in virulence is likely multifactorial. First, as our results show, functional loss of the agr global regulator is associated with impaired virulence in the G. mellonella model. The agr locus of S. aureus is a quorum-sensing gene cluster that up-regulates production of secreted virulence factors, including the α-, β-, and δ-hemolysins [4], and has been shown to be important for S. aureus virulence in mammals [12]. Furthermore, the association between loss of agr function and reduced susceptibility to vancomycin in S. aureus has now been well described [4]. The G. mellonella survival curve analysis of pairs A5937/A5940 (figure 2H) and RN6607/RN9120 (figure 2E), all of which had very similar growth kinetics, strongly supports a role for agr dysfunction in attenuating virulence in S. aureus. However, the progenitor strains from the other clinical pairs had evidence of impaired agr function (table 1). Thus, apart from agr, other genes that may affect S. aureus virulence are likely involved in the genetic evolution of the hVISA/VISA phenotype, and these may include mutations in vraSR and graR[14].
We also demonstrated that some S. aureus strains that are less susceptible to vancomycin have impaired growth during the first few hours of incubation. The structural and metabolic changes associated with the development of a hVISA/VISA strain are probably costly to S. aureus fitness. However, although slower growth may contribute to attenuated virulence, it is clear from our findings that it is not the full explanation. More specifically, the clinical pair with the greatest difference in growth kinetics (A6224/A6226) had the smallest difference in killing of G. mellonella, and, conversely, the pair with minimal difference in growth (A5937/A5940) had larger differences in G. mellonella killing (figure 2H and 2I).
To date, the mechanism of G. mellonella killing by S. aureus is unknown. The insect has cellular and humoral immune response pathways that are mediated by hemocytes and antimicrobial peptides, respectively. Interestingly, in preliminary experiments, we observed a higher staphylococcal burden in tissue at 8 h after inoculation for the more pathogenic A8090 strain than for its less pathogenic pair (A8094), suggesting that host immune responses are probably important for the differences in G. mellonella killing by S. aureus. Further research on host response is ongoing.
In conclusion, we have demonstrated the utility of the nonmammalian model system, G. mellonella, for assessing the virulence of S. aureus. Using an agr knockout mutant, we have shown an important correlation between the pathogenesis of S. aureus infection in G. mellonella and that seen in mammalian models. Finally, in all strains studied that have developed the hVISA/VISA phenotype, either in vitro or in clinical circumstances, virulence toward G. mellonella is attenuated as the vancomycin MIC increases.
Acknowledgments
Financial support: National Institutes of Health (K08 award AI63084 to E.M.); Ellison Medical Foundation (New Scholar Award in Global Infectious Diseases to E.M.). This work was supported through a research grant from Cubist Pharmaceuticals (grant to G.M.E.).
Footnotes
Potential conflicts of interest: G.M.E. has served as a consultant for and has received research funding support from Cubist Pharmaceuticals and Pfizer; R.C.M. has served as consultant for Pfizer, Cubist, and Wyeth and has received research funding support from Cubist Pharmaceuticals; E.M. has served as a consultant for Biogen Idec, has received research support from Astellas Pharma, and is a member of the speakers’ bureau for Pfizer. All other authors report no potential conflicts.
References
- 1.Howden BP, Ward PB, Charles PG, et al. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis. 2004;38:521–8. doi: 10.1086/381202. [DOI] [PubMed] [Google Scholar]
- 2.Hiramatsu K, Aritaka N, Hanaki H, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–3. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 3.Mwangi MM, Wu SW, Zhou Y, et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci USA. 2007;104:9451–6. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakoulas G, Eliopoulos GM, Moellering RC, Jr, et al. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 2002;46:1492–502. doi: 10.1128/AAC.46.5.1492-1502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004;38:448–51. doi: 10.1086/381093. [DOI] [PubMed] [Google Scholar]
- 6.Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 7.Sakoulas G, Eliopoulos GM, Fowler VG, Jr, et al. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob Agents Chemother. 2005;49:2687–92. doi: 10.1128/AAC.49.7.2687-2692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aperis G, Fuchs BB, Anderson CA, Warner JE, Calderwood SB, Mylonakis E. Galleria mellonella as a model host to study infection by the Francisella tularensis live vaccine strain. Microbes Infect. 2007;9:729–34. doi: 10.1016/j.micinf.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mylonakis E, Moreno R, El Khoury JB, et al. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun. 2005;73:3842–50. doi: 10.1128/IAI.73.7.3842-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jander G, Rahme LG, Ausubel FM. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J Bacteriol. 2000;182:3843–5. doi: 10.1128/jb.182.13.3843-3845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyon GJ, Mayville P, Muir TW, Novick RP. Rational design of a global inhibitor of the virulence response in Staphylococcus aureus, based in part on localization of the site of inhibition to the receptor-histidine kinase, AgrC. Proc Natl Acad Sci USA. 2000;97:13330–5. doi: 10.1073/pnas.97.24.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelnour A, Arvidson S, Bremell T, Ryden C, Tarkowski A. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun. 1993;61:3879–85. doi: 10.1128/iai.61.9.3879-3885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sieradzki K, Leski T, Dick J, Borio L, Tomasz A. Evolution of a vancomycin-intermediate Staphylococcus aureus strain in vivo: multiple changes in the antibiotic resistance phenotypes of a single lineage of methicillin-resistant S. aureus under the impact of antibiotics administered for chemotherapy. J Clin Microbiol. 2003;41:1687–93. doi: 10.1128/JCM.41.4.1687-1693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neoh HM, Cui L, Yuzawa H, Takeuchi F, Matsuo M, Hiramatsu K. Mutated response regulator graR is responsible for phenotypic conversion of Staphylococcus aureus from heterogeneous vancomycin-intermediate resistanceto vancomycin-intermediate resistance. Antimicrob Agents Chemother. 2008;52:45–53. doi: 10.1128/AAC.00534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


