Summary
Background
A poor biological response to clopidogrel is associated with an increased risk of major cardiovascular ischemic events (MACE). Paraoxonase 1 (PON1) enzyme activity is modulated by the PON1-Q192R variant (rs662) and was recently suggested to be strongly involved in clopidogrel bioactivation, but the influence of the PON1-Q192R variant on the risk of MACE in clopidogrel-treated patients is controversial.
Objectives
To determine whether the PON1-Q192R variant influences clopidogrel biological responsiveness and the risk of MACE in patients treated with clopidogrel.
Methods
Systematic review and meta-analysis of studies of the association between the PON1-Q192R polymorphism and the biological response to clopidogrel and/or the risk of MACE during clopidogrel administration.
Results
Seventeen studies were included. In the 12 studies of the biological response to clopidogrel (n = 5302 patients), there was no significant difference between 192QQ and 192QR + 192RR subjects, whatever the laboratory method used (global mean standardized difference = 0.10 [−0.06; 0.25], P = 0.22). Eleven studies assessed the risk of MACE, four using a case–control design (n = 2739 patients) and seven a prospective design (n = 5353 patients). Overall, MACE occurred in 19% of patients in case–control studies and in 6% of patients in prospective cohort studies, with no significant difference between 192QQ and 192QR + 192RR patients (OR = 1.28 [0.97; 1.68], P = 0.08). Similar results were obtained when study design was taken into account. Heterogeneity was mainly driven by one publication.
Conclusions
This meta-analysis suggests that the PON1-Q192R polymorphism has no major impact on the risk of MACE and does not alter the biological response to clopidogrel in clopidogrel-treated patients.
Keywords: clopidogrel, ischemic events, paraoxonase-1, platelet function
Antiplatelet drugs are part of the recommended first-line treatment for atherothrombosis [1,2]. Current guidelines recommend a combination of aspirin and clopidogrel to prevent recurrent ischemic events in patients with an acute coronary syndrome (ACS) and for patients undergoing percutaneous coronary interventions (PCI) [3]. However, the biological response to clopidogrel is highly variable [4], and poor responsiveness is associated with a higher risk of recurrent ischemic events in cardiovascular patients [5]. This variability is tightly linked to the efficiency of the biological process through which clopidogrel, a pro-drug, is activated. Indeed, clopidogrel requires enzymatic bioactivation into its active thiol metabolite before interacting with the P2Y12 receptor on blood platelets. Clopidogrel bioactivation is a two-step process in which the cytochrome P450 (CYP) system, especially isoenzyme CYP2C19, plays a major role [6]. Several CYP2C19 gene variants modulate the biological response to clopidogrel [7-9] and the CYP2C19*2 [rs4244285] that leads to a loss-of-function phenotype has been linked to an increased risk of cardiovascular events [10]. Yet this polymorphism explains < 10% of the observed variability of clopidogrel responsiveness in cardiovascular patients [8,11,12], which is at odds with the marked heritability of this phenotype (h2 = 0.73) observed in a familial study [13].
More recently, paraoxonase-1 (PON1), an esterase synthesized in the liver and present in the serum, was reported to be strongly involved in clopidogrel bioactivation. A PON1 genetic variant (Q192R, rs662, A576G) was reported to account for > 70% of the variability of clopidogrel responsiveness, with the PON1-192Q allele being associated with an increased risk of recurrent ischemic events in clopidogrel-treated patients. Of note, the authors found no evidence for the involvement of CYP2C19 in any of the steps of clopidogrel metabolism [14]. However, these associations were recently challenged by the results of several independent studies that showed no influence of the Q192R polymorphism on the biological response to clopidogrel or on the risk of ischemic events. Some studies have shown a trend towards an increased risk of ischemic events [15,16], or significantly increased platelet reactivity, in 192QQ patients [17]. As the failure of most studies to show clinical or biological effect of the PON1-Q192R genotype might have been due to a lack of statistical power, we conducted a systematic review of the literature and meta-analysis of summarized data to determine the influence of the PON1-Q192R polymorphism on clopidogrel biological responsiveness and the risk of recurrent ischemic events in clopidogrel-treated cardiovascular patients.
Methods
We followed published guidelines for meta-analyses of observational studies and their reporting [18].
Search strategy
The search was based on electronic databases (Medline, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials), and abstracts of international meetings held in 2011, with no limitations on the type of publication or language. The electronic database search was last updated on 10 January 2012. A free-text search was conducted will the following key-word combination: (‘PON1’ or ‘PON-1’ or ‘paraoxonase’) and ‘clopidogrel’. Articles were selected on the basis of the abstract, before examining the full text. In addition, the reference lists of selected articles were hand-searched to identify additional relevant reports. The reviewers were not blinded to the journal, authors or institution of the publications, as this has been shown to be unnecessary [19].
Inclusion criteria
Data extraction
To assess the specificity and characteristics of the identified studies, we abstracted information from each report on the year of publication, the total sample size, the study design, the duration and completeness of follow-up in prospective cohort studies, the type of clinical endpoint, blinding of clinicians to PON1 genotyping results, and study population characteristics (consecutive inclusions, age, sex, vascular risk factors, patients with acute events or stable ischemic disease, and drug compliance monitoring).
Exposed patients were defined as 192QQ. This arbitrary choice was related to the lower frequency of the R allele. Clinical outcome was defined in terms of major adverse cardiovascular events (MACE), including stent thrombosis, acute coronary syndromes, myocardial infarction and revascularization, and was expressed as the number of patients concerned in each genotype group. These numbers were extracted from the articles to prepare 2 × 2 tables. Biological outcome was defined in terms of platelet function test results, expressed as continuous variables. When residual platelet reactivity was evaluated at several time-points in pharmacodynamic studies, we included only the time-point closest to the loading dose in order to minimize heterogeneity. When two or more platelet reactivity tests were used in a given study, we only included the results of the test most specific for the inhibition of the P2Y12 receptor by clopidogrel (VASP > Verify-NowP2Y12 > ADP-induced aggregation > other aggregation-based assays). When different ADP concentrations were used for ADP-induced aggregation, we selected the concentration (20 μm) most frequently used in clinical studies, again to minimize heterogeneity. When biological test data were available as the mean and standard deviation (SD) for each of the three PON1 genotypes (192QQ, 192QR and 192RR), we used the Huygens theorem (decomposition of the total variance into an intra-stratum and an extra-stratum variance) to compute the mean and SD for the 192QQ group and the 192QR + 192RR group. When the published data were not sufficient to compute the 2 × 2 table for MACE or when the mean and SD of platelet function test results were not provided, the corresponding author was contacted and kindly requested to provide the required summarized data.
Two authors (PF and JLR) each independently implemented the search strategy, selected the studies and recorded the abstracted data. Disagreements on the study selection or data extraction were resolved by discussion among three authors (CC, PF and JLR).
Assessment of study quality
To estimate the risk of bias, we predefined a customized quality assessment tool based on eight methodological items that were evaluated on a scale from A to C [5]. The mode of patient inclusion was ranked A if consecutive and B if not consecutive or unclear. Quality of follow-up was based on the percentage of patients lost to follow-up (A, < 10%; B, 10–20%; C, more than 20%), identical follow-up and identical clopidogrel treatment for all included patients (A, yes; B, unclear; C, not identical), and evaluation of compliance (A, evaluated or in-hospital study with no clinical outcome; B, unclear; C, not evaluated). Two modes of evaluation were considered: blinding of clinicians to the PON1 genotype (A, blinded; B, unclear or not blinded) and adjudication of clinical events by independent clinicians (A, yes; B, unclear or no independent adjudication).
The overall quality of studies was defined as follows: if a study had one C or more, its overall quality was C. If all items were rated A, then overall quality was A. All other studies were rated B.
Statistical analysis
Descriptive data on the patients were recorded for each study as the mean and standard deviation (SD) or percentage.
The odds ratio (OR) for ischemic events associated with the 192QQ genotype was assessed in each study, along with its 95% confidence interval. The global OR was computed by using the method of the inverse of the variance with random effects [20], and the hypothesis OR = 1 was tested. Heterogeneity was tested with the Cochran Q statistic and measured with the I-squared statistic (I2). The analysis was stratified for the case-control or prospective cohort study design. In case of heterogeneity (Cochran’s test with P < 0.10 or I2 > 50%), predefined potential factors of heterogeneity were tested by meta-regression [21] or by comparing subgroups with random effects. For each study the candidate factors included the design, mean age, sex ratio, prevalence of diabetes, mean BMI, length of follow-up in prospective studies, frequency of stent thrombosis, and clinical setting (acute vs. stable patients).
To test for an association between platelet reactivity using different platelet function assays and the PON1 genotype, the standardized mean difference was used for meta-analysis; this was preferred to the mean difference because the test results are from different assays and were originally expressed with different units on different scales. The pooled standardized mean difference was computed for each type of platelet function assay by using the method of the inverse of the variance with random effects. As described above, in case of heterogeneity, predefined potential factors of heterogeneity were tested, including the type of platelet function assay.
Sensitivity analyses were also performed for both the global OR for MACE and the global standardized mean difference for platelet reactivity assays: (i) the pooled results, I2 and Cochran’s test were assessed n times, the studies being removed one by one; (ii) the pooled results were assessed using a dominant model (192QQ + 192QR vs. 192RR) and comparing homozygotes (192QQ vs. 192RR); and (iii) to check the robustness of the overall results of the meta-analysis with respect to studies with missing summarized data (not included in the meta-analysis), two separate meta-analyses were performed with extreme assumptions for the OR or the standardized mean difference.
The potential publication bias was explored for both the OR and the standardized mean difference by visual interpretation of the funnel plot [22], and the asymmetry of the funnel plot was checked with Egger’s test [23]. The impact of funnel plot asymmetry on the results of the meta-analyses was evaluated by using the trim-and-fill method, which consists of adding missing studies virtually in order to obtain symmetry, and assessing the pooled results, including those of these missing studies [24].
Data were analyzed by using Comprehensive Meta Analysis Version 2 (BioStat, Englewood, NJ, USA) and Review Manager (RevMan) Version 5.1. (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011). Significance was assumed at P < 0.05 in all analyses.
Results
Selection and characteristics of the studies
The flow of references through the review is shown in Fig. 1. There were 11 449 patients enrolled in the 17 selected studies [12,14-17,25-36], which were all published in 2011 or 2012, most data being derived from existing cohorts. Details of the 17 studies are shown in Table 1. Excepting the few studies of healthy subjects, the studies were quite homogenous with respect to the patients’ mean age (61–68 years in most studies), mean BMI (26–30 kg m−2), prevalence of diabetes (21–30%), length of follow-up (≥ 12 months in all but one of the prospective cohort studies) and the PON1-192Q allelic frequency (0.64–0.73). In contrast, the studies differed markedly with respect to the type of assay used to assess platelet reactivity and the clinical setting (acute coronary syndrome or stable disease).
Fig. 1.
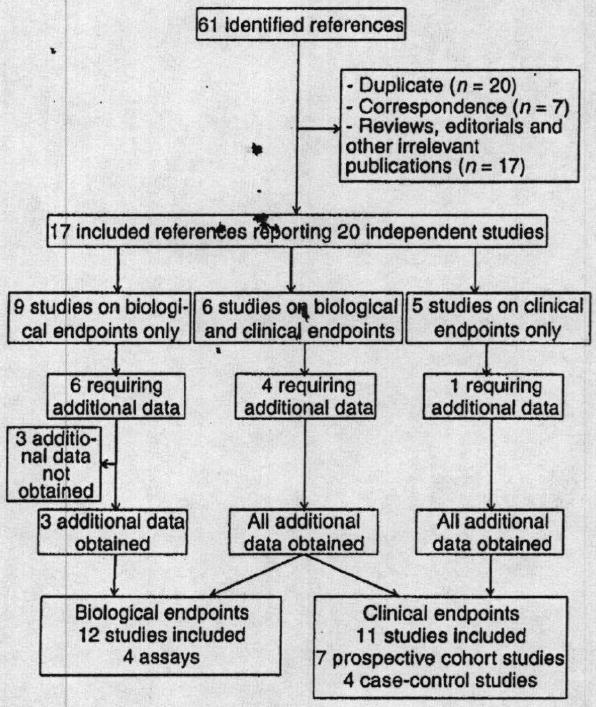
Flow chart of the meta-analysis.
Table 1.
Main characteristics of published studies
| Study | n | Design | Mean age (y) | Male (%) | Diabetics (%) | BMI (kg m−2) | Clinical setting | % of ST | Follow-up (months) | Platelet reactivity assay | % of poor responders | 192Q allele frequency (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bouman 2011a | 112 | Case-cohort | 61.2 ± 8.5 | 79.5 | 25.9 | 27.0 + 3.2 | 41 ST vs. 71 controls | 37 | 18 | LTA (ADP 20 μm) | NA | 66.9 |
| Bouman 2011b | 1982 | Cohort | 62.2 ± 10.2 | 71.3 | 24.3 | 27.1 + 4.3 | ACS | 2.2 | 12 | No | NA | 63.9 |
| Campo 2011 | 300/281 | Cohort | 66 ± 13 | 77.0 | 23.7 | 27.0 ± 4 | PCI (61% NSTEMI) | 1.3 | 12 | VerifyNow | 13 | 66.7 |
| Cayla 2011 | 123/246 | Case-cohort | 62.7 ± 12.2 | 80.5 | 25.2 | 26.6 ± 4.1 | ST vs. non-ST | 50 | – | No | – | 67.1 |
| Cuisset 2011 | 482 | Cohort | 63.0 ± 10 | 81.0 | 28.0 | 26.3 ± 4.0 | ACS | – | No | LTA (ADP 10 μm) and VASP | NA | 69.9 |
| Delaney 2012 | 693 | Case-control | 68.3 ± 11.7 | 63.5 | 34.8 | 30.3 ± 6.4 | MI and PCI patients | 1.7 | – | – | – | 69.0 |
| Dery 2011 | 213 | Cohort | 61.1 ± 9.8 | 83.1 | 9.8 | 28 ± 4.2 | 30 days post-PCI | – | – | LTA (ADP 5 μm), VASP | NA | 70.2 |
| Fontana 2011 | 538 | Cohort | 62 ± 12.2 | 81.9 | 21.2 | 26.5 ± 3.9 | Stable | – | – | LTA (ADP 20 μm), VASP | 49.6 (VAS P) | 70.8 |
| Hulot 2011 | 371 | Cohort | 40.3 ± 5.5 | 84.6 | 10.5 | 25.9 ± 4 | Stable young survivors of MI | 4.8 | 96 | VerifyNow | NA | 65.2 |
| Lewis 2011a | 566 | Cohort | 45.5 ± 13.2 | 48.7 | 0.9 | 27.0 ± 4.7 | Healthy subjects | – | – | LTA (ADP 20 μm) | NA | 68.0 |
| Lewis 2011b | 210 | Cohort | 64.3 ± 11.4 | 59.9 | 36.6 | 30.4 ± 6.6 | Non-emergent PCI | NA | 12 | LTA (ADP 20 μm) in 180 patients | NA | NA |
| Rideg 2011 | 189 | Cohort | 61.8 ± 8.4 | 61.4 | 37.6 | 29.4 ± 5.2 | Non-emergent PCI | 0.5 | 12 | LTA (ADP 5 μm) and VASP | NA | 70.9 |
| Sibbing 2011a* | 1524 | Cohort | 67.5 ± 10.5 | 77.4 | 28.2 | 27.5 ± 4.4 | PCI (67% stable) | – | – | MEA | 20 | 72.7 |
| Sibbing 2011b | 127/1439 | Case-control | 67.6 ± 10.5 | 77.5 | 29.0 | NA | Cases with ST | 8.1 | – | No | – | NA |
| Simon 2011 | 1538 | Cohort | 64.4 ± 13.1 | 74.7 | 29.7 | 27.4 ± 4.7 | ACS with PCI only | NA | 12 | – | – | 66.7 |
| Trenk 2011 | 760 | Cohort | 66.3 ± 9.2 | 78.3 | 24.3 | 27.7 ± 3.9 | Elective PCI | 2.1 | 12 | LTA (ADP 5 and 20 μm), FACS | NA | 70.5 |
| Tselepis 2011 | 74 | Cohort | 63.3 ± 8.6 | 70.3 | NA | NA | ACS | – | – | LTA (ADP 5 and 10 μm), VASP, FACS | 23 (at 5 days with VASP) | 70.3 |
ACS, acute coronary syndrome; ADP, adenosine diphosphate; LTA, light transmission aggregometry; MI, myocardial infarction; NSTEMI, non-ST elevation myocardial infarction; PCI, percutaneous coronary intervention; ST, stent thrombosis.
Used for platelet reactivity data only.
Regarding methodological quality (Table 2), it was unclear in most studies whether or not compliance was assessed and whether an independent adjudicating committee was used for MACE. Patient follow-up and the clopidogrel regimen were identical within each prospective cohort study but one, and losses to follow-up were lower than 3% in all prospective cohort studies, apart from one study in which follow-up information was unclear.
Table 2.
Methodological quality assessment and risk of bias
| Study | Consecutive patients | Losses to follow-up (%) | Identical follow-up and clopidogrel treatment | Assessment of compliance | Clinician blinding to platelet reactivity | Independent adjudicating committee | Quality score |
|---|---|---|---|---|---|---|---|
| Bouman 2011a | Unclear | – | – | Unclear | – | Unclear | B |
| Bouman 2011b | Unclear | 2.8 | Yes | Unclear | – | Unclear | B |
| Campo 2011 | Unclear | 0 | Yes | Unclear | Unclear | Unclear | B |
| Cayla 2011 | Unclear | – | – | Yes | – | No | B |
| Cuisset 201l | Unclear | – | – | Yes | – | – | B |
| Delaney 2012 | Yes | – | – | Unclear | – | Yes | B |
| Dery 2011 | No | – | – | Unclear | – | – | B |
| Fontana 2011 | Yes | – | – | Yes | – | – | A |
| Hulot 2011 | Yes | 0 | Yes | Yes | – | Yes | A |
| Lewis 201la | – | – | – | Unclear | – | – | B |
| Lewis 2011b | Unclear | Unclear | Yes | Yes | Yes | Unclear | B |
| Rideg 2011 | Unclear | Unclear | No | Yes | Yes | Yes | C |
| Sibbing 2011a | Yes | 1.4 | Yes | Yes | Yes | Yes | A |
| Sibbing 2011b | Unclear | – | – | Unclear | – | Unclear | B |
| Simon 2011 | Yes | 1% | Yes | Unclear | – | Unclear | B |
| Trenk 2011 | Yes | < 1% | Yes | Unclear | Unclear | Yes | B |
| Tselepis 2011 | Yes | – | – | Unclear | – | – | B |
As shown in the flow chart in Fig. 1, summarized data could not be abstracted from 10 studies, corresponding to five published papers [16,26-28,32] and four conference abstracts [33-36]. All summarized data required to compute a 2 × 2 table for MACE were kindly provided by the corresponding author of the published papers. Similarly, the means and standard deviation of platelet function assay results could not be retrieved from the publications of eight studies [16,27,28,33-36]. In three instances of studies presented at conferences but not yet published, summarized data could not be obtained from the authors (n = 1575) [34-36].
Clinical outcome
Eleven studies assessed the risk of MACE, four in a case–control design (n = 2739) and seven in a prospective design (n = 5353). MACE cases represented 19% of patients in the case–control studies and 6% of patients in the prospective cohorts. There was no significant difference in the incidence of MACE between the 192QQ patients and the 192QR + 192RR patients (OR = 1.28 [0.97; 1.68] P = 0.08) (Fig. 2). Statistical heterogeneity was found (Cochran P = 0.004, I2 = 62%), but there was no significant subgroup difference in OR between the two types of study design (P = 0.55 and I2 = 0%).
Fig. 2.
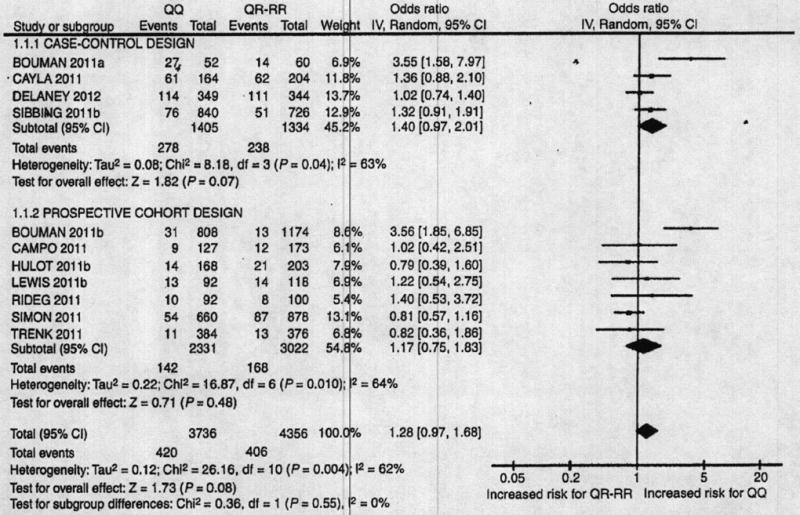
PON1-Q192R and risk of MACE. The odds ratios (ORs) for major adverse cardiovascular events (MACE) are presented for PON1 192QQ compared with 192QR + RR patients. The analysis is stratified by study design. The size of the squares corresponds to the weight of the study in the meta-analysis. Horizontal lines represent corresponding 95% confidence intervals (CIs). The global ORs are depicted as black diamonds.
Platelet reactivity
Four different types of platelet function assay were used (VASP, VerifyNowP2Y12, LTA, and impedance whole-blood aggregometry). For these four assays, the pooled standardized mean difference was never significantly different from zero between 192QQ and 192QR + 192RR subjects (Fig. 3). The pooled standardized mean difference for all platelet function tests combined was close to 0 (−0.02 [−0.10; 0.06], P = 0.68). Statistical heterogeneity was found (Cochran P < 0.00001, I2 = 86%), but there was no significant difference in the standardized mean difference between PON1 genotypes across the different tests (P = 0.31 and I2 = 17%).
Fig. 3.
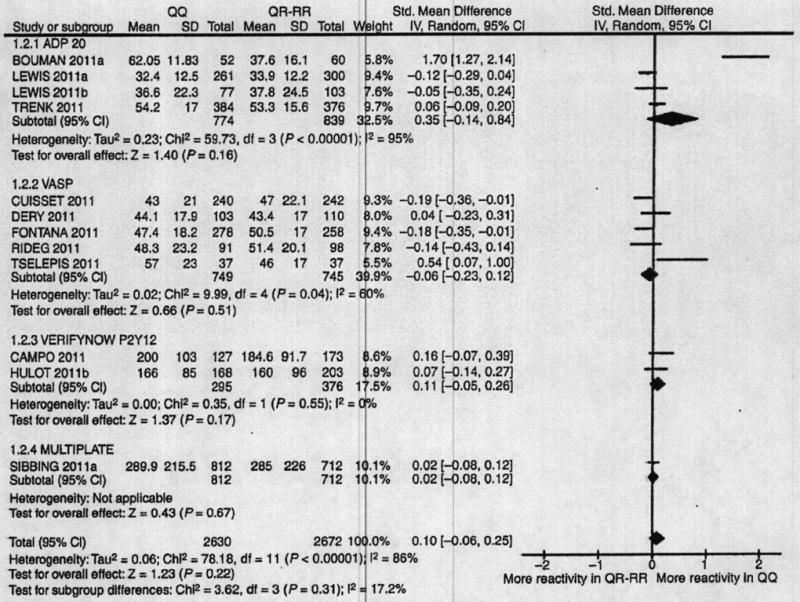
PON1-Q192R and platelet reactivity. The standardized mean differences in the platelet reactivity assay results between PON1 192QQ and 192QR + RR patients are shown. The analysis is stratified by the types of assays. The size of the squares corresponds to the weight of the study in the meta-analysis. Horizontal lines represent corresponding 95% confidence intervals (CIs). The global standardized mean differences are depicted as black diamonds.
Publication bias, sensitivity analyses and meta-regression
No publication bias was detected on funnel plots or with Egger’s test (P = 0.30 for OR and P = 0.13 for the standardized mean difference). Trim-and-fill analysis showed that the pooled estimates were not sensitive to the missing studies on the left part of the funnel plot (data not shown).
The results of the meta-analyses were similar when patients were grouped in 192QQ + 192QR vs. 192RR or in 192QQ vs. 192RR. The tested potential heterogeneity factors (mean age, sex ratio, prevalence of diabetes, mean BMI, acute or stable patients, length of follow-up and frequency of stent thrombosis in prospective studies) included in meta-regressions were not significantly associated with the OR or the standardized mean difference. In the five studies [14,16,25,29] reporting analyses adjusted on potential individual confounders for the risk of MACE, the results were only marginally modified. When considering the risk of MACE most of the statistical heterogeneity was explained by two studies reported in one publication [14]. When each of these studies was removed, I2 fell from 63% to 0% for the group of case–control studies and from 64% to 0% for the group of prospective studies, whereas removal of any of the other studies only marginally affected the level of heterogeneity (from 63% to 80% and from 64% to 70% in case–control and prospective studies, respectively). Removal of both the studies conducted by Bouman et al. yielded a global OR of 1.06 ([0.90; 1.24], P = 0.51), without statistical heterogeneity. When considering the platelet reactivity test results, most of the statistical heterogeneity was explained by one study [14]. When this study was removed, I2 fell from 86% to 48% whereas removal of any of the other studies did not affect the level of heterogeneity (I2 = 86%–87%).
To study the potential impact of platelet reactivity data missing from three studies totalling 1544 patients, a sensitivity analysis was performed with the following extreme hypotheses: the mean difference and standard deviation in platelet assay results between PON1-192QQ and 192QQ + RR subjects in each missing study was set to the highest possible standardized mean difference, knowing that the differences were reported as statistically non-significant in each of the three missing studies. Assuming that all differences were positive, this corresponded to standardized mean differences of 0.42 (0.00; 0.85), 0.32 (0.00; 0.63) and 0.11 (0.00; 0.22), for the studies of 88 [36], 157 [34] and 1299 patients [35], respectively. Following this extreme hypothesis, the pooled standardized mean difference would be 0.12 (−0.01; 0.25) (P = 0.07). Assuming all three differences were negative, the pooled standardized mean difference would be 0.03 (−0.10; 0.16) (P = 0.67).
Discussion
Inherited factors play a major role in platelet aggregation [37] and familial studies have shown a high degree of heritability of the platelet response to clopidogrel (h2 = 0.73) [13]. This has led to attempts to identify the genetic factors underlying the variable biological response to clopidogrel. Hepatic CYP 2C19 gene variants associated with loss of function have been identified as the main contributors to this variability [6]. However, the proportion of the variability explained by the most frequent loss-of-function allele of CYP 2C19 (2C19*2, rs4244285) is only 12% in healthy volunteers and below 10% in cardiovascular patients [8,11,12] and its role in predicting cardiovascular events is controversial [38]. A new player in clopidogrel pharmacogenetics was recently reported by Bouman et al. [14], who identified PON1 as a potential major determinant of the efficiency of clopidogrel bioactivation and this drug’s clinical efficacy in patients of European descent.
The results of our meta-analysis of 17 studies (11 449 patients) do not support a major biological or clinical influence of PON1. Indeed, the PON1-Q192R polymorphism was associated neither with platelet reactivity, regardless of the test used, nor with the risk of cardiovascular events in patients treated with clopidogrel, independently of the study design (case–control or prospective cohort). These results are in line with the lack of correlation between PON1 activity and P2Y12 receptor inhibition (evaluated with the specific VASP assay) in 538 stable cardiovascular patients [12] as well as with the lack of correlation between PON1 activity and clopidogrel active metabolite [39] or other in vitro assays showing no impact of PON1 inhibition on the production of the clopidogrel active metabolite [40]. The trend towards an association between the PON1-Q192R polymorphism and MACE observed in our meta-analysis was mainly driven by Bouman’s study, which accounted for most of the observed heterogeneity. No publication bias was detected by visual inspection of the funnel plot or by statistical analysis, although the tests used lack power when the number of studies is low.
For meta-analyses, it is strongly recommended to investigate the influence of potential heterogeneity factors in order to avoid simplistic and potentially misleading conclusions [41], but, owing to the danger of over-interpretation, the choice of potential factors should be limited and based on ‘reasonable suspicion’. Prior to conducting our meta-analysis, we defined a limited number of potential heterogeneity factors. Overall, the statistical heterogeneity was not explained by any of the factors tested in meta-regressions. These meta-regressions, conducted at the study level, do not exclude a potential interaction of patient-related factors, and further patient-level analysis is needed to reach a firm interpretation. On investigating study-level factors, we found that the heterogeneity was mainly related to two independent studies published in one paper [14]. Indeed, a sensitivity analysis, in which we removed one study at a time, showed that a large part of the statistical heterogeneity was associated with the two studies by Bouman et al. [14], whereas heterogeneity was not noticeably affected when any of the other studies were removed.
The reasons for the discrepancy between the original work of Bouman el al. [14] and most subsequent studies are unclear. Differences in populations may be an issue; indeed, the frequency of the Q192 allele is somewhat lower in their cohort study (63.9%) than in any of the other studies [12,15-17,25-33,42]. A recent in vitro study toy Dansette et al. [43] clearly demonstrates that PON1 is involved in clopidogrel metabolism, but the resulting metabolite is a minor isomer by comparison with the active metabolite produced by cytochromes P450. Dansette et al. suggested that the analytical method used by Bouman et al. was not sufficiently selective to distinguish among the different isomers of the active metabolite, and that it preferentially detected the inactive metabolite produced by PON1. The observed clinical differences between PON1 gene variants may be due to a clopidogrel-unrelated mechanism, as PON1 plays a pivotal role in atherogenesis by protecting low-density lipoprotein from oxidation [44]. This may partly explain the trend towards an association of the PON1-Q192R variant with MACE, best seen in two studies [15,16] in addition to Bouman’s studies (Fig. 2).
Differences in clinical endpoints across studies might also be involved. Indeed, Bouman et al. [14] found the strongest association with definite stent thrombosis, while the association with all MACE was smaller. The CYP2C19*2 variant also seems to mainly affect the risk of stent thrombosis. In our meta-analysis, most of the studies addressing the association of the PON1-Q192R variant with clinical events involved composite endpoints, with a low incidence of stent thrombosis, possibly leading to an underestimation of the clinical importance of the PON1 genotype (Table 1). Another potential bias is ethnic heterogeneity across the different studies, resulting in genetic differences that might have influenced the response to clopidogrel [45]. Drug–drug interactions, mainly via CYP3A4/5, are another important issue when analyzing the variability of clopidogrel responsiveness [46]. For example, platelet inhibition by clopidogrel is attenuated by co-administration of ketoconazole, a known CYP3A4/5 inhibitor [47], or atorvastatin, a competitive inhibitor [48], though it is debated for this latter drug. Conversely, increased platelet inhibition is observed in hyporesponsive patients when CYP3A4/5 activity is induced by St John’s Wort, an herbal remedy used for the treatment of depression [49].
Significant pharmacokinetic interactions also exist with proton pump inhibitors, probably through a CYP2C19 inhibition [50-52]. Hence, an imbalance of co-medication with drugs affecting cytochrome P450 activities across genotype groups might have influenced the findings. Finally, as recently suggested in a study of 74 patients with ACS, the PON1-Q192R variant may modulate the clopidogrel response (and possibly clinical outcome) in a subset of patients considered to be good responders to this drug [17]. Only a few of the papers describing the studies that we included in our meta-analysis mentioned the prevalence of good responders, meaning that this issue could not be addressed by meta-regression. However, analysis of the ADRIE study data restricted to patients treated with clopidogrel and with a platelet reactivity index below 50% (n = 261) gave results similar to those obtained for the entire population (data not shown) [12].
Study limitations
First, most of the studies included in this meta-analysis were observational, being based on prospective or retrospective registries. However, we applied the MOOSE statement checklist [18], allowing careful selection of studies with systematic use of a quality score, and we provided an indication of statistical uncertainty of findings using sensitivity analyses that are key factors to control for this limitation. Second, although analysis of statistical heterogeneity is recommended, it is well known that meta-regressions may have limited relevance in meta-analyses of summarized data and certainly limited power with the number of studies involved in each meta-regression. However, study-level sub-group analyses yielded similar conclusions irrespective of the biological test used to assess clopidogrel responsiveness and the study design (case–control or prospective cohort). Finally, information on clopidogrel use at the time of the event was incomplete.
Conclusion
The results of this meta-analysis do not support the PON1-Q192R polymorphism as a major determinant of the biological response to clopidogrel or as a risk factor for MACE in clopidogrel-treated patients. As CYP2C19*2 plays only a minor role in the variability of clopidogrel responsiveness, further studies of clopidogrel pharmacogenetics are needed before implementing CYP2C19 genotyping in routine clinical practice [53].
Appendix
The PON1 meta-analysis study group includes, in addition to the authors of the present paper, D. Aradi, J. Delaney, J.-P. Déry, P. Gurbel, J. Lewis, D. Sibbing, D. Taubert, D. Trenk and the Geneva Platelet Group members who contributed to this work: V. Ancrenaz, J. Desmeules, A. Perrier, A. Poncet, J.-C. Sanchez and A. Zufferey.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Patrono C, Andreotti F, Arnesen H, Badimon L, Baigent C, Collet JP, De Caterina R, Gulba D, Huber K, Husted S, Kristensen SD, Morais J, Neumann FJ, Rasmussen LH, Siegbahn A, Steg PG, Storey RF, van de Werf F, Verheugt F. Antiplatelet agents for the treatment and prevention of atherothrombosis. Eur Heart J. 2011;32:2922–32. doi: 10.1093/eurheartj/ehr373. [DOI] [PubMed] [Google Scholar]
- 2.Fontana P, Reny JL. New antiplatelet strategies in atherothrombosis and their indications. Eur J Vasc Endovasc Surg. 2007;34:10–17. doi: 10.1016/j.ejvs.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Antman EM, Califf RM, Chavey WE, 2nd, Hochman JS, Levin TN. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/Non-ST-elevation myocardial infarction: a report of the American college of cardiology foundation/American heart association task force on practice guidelines developed in collaboration with the American academy of family physicians, society for cardiovascular angiography and interventions, and the society of thoracic surgeons. J Am Coll Cordial. 2011;57:e215–367. doi: 10.1016/j.jacc.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Mallouk N, Labruyere C, Reny JL, Chapelle C, Piot M, Fontana P, Gris JC, Delavenne X, Mismetti P, Laporte S. Prevalence of poor biological response to clopidogrel. A systematic review. Thromb Haemost. 2012;107:494–506. doi: 10.1160/TH11-03-0202. [DOI] [PubMed] [Google Scholar]
- 5.Combescure C, Fontana P, Mallouk N, Berdague P, Labruyere C, Barazer I, Gris JC, Laporte S, Fabbro-Peray P, Reny JL. Clinical implications of clopidogrel non-response in cardiovascular patients: a systematic review and meta-analysis. J Thromb Haemost. 2009;8:923–33. doi: 10.1111/j.1538-7836.2010.03809.x. [DOI] [PubMed] [Google Scholar]
- 6.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–9. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 7.Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, Aiach M, Lechat P, Gaussem P. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidorel responsiveness in healthy subjects. Blood. 2006;108:2244–7. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 8.Fontana P, Hulot JS, De Moerloose P, Gaussem P. Influence of CYP2C19 and CYP3A4 gene polymorphisms on clopidogrel responsiveness in healthy subjects. J Thromb Haemost. 2007;5:2153–5. doi: 10.1111/j.1538-7836.2007.02722.x. [DOI] [PubMed] [Google Scholar]
- 9.Harmsze AM, van Werkum JW, Hackeng CM, Ruven HJ, Kelder C, Bouman HJ, Breet NJ, ten Berg JM, Klungel OH, de Boer A, Deneer VH. The influence of CYP2C19*2 and *17 on on-treatment platelet reactivity and bleeding events in patients undergoing elective coronary stenting. Pharmacogenet Genomics. 2012;22:169–75. doi: 10.1097/FPC.0b013e32834ff6e3. [DOI] [PubMed] [Google Scholar]
- 10.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–30. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, Buttner HJ, Neumann FJ. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol. 2010;55:2427–34. doi: 10.1016/j.jacc.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Fontana P, James R, Barazer I, Berdague P, Schved JF, Rebsamen W, Vuilleumier N, Reny JL. Relationship between paraoxonase-1 activity, its Q192R genetic variant and clopidogrel responsiveness in the ADRIE study. J Thromb Haemost. 2011;9:1664–6. doi: 10.1111/j.1538-7836.2011.04409.x. [DOI] [PubMed] [Google Scholar]
- 13.Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–57. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouman HJ, Schomig E, van Werkum JW, Velder J, Hackeng CM, Hirschhauser C, Waldmann C, Schmalz HG, ten Berg JM, Taubert D. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med. 2011;17:110–16. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 15.Cayla G, Hulot JS, O’Connor SA, Pathak A, Scott SA, Gruel Y, Silvain J, Vignalou JB, Huerre Y, de la Briolle A, Allanic F, Beygui F, Barthelemy O, Montalescot G, Collet JP. Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. JAMA. 2011;306:1765–74. doi: 10.1001/jama.2011.1529. [DOI] [PubMed] [Google Scholar]
- 16.Sibbing D, Koch W, Massberg S, Byrne RA, Mehilli J, Schulz S, Mayer K, Bernlochner I, Schomig A, Kastrati A. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur Heart J. 2011;32:1605–13. doi: 10.1093/eurheartj/ehr155. [DOI] [PubMed] [Google Scholar]
- 17.Tselepis AD, Tsoumani ME, Kalantzi KI, Dimitriou AA, Tellis CC, Goudevenos IA. Influence of high-density lipoprotein and paraoxonase-1 on platelet reactivity in patients with acute coronary syndromes receiving clopidogrel therapy. J Thromb Haemost. 2011;9:2371–8. doi: 10.1111/j.1538-7836.2011.04541.x. [DOI] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.Berlin JA. Does blinding of readers affect the results of meta-analyses? University of Pennsylvania Mcta-analysis Blinding Study Group. Lancet. 1997;350:185–6. doi: 10.1016/s0140-6736(05)62352-5. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Altman DG. Systematic Reviews in Health Care. Meta-Analysis in Context. London: BMJ Books; 2001. [Google Scholar]
- 22.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Simon T, Steg PG, Beequemont L, Verstuyft C, Kotti S, Schiele F, Ferrari E, Drouet E, Grollier G, Danchin N. Effect of paraoxonase-1 polymorphism on clinical outcomes in patients treated with clopidogrel after an acute myocardial infarction. Clin Pharmacol Ther. 2011;90:561–7. doi: 10.1038/clpt.2011.193. [DOI] [PubMed] [Google Scholar]
- 26.Rideg O, Komocsi A, Magyarlaki T, Tokes-Fuzesi M, Miseta A, Kovacs GL, Aradi D. Impact of genetic variants on post-clopidogrel platelet reactivity in patients after elective percutaneous coronary intervention. Pharmacogenomics. 2011;12:1269–80. doi: 10.2217/pgs.11.73. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JP, Fisch AS, Ryan K, O’Connell JR, Gibson Q, Mitchell BD, Shen H, Tanner K, Horenstein RB, Pakzy R, Tantry US, Bliden KP, Gurbel PA, Shuldiner AR. Paraoxonase 1 (PON1) gene variants are not associated with clopidogrel response. Clin Pharmacol Ther. 2011;90:568–74. doi: 10.1038/clpt.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trenk D, Hochholzer W, Fromm MF, Zolk O, Valina CM, Stratz C, Neumann FJ. Paraoxonase-1 Q192R polymorphism and antiplatelet effects of clopidogrel in patients undergoing elective coronary stent placement. Circ Cardiovasc Genet. 2011;4:429–36. doi: 10.1161/CIRCGENETICS.111.960112. [DOI] [PubMed] [Google Scholar]
- 29.Hulot JS, Collet JP, Cayla G, Silvain J, Allanic F, Bellemain-Appaix A, Scott SA, Montalescot G. CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics, and clinical efficacy in post-myocardial infarction patients. Circ Cardiovasc Interv. 2011;4:422–8. doi: 10.1161/CIRCINTERVENTIONS.111.963025. [DOI] [PubMed] [Google Scholar]
- 30.Cuisset T, Morange PE, Quilici J, Bonnet JL, Gachet C, Alessi MC. Paraoxonase-1 and clopidogrel efficacy. Nat Med. 2011;17:1039. doi: 10.1038/nm.2367. [DOI] [PubMed] [Google Scholar]
- 31.Campo G, Ferraresi P, Marchesini J, Bernardi F, Valgimigli M. Relationship between paraoxonase Q192R gene polymorphism and on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention. J Thromb Haemost. 2011;9:2106–8. doi: 10.1111/j.1538-7836.2011.04457.x. [DOI] [PubMed] [Google Scholar]
- 32.Delaney JT, Ramirez AH, Bowton E, Pulley JM, Basford MA, Schildcroul JS, Shi Y, Zink R, Oetjens M, Xu H, Cleator JH, Jahangir E, Ritchic MD, Masys DR, Roden DM, Crawford DC, Denny JC. Predicting clopidogrel response using DNA samples linked to an electronic health record. Clin Pharmacol Ther. 2012;91:257–63. doi: 10.1038/clpt.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dery JPP, Khalil A, Dery U, Roy M, Tricoci P, Becker R, Rinfret S, Larose E, Barbeau G, Delarochelliere R, Rodes-Cabau J, Bertrand OF. Paraoxonase-1 (PON1) activity and PON1 polymorphisms have no effect on platelet reactivity in patients treated with clopidogrel. J Am Coll Cardiol. 2011;58:B40. [Google Scholar]
- 34.Karunakaran A, Judge H, Morton A, Milano A, Arnold J, Chico TJ, Flaherty L, Baker D, Daly R, Storey RF. Cytochrome P450 2C19 loss-of-function genetic variants but not paraoxonase-1 activity modulate P2Y12 blockade in response to clopidogrel therapy. J Am Coll Cardiol. 2011;58:B45. [Google Scholar]
- 35.Park JJ, Park KW, Kang J, Jeon KH, Kang SH, Ahn HS, Yang HM, Lee HY, Kang HJ, Koo BK, Oh BH, Park YB, Kim HS. Genetic determinants of clopidogrel responsiveness in Koreans. J Am Coll Cardiol. 2011;58:B41. doi: 10.1016/j.ijcard.2012.09.075. [DOI] [PubMed] [Google Scholar]
- 36.So D, Goncalves S, Roberts J, Stewart A, Al-turbak H, Le May M, Glover C, Marquis J, Dick A, O’Brien E, Froeschl M, Tran L, Szymanska I, Labinaz M. Genetic testing for CYP2C19null2 but not for PON-1 QQ carrier status predicts high on-clopidogrel platelet reactivity in patients undergoing percutaneous coronary interventions. Can J Cardiol. 2011;27:S183. [Google Scholar]
- 37.O’Donnell CJ, Larson MG, Feng D, Sutherland PA, Lindpaintner K, Myers RH, D’Agostino RA, Levy D, Tofler GH. Genetic and environmental contributions to platelet aggregation: the Framingham heart study. Circulation. 2001;103:3051–6. doi: 10.1161/01.cir.103.25.3051. [DOI] [PubMed] [Google Scholar]
- 38.Bauer T, Bouman HJ, van Werkum JW, Ford NF, ten Berg JM, Taubert D. Impact of CYP2C19 variant genotypes on clinical efficacy of antiplatelet treatment with clopidogrel: systematic review and meta-analysis. BMJ. 2011;343:d4588. doi: 10.1136/bmj.d4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong IY, Crown N, Suen CM, Schwarz UI, Dresser GK, Knauer MJ, Sugiyama D, Degorter MK, Woolsey S, Tirona RG, Kim RB. Clarifying the importance of CYP2C19 and PON1 in the mechanism of clopidogrel bioactivation and in vivo antiplatelet response. Eur Heart J. 2012 doi: 10.l093/curheartj/ehs042. [DOI] [PubMed] [Google Scholar]
- 40.Anerenaz V, Desmeules J, James R, Fontana P, Reny JL, Dayer P, Daali Y. Paroxonase-1 is not a major bioactivation pathway of clopidogrel in vitro. Br J Pharmacol. 2012 doi: 10.1111/j.1476-5381.2012.01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ. 1994;309:1351–5. doi: 10.1136/bmj.309.6965.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–76. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dansette PM, Rosi J, Bertho G, Mansuy D. Cytochromes P450 catalyze both steps of the major pathway of clopidogrel bioactivition, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer. Chem Res Toxicol. 2012;25:348–56. doi: 10.1021/tx2004085. [DOI] [PubMed] [Google Scholar]
- 44.Wheeler JG, Keavney BD, Watkins H, Collins R, Dancsh J. Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: meta-analysis of 43 studies. Lancet. 2004;363:689–95. doi: 10.1016/S0140-6736(04)15642-0. [DOI] [PubMed] [Google Scholar]
- 45.Talameh JA, McLeod HL. PON1 Q192R and clopidogrel: a case of the winner’s curse or inadequate replication? Clin Pharmacol Ther. 2011;90:771–4. doi: 10.1038/clpt.2011.226. [DOI] [PubMed] [Google Scholar]
- 46.Ancrenaz V, Daali Y, Fontana P, Besson M, Samer C, Dayer P, Desmeules J. Impact of genetic polymorphisms and drug-drug inter actions on clopidogrel and prasugrel response variability. Curr Drug Metab. 2010;11:667–77. doi: 10.2174/138920010794233521. [DOI] [PubMed] [Google Scholar]
- 47.Farid NA, Payne CD, Small DS, Winters KJ, Ernest CS, 2nd, Brandt JT, Darstein C, Jakubowski JA, Salazar DE. Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther. 2007;81:735–41. doi: 10.1038/sj.clpt.6100139. [DOI] [PubMed] [Google Scholar]
- 48.Lau WC, Waskell LA, Watkins PB, Neer CJ, Horowitz K, Hopp AS, Tait AR, Carville DG, Guyer KE, Bates ER. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug–drug interaction. Circulation. 2003;107:32–7. doi: 10.1161/01.cir.0000047060.60595.cc. [DOI] [PubMed] [Google Scholar]
- 49.Lau WC, Welch TD, Shields T, Rubenfire M, Tantry US, Gurbel PA. The effect of St John’s Wort on the pharmacodynamic response of clopidogrel in hyporesponsive volunteers and patients: increased platelet inhibition by enhancement of CYP3A4 metabolic activity. J Cardiovasc Pharmacol. 2011;57:86–93. doi: 10.1097/FJC.0b013e3181ffe8d0. [DOI] [PubMed] [Google Scholar]
- 50.Sibbing D, Morath T, Stegherr J, Braun S, Vogt W, Hadamitzky M, Schomig A, Kastrati A, von Beckerath N. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost. 2009;101:714–19. [PubMed] [Google Scholar]
- 51.Juurlink DN. Proton pump inhibitors and clopidogrel: putting the interaction in perspective. Circulation. 2009;120:2310–12. doi: 10.1161/CIRCULATIONAHA.109.907295. [DOI] [PubMed] [Google Scholar]
- 52.Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, Mansourati J, Mottier D, Abgrall JF, Boschat J. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51:256–60. doi: 10.1016/j.jacc.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 53.Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306:2704–14. doi: 10.1001/jama.2011.1880. [DOI] [PubMed] [Google Scholar]


