SUMMARY
Stringent control of NF-κB and mitogen-activated protein kinase (MAPK) signaling is critical during innate immune responses. TGF-β activated kinase-1 (TAK1) is essential for NF-κB activation in T and B cells but has precisely the opposite activity in myeloid cells. Specific deletion of TAK1 (Map3k7ΔM/ΔM) led to development of splenomegaly and lymphomegaly associated with neutrophilia. Compared with wild-type cells, TAK1-deficient neutrophils enhanced the phosphorylation of the kinases IKK, p38, and JNK and the production of interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), and reactive oxygen species (ROS) after lipopolysaccharide (LPS) stimulation. Map3k7ΔM/ΔM mice were significantly more susceptible to LPS-induced septic shock and produced higher amounts of IL-1β, IL-6, and TNF-α in plasma than do wild-type mice. Specific ablation of p38 rescued the phenotype and functional properties of Map3k7ΔM/ΔM mice. Our findings identify a previously unrecognized role of TAK1 as a negative regulator of p38 and IKK activation in a cell type-specific manner.
INTRODUCTION
Recognition of cytokines and pattern-associated molecular patterns (PAMPs) by innate immune receptors triggers immune responses involving several signaling molecules (Han and Ulevitch, 2005; Hayden and Ghosh, 2008; Kawai and Akira, 2007). Transforming growth factor-β activated kinase-1 (TAK1, Map3k7), a member of the mitogen-activated protein kinase kinase kinase (MAP3K) family, is essential in innate and adaptive immune signaling cascades (Ninomiya-Tsuji et al., 1999; Wang et al., 2001). TAK1 activation is triggered by diverse stimuli including proinflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor (TNF), and Toll-like receptor (TLR) ligands and culminates in downstream activation of nuclear factor-κB (NF-κB), p38, JNK, and ERK mitogen-activated protein kinase (MAPK) signaling pathways (Adhikari et al., 2007; Shim et al., 2005). Activation of the NF-κB pathway is initiated by ligand activation of TLRs or IL-1 receptor (IL-1R) resulting in recruitment of adaptor proteins including MyD88, IRAK1, IRAK4, and TRAF6 to the Toll-IL-1R homology cytoplasmic domain of the receptors (Kawai and Akira, 2007). TAK1 with its associated proteins TAB1, TAB2, and TAB3 play a pivotal role in activating IκB kinase (IKK) and MAPK signaling cascades (Adhikari et al., 2007; Bhoj and Chen, 2009). Recent studies have defined an essential role of TAK1 in T cell receptor (TCR)- and B cell receptor (BCR)-induced activation of NF-κB and in the survival and development of immune cells, including mature B cells and T cells (Liu et al., 2006; Sato et al., 2005, 2006; Schuman et al., 2009; Tang et al., 2008; Wan et al., 2006). In B cells, TAK1 is required for B cell development and NF-κB and MAPK activation induced by cytokines, TLR ligands, and BCR stimuli (Sato et al., 2005; Schuman et al., 2009). T cell-specific ablation of TAK1 impairs T cell survival in the periphery and TAK1 is also essential for TCR-mediated NF-κB and MAPK activation (Liu et al., 2006; Sato et al., 2006; Wan et al., 2006). Multilineage hematopoietic cell survival also requires TAK1 as indicated by studies where TAK1 deletion triggers uncontrolled apoptosis in hematopoietic cells (Tang et al., 2008). However, the specific function, if any, of TAK1 in myeloid cell development and TLR-induced innate immunity remains largely unknown.
The mammalian innate immune system is evolutionarily conserved and serves as an initial defense mechanism against harmful pathogens by activating immune cells such as macrophages, neutrophils, and dendritic cells (Akira et al., 2006). Myeloid cells (Gr-1+CD11b+ neutrophils and F4/80+CD11b+ macrophages) arise from common myeloid and granulocyte-monocyte progenitors and are crucial for mounting effective host defense against microbial invasion (Geissmann et al., 2010). Neutrophils and macrophages function as professional phagocytic cells that mediate host defense by recognizing and destroying foreign infectious pathogens (Kantari et al., 2008). Pattern recognition receptors of the innate immune system including TLR that are expressed by myeloid cells are critical for immune surveillance, pathogen recognition, and activation of proinflammatory pathways (Janeway and Medzhitov, 2002). After recognition of microbial pathogens by myeloid cells, proinflammatory cytokines such as IL-6, TNF-α, IL-1, IL-12, IL-15, and IL-18 (Cohen, 2006) are released that initiate and amplify inflammatory signaling cascades. Because of the importance of myeloid cells in innate and adaptive immunity, in the present study we describe a role for TAK1 as a negative rather than a positive regulator of NF-κB and MAPK signaling in neutrophils. Myeloid cell-specific TAK1 deletion resulted in splenomegaly and enlargement of lymph nodes and enhanced rather than reduced the phosphorylation of IKK, p38, and JNK. Consistent with these results, TAK1-deficient mice produced larger amounts of proinflammatory cytokines and were more susceptible to LPS-induced septic shock compared to wild-type (WT) mice. Importantly, we provide genetic evidence that specific deletion of p38α in myeloid cells rescued the phenotypes and functional properties observed in TAK1-deficient mice. We also have shown that three distinct mechanisms are involved in p38 activation. These results identify a previously unrecognized role of TAK1 as a cell type-specific negative regulator of the TLR4-induced NF-κB and p38 signaling pathways during myeloid cell homeostasis.
RESULTS
Generation and Characterization of Myeloid Cell-Specific TAK1-Deficient Mice
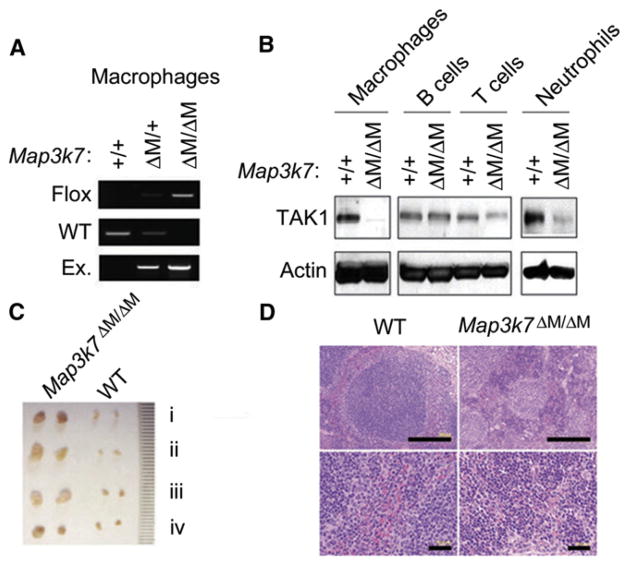
To investigate the role of TAK1 in myeloid lineage development and innate immune response, we specifically ablated TAK1 in myeloid cells by crossing Map3k7flox/flox mice with mice expressing the lysozyme promoter-driven cre recombinase gene (Lyz2-Cre). The resultant heterozygous Map3k7flox/+ × Lyz2-Cre and homozygous Map3k7flox/flox × Lyz2-Cre mice were designated Map3k7ΔM/+ and Map3k7ΔM/ΔM mice, respectively. Selective deletion of Map3k7 in myeloid cells of Map3k7ΔM/ΔM mice was demonstrated by polymerase chain reaction (PCR) and immunoblot analyses (Figures 1A and 1B). Although there were no phenotypic or functional differences between wild-type (WT) Map3k7+/+ and heterozygous Map3k7ΔM/+ mice, Map3k7ΔM/ΔM mice developed splenomegaly (data not shown) and profound enlargement of all lymph nodes (LNs) (Figure 1C). Gross examination of tissues showed that spleens and LNs from Map3k7ΔM/ΔM mice were at least three times larger than those from control mice. The total number of splenocytes and the weight of spleens were markedly increased in Map3k7ΔM/ΔM mice (Figures S1A and S1B available online). Histological analysis revealed extramedullary hematopoiesis in the spleens of Map3k7ΔM/ΔM mice (Figure 1D, top). Splenic architecture was grossly disorganized, with loss of the marginal zone barrier and massive myeloid hyperplasia in the red pulp (Figure 1D, bottom). Hematologic analysis of peripheral blood in Map3k7ΔM/ΔM mice revealed leukocytosis and granulocytosis but decreased erythropoiesis and hemoglobin (Table S1).
Figure 1. Characterization and Phenotypic Analysis of Map3k7ΔM/ΔM Mice.
(A) PCR analysis of Map3k7 deletion with macrophages from WT and Map3k7ΔM/ΔM mice.
(B) Immunoblot analysis of TAK1 protein expression with anti-TAK1 in macrophages, B cells, T cells, and neutrophils.
(C) Lymphadenopathy in Map3k7ΔM/ΔM mice compared with WT control. Inguinal (i), axillary (ii), superficial cervical (iii), and mesenteric (iv) lymph nodes were examined.
(D) H&E staining of spleen sections from WT and Map3k7ΔM/ΔM mice.
Data shown are representative of at least five independent experiments. See also Table S1 and Figure S1.
Loss of TAK1 Increases the Proliferative Activity of Neutrophils but Promotes Apoptosis in Macrophages
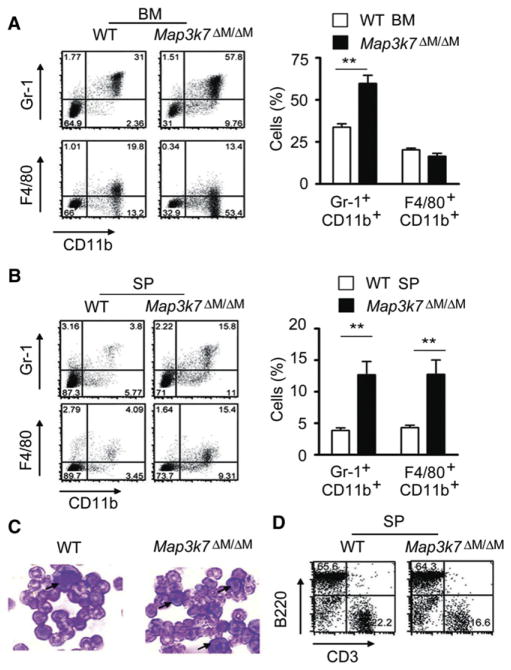
Flow cytometric analysis of different cell populations in the bone marrows and spleens from WT and TAK1-deficient mice indicated that the percentages of Gr-1+CD11b+ neutrophils were markedly increased in the bone marrow and spleen (Figures 2A and 2B). The percentages of F4/80+CD11b+ macrophages were also increased in TAK1-deficient spleens (Figure 2B). Bone marrow smears revealed an increase in immature neutrophils in Map3k7ΔM/ΔM mice compared to WT mice (Figure 2C). These results indicate that Gr-1+CD11b+ neutrophils are strikingly increased in Map3k7ΔM/ΔM mice, in contrast to the reduction of T cells or mature B cells observed in mice with specific ablation of TAK1 in lymphoid cells (Liu et al., 2006; Sato et al., 2005, 2006; Schuman et al., 2009; Wan et al., 2006) and in hematopoietic cells (Tang et al., 2008). Because myeloid-derived suppressor cells (MDSC) expressing Gr-1 (Ly-6C and/or Ly-6G) and CD11b molecules are known to dampen immune response by inhibiting CD4+ and CD8+ T lymphocyte function in cancer (Marigo et al., 2008), we tested whether Gr-1+CD11b+ cells in Map3k7ΔM/ΔM mice had suppressive function. A T cell proliferation assay showed that Gr-1+CD11b+ cells isolated from Map3k7ΔM/ΔM mice had no suppressive effect on naive CD4+ T cell proliferation (Figure S1C).
Figure 2. TAK1 Ablation Increases Gr-1+CD11b+ Neutrophils in the Bone Marrow and Spleen.
(A and B) Flow cytometric analysis of Gr-1+CD11b+ neutrophil and F4/80+CD11b+ macrophage populations in bone marrow (A) and spleen (B) of WT and Map3k7ΔM/ΔM mice, with anti-Gr-1, anti-F4/80, and anti-CD11b. Results plotted as mean ± SD. **p < 0.01. BM, bone marrow; SP, spleen.
(C) Bone marrow smear and Wright-Giemsa staining of bone marrow cells visualized by light microscopy.
(D) T and B lymphocyte analysis in spleen via anti-CD3 and anti-B220 staining and flow cytometry.
Results are representative of at least four independent experiments. See also Figure S1.
Consistent with the results of blood counts (Table S1), splenic CD3+ T and B220+ B cell populations were comparable between Map3k7ΔM/ΔM and WT mice (Figure 2D). We further investigated whether the development of splenomegaly and lymphomegaly in Map3k7ΔM/ΔM mice affected T cell activation in the periphery and found that both activated (CD69+ or CD44+) T cell populations were elevated in the spleens and LNs of Map3k7ΔM/ΔM mice compared with WT mice (Figures S1D and S1E). Intracellular staining revealed that interferon-γ (IFN-γ)-producing CD4+ and CD8+ T cells were also increased in the spleens and LNs of Map3k7ΔM/ΔM mice compared with WT mice (Figure S1F). These data suggest that TAK1 deletion in myeloid cell lineage does not alter the numbers of T cells, but markedly increases the activated T cell population in Map3k7ΔM/ΔM mice, probably because of the proinflammatory cytokine environment.
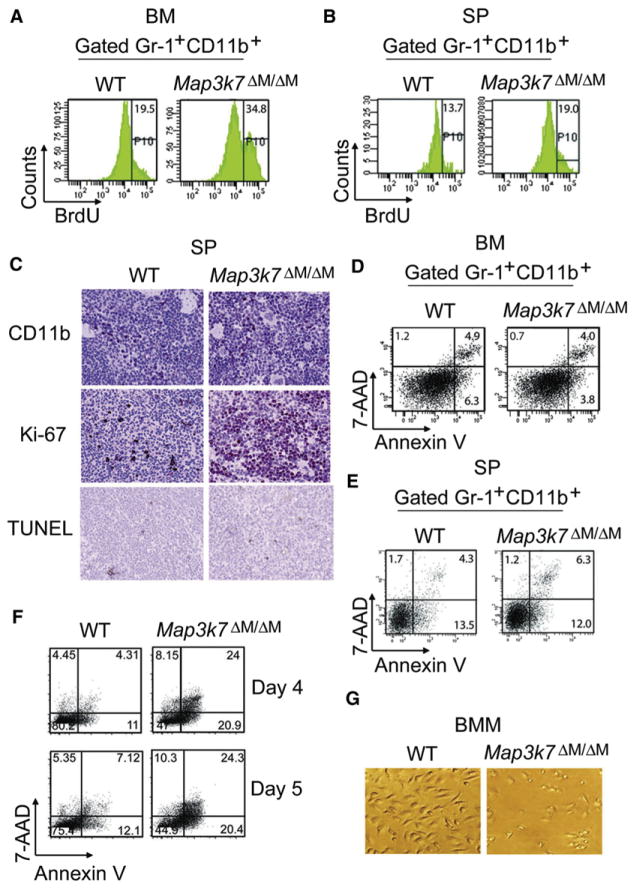
To determine whether cell proliferation or apoptosis accounted for the observed differences in myeloid cell numbers between Map3k7ΔM/ΔM and WT mice, bromodeoxyuridine (BrdU) pulse labeling and flow cytometry were performed. The results in Figures 3A and 3B show that BrdU-positive proliferating Gr-1+CD11b+ cells were dramatically increased in bone marrows and spleens of TAK1-deficient mice compared with WT mice, suggesting that TAK1 deletion increases the proliferation of neutrophils. Consistent with these observations, immunohistochemical staining of spleen sections with anti-CD11b and anti-Ki-67 showed that the numbers of CD11b-positive or Ki-67-positive cells were much higher in Map3k7ΔM/ΔM than in WT mice (Figure 3C). Because TAK1 ablation in T cells, hematopoietic cells, intestinal epithelia, keratinocytes, and hepatocytes increases apoptosis (Bettermann et al., 2010; Inokuchi et al., 2010; Kajino-Sakamoto et al., 2008; Liu et al., 2006; Omori et al., 2006; Sato et al., 2006; Schuman et al., 2009; Tang et al., 2008; Wan et al., 2006), we examined the effect of TAK1 loss on myeloid cell survival in vitro and in vivo. We did not observe any difference in terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells in spleen sections between Map3k7ΔM/ΔM and WT mice (Figure 3C). Annexin V staining and flow cytometry analysis of gated Gr-1+CD11b+ cells also showed equal or less apoptosis in neutrophils from spleen and bone marrow of Map3k7ΔM/ΔM mice compared with WT mice (Figures 3D and 3E), suggesting that TAK1 deletion had a major effect on neutrophil proliferation but not on its apoptosis.
Figure 3. Proliferation and Apoptosis Analyses of TAK1-Deficient Neutrophils and Macrophages.
(A and B) BrdU pulse labeling of mice and flow cytometric analysis of bone marrow (A) and spleen (B) cells via anti-BrdU. Gr-1+CD11b+ cells were gated to determine the percentage of BrdU-positive cells.
(C) Immunohistochemistry was performed on spleen tissues with anti-CD11b and anti-Ki-67, and TUNEL staining was done to detect apoptotic cells.
(D–F) Apoptosis was assayed with Annexin V/7-AAD apoptosis staining and flow cytometry analysis of bone marrow (D), spleen (E), and bone-marrow-derived macrophages (BMM) cultured with 10% M-CSF conditioned medium (F). Gr-1+CD11b+ bone marrow and spleen cells and F4/80+CD11b+ BMM cells were gated to determine percentage of apoptotic cells.
(G) Photographs of BMM were visualized by light microscopy.
Results are representative of at least five independent experiments. See also Figure S2.
We next examined the survival of cultured bone marrow-derived macrophages (BMM) from Map3k7ΔM/ΔM and WT mice and found that BMM from TAK1-deficient mice died after 3 days of culture (Figures 3F and 3G), even with different amounts of conditioned medium (Figure S2A). It appeared that apoptosis mainly contributed to cell death or the reduction of cell numbers (Figures S2A and S2B), because carboxyfluorescein succinimidyl ester (CFSE)-labeling experiments showed that the viable TAK1-deficient differentiating cells divided only slightly slower than did WT cells (Figure S2C). In contrast, we did not observe increased apoptosis in neutrophils cultured in medium with or without granulocyte-colony stimulating factor (G-CSF) (Figure S2D). These results suggest that TAK1 loss enhances the proliferation and growth of neutrophils but causes increased apoptosis in macrophages. Because TAK1-deficient BMM undergo apoptosis, we used freshly isolated peritoneal macrophages for further experiments, because there was no difference in cell survival between freshly isolated WT and TAK1-deficient cells (Figure S2E).
TAK1 Negatively Regulates IKK, p38, and JNK Activation in Neutrophils
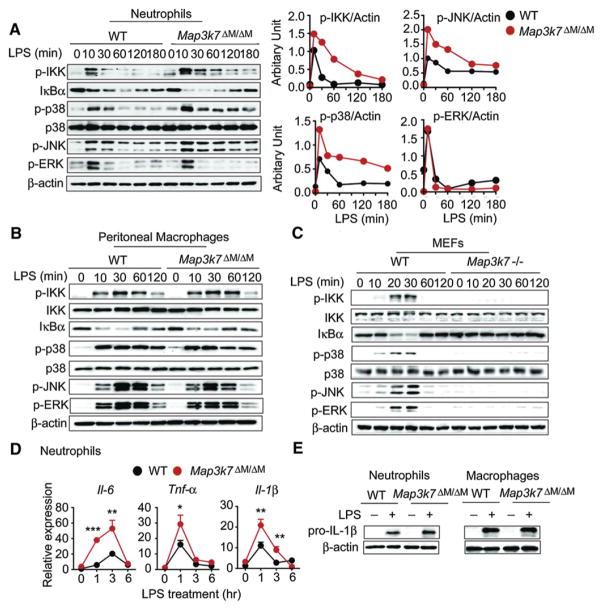
To determine the function of TAK1 in innate immune signaling pathways in myeloid cells, we isolated neutrophils from WT and Map3k7ΔM/ΔM mice, treated them with LPS, and performed immunoblot analysis with specific antibodies. We unexpectedly found that IKK phosphorylation was enhanced and remained high for a sustained period in TAK1-deficient neutrophils, compared with WT cells (Figure 4A). Consistently, IκBα degradation was more robust in Map3k7ΔM/ΔM cells compared to WT cells (Figure 4A). More importantly, we found that phosphorylation of p38 and JNK was also markedly enhanced rather than reduced in LPS-treated TAK1-deficient neutrophils, compared with WT cells (Figure 4A). In contrast, there was no appreciable increase in phosphorylation of ERK between TAK1-deficient and WT cells (Figure 4A). Densitomeric quantification of these results is shown in Figure 4A. Phosphorylation of IKK, p38, JNK, and ERK was comparable or slightly reduced in TAK1-deficient macrophages, compared with WT cells after LPS stimulation (Figure 4B). Similarly, IκBα degradation was also slightly reduced in Map3k7ΔM/ΔM cells compared to WT cells (Figure 4B). Consistent with previous findings with TAK1-null mouse embryonic fibroblasts (MEFs) (Sato et al., 2005; Shim et al., 2005), we found that phosphorylation of IKK, p38, JNK, and ERK was completely abolished in TAK1-deficient MEFs, compared to WT MEFs (Figure 4C). Taken together, these data indicate that TAK1 acts as a negative regulator of NF-κB, p38, and JNK activation in neutrophils.
Figure 4. Effects of Map3k7 Ablation on NF-κB and MAPK Signaling Pathways Is Cell Type Specific.
(A–C) WT or TAK1-deficient neutrophils (A), peritoneal macrophages (B), or MEFs (C) were treated with LPS for the indicated time points, followed by immunoblot analysis of phosphorylated IKK (P-IKK), P-p38, P-JNK, P-ERK, p38, and IκBα with cell lysates.
(A) Right: Quantitative comparison of activation of signaling molecules between WT or Map3k7ΔM/ΔM neutrophils by densitometric scanning of blots.
(D) IL-6, TNF-α, and IL-1β mRNA expression in neutrophils treated with LPS (100 ng/ml) for indicated time points determined by real-time PCR. Results are plotted as mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001.
(E) Immunoblot analysis of pro-IL1β in neutrophils and macrophages treated with LPS for 2 hr and 6 hr, respectively.
Results are representative of at least five independent experiments. See also Figure S2.
To further assess expression of downstream NF-κB-responsive cytokine genes, we performed real-time PCR analysis and found that expression of IL-6, TNF-α, and IL-1β mRNA was markedly increased in TAK1-deficient neutrophils compared with WT cells (Figure 4D). By contrast, mRNA expression of these genes in Map3k7ΔM/ΔM macrophages was similar or lower than those in WT cells (Figure S2F). Furthermore, pro-IL-1β protein expression was increased in Map3k7ΔM/ΔM neutrophils compared with WT cells, but no difference was observed between Map3k7ΔM/ΔM macrophages and WT cells (Figure 4E). These results suggest that TAK1 ablation increases NF-κB, p38, and JNK signaling as well as expression of their downstream target cytokine genes in neutrophils.
TAK1 Deletion Enhances Proinflammatory Cytokine Production in Neutrophils
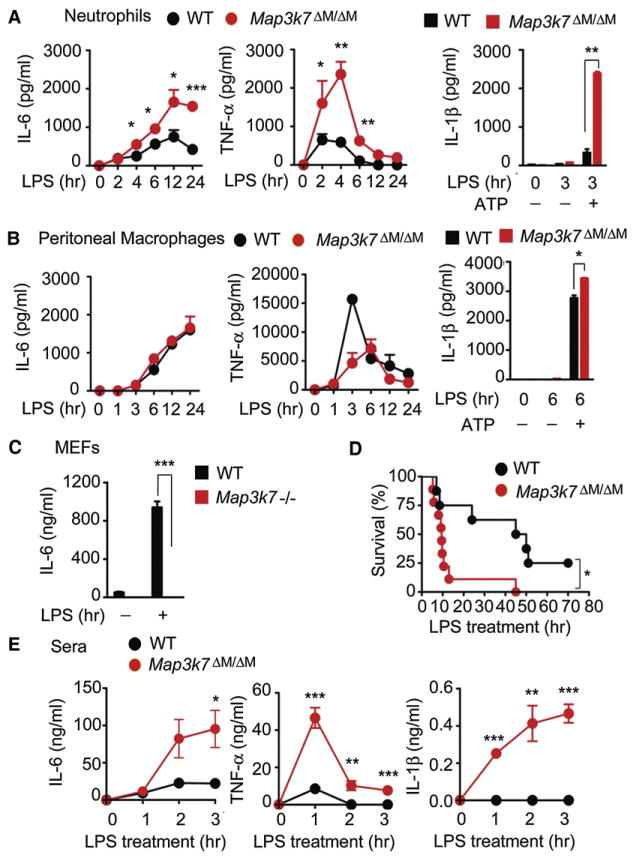
Given these unexpected findings, we reasoned that TAK1-deficient neutrophils would produce more proinflammatory cytokines than control cells after LPS treatment. Indeed, neutrophils from Map3k7ΔM/ΔM mice produced significantly more IL-6 and TNF-α than those from WT mice (Figure 5A). Because IL-1β release requires the processing of pro-IL-1β by inflammasome-activated capsase-1 (Schroder and Tschopp, 2010), we measured IL-1β secretion from WT and TAK1-deficient neutrophils after LPS with or without ATP treatment. Although neutrophils could produce IL-1β after LPS treatment alone, LPS plus ATP treatment markedly increased IL-1β production in WT and TAK1-deficient cells (Figures 5A and S2G). Furthermore, TAK1-deficient neutrophils produced significantly more IL-1β than did WT cells after LPS treatment with or without ATP (Figures 5A and S2G). By contrast, peritoneal macrophages from TAK1-deficient mice produced similar amounts of IL-6 and lower amounts of TNF-α than found in WT cells after LPS treatment (Figure 5B). Although neither WT nor TAK1-deficient peritoneal macrophages secreted IL-1β after LPS treatment alone, TAK1-deficient peritoneal macrophages produced more IL-1β than WT controls after LPS plus ATP treatment (Figures 5B and S2G), suggesting that activation of inflammasome-mediated capsase-1 by ATP is required for IL-1β secretion from macrophages. In contrast to WT MEF cells, which produced robust amounts of IL-6 after LPS treatment, TAK1-deficient MEF cells failed to release IL-6 after LPS treatment (Figure 5C), further suggesting that TAK1 functions differently in a cell type-specific manner.
Figure 5. Effects of TAK1 Ablation on Proinflammatory Cytokine Production in a Cell Type-Specific Manner.
(A and B) IL-6, TNF-α, and IL-1β secretion by neutrophils (A) or peritoneal macrophages (B) treated with or without LPS (100 ng/ml) for indicated time points was measured by ELISA. IL-1β production was stimulated with ATP (5 mM) for 1 hr.
(C) ELISA measurement of IL-6 production by MEFs after LPS (1 μg/ml) treatment for 24 hr.
(D) Survival of WT and Map3k7ΔM/ΔM mice (n = 10 per group) after high-dose LPS (30 mg/kg) challenge. (E) Plasma concentrations of IL-6, TNF-α, and IL-1β in mice at various times after LPS treatment.
Data shown are the mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001. Data shown are representative of at least five independent experiments. See also Figure S2.
To gain further insight into the physiological function of TAK1, we injected WT and Map3k7ΔM/ΔM mice with a high dose of LPS (30 mg/kg) and monitored their survival. Eighty percent of Map3k7ΔM/ΔM mice died from LPS-induced endotoxic shock within 10 hr, compared to only 25% of the WT group, whereas the remaining mice survived for up to 40 hr after LPS treatment (Figure 5D). Consistent with this observation, Map3k7ΔM/ΔM mice had markedly elevated serum concentrations of proinflammatory cytokines such as IL-6, TNF-α, and IL-1β after LPS treatment, compared with WT mice (Figure 5E). To exclude the possibility that macrophages contribute to susceptibility of Map3k7ΔM/ΔM mice to LPS, we depleted macrophages in Map3k7ΔM/ΔM mice with clodronate-containing liposomes followed by LPS treatment and found no difference in LPS-induced septic shock in mice treated with control liposomes and clodronate (Figure S2H), suggesting that macrophages may not be critical in LPS-induced septic shock in Map3k7ΔM/ΔM mice. These results provide in vivo evidence that TAK1 deletion in myeloid cells enhances the sensitivity and severity of LPS-induced septic shock and is associated with increased serum concentrations of proinflammatory cytokines, reinforcing the apparent role of TAK1 as a negative regulator of innate immune response.
Genetic Ablation of p38 in Myeloid Cells Rescues the Phenotypes of Map3k7ΔM/ΔM Mice
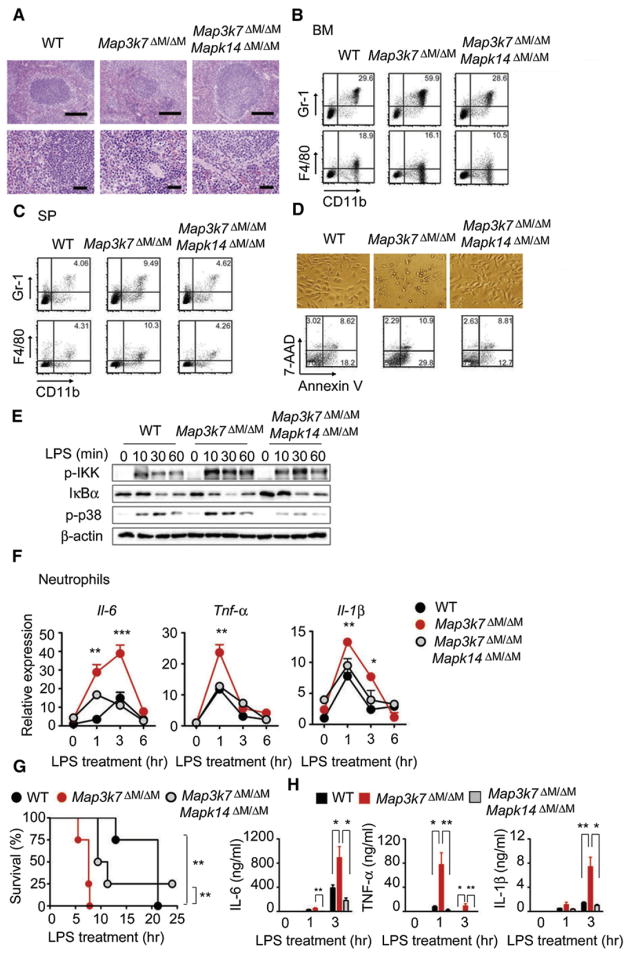
It has been reported that downregulation of NF-κB activation, resulting from IKKβ (encoded by Ikbkb) deficiency in myeloid cells, enhances IL-1β production through a caspase-1-independent mechanism that modulates NF-κB-dependent expression of protease inhibitors in neutrophils (Greten et al., 2007). However, this mechanism could not explain the enhanced production of IL-1β in Map3k7ΔM/ΔM neutrophils, because IKK activation was increased rather than decreased in these cells after LPS treatment. Furthermore, this proposed mechanism does not account for the aberrant production of IL-6 and TNF-α in neutrophils. Because TAK1 ablation markedly enhances activation of p38, which is known to play a crucial role in inflammatory responses and translational regulation of IL-1β, IL-6, and TNF-α (Han and Ulevitch, 2005; Kang et al., 2008), we reasoned that specific deletion of p38 (encoded by Mapk14) could rescue the phenotype and function of Map3k7ΔM/ΔM mice. To test this possibility, we generated Map3k7flox/flox × Mapk14flox/flox × Lyz2-Cre double-deletion (Map3k7ΔM/ΔMMapk14ΔM/ΔM) mice. Both PCR and immunoblot analyses confirmed the deletion of TAK1 and p38 in macrophages and neutrophils (Figures S3A–S3C). Gross phenotypic analysis revealed that the spleen size of Map3k7ΔM/ΔM Mapk14ΔM/ΔM mice was reduced to a size comparable to WT spleen, thus rescuing the splenomegaly phenotype observed in Map3k7ΔM/ΔM mice (data not shown). Specific ablation of Mapk14 also restored disorganized splenic architectures observed in Map3k7ΔM/ΔM mice (Figure 6A). In agreement with these observations, the percentage (59.9%) of Gr-1+CD11b+ cells in the bone marrow of Map3k7ΔM/ΔM mice was reduced to 28.6% in Map3k7ΔM/ΔMMapk14ΔM/ΔM mice, similar to 29.6% in WT mice (Figure 6B). Similarly, the percentage (9.5%) of neutrophils in Map3k7ΔM/ΔM spleen was decreased to 4.6% in Map3k7ΔM/ΔMMapk14ΔM/ΔM spleen (Figure 6C). Interestingly, specific ablation of Mapk14 in Map3k7ΔM/ΔM mice reduced the population of activated CD69+ and IFN-γ-producing T cells to the same percentages observed in WT mice (Figures S3D and S3E). The notion that Mapk14 deletion can reduce the hyperproliferation of neutrophils in Map3k7ΔM/ΔM mice was further supported by results from in vivo BrdU pulse-labeling experiments (Figure S3F). We found that apoptosis was similar in Gr-1+CD11b+ neutrophils from WT, Map3k7ΔM/ΔM, and Map3k7ΔM/ΔMMapk14ΔM/ΔM bone marrows and spleens (Figure S3G). In contrast, Mapk14 ablation restored the survival of cultured TAK1-deficient BMMs similar to WT cells, as well as cell proliferation based on CFSE labeling (Figures 6D, S3H, and S3I). These results indicate that Mapk14 ablation reduces proliferative expansion of neutrophils in the bone marrow and spleen, meanwhile improving the survival of BMMs, thus rescuing the splenomegaly, disorganized splenic histology and hyperplasia, and abnormal cellularity observed in Map3k7ΔM/ΔM mice.
Figure 6. p38 Ablation Rescues Splenomegaly Phenotype and Increased Proinflammatory Signaling in TAK1-Deficient Mice.
(A) H&E staining of spleen sections from WT, Map3k7ΔM/ΔM, and Map3k7ΔM/ΔMMapk14ΔM/ΔM mice. Top, 100× magnification; bottom, 400× magnification.
(B and C) FACS analysis of Gr-1+CD11b+ neutrophils and F4/80+CD11b+ macrophages in bone marrows (B) and spleens (C) from WT, Map3k7ΔM/ΔM, and Map3k7ΔM/ΔMMapk14ΔM/ΔM mice.
(D) Photographs of cultured BMM were visualized by light microscopy and apoptotic cells were analyzed by Annexin V/7-AAD staining and flow cytometry. Results are representative of at least three independent experiments.
(E) Immunoblot analysis of P-IKK, IκBα, and P-p38 in neutrophils treated with LPS for the indicated time points.
(F) Real-time PCR analysis of IL-6, TNF-α, and IL-1β mRNA expression in neutrophils treated with LPS for the indicated time points.
(G) Survival of WT (n = 4), Map3k7ΔM/ΔM (n = 4), and Map3k7ΔM/ΔMMapk14ΔM/ΔM (n = 4) mice treated with high-dose LPS (30 mg/kg).
(H) Serum concentrations of IL-6, TNF-α, and IL-1β were measured at 1 and 3 hr after LPS challenge.
Data shown in (G) and (H) are the mean ± SD.
*p < 0.05; **p < 0.01; ***p < 0.001. Data shown are representative of at least five independent experiments. See also Figure S3.
Specific Deletion of Mapk14 Restores NF-κB Signaling and Proinflammatory Cytokine Production in Map3k7ΔM/ΔM Mice
We next tested whether the elevated NF-κB signaling and proinflammatory cytokine production seen in Map3k7ΔM/ΔM mice were also reduced in Map3k7ΔM/ΔMMapk14ΔM/ΔM mice. In response to LPS stimulation, Mapk14 deletion reduced the LPS-induced elevated IKK phosphorylation as well as IκBα degradation observed in TAK1-deficient neutrophils (Figures 6E and S3J). Consistent with these observations, mRNA and protein expression of IL-1β, IL-6, and TNF-α detected in Map3k7ΔM/ΔMMapk14ΔM/ΔM neutrophils were either partially or completely restored to similar amounts observed in WT cells (Figures 6F and S3K). These results suggest that Mapk14 deletion restores the increased IKK phosphorylation and cytokine production in TAK1-deficient neutrophils to those in WT cells.
To determine the sensitivity of Map3k7ΔM/ΔM Mapk14ΔM/ΔM mice to LPS-induced septic shock, we treated WT, Map3k7ΔM/ΔM, and Map3k7ΔM/ΔMMapk14ΔM/ΔM mice with LPS and monitored their survival. As indicated in Figure 6G, Map3k7ΔM/ΔMMapk14ΔM/ΔM mice had intermediate survival times compared with the WT and Map3k7ΔM/ΔM groups, thus partially rescuing mice from LPS-induced endotoxin shock. Consistent with this observation, the plasma concentrations of IL-6, TNF-α, and IL-1β in Map3k7ΔM/ΔM Mapk14ΔM/ΔM mice were completely restored to those in WT mice, compared with Map3k7ΔM/ΔM mice (Figure 6H). Taken together, these results clearly indicate that Mapk14 ablation in myeloid cells can offset the increased production of inflammatory cytokines and reduce the hypersensitivity to LPS-induced septic shock seen in Map3k7ΔM/ΔM mice.
Multiple Signaling Pathways Lead to Increased Phosphorylation of p38
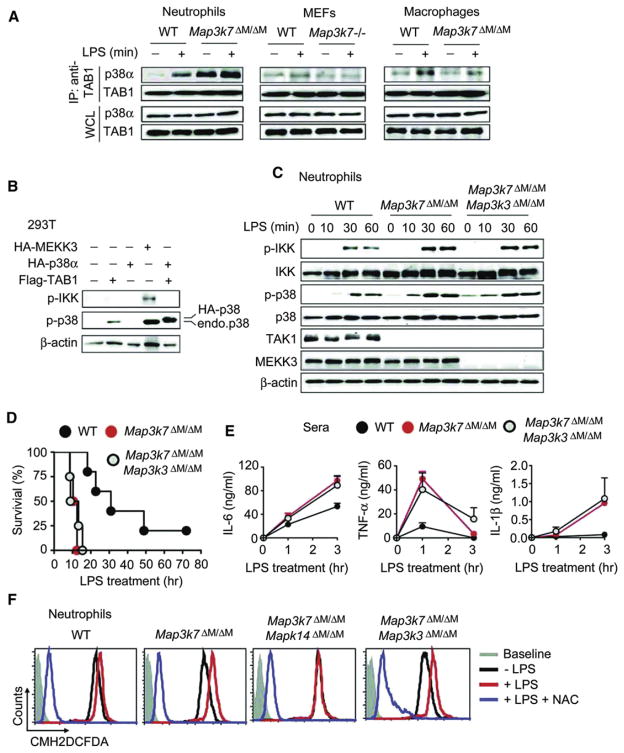
We next sought to determine how p38 phosphorylation was enhanced in TAK1-deficient neutrophils. Phosphorylation of p38 is generally thought to be controlled by the activated TAK1 and TAB1-3 complex or MAPK-ERK kinase kinase (MEKK3, Map3k3) (Ge et al., 2002). It has been reported that in addition to its binding to TAK1 with high affinity, TAB1 can also directly bind to p38α, resulting in enhanced phosphorylation of p38α (Ge et al., 2002). Thus, we reasoned that loss of TAK1 in neutrophils might allow free TAB1 to bind to p38, thus increasing the interaction between TAB1 and p38. Indeed, we observed a stronger TAB1-p38 interaction in untreated TAK1-deficient neutrophils compared with WT cells (Figure 7A). Although this interaction was increased in WT neutrophils after LPS treatment, a more robust increase in TAB1-p38 interaction was observed in TAK1-deficient neutrophils (Figure 7A). By contrast, we observed little or only weak TAB1-p38 interaction in WT and TAK1-deficient MEFs, although this interaction was only slightly increased in WT and TAK1-deficient macrophages after LPS treatment (Figure 7A). To determine the functional significance of TAB1-p38 interaction, we found that TAB1 could activate endogenous and exogenous p38 phosphorylation but did not activate IKK phosphorylation, whereas MEKK3 could stimulate both IKK and p38 phosphorylation (Figure 7B). These results suggest that interaction between TAB1 and p38 is cell type specific and is markedly enhanced in TAK1-deficient neutrophils but not in TAK1-deficient MEFs and macrophages.
Figure 7. Multiple Pathways Are Involved in p38 Activation in TAK1-Deficient Neutrophils.
(A) Interaction between TAB1 and p38 in neutrophils, peritoneal macrophages, and MEFs. Cells were treated with or without LPS for 15 min and cell lysates were immunoprecipitated (IP) with anti-TAB1 followed by immunoblotting with anti-p38α and anti-TAB1.
(B) 293T cells were transfected with Flag-TAB1, HA-p38α, HA-MEKK3, or empty vector alone, followed by immunoblot analysis of cell lysates with indicated antibodies.
(C) WT, Map3k7ΔM/ΔM, and Map3k7ΔM/ΔMMap3k3ΔM/ΔM neutrophils were treated with LPS, and cell lysates were immunoblotted with indicated antibodies.
(D) Survival of WT (n = 5), Map3k7ΔM/ΔM (n = 4), and Map3k7ΔM/ΔMMap3k3ΔM/ΔM (n = 4) mice treated with high-dose LPS (25 mg/kg). Serum concentrations of IL-6, TNF-α, and IL-1β were measured at 0, 1, and 3 hr after LPS injection.
(E) Serum concentrations of IL-6, TNF-α, and IL-1β were measured at 1 hr and 3 hr after LPS challenge.
(F) WT, Map3k7ΔM/ΔM, Map3k7ΔM/ΔMMapk14ΔM/ΔM, and Map3k7ΔM/ΔMMap3k3ΔM/ΔM neutrophils were pretreated with or without NAC (5 mM) for 30 min followed by LPS stimulation for 3 hr. ROS production was measured by staining cells with CM-H2DCFDA for 30 min followed by flow cytometry.
Results are representative of at least three independent experiments. See also Figure S4.
We next sought to determine the relative contribution of MEKK3 to increased p38 phosphorylation, because MEKK3 is an important kinase upstream of p38 signaling (Huang et al., 2004). To test this prediction, we simultaneously deleted Map3k7 and Map3k3 by generating Map3k7flox/flox × Map3k3flox/flox × Lyz2-Cre (Map3k7ΔM/ΔMMap3k3ΔM/ΔM) mice. Specific deletion of Map3k3 partially reduced the splenomegaly observed in Map3k7ΔM/ΔM mice (Figure S4A), suggesting that Map3k3 ablation affects the phenotype of Map3k7ΔM/ΔM mice in developmental processes. However, specific ablation of Map3k3 failed to reduce the increased phosphorylation of IKK and p38 observed in Map3k7ΔM/ΔM mice (Figure 7C). Consistent with this observation, the sensitivity of Map3k7ΔM/ΔM Map3k3ΔM/ΔM mice to LPS-induced septic shock was almost identical to that of Map3k7ΔM/ΔM mice, which showed increased sensitivity to LPS-induced septic shock compared with WT mice (Figure 7D). We also observed that the plasma concentrations of IL-6, TNF-α, and IL-1β in Map3k7ΔM/ΔMMap3k3ΔM/ΔM mice were similar to those in Map3k7ΔM/ΔM mice but were higher than those in WT mice (Figure 7E). These results suggest that specific deletion of Map3k3 partially reduces the splenomegaly of Map3k7ΔM/ΔM mice but fails to reduce increased phosphorylation of IKK and p38 after LPS treatment or increased sensitivity to LPS-induced septic shock in Map3k7ΔM/ΔM mice.
Recent studies show that reactive oxygen species (ROS) can also stimulate phosphorylation of p38 and IKK (Kulisz et al., 2002; Matsuzawa et al., 2005). In particular, LPS-induced production of ROS selectively activates p38 phosphorylation via an ASK1 (apoptosis signaling regulated kinase 1)-dependent manner (Matsuzawa et al., 2005). To test whether TAK1 deletion may result in increased ROS production, we treated neutrophils and macrophages with LPS for 30 and 180 min and then incubated cells with CM-H2DCFDA for 30 min. ROS production in cells without LPS served as control. Flow cytometry analysis revealed markedly increased ROS production in TAK1-deficient neutrophils, compared to WT cells after LPS treatment (Figure S4B). In contrast, ROS production was lower in TAK1-deficient macrophages than in WT cells after LPS stimulation (Figure S4C). To determine whether specific deletion of Mapk14 or Map3k3 affects ROS production in TAK1-deficient neutrophils, we isolated neutrophils from WT, Map3k7ΔM/ΔM, Map3k7ΔM/ΔM Mapk14ΔM/ΔM, and Map3k7ΔM/ΔMMap3k3ΔM/ΔM mice and then measured ROS production after LPS treatment. As shown in Figure 7F, LPS-induced ROS production in TAK1-deficient neutrophils was much higher than that in WT cells. However, Mapk14 but not Map3k3 deletion reduced the amounts of LPS-induced ROS production in TAK1-deficient neutrophils to the amounts seen without LPS treatment, consistent with reduced IKK phosphorylation in Map3k7ΔM/ΔMMapk14ΔM/ΔM neutrophils (Figure S3J). Treatment of cells with the ROS inhibitor N-acetyl-L-cysteine (NAC) completely abolished total (LPS-dependent and -independent) ROS production and phosphorylation of IKK and p38 in all cells regardless of the source (Figures 7F and S4D). To determine whether ROS production is responsible for increased IKK phosphorylation, we treated neutrophils from WT, Map3k7ΔM/ΔM, and Map3k7ΔM/ΔMMapk14ΔM/ΔM mice with an organic H2O2 (tertiary butyl hydroperoxide, tBHP) and found that exogenous ROS treatment rapidly increased IKK and p38 phosphorylation as well as NF-κB-responsive genes such as IL-6 in neutrophils, regardless of the state of TAK1 and p38 (Figures S4E and S4F). Furthermore, tBHP treatment restored IKK phosphorylation in Map3k7ΔM/ΔMMapk14ΔM/ΔM neutrophils to similar amounts observed in Map3k7ΔM/ΔM neutrophils, compared with those in cells treated with LPS alone (Figure S4E). These results clearly suggest that ROS production is responsible for increased IKK phosphorylation.
DISCUSSION
In this study we have shown that specific deletion of Map3k7 in the myeloid lineage leads to splenomegaly, lymphadenopathy, and increased percentages of Gr-1+CD11b+ neutrophils. Hyper-proliferation and increased inflammatory cytokine production in TAK1-deficient neutrophils, but not macrophages, stands in sharp contrast to the increased cell death observed in mice with conditional inactivation of TAK1 in hematopoietic lineages, liver, and intestine epithelial cells (Bettermann et al., 2010; Inokuchi et al., 2010; Kajino-Sakamoto et al., 2008; Liu et al., 2006; Tang et al., 2008). Importantly, we have demonstrated that Map3k7 deletion in neutrophils enhanced activation of NF-κB, p38, and JNK signaling after LPS stimulation. Notably, NF-κB and p38 signaling was only slightly reduced in TAK1-deficient peritoneal macrophages after TLR stimulation, in contrast to TAK1-deficient MEFs, where NF-κB and p38 signaling and cytokine response were completely abolished. However, Map3k7 deletion caused the apoptotic death of BMMs after 3 days of culture. Taken together, these results clearly indicate that TAK1 plays important roles in NF-κB, p38, and JNK signaling pathways in a cell type-specific manner: TAK1 functions as a negative regulator of the IKK, p38, and JNK signaling pathways in neutrophils but serves as an essential and positive regulator in macrophages and fibroblasts.
We have further shown that TAK1-deficient Gr-1+CD11b+ neutrophils produce more IL-1β, IL-6, and TNF-α than WT cells after LPS treatment. Although the numbers of B and T cells were not changed in Map3k7ΔM/ΔM mice, further experiments showed elevated percentages of activated T cells, suggesting that larger amounts of proinflammatory cytokines may promote T cell activation in Map3k7ΔM/ΔM mice. By contrast, IL-6 and TNF-α production in TAK1-deficient macrophages was similar or reduced after LPS treatment, but IL-1β production was increased in TAK1-deficient peritoneal macrophages compared with WT cells, which might be due to increased cell apoptosis, as shown in IKKβ-deficient macrophages (Greten et al., 2007). Although most negative regulators of innate immune signaling molecules, including A20, NLRC5, and NLRX1, inhibit NF-κB signaling in most cell types (Allen et al., 2011; Cui et al., 2010; Wertz et al., 2004; Xia et al., 2011), cell type-specific regulators have been reported for other innate immune adaptor molecules like RIG-I and MDA5, as well as IRF7 (Honda and Taniguchi, 2006; Kato et al., 2005, 2006). TRAF family member-associated NF-κB activator (TANK) has been implicated in positive regulation of transcriptional factors IRF3 and NF-κB. However, a recent study showed that TANK-deficient mice develop splenomegaly and lymphadenopathy and enhance NF-κB signaling by promoting the ubiquitination of TRAF6 upon TLR stimulation, thus serving as a negative regulator of TLR signaling (Matsushita et al., 2009). In light of these findings, we conclude that TAK1 is another crucial (either positive or negative) regulator of NF-κB and MAPK signaling in a cell type-specific manner.
Downregulation of NF-κB activation resulting from specific Ikbkb (IKKβ) inactivation in myeloid cells has been shown to enhance IL-1β production and promote rapid LPS-induced septic shock through a mechanism involving NF-κB-dependent modulation of protease inhibitors (Greten et al., 2007). However, Ikbkbflox/flox × Lyz2-Cre mice do not develop phenotypes such as splenomegaly and neutrophilia observed in Map3k7ΔM/ΔM mice (Greten et al., 2007). Because we found that TAK1-deficient neutrophils increased NF-κB and MAPK signaling and produced more IL-1β, IL-6, and TNF-α compared with WT cells, NF-κB-dependent expression of protease inhibitors, which explains the increased IL-1β processing in IKKβ-deficient neutrophils (Greten et al., 2007), could not fully explain the enhanced production of IL-1β in TAK1-deficient neutrophils. Therefore, we sought to identify alternative mechanisms. We have provided compelling genetic evidence that Mapk14 deletion in myeloid cells rescues the observed phenotypes and the inflammatory cytokine responses in Map3k7ΔM/ΔM mice. Thus, it appears that increased activation of p38 in TAK1-deficient cells is one possible reason for the increased production of IL-1β, IL-6, and TNF-α. These results are supported by previous findings that p38 plays a critical role in inflammatory responses and translational regulation of IL-1β, IL-6, and TNF-α (Han and Ulevitch, 2005; Kang et al., 2006). Consistent with these observations, the hypersensitivity to LPS-induced septic shock in Map3k7 and Mapk14 double-ablated mice was reduced, compared with TAK1 single mutant mice. Thus, our results suggest that although TAK1 deletion affects the phosphorylation of IKK, p38, and JNK in neutrophils, its effect on p38 appears to be critical for the observed phenotypes and cytokine production in Map3k7ΔM/ΔM mice.
To identify the molecular mechanisms by which TAK1 deletion in neutrophils increases the phosphorylation of p38, we have provided several lines of evidence that p38 can be activated and phosphorylated by three distinct signaling pathways. First, several studies have shown that whereas TAB1 binds to TAK1 with high affinity, TAB1 can also directly bind to p38α, resulting in enhanced phosphorylation and activation of p38α (Ge et al., 2002). To determine how TAK1 deletion affects p38 activation in neutrophils, we found that TAB1-p38 interaction was markedly increased in unstimulated and LPS-stimulated TAK1-deficient neutrophils. By contrast, we did not observe such an interaction in MEFs or macrophages even after LPS stimulation. In addition, we have shown that TAB1 can functionally stimulate p38, but not IKK, phosphorylation. Thus, it is possible that loss of TAK1 in neutrophils may free TAB1 to bind and stimulate p38 phosphorylation, but not in MEFs or macrophages. Second, because MEKK3 can also activate IKK and p38 phosphorylation, we generated Map3k7 and Map3k3 double-ablated mice. Specific deletion of Map3k3 partially reduced the splenomegaly observed in Map3k7ΔM/ΔM mice, suggesting that MEKK3 may partially contribute to the developmental phenotype of Map3k7ΔM/ΔM mice. However, neither increased IKK and p38 phosphorylation after LPS stimulation nor increased sensitivity to LPS-induced septic shock observed in Map3k7ΔM/ΔM mice changed in Map3k7ΔM/ΔMMap3k3ΔM/ΔM mice. This would indicate that MEKK3 might not be primarily responsible for increased IKK and p38 phosphorylation in TAK1-deficient neutrophils after LPS treatment. Third, our results showed that specific deletion of p38 reduced the LPS-induced elevated IKK phosphorylation as well as IκBα degradation observed in TAK1-deficient neutrophils. Thus, there are two key questions that remain to be addressed. (1) How is IKK activated? (2) How does p38 affect IKK activation in TAK1-deficient neutrophils after LPS treatment? To address these issues, we show that TAK1 deletion led to an increase in LPS-induced ROS production in neutrophils, but not in macrophages. Several recent studies demonstrate that LPS-induced ROS can promote IKK and p38 phosphorylation (Asehnoune et al., 2004; Ito et al., 2006; Kulisz et al., 2002; Matsuzawa et al., 2005). The fact that p38 phosphorylation by LPS-induced ROS selectively requires ASK1, but not MEKK3 adaptor molecule (Matsuzawa et al., 2005; Su, 2005), may explain why Map3k3 deletion failed to reduce increased IKK and p38 phosphorylation observed in Map3k7ΔM/ΔM mice. Indeed, treatment of neutrophils with NAC completely inhibited ROS production and abolished IKK and p38 phosphorylation. Conversely, treatment of neutrophils with exogenous H2O2 (tBPH) activated IKK phosphorylation, regardless of genetic state of TAK1 and/or p38. Our results further show that increase in LPS-induced ROS production after treatment required active p38, because deletion of p38 failed to increase ROS production, thus reducing IKK phosphorylation. However, this phenomenon could be reversed by treatment of neutrophils with tBPH, suggesting that ROS production is important for IKK phosphorylation. Although TAK1 deletion in keratinocytes also increases ROS production, the mechanisms responsible for increased ROS production may be different (Lam et al., 2011). Thus, further studies are needed to elucidate how TAK1 ablation affects ROS production induced by different stimuli.
On the strength of the above findings and published results, we propose a working model that TAK1 ablation enhances rather than diminishes innate immune signaling in neutrophils (Figure S5). Upon LPS treatment, both WT and TAK1-deficient neutrophils produce LPS-specific ROS (in addition to normal metabolic ROS), which activates IKK and p38 phosphorylation in neutrophils, regardless of the state of TAK and p38. However, the differences in IKK and p38 phosphorylation between WT and TAK1-deficient neutrophils after LPS treatment may begin with the intrinsic shift from TAB1 interaction with TAK1 to TAB interaction with p38 in TAK1-deficient neutrophils. Free TAB1 in TAK1-deficient neutrophils enhances or amplifies p38 phosphorylation, in contrast to TAB1 in WT cells, which mainly binds to TAK1. This delicate shift may lead to increased phosphorylation of p38 and production of downstream responsive genes such as IL-6 in TAK1-deficient neutrophils. Increased production of proinflammatory cytokines may further increase ROS production through cytokine receptor signaling pathways, thus forming a positive regulatory mechanism or loop (ROS/p38/ROS). It should be noted that this is an active and ongoing process. The difference in IKK phosphorylation between WT and TAK1-deficient neutrophils after LPS treatment may be due to differences in ROS production that induces IKK phosphorylation. Specific deletion of p38 disrupts this positive feedback regulatory loop, thus reducing ROS production, restoring IKK phosphorylation in TAK1-deficient neutrophils to similar amounts observed in WT cells, and rescuing the phenotypes observed in Map3k7ΔM/ΔM mice. By contrast, specific deletion of Map3k3 fails to do so, although MEKK3 can activate both IKK and p38 activation during developmental processes.
In conclusion, we clearly demonstrate that TAK1 negatively regulates proinflammatory signaling cascades and cytokine production in neutrophils, but not in macrophages, thus providing genetic and biochemical evidence that TAK1 regulates NF-κB and MAPK signaling pathways in a cell type-specific manner. More importantly, p38 is a key downstream signaling molecule that controls inflammatory signaling and maintains homeostasis of innate immune cells, particularly in Map3k7ΔM/ΔM mice. We also provide evidence that increased ROS production and increased TAB1-p38 interaction in TAK1-deficient neutrophils may contribute to increased p38 and IKK phosphorylation as well as the production of proinflammatory cytokines after LPS stimulation, whereas MEKK3 may be involved in controlling homeostasis of immune responses during development. Thus, these findings identify a previously unrecognized role of TAK1 in negative regulation of innate immune signaling in neutrophils and could have important implications for development of therapies for inflammation-associated diseases, including cancer.
EXPERIMENTAL PROCEDURES
Animals
Map3k7flox/flox mice (gift from M.D. Schneider, Baylor College of Medicine) were crossed with lysozyme-Cre (Lyz2-Cre) mice (Jackson Laboratory) to obtain Map3k7ΔM/ΔM mice in the C57BL/6 background. To generate Map3k7ΔM/ΔMMapk14ΔM/ΔM mice, Map3k7ΔM/ΔM mice were bred with Mapk14flox/flox mice (provided by Y. Wang, UCLA, and H. Jiang, Boehringer-Ingelheim) (Engel et al., 2005). To generate Map3k7ΔM/ΔMMap3k3ΔM/ΔM mice, Map3k7ΔM/ΔM mice were bred with Map3k3flox/flox mice. Studies were performed with 8- to 12-week-old mice. Animal experiments were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. To study in vivo endotoxicity, mice received i.p. injection of LPS (25–30 mg/kg) and blood samples were collected to assay plasma cytokine concentrations.
Primary Cell Cultures
Bone marrow-derived macrophages (BMM) were generated by flushing bone marrow cells from femurs and tibiae of mice. Cells were cultured in RPMI media containing 10% FBS and 1% (volume/volume) penicillin-streptomycin supplemented with 10% M-CSF conditioned media. Peritoneal macrophages were obtained by injecting mice with 4% (v/v) thioglycollate (Beckton Dickson), and peritoneal cavities were flushed after 3 days with RPMI media/2% FBS. TAK1 WT and deficient MEFs were a generous gift from D. Zhang (Texas A&M University). Neutrophils were obtained from the peritoneal cavity by injecting mice with 2 ml 4% (v/v) thioglycollate for 4 hr followed by collection of peritoneal exudates with RPMI media/2% FBS. Cells were stained with anti-Gr-1-PE (eBioscience) and purified with PE-positive selection magnetic beads (Stem Cell Technologies).
Immunoprecipitation and Immunoblot Analyses
For immunoprecipitation, cell lysates were incubated with indicated antibodies and protein A and G beads (Pierce) overnight. Beads were washed four times with lysis buffer, and immunoprecipitates were eluted with SDS loading buffer. Immunoblotting was performed by resolving protein lysates on SDS-PAGE gels, followed by a transfer to PVDF membranes (Bio-Rad) and then incubating membranes with indicated antibodies overnight. For all blots, LumiGlo Chemiluminescent Substrate System from KPL (Gaithersburg, MD) was used for protein detection.
Flow Cytometry
Cell surface staining, intracellular cytokine staining, flow cytometry, and cell sorting were performed as described previously (Wang et al., 2004; Ivanov et al., 2006; Miyahara et al., 2008). Single-cell suspensions were obtained from tissues and stained for 30 min with indicated antibodies. Flow cytometric analysis was performed with BD FACS Calibur or BD FACS Aria (Becton Dickson).
Cytokine Release Assay
Cell supernatants or sera were collected at indicated time points after stimulation with LPS with or without ATP. Cytokine concentrations were measured by ELISA with anti-mouse IL-6 antibodies (Thermo Scientific), TNF-α and IL-1β ELISA kits purchased from R&D Systems. ELISA assays were performed according to the manufacturer’s instructions.
ROS Measurement and H2O2 Treatment
Neutrophils were pretreated with or without NAC (5 mM) for 30 min followed by stimulation with LPS (100 ng/ml) for 0, 10, 30 min, and 3 hr at 37°C. Cells were harvested and incubated with or without CM-H2DCFDA (10 μM) in PBS for 30 min at 37°C and cells were analyzed by flow cytometry. Similarly, neutrophils from WT, Map3k7ΔM/ΔM, or Map3k7ΔM/ΔMMapk14ΔM/ΔM mice were treated with an organic form of H2O2 (tertiary butyl hydroperoxide, tBHP), LPS, or both for 0, 10, and 30 min and then used to determine IKK and p38 phosphorylation by immunoblot.
Hematological and Histological Analysis
Complete blood counts were performed with an automated hematology analyzer (Hemavet 850; Drew Scientific). For histology, tissues were formalin-fixed, processed, and paraffin-embedded. H&E staining was done by the Breast Cancer Center (Baylor College of Medicine).
Statistical Analysis
Data are represented as mean ± SD when indicated and Student’s t test was used for all statistical analyses with the GraphPad Prism 4.0 software. Differences were considered significant when p value was less than 0.05.
Supplementary Material
Acknowledgments
We would like to thank M. Schneider (Baylor College of Medicine) for TAK1flox/flox mice; Y. Wang (UCLA) and H. Jiang (Boehringer-Ingelheim) for p38flox/flox mice; and D. Zhang (Texas A&M University) for TAK1-deficient MEFs. This work was in part supported by grants from NCI, NIH, and Cancer Research Institute. J.C. was partially supported by NNSFC (31000394).
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, five figures, and one table and can be found with this article online at doi:10.1016/j.immuni.2011.12.010.
References
- Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, Gris D, Roney KE, Zimmermann AG, Bowzard JB, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways. Immunity. 2011;34:854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, Weber A, Weiskirchen R, Liedtke C, Gassler N, et al. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell. 2010;17:481–496. doi: 10.1016/j.ccr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140:2646–2706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- Cui J, Zhu L, Xia X, Wang HY, Legras X, Hong J, Ji J, Shen P, Zheng S, Chen ZJ, Wang RF. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell. 2010;141:483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, Luo Y, Han J. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Göktuna SI, Neuenhahn M, Fierer J, Paxian S, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol. 2005;6:1198–1205. doi: 10.1038/ni1274. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- Huang Q, Yang J, Lin Y, Walker C, Cheng J, Liu ZG, Su B. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat Immunol. 2004;5:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- Inokuchi S, Aoyama T, Miura K, Osterreicher CH, Kodama Y, Miyai K, Akira S, Brenner DA, Seki E. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci USA. 2010;107:844–849. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Kajino-Sakamoto R, Inagaki M, Lippert E, Akira S, Robine S, Matsumoto K, Jobin C, Ninomiya-Tsuji J. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol. 2008;181:1143–1152. doi: 10.4049/jimmunol.181.2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Seit-Nebi A, Davis RJ, Han J. Multiple activation mechanisms of p38alpha mitogen-activated protein kinase. J Biol Chem. 2006;281:26225–26234. doi: 10.1074/jbc.M606800200. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Chen J, Otsuka M, Mols J, Ren S, Wang Y, Han J. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J Immunol. 2008;180:5075–5082. doi: 10.4049/jimmunol.180.7.5075. [DOI] [PubMed] [Google Scholar]
- Kantari C, Pederzoli-Ribeil M, Witko-Sarsat V. The role of neutrophils and monocytes in innate immunity. Contrib Microbiol. 2008;15:118–146. doi: 10.1159/000136335. [DOI] [PubMed] [Google Scholar]
- Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kulisz A, Chen N, Chandel NS, Shao Z, Schumacker PT. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1324–L1329. doi: 10.1152/ajplung.00326.2001. [DOI] [PubMed] [Google Scholar]
- Lam CR, Tan MJ, Tan SH, Tang MB, Cheung PC, Tan NS. TAK1 regulates SCF expression to modulate PKBα activity that protects keratinocytes from ROS-induced apoptosis. Cell Death Differ. 2011;18:1120–1129. doi: 10.1038/cdd.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HH, Xie M, Schneider MD, Chen ZJ. Essential role of TAK1 in thymocyte development and activation. Proc Natl Acad Sci USA. 2006;103:11677–11682. doi: 10.1073/pnas.0603089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, Akira S. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458:1185–1190. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–592. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC, Ninomiya-Tsuji J. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Tsujimura T, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Takeuchi O, Akira S. TAK1 is indispensable for development of T cells and prevention of colitis by the generation of regulatory T cells. Int Immunol. 2006;18:1405–1411. doi: 10.1093/intimm/dxl082. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Schuman J, Chen Y, Podd A, Yu M, Liu HH, Wen R, Chen ZJ, Wang D. A critical role of TAK1 in B-cell receptor-mediated nuclear factor kappaB activation. Blood. 2009;113:4566–4574. doi: 10.1182/blood-2008-08-176057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B. Linking stress to immunity? Nat Immunol. 2005;6:541–542. doi: 10.1038/ni0605-541. [DOI] [PubMed] [Google Scholar]
- Tang M, Wei X, Guo Y, Breslin P, Zhang S, Zhang S, Wei W, Xia Z, Diaz M, Akira S, Zhang J. TAK1 is required for the survival of hematopoietic cells and hepatocytes in mice. J Exp Med. 2008;205:1611–1619. doi: 10.1084/jem.20080297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7:851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- Xia X, Cui J, Wang HY, Zhu L, Matsueda S, Wang Q, Yang X, Hong J, Songyang Z, Chen ZJ, Wang RF. NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity. 2011;34:843–853. doi: 10.1016/j.immuni.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.