Abstract
Objective
Subjects with peripheral artery disease (PAD) are at increased risk of cardiovascular morbidity and mortality, perhaps in part, related to increased levels of inflammation, platelet activity, and lipids. We therefore sought to investigate the relationship between PAD and levels of inflammatory, platelet, and lipid biomarkers and the treatment effect of darapladib, a novel lipoprotein-associated phospholipase A2 (Lp-PLA2) inhibitor.
Methods
This is a post hoc analysis of the 959 patients with coronary disease or their risk equivalent receiving atorvastatin who were randomized to receive darapladib or placebo to examine the effects of an Lp-PLA2 inhibitor on the biomarkers of cardiovascular risk. We conducted an exploratory analysis evaluating the levels of biomarkers in subjects with PAD (n = 172) compared with those without PAD (n = 787).
Results
After adjustment for age, sex, smoking, body mass index, and diabetes, subjects with PAD had greater levels of matrix metalloproteinase-9 (between group comparisons 22%, 95% confidence interval [10–31], P < .01), myeloperoxidase (12% [2–20], P = .01), interleukin-6 (13% [4–21], P = .01), adiponectin (17% [7–26], P < .01), intercellular adhesion molecule-1 (7% [2–11], P < .01), osteoprotegrin (6% [1–10], P = .02), CD40 ligand (15% [1–28], P = .04), high-sensitivity C-reactive protein (17% [1–31], P = .04), and triglycerides (11% [0.2–21], P = .05). No significant difference was detected for Lp-PLA2 activity, P-selectin, urinary 11-dehydrothroboxane B2, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol between subjects with and without PAD. Darapladib produced highly significant inhibition of Lp-PLA2 activity when compared with placebo at weeks 4 and 12 (P < .01) in patients with and without PAD.
Conclusions
Subjects with PAD had elevated levels of matrix metalloproteinase-9, myeloperoxidase, interleukin-6, adiponectin, intercellular adhesion molecule-1, osteoprotegrin, CD40 ligand, high-sensitivity C-reactive protein, and triglycerides compared with those without PAD. Darapladib, a novel Lp-PLA2 inhibitor, was equally effective in reducing Lp-PLA2 activity levels in subjects with and without PAD.
Peripheral arterial disease (PAD) is a common manifestation of atherosclerosis and is a highly prevalent condition in the United States, affecting approximately 27 million people in North America and Europe.1 Persons with PAD are at significantly increased risk for cardiovascular morbidity and mortality and have impaired function and quality of life.2 Targeted therapies aimed at reducing symptom onset and disease progression have had limited success, partially because the pathophysiology of this condition is relatively understudied compared with coronary and cerebrovascular arterial disease. Emerging data suggest that the peripheral vasculature differs from the coronary and cerebrovascular beds.1,3,4 A more thorough understanding of the pathophysiology of PAD is required to provide the basis for novel diagnostic and therapeutic strategies.5
Chronic inflammation, endothelial dysfunction, and increased platelet activity contribute to the development and consequences of atherothrombosis.6 Biomarkers are generally considered to provide independent diagnostic and prognostic value by reflecting an underlying disease state. Numerous prospective studies have shown independent associations of serum levels of biomarkers of inflammation such as C-reactive protein (CRP) with myocardial infarction, stroke, and all-cause mortality.7 Recent studies suggest that, beside CRP, other biomarkers such as cytokines (interleukin [IL]-1, IL-6, IL-8, monocyte chemoattractant protein-1), soluble CD40 ligand, serum amyloid A, selectins (E-selectin, P-selectin), myeloperoxidase (MPO), matrix metalloproteinases (MMPs), cellular adhesion molecules (intercellular adhesion molecule 1 [ICAM-1], vascular adhesion molecule 1 [VCAM-1]), placental growth factor (PlGF), and A(2) phospholipases may have a potential role in the diagnosis and risk stratification of patients with coronary disease. There is a relative paucity of data on the cumulative relationship between a wide array of biomarkers and PAD in a single cohort.8 In particular, polyvascular disease that includes atherosclerosis of multiple vascular beds is associated with higher risk of recurrent ischemic events and warrants special attention.9–11 To address these issues, we evaluated a panel of inflammatory, platelet, and lipid biomarkers as independent predictors of symptomatic PAD in a cohort of patients with coronary disease or their risk equivalent receiving atorvastatin who were randomized to receive darapladib or placebo.12 Darapladib is a selective lipoprotein-associated phospholipase A2 (Lp-PLA2) inhibitor that is under investigation for its potential to stabilize high-risk atherosclerotic plaques and potentially reduce cardiovascular events.12,13 The biomarkers evaluated in the present study included a broad panel of soluble inflammatory, platelet, and lipid-related biomarkers.
Methods
The current study included 959 patients enrolled in a multicenter, randomized, double-blind, placebo-controlled, parallel-group study that was conducted in 110 sites in 15 countries from 2005 to 2006. This study evaluated the ability of darapladib to produce sustained inhibition of plasma Lp-PLA2 activity in subjects with stable coronary heart disease (CHD) or CHD-risk equivalent receiving concomitant atorvastatin therapy. All eligible subjects were initially randomized to double-blind atorvastatin 20 or 80 mg once daily. After 4 weeks, subjects who tolerated atorvastatin therapy and achieved low-density lipoprotein cholesterol (LDL-C) levels of ≤115 mg/dL were then randomized to concomitant administration of darapladib or placebo once daily for 12 weeks. Ethics committees approved the protocol, and all subjects provided written informed consent before enrollment in the study.
The details of the trial design have been published previously.12 Briefly, the study included subjects aged 18 to 80 years with stable CHD or CHD-risk equivalent (defined as diabetes mellitus requiring hypoglycemic medication, carotid stenosis >50%, prior carotid surgery or stenting, PAD, or a cluster of risk factors resulting in 10-year risk for coronary events >20% according to Framingham Risk Score).
Assessments
Blood samples were collected in the fasting state at baseline (following randomization to atorvastatin 20 or 80 mg, but before randomization to darapladib or placebo) and at 4 and 12 weeks of dosing with darapladib or placebo. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), calculated LDL-C, and triglycerides were assessed. In addition, plasma Lp-PLA2 activity, inflammatory biomarkers, and platelet-related biomarkers were measured at baseline and at 4 and 12 weeks. Plasma Lp-PLA2 activity and platelet-related biomarkers were also measured 2 weeks after discontinuing darapladib or placebo. Details regarding the analysis of the biomarkers were described previously.12
Statistical analysis
A retrospective exploratory statistical analysis was conducted to characterize the baseline demographic and biomarker characteristics for patients with and without PAD. Analysis of variance was conducted to compare baseline characteristics in patients with and without PAD, adjusting for statin dose. Multivariate analysis of covariance was conducted to assess several biomarker responses and their change from baseline at discrete time points comparing patients with and without PAD as well to assess darapladib's effect within these patient subgroups. No adjustment was made for multiple testing because of the exploratory nature of the investigation.
Results
Among the 959 participants with coronary disease or their risk equivalent, 172 (17.9%) had a medical history of PAD. Table I shows baseline characteristics. Subjects with PAD were older (65 vs 62 years, P < .01), were less frequently diabetic (35% vs 52%, P < .01), had lower body mass index (29 vs 30 kg/m2, P = .02), had lower diastolic blood pressure (76 vs 78 mm Hg, P = .02), and were more likely to be current smokers (19% vs 13%, P = .03). There was no difference in sex, systolic blood pressure, or concomitant medications between the groups.
Table I.
Baseline characteristics of participants with and without PAD
| Overall (N = 959) |
Subjects with PAD (n = 172) |
Subjects without PAD (n = 787) |
P | |
|---|---|---|---|---|
| Demographic data | ||||
| Age (y), mean* | 63 ± 9 | 65 ± 8 | 62 ± 9 | .0005 |
| Men/women (%) | 71/29 | 74/26 | 71/29 | .4232 |
| Body mass index (kg/m2), mean* | 30 ± 6 | 29 ± 5 | 30 ± 6 | .0167 |
| Systolic blood pressure* | 133 ± 15 | 133 ± 15 | 133 ± 15 | .9834 |
| Diastolic blood pressure* | 78 ± 9 | 76 ± 10 | 78 ± 9 | .0163 |
| Heart rate* | 67 ± 11 | 68 ± 12 | 67 ± 10 | .0736 |
| Risk factors (%) | ||||
| Diabetes† | 49 | 35 | 52 | <.0001 |
| Hypertension | 74 | 75 | 74 | .7697 |
| Dyslipidemia | 61 | 65 | 60 | .2871 |
| Current smoking | 14 | 19 | 13 | .0265 |
| Medical history (%) | ||||
| CHD | 54 | 61 | 52 | .0163 |
| PAD | 18 | 100 | 0 | |
| Stroke or transient ischemic attack | 6 | 12 | 5 | .0007 |
| No documented vascular disease‡ | 39 | 0 | 48 | |
| Concomitant medications (%) | ||||
| Antiplatelet | 83 | 84 | 82 | .4287 |
| Atorvastatin | 100 | 100 | 100 | |
| ACE inhibitor or ARB | 68 | 67 | 68 | .9136 |
| β-Blocker | 48 | 50 | 48 | .6418 |
| Thiazolidinedione | 10 | 8 | 10 | .4255 |
ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Presented as arithmetic mean ± SD.
Denotes diabetes requiring pharmacotherapy.
Refers to patients with Framingham Risk Score >20%.
Table II demonstrates the baseline lipid, inflammatory, and platelet biomarkers in subjects with and without PAD. Levels of total cholesterol, LDL-C, HDL-C, triglycerides, Lp-PLA2 activity, and P-selectin were not different between the groups. Subjects with PAD had significantly higher levels of IL-6, MMP-9, adiponectin, ICAM-1, and osteoprotegrin compared with subjects without PAD. Levels of high-sensitivity CRP (hs-CRP), MPO, CD40 ligand were higher in the PAD group; however, these results did not reach statistical significance.
Table II.
Baseline biomarkers of participants with and without PAD
| Overall (N = 959) | Subjects with PAD (n = 172) |
Subjects without PAD (n = 787) |
P | |
|---|---|---|---|---|
| Baseline lipid and HbA1c values* | ||||
| Total cholesterol (mg/dL) | 142 ± 28 | 142 ± 31 | 142 ± 28 | .8373 |
| LDL-C (mg/dL) | 67 ± 22 | 67 ± 22 | 68 ± 21 | .6846 |
| HDL-C (mg/dL) | 50 ± 13 | 50 ± 14 | 50 ± 13 | .7620 |
| Triglycerides (mg/dL) | 127 ± 65 | 132 ± 76 | 126 ± 63 | .1760 |
| HbA1c (%) | 6.7 ± 1.04 | 6.5 ± 0.97 | 6.7 ± 1.05 | .0535 |
| Baseline Inflammatory biomarker values† | ||||
| Lp-PLA2 activity (nmol min−1 mL−1) | 123 (107, 145) | 125 (109, 149) | 123 (107, 145) | .3056 |
| hs-CRP (mg/L) | 1.17 (0.6, 2.5) | 1.32 (0.6, 2.6) | 1.14 (0.6, 2.4) | .1252 |
| IL-6 (ng/L) | 2.53 (1.7, 3.5) | 2.88 (2.0, 4.2) | 2.46 (1.7, 3.3) | .0035 |
| Myeloperoxidase (pmol/L) | 556 (380, 755) | 600 (422, 826) | 547 (375, 743) | .0660 |
| Matrix metalloproteinase-9 (µg/L) | 499 (273, 877) | 593 (323, 1050) | 481 (260, 830) | .0017 |
| Adiponectin (ng/mL) | 6.73 (4.2, 10.6) | 7.9 (4.9, 13.1) | 6.5 (4.2, 10.2) | .0017 |
| ICAM-1 (µg/L) | 262 (224, 300) | 280 (235, 327) | 259 (222, 295) | .0011 |
| Osteoprotegerin (pmol/L) | 577 (4.7, 7.0) | 6.17 (5.0, 7.0) | 5.69 (4.6, 7.0) | .0046 |
| Baseline platelet biomarker values† | ||||
| P-selectin (µg/L) | 49 (38, 62) | 50 (39, 63) | 49 (37, 61) | .3631 |
| CD40L (pg/mL) | 375 (188, 669) | 411 (216, 707) | 367 (181, 654) | .1511 |
| U-11-dehydro-TxB2 (ng/mmol Cr) | 56 (35, 81) | 58 (35, 88) | 55 (34, 80) | .3657 |
HbA1c, Hemoglobin A1c; TxB2, thromboxane B2.
Presented as arithmetic mean ± SD.
Presented as geometric mean (interquartile range: 25th, 75th percentile).
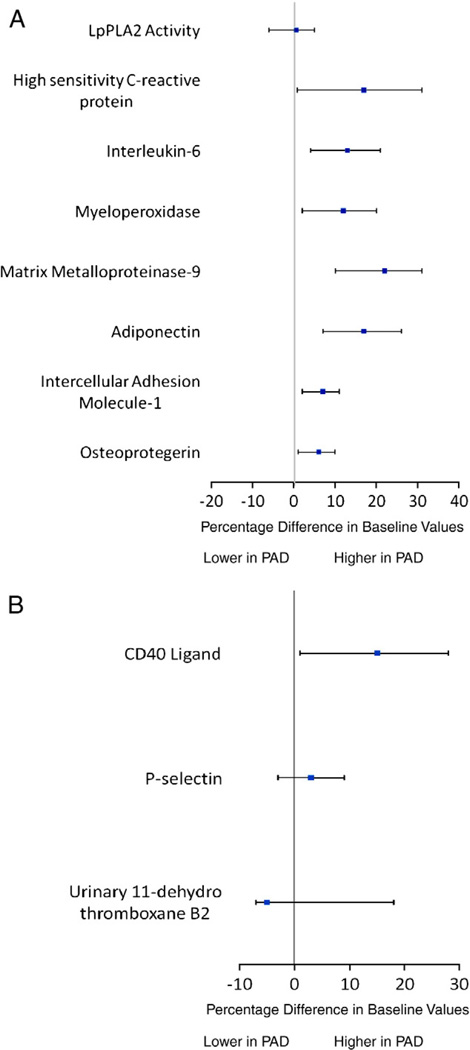
Figure 1 shows the results of baseline inflammatory and platelet biomarkers in subjects with versus without PAD, after multivariable adjustment. After adjustment for age, sex, body mass index, smoking status, diabetic status, and randomized statin dose, participants with PAD had higher levels of hs-CRP (17%, 95% confidence interval [CI] 0.8–31, P = .04), IL-6 (13%, 95% CI 4–21, P < .01), MMP-9 (22%, 95% CI 10–31, P < .01), adiponectin (17%, 95% CI 7–26, P < .01), ICAM-1 (7%, 95% CI 2–11, P < .01), MPO (12%, 95% CI 2–20, P = .01), and osteoprotegrin (6%, 95% CI 1– 10, P = .02) compared with subjects without PAD (Figure 1, A). In addition, CD40 ligand (15%, 95% CI 1–28, P = .04) and triglycerides (11%, 95% CI 0.2–21, P = .05) were significantly higher in subjects with PAD (Figure 1, B). Levels of other platelet biomarkers (urinary 11-dehydro-TxB2 [7%, 95% CI −5 to 18, P = .24] and P-selectin [3%, −3 to 9, P = .34]) were higher in subjects with PAD, but the difference was not statistically significant. Even after adjustment, levels of Lp-PLA2 (−0.6%, 95% CI −6 to 5, P = .82), total cholesterol (0.5%, 95% CI −5 to 4, P = .83), LDLC (−2%, 95% CI −5 to 2, P = .35), and HDL-C (−1%, 95% CI −3 to 1, P = .30) were not different between the groups with and without PAD.
Figure 1.
Peripheral arterial disease status and levels of inflammatory biomarkers (A) and platelet biomarkers (B), after multivariable adjustment. Adjusted for age, sex, body mass index, statin use, smoking, and diabetic status.
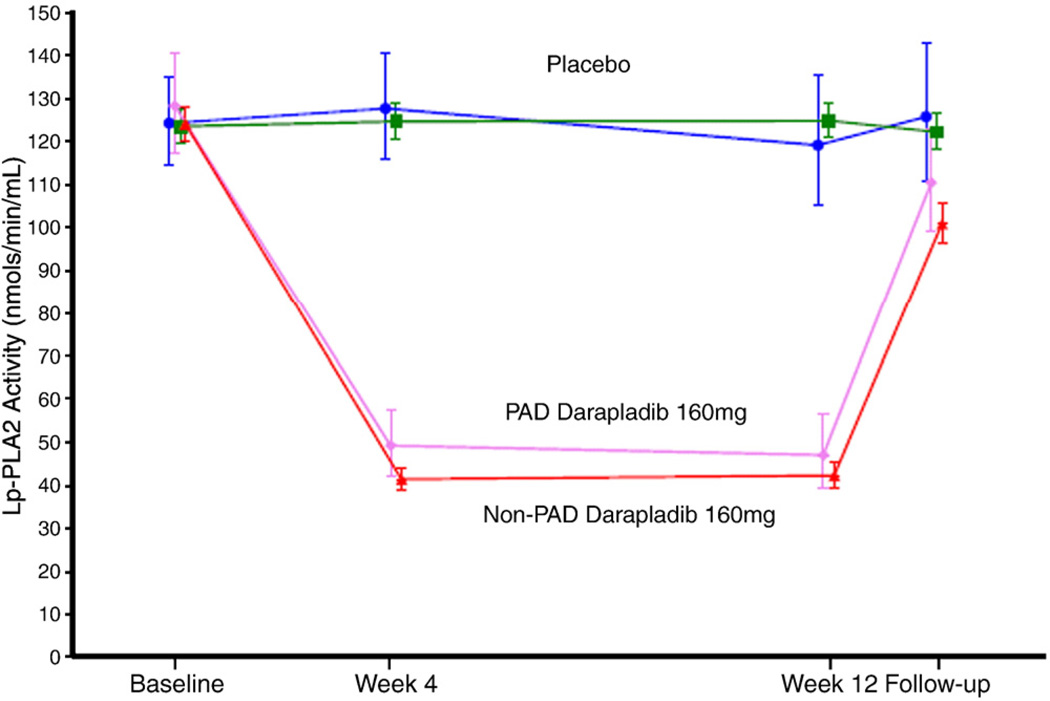
As shown in Table II and Figure 1, A, baseline Lp-PLA2 activity was comparable among subjects with and without PAD. Of note, darapladib 160 mg produced highly significant inhibition of Lp-PLA2 activity when compared with placebo at weeks 4 and 12 (P < .01) in patients with and without PAD in the setting of intensive statin therapy (Figure 2). In both groups, there was a clear dose-dependent reduction of Lp-PLA2 activity after 4 weeks of darapladib dosing (first measurement after randomization), which was also observed at 12 weeks (P < .001 for all doses) (data not shown). In the PAD group, the observed inhibition of Lp-PLA2 activity was sustained at approximately 44%, 58%, and 65% for darapladib 40, 80, and 160 mg, respectively. In the non-PAD group, the observed inhibition of Lp-PLA2 activity was sustained at approximately 43%, 55%, and 67% for darapladib 40, 80, and 160 mg, respectively. Following darapladib discontinuation in both groups, levels of Lp-PLA2 activity returned toward baseline values.
Figure 2.
Effect of darapladib on Lp-PLA2 in patients with and without PAD. The results are presented as geometric means with 95% CI. Darapladib produced highly significant inhibition of Lp-PLA2 activity when compared with placebo at weeks 4 and 12 (P < .01) in patients with and without PAD.
Discussion
There are 2 important findings of this analysis of patients with CHD or their risk equivalent receiving background atorvastatin treatment. First, subjects with PAD differed from those with CHD or CHD-risk equivalent but without PAD. In conjunction with differences in clinical characteristics, the presence of PAD was associated with higher levels of MMP-9, MPO, IL-6, adiponectin, ICAM-1, osteoprotegrin, CD40 ligand, hs-CRP, and triglycerides compared with those without PAD, after multivariable adjustment. Second, darapladib (an Lp-PLA2 inhibitor) was effective at potently reducing Lp-PLA2 irrespective of PAD and baseline differences in other circulating biomarkers.
These observations suggest the importance of inflammatory, platelet, and lipid biomarkers in the pathophysiology of PAD. Higher inflammatory burden observed in this and other studies of PAD patients8,14–17 could be mediated by reverse causation due to larger disease burden and often delayed diagnosis of PAD as compared with CHD alone. Alternatively, differences in biomarkers may reflect a more aggressive disease process in patients with PAD.10,11 It is also important to underscore that at least some biomarkers could have originated outside atherosclerotic lesions. Although, abdominal obesity has been linked to higher circulating interleukin levels and CRP, patients with PAD in this study had lower body mass index, lower frequency of diabetes, and higher levels of adiponectin compared with those without PAD, making this a less likely explanation. There are several reported studies evaluating hs-CRP16–20 and some reports evaluating other proatherosclerotic risk factors in patients with PAD. However, there are few published reports comparing the combination of numerous inflammatory, platelet, and lipid biomarkers simultaneously in patients with PAD. Our results confirm previous studies indicating that hs-CRP, MPO,21 IL -6,17 ICAM-1,22 CD40 ligand,23 and triglycerides are elevated in patients with PAD. Several studies including the present one noted a higher level of adiponectin in patients with PAD,24–26 although this is far from certain.15 We are unaware of any studies comparing all of the reported biomarkers simultaneously in those with CHD and PAD compared with those with CHD and no evidence of PAD.
Inflammation, platelets, metabolic, and lipid abnormalities all play a prominent role in the development and complications of atherosclerosis.27 In addition to improving the lipid and lipoprotein profile, statins may have “pleiotropic” effects by decreasing inflammation, improving endothelial function, and inhibiting platelet function.28 Nonetheless, patients continue to experience adverse cardiovascular events even after high-dose statins and attainment of the LDL-C goal. In patients following acute coronary syndrome enrolled in the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE-IT-TIMI 22) study, in which LDL was <70 mg/dL, there was a 22% event rate in the atorvastatin group at 2 years after enrollment.29 Great interest has therefore emerged to further stratify patients and identify those who would benefit from more aggressive risk reducing therapies. One such biomarker that may be used to stratify and target is Lp-PLA2. Lipoprotein-associated phospholipase A2 is produced in atherosclerotic plaque, and it is specifically linked to the causal pathway of plaque inflammation and presumably rupture.30 A recent meta-analysis found that Lp-PLA2 was associated with the risk of CHD and vascular death.31 A study in a hyperlipidemic, diabetic pig model demonstrated a marked reduction in atherosclerosis with inhibition of Lp-PLA2.13 A phase 2 clinical trial in 959 patients with CHD or CHD-risk equivalents demonstrated that darapladib was effective at producing sustained inhibition of plasma Lp-PLA2 activation in patients on atorvastatin therapy. The current study is a post hoc analysis of this phase 2 trial studying high-risk patients with a diagnosis of PAD. Despite a more aggressive baseline risk factor profile as demonstrated with significantly higher levels of biomarkers, darapladib was equally effective at reducing Lp-PLA2 in patients with and without PAD.
As noted, there was no difference in Lp-PLA2 activity, LDL-C, and total cholesterol between patient with and without PAD. This observation most likely reflects that both groups of patients were placed on medium- and high-dose atorvastatin therapy before measuring these biomarkers. Because Lp-PLA2 primarily circulates bound to LDL particles,32 it is not surprising that this biomarker did not differ among PAD and non-PAD patients on a background of statin therapy.
Limitations
There are several potential limitations to keep in mind when interpreting the results of this study. First, the diagnosis of PAD was ascertained and recorded by medical history. Given the universal underdiagnosis of PAD33,34 and the inclusion in this analysis of patients with documented PAD, it is likely that our study included asymptomatic PAD in the comparator group, and therefore, this report may actually underestimate the impact of PAD on biomarkers. Second, the results for inflammatory and platelet biomarkers are likely underestimated due to protocol-driven requirements that patients are all treated with aspirin and statin therapy, respectively. In addition, because of its nonrandomized, post hoc nature, there may remain significant unrecognized differences between groups, even after correction for the observed differences. Finally, because this study had no clinical follow-up period, it is unknown whether the administration of an Lp-PLA2 inhibitor would decrease clinical end points. This is an area of active investigation that is being addressed in the STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY (STABILITY) trial, a phase III study multicenter, randomized, double-blind, placebo-controlled event-driven outcomes study in patients with chronic CHD. The trial is expected to be completed in 2012, and the results determine whether inhibiting Lp-PLA2 activity in circulation and/or atherosclerotic plaques confers clinical benefit on cardiovascular risk.
Conclusions
Among patients with stable CHD or CHD-risk equivalent receiving concomitant atorvastatin therapy, PAD is associated with a higher level of MMP-9, MPO, IL -6, adiponectin, ICAM-1, osteoprotegrin, CD40 ligand, hs-CRP, and triglycerides compared with those without PAD. The association between PAD and these biomarkers underscores the high risk detected in patients with a diagnosis of PAD. Despite the higher level of baseline biomarkers in patients with PAD, darapladib was equally effective at reducing Lp-PLA2 activity in patients with and without PAD.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Mohler ER., III Peripheral arterial disease: identification and implications. Arch Intern Med. 2003;163:2306–2314. doi: 10.1001/archinte.163.19.2306. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, National Heart, Lung, and Blood Institute, Society for Vascular Nursing, TransAtlantic Inter-Society Consensus, Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg RD, Aird WC. Vascular-bed–specific hemostasis and hypercoagulable states. N Engl J Med. 1999;340:1555–1564. doi: 10.1056/NEJM199905203402007. [DOI] [PubMed] [Google Scholar]
- 5.Mohler E, III, Giri J. Management of peripheral arterial disease patients: comparing the ACC/AHA and TASC-II guidelines. Curr Med Res Opin. 2008;24:2509–2522. doi: 10.1185/03007990802274379. [DOI] [PubMed] [Google Scholar]
- 6.Mohler ER., III Therapy insight: peripheral arterial disease and diabetes—from pathogenesis to treatment guidelines. Nat Clin Pract Cardiovasc Med. 2007;4:151–162. doi: 10.1038/ncpcardio0823. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM. C-reactive protein: eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clin Chem. 2009;55:209–215. doi: 10.1373/clinchem.2008.119214. [DOI] [PubMed] [Google Scholar]
- 8.Cooke JP, Wilson AM. Biomarkers of peripheral arterial disease. J Am Coll Cardiol. 2010;55:2017–2023. doi: 10.1016/j.jacc.2009.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger JS, Petersen JL, Brown DL. Vascular disease burden and in-hospital outcomes among patients undergoing percutaneous coronary intervention in New York State. Circ Cardiovasc Interv. 2009;2:317–322. doi: 10.1161/CIRCINTERVENTIONS.108.847459.108.847459. [DOI] [PubMed] [Google Scholar]
- 10.Alberts MJ, Bhatt DL, Mas JL, et al. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J. 2009;30:2318–2326. doi: 10.1093/eurheartj/ehp355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 12.Mohler ER, III, Ballantyne CM, Davidson MH, et al. The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2008;51:1632–1641. doi: 10.1016/j.jacc.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 13.Wilensky RL, Shi Y, Mohler ER, III, et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med. 2008;14:1059–1066. doi: 10.1038/nm.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoogeveen RC, Morrison A, Boerwinkle E, et al. Plasma MCP-1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis Risk in Communities study. Atherosclerosis. 2005;183:301–307. doi: 10.1016/j.atherosclerosis.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Iwashima Y, Horio T, Suzuki Y, et al. Adiponectin and inflammatory markers in peripheral arterial occlusive disease. Atherosclerosis. 2006;188:384–390. doi: 10.1016/j.atherosclerosis.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 17.Tzoulaki I, Murray GD, Lee AJ, et al. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976–983. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 18.Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623–2629. doi: 10.1161/CIRCULATIONAHA.105.608679. [DOI] [PubMed] [Google Scholar]
- 19.Musicant SE, Taylor LM, Jr, Peters D, et al. Prospective evaluation of the relationship between C-reactive protein, d-dimer and progression of peripheral arterial disease. J Vasc Surg. 2006;43:772–780. doi: 10.1016/j.jvs.2005.12.051. [discussion 780]. [DOI] [PubMed] [Google Scholar]
- 20.Shankar A, Li J, Nieto FJ, et al. Association between C-reactive protein level and peripheral arterial disease among US adults without cardiovascular disease, diabetes, or hypertension. Am Heart J. 2007;154:495–501. doi: 10.1016/j.ahj.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 21.Brevetti G, Schiano V, Laurenzano E, et al. Myeloperoxidase, but not C-reactive protein, predicts cardiovascular risk in peripheral arterial disease. Eur Heart J. 2008;29:224–230. doi: 10.1093/eurheartj/ehm587. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan AD, Rifai N, Ridker PM. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation. 2002;106:820–825. doi: 10.1161/01.cir.0000025636.03561.ee. [DOI] [PubMed] [Google Scholar]
- 23.Blann AD, Tan KT, Tayebjee MH, et al. Soluble CD40L in peripheral artery disease. Relationship with disease severity, platelet markers and the effects of angioplasty. Thromb Haemost. 2005;93:578–583. doi: 10.1160/TH04-09-0586. [DOI] [PubMed] [Google Scholar]
- 24.Dieplinger B, Haltmayer M, Poelz W, et al. Value of adiponectin as predictor of 5-year all-cause mortality in patients with symptomatic peripheral arterial disease: results from the Linz Peripheral Arterial Disease (LIPAD) study. Clin Chim Acta. 2009;408:87–91. doi: 10.1016/j.cca.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Dieplinger B, Poelz W, Haltmayer M, et al. Association of adiponectin and amino terminal proBNP in peripheral arterial disease. Clin Chim Acta. 2007;377:192–197. doi: 10.1016/j.cca.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Komai H, Shibata R, Juri M, et al. Plasma adiponectin as a predictive factor of survival after a bypass operation for peripheral arterial disease. J Vasc Surg. 2009;50:95–99. doi: 10.1016/j.jvs.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 27.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 28.Davidson MH. Clinical significance of statin pleiotropic effects: hypotheses versus evidence. Circulation. 2005;111:2280–2281. doi: 10.1161/01.CIR.0000167560.93138.E7. [DOI] [PubMed] [Google Scholar]
- 29.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 30.Braun LT, Davidson MH. Lp-PLA2: a new target for statin therapy. Curr Atheroscler Rep. 2010;12:29–33. doi: 10.1007/s11883-009-0074-y. [DOI] [PubMed] [Google Scholar]
- 31.Thompson A, Gao P, Orfei L, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zalewski A, Nelson JJ, Hegg L, et al. Lp-PLA2: a new kid on the block. Clin Chem. 2006;52:1645–1650. doi: 10.1373/clinchem.2006.070672. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch AT, Murphy TP, Lovell MB, et al. Gaps in public knowledge of peripheral arterial disease: the first national PAD public awareness survey. Circulation. 2007;116:2086–2094. doi: 10.1161/CIRCULATIONAHA.107.725101. [DOI] [PubMed] [Google Scholar]