Abstract
Background
Acute exacerbations in allergic asthmatics may lead to impaired ability to clear mucus from the airways, a key factor in asthma morbidity.
Objective
The purpose of this study was to determine the effect of inhaled house dust mite challenge on regional deposition of inhaled particles and mucociliary clearance (MCC) in allergic asthmatics.
Methods
We used gamma scintigraphy (inhalation of 99mTc -sulfur colloid particles) to measure regional particle deposition and MCC in allergic asthmatics (n=12) 4hr following an inhaled dust mite allergen challenge (Dermatophagoides farinae extract; PDmax = fall in FEV1 of 10%) for comparison to baseline non-challenge measures.
Results
In responders (n=9 PDmax dose), lung function returned to pre-challenge values by 3 hours but was significantly decreased at 6 and 24 hours in 3 of the responders (i.e. late phase response) and induced sputum eosinophils were increased at 24 hours post-challenge (p < 0.05). Responders showed enhanced bronchial airway deposition of inhaled particles (p < 0.05) and slowed clearance from the central lung zone (p < 0.01) at 4 hrs post-challenge compared to baseline (no allergen challenge) that was predicted by the PDmax allergen concentration (r = − 0.70, p < 0.05). The fall in lung function at 24 hours post challenge correlated with reduced MCC from the central lung zone (r = − 0.78, p < 0.02) and PDmax. Non-responders (n=3) had no change in lung function, regional deposition or MCC post-challenge vs. baseline.
Conclusions and clinical relevance
These data suggest that regional deposition and clearance of inhaled particles may be sensitive for detecting mild airway obstruction associated with early and late-phase allergen-induced effects on mucus secretions. The study was listed on clinicaltrials.gov (NCT00448851).
Keywords: dust-mite allergen, particle inhalation, airway deposition, mucus
Introduction
Acute exacerbation of asthma is a leading cause of morbidity and mortality associated with this disease. While change in spirometry is the most validated physiological endpoint for defining acute asthma exacerbation, other physiological changes play a role in this disease. Severe exacerbations of asthma are associated with inability to improve lung function with β-agonists and mucus accumulation in the airways, impeding airflow. Mucus accumulation likely results from hypersecretion of mucus and failure of the mucociliary apparatus to effectively clear this mucus and airway debris. Asthmatics are thought to have impaired ability to clear mucus from their airways [1], especially during acute exacerbations [2]. The mechanisms that account for acute impairment of mucociliary clearance (MCC) are poorly understood and few experimental models have been developed to study the relationship between inflammation and MCC in humans.
One model for investigation of asthma exacerbation is inhaled allergen challenge. Allergen challenge in allergic asthmatics often induces both an immediate phase response that occurs within minutes then rapidly resolves, and a late-phase decrease in lung function that occurs 2–8 hours later and is associated with increased airway inflammation [3,4]. Ragweed antigen challenge has been shown to decrease tracheal mucus velocity in asthmatic patients immediately following challenge [5], and in allergic animals up to 2 days post challenge [6,7]. However, there have been few studies to assess mucociliary function from the whole lung in asthmatics following allergen challenge.
The relationship between airway obstruction, inflammation, and mucociliary clearance is poorly understood in allergic asthmatics as these endpoints have not been simultaneously assessed in the same study. In the current study, we employed inhalational challenge using house dust mite (HDM, Dermatophagoides farinae) allergen as a model of acute exacerbation. We assessed regional lung deposition and clearance of inhaled, radiolabeled particles by gamma scintigraphy 4 hours after challenge and inflammatory cell content of airway sputum recovered by hypertonic saline induction 24 hours after challenge.
Whole lung MCC measurements are highly dependent on the regional particle deposition pattern in the lung [8] that, in turn, might vary with airway changes induced by allergen challenge. Thus, while a confounder for MCC comparisons, these changes in deposition heterogeneity may also be a very sensitive indicator of mild, heterogeneous bronchoconstriction. Previous scintigraphy studies have shown both an increase in central airway deposition [9] and increased “patchiness” of particle deposition [10] associated with induced bronchoconstriction.
To confirm that an individual was responsive to allergen, we assessed the change in FEV1 from baseline measurements (obtained immediately prior to challenge). Our primary hypotheses were that in allergen responders, regional particle deposition would be more heterogenous and mucociliary clearance depressed as part of a late phase reaction to allergen challenge compared to baseline measures made during an earlier study visit. We also hypothesized that increases in eosinophils would correlate with changes in MCC and particle deposition as well as changes in spirometry. This report summarizes the development of a model of allergen induced asthma exacerbation that assesses MCC, particle deposition and airway inflammation.
Methods
Subjects
Twelve (6M/6F) mild allergic, non-smoking asthmatics ages 20–39 with skin sensitivity to HDM and normal baseline lung function (FEV1 %pred > 80, FEV1/FVC ratio >.70) (without use of bronchodilating medications for 12 hours) were studied. Subjects had to have a history of episodic wheezing, chest tightness, or shortness of breath consistent with asthma, or physician diagnosed asthma. All subjects took only albuterol as needed and none were on chronic asthma therapy (ie no daily LABA, inhaled steroids etc). Pre- and post-bronchodilator spirometry, and a standard graded dose methacholine bronchoprovocation challenge test [11,12] to determine non-specific bronchial reactivity was performed on each subject during a screening visit. A provocative methacholine concentration of 10 mg/ml or less producing a 20% fall in FEV1 (PC20 methacholine) was required for subject inclusion. Informed written consent was obtained from all subjects prior to their participation in the study that was approved by the Biomedical Institutional Review Board of the University of North Carolina at Chapel Hill. The study was listed on clinicaltrials.gov (NCT00448851).
Study design
Figure 1 illustrates the timeline of measurements on the baseline and challenge study days. During the subject’s baseline visit we measured mucociliary clearance (MCC) of inhaled, radiolabeled particles by gamma scintigraphy [13, 14]. The subject returned the next day for a follow-up gamma camera scan (24 hour retention) and an induced sputum sample was collected [14,15]. At least 2 days after the baseline visit the subject returned for their allergen challenge study visit. At 4 hours post allergen challenge we measured MCC, and then the following day, the subject returned for the 24 hour retention scan, spirometry, and induced sputum procedure. Subjects were monitored overnight in the CTRC inpatient unit from the time of the challenge until the 24-hour follow-up scan. Comparisons were made with MCC obtained from the same subject during the baseline visit, and sputum samples were analyzed and compared at 24 hours post challenge versus the baseline sample.
Figure 1.
Schematic of study design showing order and timing of procedures for baseline and allergen challenge study days. *Post challenge spirometry on the challenge day was performed at 10 min, 20 min, 30 min, 1 hr, 90 min, 2 hr, 3 hr, and 6 hr post end of challenge.
Inhaled allergen challenge
Each subject inhaled sequential doses of inhaled HDM extract (D. farinae, Greer®, Lenoir, NC) delivered as 5 inhalations from a Devilbiss 646 nebulizer (mass median aerodynamic diameter of 5um, GSD = 2.0 [16]) at concentrations of zero (saline control), 0.25, 0.50, 1.0, 2.0, 4.0, 8.0, 16, 32, 64, 125, 250, 500, 1000, and 2000 Allergen Units (AU)/mL (figure 3). For each of the breaths of inhaled HDM at each dose, subjects were instructed to perform a full rapid inhalation to total lung capacity with a 5 second breath hold. FEV1 was measured prior to and 10 minutes after each aerosol inhalation. The FEV1 following saline challenge and prior to the first dose of antigen was considered the baseline value. If the FEV1 declined by less than 10% of baseline after a given concentration of allergen had been inhaled, the next higher concentration was given. If the decline in FEV1 was between 10% and 15%, spirometry was repeated each 5 minutes for 15 minutes or until a clear nadir in the decline had been reached. If the nadir after 15 minutes was a decline in FEV1 of less than a 10 %, the next higher concentration was administered, and if it was a decline of 10–15%, the challenge was stopped. Once the challenge was stopped, the FEV1 was repeated every 10 minutes until 30 minutes, each 30 minutes until 2 hours, at 3 and 6 hours, and again the next day. It was not measured at 4 and 5 hrs post challenge in order to avoid forced exhalation maneuvers during and immediately prior to the MCC scanning. A few responders received albuterol by MDI during the first 30 minutes following the challenge. Nonresponders as well as those with only small drops in FEV1 or milder symptoms were not given albuterol at the end of the challenge.
Figure 3.
FEV1 as % of initial saline dose as a function of allergen dose in the 9 patients who had at least a 10% drop associated with the challenge. Each symbol represents data from one patient. Those patients who had a late phase response (LPR), i.e. a > 10% fall in FEV1 from pre-challenge at 6 and 24 hours post challenge, are indicated with dashed lines.
Regional Particle Deposition and Mucociliary Clearance (MCC)
The procedure we used for measuring MCC in humans has been described in detail previously [14,15]. A xenon (133Xe) equilibrium lung scan was recorded for each subject on their baseline visit to allow the creation of suitable regions of interest (ROIs) for determining regional lung deposition and MCC. For each measure of MCC, the subject inhaled an aerosol (mass median aerodynamic diameter of 5um, GSD = 2.0) of sulfur colloid labelled with 99mTc (99mTc -SC) (40 microcuries) (CIS-US, Inc.) from a Devilbiss 646 nebulizer. While breathing the radiolabeled aerosol the subject matched his/her tidal flow and breathing rate at 500ml/sec and 30/min respectively by following a visual flow signal while breathing in time to a metronome. Immediately following inhalation of radioaerosol (duration of less than 2 minutes), an initial deposition scan was recorded (sum of two 2-min images) and then continuous two minute images were recorded for a period of two hours to monitor clearance of particles from the lung as the subject remained seated in front of the gamma camera. The subject returned the following day after the radiolabeled aerosol exposure to obtain a 30-minute scan of 24-hour lung activity/retention.
Only the right lung was used to analyze both regional deposition and MCC because of the potential overlap of stomach and lung activity on the left side. To assess central (C) vs. peripheral (P) deposition (figure 2), two outline regions of interest (ROI) were created over the right 133Xe lung image; 1) a rectangular region around the entire right lung and 2) a central (C) ROI, with dimensions equal to half the whole lung ROI’s width and one-half its height. The C region was positioned on the medial boundary of the lung, centered by height, 25% of the area of the whole lung ROI. The peripheral region (P) is the area lying between the central and whole lung outline. These regions were displayed over the initial aerosol scans to determine the initial counts in each region. We then calculated the ratio of central to peripheral counts, (C/P)Tc, and normalized this ratio by dividing by the central-to-peripheral ratio for the 133Xe scan,
(C/P)Xe; (C/P)Tc/(C/P)Xe = C/P.
Figure 2.
Central (C) vs. Peripheral (P) deposition and clearance. Schematic (left) illustrating C and P regions based on outline of Xenon Equilibrium image (center).
A deposition image from the same individual is shown on right.
This normalization was done to account for the difference in relative lung areas and thickness between the central and peripheral regions. C/P provides an index of relative deposition between the two regions. A C/P of 1.0 reflects equal deposition in each region. However, because the central region outlines both bronchial airways and lung parenchyma surrounding these airways, a C/P of near unity reflects primarily deposition in the pulmonary airspaces distal to anatomic dead space. Increases in C/P to values greater than unity reflect an increase in central vs. peripheral deposition primarily as a result of increased bronchial deposition.
Another measure of regional deposition heterogeneity is the skew of the histogram distribution (counts/pixel vs. #pixels) [17] within the right whole lung ROI, increasing with increased frequency of “hot spots” in the lung. These hot spots are presumed due to increased deposition within bronchial airways throughout the lung so that skew is independent of the specific region within the lung (e.g. central vs. peripheral). To determine skew, frequency distribution histograms were constructed from the right lung deposition images, with the number of pixels with a given count value (expressed as a fraction of total pixels) on the y axis and the count values on the x axis (figure 4). These histograms were analyzed for skew (a measure of histogram symmetry, the third moment about the mean of the histogram) [16]. Heterogeneity of deposition increases with increasing skew (i.e. more pixels with high counts/pixel).
Figure 4.
Baseline vs. post challenge. (C/P = 1.08 vs. 1.72 and Skew = 1.27 vs. 1.56 respectively).
The whole lung ROI bordering the right lung was used to determine, by computer analysis, the whole lung retention (decay and background corrected) as a fraction of the initial counts in the right lung, over the two-hour clearance period at 10 min intervals (two-2 min images summed for each 10 min time point, e.g. images 1 and 2 for initial time 0 and images 6 and 7 for time 10 min). Similarly, the twenty-four hour retention (R24) was calculated. For these experiments we assumed that R24 primarily represented the fraction of aerosol initially deposited in the alveolar region [18, 19, 20, 21], or conversely that 24Hr Clear represented the deposition in the bronchial airways that could be cleared by ciliary action [18]. To determine tracheobronchial (TB) retention (Rt) vs. time for the initial 2-hour period of observation, R24 was subtracted from the retention measurements during the initial 2 hour clearance period and re-normalized (divided) by (1 – R24), i.e.
TB Rt = (Rt – R24)/(1-R24) where t is time between 0 and 2 hours.
Finally, both central (C) and peripheral (P) TB retention vs. time were also determined from the respective regions described above to allow comparisons of MCC from a region with a preponderance of large, bronchial airways (C) to a region lacking in such airways (P). For each retention vs. time data set (e.g. mean data shown in figure 5), the average retention over the 2 hour period of observation (Ave120 Ret) were computed (i.e. average of the 10 minute retention values from 10 to 120 minutes).
Figure 5.
Whole lung tracheobronchial (TB) retention vs. time for responders, baseline vs. 4 hours post challenge.
Induced Sputum, Cell Counts Assessments
This procedure has been described in detail previously [15]. The numbers and percent of airway eosinophils and neutrophils in sputum were assessed and compared for baseline vs. allergen challenge study visits.
Statistical methods
Comparisons between baseline and post-challenge measurements were analyzed using nonparametric statistics for paired samples (Wilcoxon signed-rank test, Stata for MacIntosh). The significance of relationships between individual variables was tested using Spearman’s correlation (Stata for MacIntosh). An overall significance level of p ≤ 0.05 was considered to be significant. All values are expressed as the mean (+/− standard deviation). Comparison of TB retention vs. time between baseline and allergen study days was made by mixed model analysis (SAS) for retention as a function of visit (i.e. baseline vs. allergen), time, time-squared and their interactions. The Ave120 Ret (described above) for post challenge retention vs. time data was used to compare relationships between MCC and other post challenge parameters.
Results
Characterization of Volunteers with Allergic Asthma
Mean FEV1 (expressed as % of predicted value) was 97± 12% prior to and 105 ± 13 post use of albuterol (bronchodilator). All subjects were responsive to methacholine, with the PD20 for methacholine ranging from 1.25 to 10 mg/ml. All subjects also demonstrated skin test reactivity to D. farinae.
Response to Inhaled Allergen
Of the 12 patients studied, nine (5M/4F) responded to inhaled allergen challenge with >10% reduction in FEV1 (individual responses depicted in figure 3). The mean (SD) PDmax for all responders was 639 (788) AU/ml. In all cases, FEV1 and FEF25–75 returned to pre-challenge values by 3 hours post-challenge (table 1). In responders, lung function significantly declined by 6 and 24 hours post-challenge relative to pre-challenge values (table 1). This was primarily determined by a late phase response (LPR), >10% reduction in FEV1 6 hours post challenge, occurring in 3 of the responders (table 1). The non-late phase responders (nLPR) all had returned to pre-challenge spirometry by 30 min post challenge and remained normal throughout the 24 hour testing period. Among the 3 late phase responders (LPR), the early response had all resolved (i.e. returned to pre challenge FEV1) by 2 hours (specifically 30 min, 90 min, and 120 min) before all dropping again at 6 hours post-challenge. The mean (SD) PDmax for nLPR and LPR was 896 (868) and 126 (107) AU/ml respectively. Three of the responders (one LPR and two nLPR) received albuterol (total of 4 puffs from a MDI (90 ug/actuation) ) during the first 30 minutes post challenge. Two additional late phase responders received albuterol by nebulizer (0.083%[2.5 mg/3 mL] solution) at approximately 7 hrs post end of challenge. One of the 2 late responders required repeated doses of 2 albuterol puffs via MDI at 10 hours, 20 hours and 24 hours post end of challenge. The other individual required 2 puffs of albuterol at 10 and 24 hours post challenge as well as albuterol via nebulizer at 20 hours post challenge. Both of these late phase responders were treated with prednisone taper at discharge on the post challenge follow-up day. The PDmax for allergen significantly predicted the reduction in FEV1 at 24 hours post challenge (R = − 0.75, p = 0.02). Within all subjects there was no significant correlation between HDM specific and non-specific (methacholine) airway reactivity (R = 0.13 for correlation of PDmax of allergen concentration vs. PC20 of methacholine concentration).
Table 1.
Mean (SD) changes in spirometry associated with allergen challenge (all values are as % saline pre- challenge) for responders (late phase (LPR) and non-late phase (nLPR)) and non-responders.
| Mean Allergen response |
3 hrs post- challenge |
6 hrs post- challenge |
24 hours post- challenge |
|
|---|---|---|---|---|
| FEV1 | ||||
| Responders (n=9) | 79 (14) | 103 (5) | 94 (11)*100 | 92 (8)# |
| nLPR (n=6) | 82 (15) | 104 (5) | (5) | 97 (2) |
| LPR (n=3) | 76 (11) | 100 (5) | 82 (10) | 82 (3) |
| Non-responders (n=3) | - | 104 (3) | 106 (3) | 102 (2) |
| FEF25-75 | ||||
| Responders | 68 (21) | 104 (11) | 86 (22)# | 80 (14)# |
| nLPR | 74 (25) | 108 (11) | 100 (10) | 89 (4) |
| LPR | 58 (4) | 96 (6) | 60 (11) | 62 (4) |
| Non-responders | - | 106 (6) | 112 (13) | 104 (7) |
P < 0.02
P < 0.01 compared to 3 hrs post challenge.
Regional Particle Deposition after Allergen Challenge
Table 2 summarizes the changes in regional particle deposition between baseline and 4-hour post allergen challenge. Among the responders skew of the deposition distribution was significantly increased (p = 0.02) after allergen challenge compared to baseline. There was also a trend for C/P to be increased among responders (p = 0.07). Figure 4 shows an example of deposition images from one responder, baseline study day vs. 4-hour post challenge, and the corresponding number vs. counts/pixel histogram for the whole right lung for each after normalizing to a common median count. Both skew of the distribution and C/P were increased in this subject for post challenge vs. baseline (values given in legend). Finally, within the responders, the percent of deposited particles cleared through 24 hours was significantly increased post challenge compared to that at baseline (p < 0.02). Again, the percent of particles cleared through 24 hours is a measure of regional deposition (not clearance rate in these subjects) and therefore reflects a greater initial deposition of particles in airways vs. alveoli for post-challenge vs. baseline [18]. Finally, using multivariate analysis, skew and 24 hour clear were both modeled as a function of presence of a LPR, study day and their interaction. As with the paired analysis, both skew and 24 hour clear were found to be a significant function of study day. Neither was significantly predicted by presence of LPR or its interaction with study day. There was a trend, however, for 24 hour clear to be predicted by an interaction of study day and presence of LPR (p = 0.10), i.e. the late phase responders tending to have the largest difference in 24 hour clear between study days (table 2).
Table 2.
Mean (SD) regional deposition and clearance indices of inhaled aerosol at 4 hours post challenge (PC) compared to baseline (Base) for responders (late phase (LPR) and non-late phase (nLPR)) and non-responders.
| Skew | C/P | 24 hr clear (%) | Central TB Ave120Ret |
|||||
|---|---|---|---|---|---|---|---|---|
| Base | PC | Base | PC | Base | PC | Base | PC | |
| Responders (n=9) | 2.00 (0.45) | 2.53* (0.78) | 1.85 (0.43) | 2.20 (0.61) | 52 (16) | 66* (12) | 0.69 (0.17) | 0.79 (0.22) |
| nLPR (n=6) | 2.17 (0.32) | 2.64 (0.68) | 2.17 (0.32) | 2.32 (0.72) | 59 (12) | 66 (14) | 0.71 (0.17) | 0.74 (0.20) |
| LPR (n=3) | 1.67 (0.54) | 2.31 (1.10) | 1.50 (0.36) | 1.97 (0.28) | 38 (12) | 66 (10) | 0.67 (0.22) | 0.88 (0.25) |
| Non-responders (n=3) | 1.75 (0.60) | 1.65 (0.65) | 2.01 (0.82) | 2.11 (1.13) | 43 (6) | 41 (13) | 0.77 (0.20) | 0.69 (0.25) |
P ≤ 0.02 compared to baseline.
Mucociliary Clearance as reflected by Particle Retention in Responders
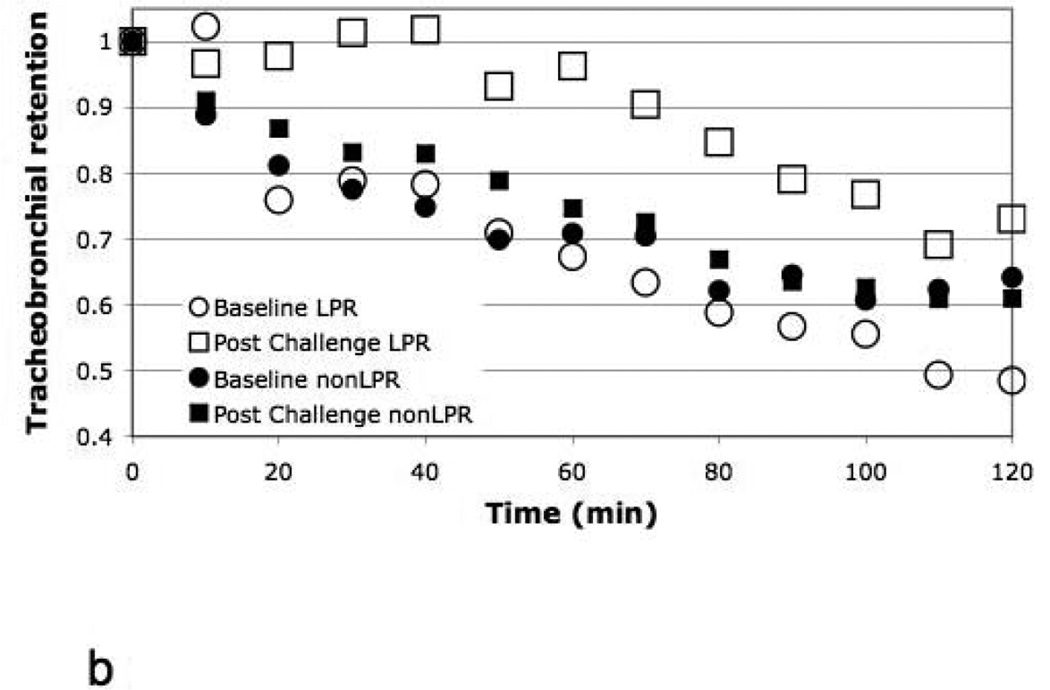
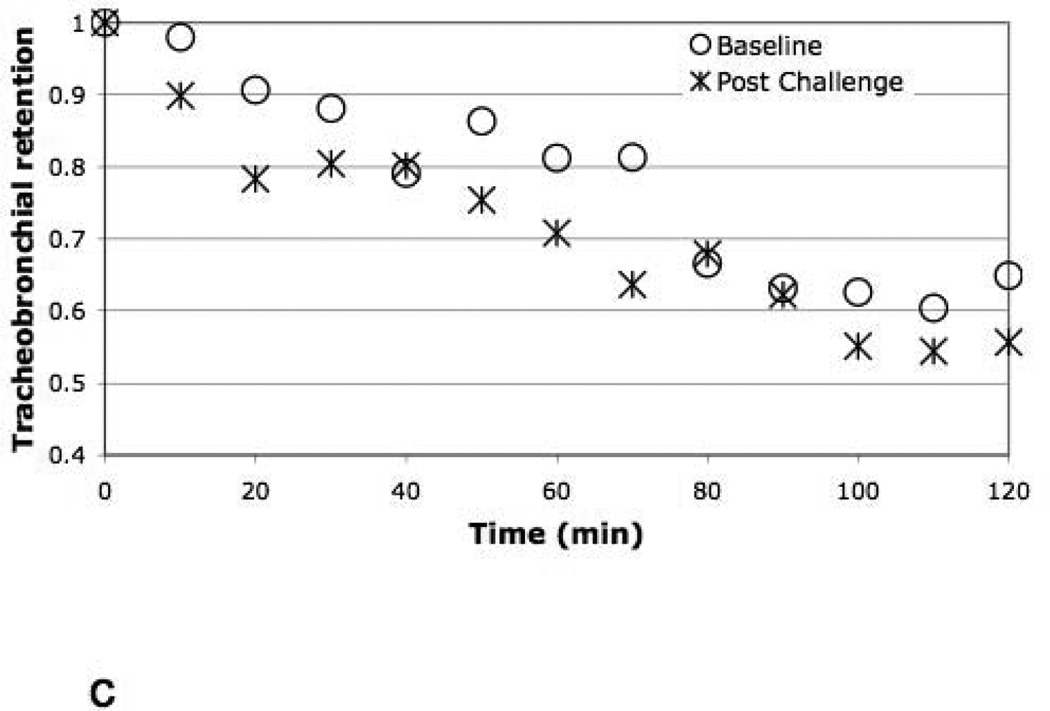
Figure 5 illustrates the mean whole lung TB retention vs. time for baseline vs. post allergen challenge for the responders, suggesting a trend (p=0.07) for a reduction in whole lung MCC (increased retention) following antigen challenge compared to baseline. There was a significant (p=0.01) slowing of central airway clearance post challenge compared to baseline in responders depicted in Figure 6a (mean Central TB Ave120Ret increased from 0.69 to 0.79 for baseline vs. allergen challenge respectively). On the other hand, there was no difference in retention vs. time from the P region between the two study days. The PDmax of allergen predicted the slowing of TB MCC in the C region between baseline and challenge, r = − 0.70, p < 0.05). This reduction in MCC from the C region also significantly correlated with the post challenge 24 hour FEV1 (as % of saline control pre-challenge) (r = − 0.78, p < 0.02, respectively). In fact, when we categorized the central TB MCC by LPR vs. nLPR (figure 6b), it was clear that the slowing of MCC post challenge was determined primarily by those patients with a LPR. When the difference in retention between study days at each time point (10–120 min) (figure 6a) was modeled as a function of both time and presence of a LPR, we found that the presence of a LPR was a significant predictor of retention difference, interacting with time (p=0.03). Finally, non-responders had no change in MCC from the whole lung central airways (figure 6c) associated with allergen challenge.
Figure 6.
a. Central (C) lung TB retention vs. time (for the C region of figure 2) for responders, baseline vs. 4 hours post challenge.
b. Central (C) lung TB retention vs. time (bottom) for LPR and nLPR at baseline vs. 4 hours post challenge. 2)
c. Central (C) lung TB retention vs. time for non-responders, baseline vs. 4 hours post challenge.
Inflammatory response to allergen challenge
Table 3 gives the differential cell counts from induced sputum samples collected at baseline and 24-hour post allergen challenge. Only 10 of the 12 subjects (7 of the responders and the 3 nonresponders) were able to provide sufficient samples for cells counts on both days for comparison. Eosinophils (EOS) were significantly increased in all subjects 24 hours following post allergen challenge. The two subjects with the highest eosinophil concentration (cells/mg) at 24 hours post challenge were both LPRs. Accordingly, within the responders the concentration of eosinophils (cells/mg) post challenge was significantly correlated with the FEV1 at 24 hours post challenge relative to the saline control prior to challenge (r = −0.93, p < 0.005), suggesting that resolution of the inflammatory response to allergen exposure was not yet normalized by 24 hrs post challenge. . Within the responders there was also a trend for eosinophil concentrations post challenge to correlate with the reduction in TB clearance from the C region (r = 0.64, p = 0.12, n=7) but it was not statistically significant.
Table 3.
Mean (SD) granulocyte cell counts from induced sputum (% and cells/mg in sample).
| EOS % cells/mg |
PMN % cells/mg |
|||
|---|---|---|---|---|
| Base | PC | Base | PC | |
| Responders (n=7) | 1.3 (2.7) | 14.6 (11)* | 28.7 (13.8) | 35.1 (12.3) |
| 12 (18) | 136 (169)* | 307 (331) | 334 (461) | |
| nonLPR (n=5) | 1.5 (3.2) | 12.1 (0.68) | 25.5 (13.8) | 30.7 (11.3) |
| 10 (22) | 57 (83) | 166 (82) | 129 (105) | |
| LPR (n=2) | 0.9 (0.1) | 20.9 (1.10) | 37.6 (10.1) | 46 (7.6) |
| 15 (6) | 335 (182) | 497 (469) | 845 (706) | |
| Non-responders | 0.8 (0.8) | 8.6 (11.3) | 56 (32) | 30 (7.7) |
| (n=3) | 5 (7) | 13 (15) | 286 (397) | 134 (150) |
P < 0.05 compared to baseline.
Discussion
In this study, we employed inhaled D. farinae allergen challenge to induce model exacerbations in mild allergic asthmatic volunteers. In addition to traditional characterization of response by changes in spirometry after challenge, we used inhalation of 99mTc -labeled sulfur colloid (99mTc -SC) particles and gamma scintigraphy to assess particle deposition pattern and both whole and regional lung mucociliary clearance (MCC). We also collected sputum 24 hours after challenge and examined changes in inflammatory cells to explore relationships between inflammatory cells and spirometric, particle deposition and MCC endpoints. Of the 12 volunteers who participated in this study, 9 were spirometric responders and 3 were non-responders to inhalational allergen challenge. Furthermore, the spirometric function of all responders had returned to normal 3 hours after the end of the allergen challenge procedure. Of the nine responders, however, 3 had an additional late phase response (LPR), i.e. a fall of >10% in FEV1 from pre-challenge values at 6 and 24 hours post challenge. These data provided us with an opportunity to determine if MCC and particle deposition, as measured by the ratio of particles deposited in the central vs. peripheral airways (C/P ratio) and skew (or lack of homogeneity of particle distribution) at 4 hours post allergen challenge, were sensitive indicators of allergen responsiveness, either the initial or late phase, compared to traditional spirometric measures.
One of the most intriguing observations was that both skew, a measure of regional particle deposition, and fraction of particles cleared through 24 hours was significantly increased in responders, with a trend for increased C/P ratio as well. Skew increases with increased heterogeneity of particle deposition in the lung that may occur with increased frequency of localized deposition areas, known as “hot spots” [16]. This increased patchiness of deposition may result from non-homogeneous mucus accumulation or local bronchial smooth muscle constriction. The percent of particles cleared through 24 hours is an estimate of percent of total particles depositing in bronchial airways. As such the increase in 24 hr clear also indicated enhanced airway deposition post allergen challenge. Inhalation of 99mTc -SC occurred 4 hours after challenge while spirometry had already been normalized 3 hours after challenge (either with or without albuterol). Furthermore, the presence of a late phase response among the early responders was not a significant predictor of changes in deposition pattern (skew). We suggest that these changes in airway deposition reflect residual effects on airway surface biology associated with the immediate response to allergen. Such effects may include increased mucus secretion, plasma exudate, and intermittent presence of allergen-induced airway edema.
We also examined the effect of allergen challenge on MCC as reflected in retention of 99mTc -SC particles, and found a significant decrease in MCC in the central region of the right lung in responders (figure 6a). On the other hand, there was no change in MCC between baseline and post-challenge for the 3 patients who had no spirometric response to allergen challenge (figure 6c), While the effects on MCC were observed an hour after spirometry had returned to normal, the depression in MCC inversely correlated with the maximal dose of allergen required to induce a response in the 9 responders. Furthermore, it was clear that the presence of a late phase response was a significant predictor of the observed slowing in central airways MCC (figure 6b). We think the most likely mechanism associated with both MCC inhibition from the central airways and enhanced “hot spot” particle deposition post allergen challenge is increased release of mucins from airway epithelial cells [22,23], regardless of the induction pathway, that are not readily or easily cleared from the airway surface. Many of the inflammatory mediators implicated in the pathophysiology of asthma have been shown to affect mucus secretion. Markedly upregulated production of MUC5AC together with stimulated secretion may contribute to airflow obstruction in asthma [22,23]. Furthermore, an imbalance in mucin release without appropriate hydration may contribute to MCC defects associated with airways disease [24].
We examined sputum eosinophilia in sputum 24 hours after challenge and 18 hours after completion of the MCC studies. Consistent with others we found a profound increase in eosinophils associated with allergen challenge in these asthmatics [25,26,27]. We further observed a trend toward a relationship between eosinophils and MCC (R = 0.64), but this did not reach statistical significance (p = 0.12). Eosinophilia also strongly correlated with FEV1 at 24 hours after challenge in responders, as well as with the PD max of allergen required to induce a response. Three of the responders received albuterol during the first 30 minutes post-challenge to ease associated chest tightness and bronchoconstriction. As a beta-adrenergic agonist, albuterol has been shown to be effective at stimulating MCC in healthy and asthmatic subjects, though its effectiveness is diminished in airways disease [28]. Thus, it might be expected that the albuterol may alleviate the effects on MCC and regional deposition associated with allergen challenge. However, to discern the effect of albuterol treatment in concert with effects of LPR on our measures would require larger numbers of subjects than studied here. .
We selected the 4–6 hour post allergen timepoints as the time to conduct scintigraphic measurements of airway function because this is the period during which late phase responses have been reported in other experimental procedures [25, 29]. This limited, however, our examination of earlier time points. Future studies should examine earlier time points (immediately after challenge) that extend to 24 hours to encompass the time frame in which clinical response to allergen occurs. Another limitation to using the combination of scintigraphy, spirometry and sputum induction is that it is necessary to perform sputum induction after other measures, as hypertonic saline and cough clearance, two components of sputum induction, may disrupt other measures. Nonetheless, using this combination of measures, we were able to show that allergen induces not only changes in lung function, but changes in particle deposition pattern and slowing of MCC. Furthermore, we have been able to associate post challenge changes in lung function with inflammatory responses.
In developing models of disease, it is important that the model actually reflect events which occur in naturally occurring disease. Messina et al [2] have shown that in severe cases of asthma exacerbations requiring hospitalization, MCC is nearly static with no discernible clearance of radiolabeled particles over a 2-hour period of observation. Using an inhalation challenge model that employed ragweed extract, Mezey et al [5] showed impairment in mucus transport in asymptomatic ragweed-sensitive asthmatics both immediately and 1 hour after challenge. Allergen challenge in animal models of allergic airways disease also show decreased TMV for several days [6, 7, 30] with no clear linkage to the time course of bronchoconstriction in the animals. Thus, the observations presented in this report are consistent with earlier animal and human studies as well as those seen in naturally occurring disease. Others have also shown that inflammation modifies MCC. Compared to non-asthmatics, MCC may actually be enhanced in mild asthmatics with normal lung function [14, 31]. However, with further progression of airway inflammation and obstruction in these patients, MCC may be depressed relative to normal [19,20, 23].
In conclusion, we used inhaled HDM allergen challenge to produce a model of asthma exacerbation in mild allergic asthmatics. We found decreases in MCC, and changes in deposition pattern of inhaled particles in asthmatics who responded to inhalational challenge. There were also changes in inflammation, most notably an association between eosinophils and lung function (FEV1) response to allergen. We propose that IgE-mediated responses to allergen modifies MCC. Lastly, we anticipate that agents interfering with IgE-mediated processes (e.g. omalizumab), or mucus clearance (e.g. hypertonic saline) may improve or even promote airway clearance during acute exacerbations of disease.
Acknowledgments
We gratefully acknowledge the statistical input of Hongtao Zhang Haibo Zhou, PhD and for the mixed model analysis of mucociliary clearance data.
The project was supported by Award Numbers RO1HL080337 from the National Heart Lung and Blood Institute, U19AI077437 from the National Institute of Allergy and Infectious Diseases, , and KL2RR025746, M01RR00046 and UL1RR025747 from the National Center of Research Resources, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Heart Lung and Blood Institute, the National Center for Research Resources of the National Institutes of Health
Abbreviations
- MCC
Mucociliary Clearance
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- FEF25-75
Forced expiratory flow from 25 to 75% vital capacity
- AU
Allergen units
- PD
provocative dose
- MDI
Metered dose inhaler
- ROI
Region of interest
- C/P
Central to peripheral ratio
- TB
tracheobronchial
- HDM
house dust mite
- 99mTc-SC
technetium labeled sulfur colloids
- CTRC
Clinical and translational research center
- LPR
late phase response
- nLPR
non-late phase response
References
- 1.Wanner A, Salathe` M, O’Riordan T. Mucociliary clearance in the airways: state of the art. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 2.Messina MS, O’Riordan TG, Smaldone GC. Changes in mucociliary clearance during acute exacerbations of asthma. Am Rev Respir Dis. 1991;143:993–997. doi: 10.1164/ajrccm/143.5_Pt_1.993. [DOI] [PubMed] [Google Scholar]
- 3.Booij-Noord H, de Vries K, Sluiter HJ, Orie NG. Late bronchial obstructive reaction to experimental inhalation of house dust extract. Clin Allergy. 1972;2(1):43–61. doi: 10.1111/j.1365-2222.1972.tb01267.x. [DOI] [PubMed] [Google Scholar]
- 4.O'Byrne PM, Dolovich J, Hargreave FE. Late asthmatic responses. Am Rev Respir Dis. 1987 Sep;136(3):740–751. doi: 10.1164/ajrccm/136.3.740. [DOI] [PubMed] [Google Scholar]
- 5.Mezey RJ, Cohn MA, Fernandez RJ, et al. Mucociliary transport in allergic patients with antigen induced bronchospasm. Am Rev Respir Dis. 1978;118:677–684. doi: 10.1164/arrd.1978.118.4.677. [DOI] [PubMed] [Google Scholar]
- 6.Wanner A, Zarzecki S, Hirsch J, et al. Tracheal mucous transport in experimental canine asthma. J Appl Physiol. 1975;39:950–957. doi: 10.1152/jappl.1975.39.6.950. [DOI] [PubMed] [Google Scholar]
- 7.Allegra L, Abraham WM, Chapman GA, Wanner A. Duration of mucociliary dysfunction following antigen challenge. J Appl Physiol. 1983;55(3):726–730. doi: 10.1152/jappl.1983.55.3.726. [DOI] [PubMed] [Google Scholar]
- 8.Ilowite JS, Smaldone GC, Perry RJ, Bennett WD, Foster WM. Relationship between tracheobronchial particle clearance rates and sites of initial deposition in man. Arch.Environ.Health. 1989;44:267–273. doi: 10.1080/00039896.1989.9935893. [DOI] [PubMed] [Google Scholar]
- 9.O'Riordan TG, Walser L, Smaldone GC. Changing patterns of aerosol deposition during methacholine bronchoprovocation. Chest. 1993 May;103(5):1385–1389. doi: 10.1378/chest.103.5.1385. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrino R, Biggi A, Papaleo A, Camuzzini G, Rodarte JR, Brusasco V. Regional expiratory flow limitation studied with Technegas in asthma. J Appl Physiol. 2001;91:2190–2198. doi: 10.1152/jappl.2001.91.5.2190. [DOI] [PubMed] [Google Scholar]
- 11.Guidelines for Methacholine and Exercise Challenge Testing – 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 12.Kehrl HR, Peden DB, Ball B, Folinsbee LJ, Horstman D. Increased specific airway reactivity of persons with mild allergic asthma after 7.6 hours of exposure to 0.16 ppm ozone. J.Allergy Clin.Immunol. 1999;104:1198–1204. doi: 10.1016/s0091-6749(99)70013-8. [DOI] [PubMed] [Google Scholar]
- 13.Bennett WD, Almond MA, Zeman KL, Johnson JG, Donohue JF. Effect of salmeterol on mucociliary and cough clearance in chronic bronchitis. Pulm Pharm Therap. 2006;19(2):96–100. doi: 10.1016/j.pupt.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Lay JC, Alexis NE, Zeman KL, Peden DB, Bennett WD. In-vivo Uptake of Inhaled Particles by Airway Phagocytes is Enhanced in Mild Asthmatics Compared to Normal Volunteers. Thorax. 2009;Vol. 64(4):313–320. doi: 10.1136/thx.2008.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexis NE, Lay JC, Zeman K, Bennett WD, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J.Allergy Clin.Immunol. 2006;117:1396–1403. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Zeman KL, Wu J, Bennett WD. Targeting aerosolized drugs to the conducting airways using very large particles and extremely slow inhalations. J Aerosol Med Pulm Drug Deliv. 2010;23(6):363–369. doi: 10.1089/jamp.2008.0711. [DOI] [PubMed] [Google Scholar]
- 17.Garrard CS, Gerrity TR, Schreiner JF, Yeates DB. The characterization of radioaerosol deposition in the healthy lung by histogram distribution analysis. Chest. 1981;80(supplement):840–842. doi: 10.1378/chest.80.6.840. [DOI] [PubMed] [Google Scholar]
- 18.Garrard CS, Mussatto DJ, Lourenco RV. Lung mucociliary transport in asymptomatic asthma: effects of inhaled histamine. J Lab Clin Med. 1989;113:190–195. [PubMed] [Google Scholar]
- 19.Bateman JR, Pavia D, Sheahan NF, et al. Impaired tracheobronchial clearance in patients with mild stable asthma. Thorax. 1983;38:463–467. doi: 10.1136/thx.38.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavia D, Bateman JR, Sheahan NF, et al. Tracheo-bronchial mucociliary clearance in asthma: impairment during remission. Thorax. 1985;40:171–175. doi: 10.1136/thx.40.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortensen J, Lange L, Nyboe J, Groth S. Lung mucociliary clearance. Eur J Nucl Med. 1994;21:953–961. doi: 10.1007/BF00238119. [DOI] [PubMed] [Google Scholar]
- 22.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med. 2009 Jan;15(1):4–11. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol. 2004 Jun;4(3):241–250. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Goralski JL, Boucher RC, Button B. Osmolytes and ion transport modulators: new strategies for airway surface rehydration. Curr Opin Pharmacol. 2010;10(3):294–299. doi: 10.1016/j.coph.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauvreau GM, Evans MY. Allergen inhalation challenge: a human model of asthma exacerbation. Contrib Microbiol. 2007;14:21–32. doi: 10.1159/000107052. [DOI] [PubMed] [Google Scholar]
- 26.Cockcroft DW, Hargreave FE, O'Byrne PM, Boulet LP. Understanding allergic asthma from allergen inhalation tests. Can Respir J. 2007;14(7):414–418. doi: 10.1155/2007/753450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ball BA, Folinsbee LJ, Peden DB, Kehrl HR. Allergen bronchoprovocation of patients with mild allergic asthma after ozone exposure. J.Allergy Clin.Immunol. 1996;98:563–572. doi: 10.1016/s0091-6749(96)70090-8. [DOI] [PubMed] [Google Scholar]
- 28.Bennett WD. Effect of β-Adrenergic Agonists on Mucociliary Clearance. Journal of Allergy and Clinical Immunology. 2002;110:S291–S297. doi: 10.1067/mai.2002.129704. [DOI] [PubMed] [Google Scholar]
- 29.Boehlecke B, Hazucha M, Alexis NE, Jacobs R, Reist P, Bromberg PA, Peden DB. Low-dose airborne endotoxin exposure enhances bronchial responsiveness to inhaled allergen in atopic asthmatics. J Allergy Clin Immunol. 2003;112:1241–1243. doi: 10.1016/j.jaci.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 30.Weissberger D, Oliver W, Jr, Abraham WM, Wanner A. Impaired tracheal mucus transport in allergic bronchoconstriction: effect of terbutaline pretreatment. J Allergy Clin Immunol. 1981 May;67(5):357–362. doi: 10.1016/0091-6749(81)90080-4. [DOI] [PubMed] [Google Scholar]
- 31.O’Riordan TG, Zwang J, Smaldone GC. Mucociliary clearance in adult asthma. Am Rev Respir Dis. 1992;146:598–603. doi: 10.1164/ajrccm/146.3.598. [DOI] [PubMed] [Google Scholar]