Abstract
Rationale
Atherosclerosis is a chronic inflammatory disease of the arterial wall. Several proinflammatory cytokines are known to promote atherosclerosis, but less is known about the physiological role of anti-inflammatory cytokines. Interleukin (IL)-27 is a recently discovered member of the IL-6/IL-12 family. The IL-27 receptor is composed of IL-27 receptor A (WSX-1) and gp130 and is required for all established IL-27 signaling pathways. The expression of the IL-27 subunit Ebi3 is elevated in human atheromas, yet its function in atherosclerosis remains unknown.
Objective
The aim of this study was to test the role of IL-27 receptor signaling in immune cells in atherosclerosis development.
Methods and Results
Atherosclerosis-prone Ldlr−/− mice transplanted with Il27ra−/− bone marrow and fed Western diet for 16 weeks developed significantly larger atherosclerotic lesions in aortic roots, aortic arches, and abdominal aortas. Augmented disease correlated with increased accumulation of CD45+ leukocytes and CD4+ T cells in the aorta, which produced increased amounts of IL-17A and tumor necrosis factor. Several chemokines, including CCL2, were upregulated in the aortas of Ldlr−/− mice receiving Il27ra−/− bone marrow, resulting in accumulation of CD11b+ and CD11c+ macrophages and dendritic cells in atherosclerotic aortas.
Conclusions
The absence of anti-inflammatory IL-27 signaling skews immune responses toward T-helper 17, resulting in increased production of IL-17A and tumor necrosis factor, which in turn enhances chemokine expression and drives the accumulation of proatherogenic myeloid cells in atherosclerotic aortas. These findings establish a novel antiatherogenic role for IL-27 receptor signaling, which acts to suppress the production of proinflammatory cytokines and chemokines and to curb the recruitment of inflammatory myeloid cells into atherosclerotic aortas.
Keywords: atherosclerosis, chemokines, cytokines, inflammation, leukocyte recruitment, vascular inflammation
Atherosclerosis is a multifactorial chronic vascular disease perpetuated by inflammation and its mediators, such as cytokines1 and chemokines.2 Atherosclerosis is characterized by accumulation of modified low-density lipoproteins in the arterial wall and formation of atherosclerotic plaques. Infiltrating monocytes take up modified low-density lipoprotein and become foam cells.3 These and other immune cells in turn produce multiple cytokines that promote chronic inflammation and progressive plaque growth.4
Previous studies identified various proinflammatory cy-tokines, such as tumor necrosis factor (TNF), interleukin (IL)-17, IL-6, IL-12, and interferon (IFN) γ, as important regulators of atherosclerosis development and progression.5–13 However, studies of negative regulators of inflammation, such as anti-inflammatory cytokines, are limited.1 Transgenic mice overexpressing IL-10 showed reduced atherosclerosis,14 whereas Apoe−/−Il10−/− double-deficient mice exhibited an increase in lesion formation.15 Systemic neutralization of transforming growth factor-β16 or its genetic deficiency16,17 resulted in increased lesion development in Apoe−/− mice.
IL-27 is a recently discovered heterodimeric member of the IL-6/IL-12 cytokine superfamily, which consists of 2 subunits: p28 and Ebi3.18 IL-27 binds to a heterodimeric IL-27 receptor (IL-27R) composed of the common gp130 (Il6st) and the unique WSX-1 (Il27ra) chains.19 Gp130 mostly activates STAT3, whereas WSX-1 activates STAT1,19 which results in a variety of signaling events, such as the T-bet–dependent induction of IL-12 receptor β chain (Il12rb2), as well as induction of pro-inflammatory IFNγ and anti-inflammatory IL-10.20 As a result, IL-27 has been reported to influence the differentiation of CD4 T-cell subtypes, including, in some cases, the stimulation of IL-10–producing type 1 T regulatory (Tr1) cells and Th1 cells, but also the inhibition of T-helper (Th) 17 cells and Th2 cells.20,21 IL-27 signaling through WSX-1 has been shown to strongly suppress Th17 cell differentiation in an STAT1-dependent fashion.22–24 IL-27R engagement also blocks STAT3 phosphorylation through a suppressor of cytokine signaling 3–dependent pathway. 25 Although WSX-1 is expressed on B cells, natural killer cells, monocytes, and macrophages, its expression is highest in CD4+ T cells, and most of the in vivo effects of Il27ra deficiency are thought to be because of altered T-cell cytokine polarization.18 In experimental autoimmune encephalitis, a mouse model of multiple sclerosis, the infusion of IL-27 reduces central nervous system inflammation by suppressing Th17 cells.24 In collagen-induced arthritis, a mouse model of rheumatoid arthritis, IL-27 administration reduced inflammatory cytokines and joint inflammation.26 Conversely, Il27ra−/− mice showed membranous glomerulonephritis and exacerbated disease in the MRL/lpr mouse model of systemic lupus erythematosus.27
The role of IL-27 in atherosclerosis has not been addressed. In 2009, Kempe et al28 reported the enhanced expression of Ebi3 subunit common to IL-27 and IL-35 in human atheromatous plaques. We reasoned that IL-27R signaling may play an important role in the regulation of aortic inflammation and atherosclerosis. Here, we report that Il27ra deficiency in hematopoietic cells leads to a significant increase in atherosclerotic lesions in aortic roots, arches, and especially in the abdominal aorta of atherosclerosis-prone Ldlr−/− mice. Absence of IL-27R in hematopoietic cells resulted in increased Th17 cells in the aorta and increased production of IL-17 and TNF. Various chemokines, including CCL2, were also upregulated, resulting in the accumulation of CD11b+ and CD11c+ inflammatory macrophages and dendritic cells (DCs) in the arterial wall. Therefore, we propose that IL-27R signaling plays an important anti-inflammatory role in the suppression of atherosclerosis development.
Methods
Mice
C57Bl/6 (B6) (000664) and Ldlr−/− (002207) mice were obtained from Jackson Labs (Bar Harbor, ME)
Il-27ra−/− (WSX-1−/−) mice were kindly provided by Amgen Inc. Il-27ra−/− mice were subsequently backcrossed into the C57Bl/6 background for 9 generations. Mice were genotyped by standard polymerase chain reaction protocols and used in age- and sex-matched groups. Animal numbers for each specific analysis are given in the Figure legends. Beginning at 8 weeks of age, female and male Ldlr−/−mice were used as bone marrow recipients. Bone marrow was isolated from either Il27ra−/− or C57Bl/6J (wild-type [wt]) mice (6–8 weeks old). After 4 weeks of reconstitution, recipient mice started to receive Western diet for 16 weeks. Littermate controls were used (ie, each cage contained both wt and Il-27ra−/− bone marrow–transplanted mice). Mice were kept in specific pathogen-free conditions in an Accreditation of Laboratory Animal Care–approved barrier facility. Blood counts were measured by an automatic analyzer (Hemavet 950FS, DREW Scientific, Oxford, CT). Alanine aminotransferase in serum was measured by kinetic colorimetric kit (Infinity ALT [GPT] reagent; Thermo Fisher Scientific Inc, Middletown, VA). Animal experiments were approved by the Animal Care Committee at the La Jolla Institute for Allergy and Immunology.
Bone Marrow Transplantation
Recipient mice (Ldlr−/−) were irradiated in 2 doses of 550 rad each (for a total 1100 rad) 3 hours apart. Femurs and tibias of donor mice (wt or Il27ra−/−) were collected, and bone marrow cells were isolated under sterile conditions. Bone marrow cells were resuspended in sterile PBS, and 300 µL of cell suspension containing 5×106 cells was intravenously injected (retro-orbitally) into recipient mice. After transplantation, recipient mice were provided with autoclaved food and autoclaved acidified water, containing antibiotic (trimethoprim-sulfamethoxazole) for 2 weeks.
Quantification of Atherosclerosis and Histological Analysis
Whole aortas were excised, fixed, and stained with Sudan IV (counterstain fast green/hematoxylin). Digital images were obtained using Moticam 1000 (Motic, Richmond, Canada) on an Olympus S267 dissection scope (Olympus, Center Valley, PA). For histological lesion size quantification, 5-µm sections were taken starting at the aortic valve plane and covering 300 µm in 50-µm intervals. Sections were stained with Oil red O/hematoxylin/light green stain. Relative collagen contents and composition were analyzed by Picrosirius red staining in polarized light. Photomicrographs were taken with a ×4 objective on a Nikon eclipse 80i microscope. Lesion size was determined using National Institutes of Health Image J and averaged over all sections in each mouse.
Histology and Immunofluorescence
For histological analysis, aortic roots were frozen in an optimal cutting temperature compound on dry ice and stored at −80°C. Five-micrometer sections were cut in the aortic valve plane, and immunostaining was performed. Frozen sections were thawed and fixed for 10 minutes in acetone at room temperature, followed by additional fixation for 8 minutes in 1% paraformaldehyde in 100 mmol/L dibasic sodium phosphate containing 60 mmol/L lysine and 7 mmol/L sodium periodate at pH 7.4 on ice. Sections were blocked using the avidin/biotin blocking kit (Vector Labs), followed by 5% normal goat serum (Caltag Laboratories) and 1% BSA (Sigma) in PBS. Sections were stained overnight at 4°C with rabbit anti-mouse smooth muscle actin (polyclonal; Abcam), rat anti-mouse CD11b-fluorescein isothiocyanate (M1/70; BD Biosciences), hamster anti-CD11c (BD Bioscience), rat anti-mouse IL-27R (provided by Dr Ghilardi, Genentech Inc), rat anti-mouse CD45 Alexa Flour 647 (Biolegend), rat anti-mouse CD4-PE (RM4-5; eBioscience), and rat anti-mouse MOMA-2 (Abcam) followed by staining with secondary antibody: donkey anti-rabbit Alexa Fluor 568 (Molecular Probes), anti-fluorescein isothiocyanate Alexa Fluor 488 (Molecular Probes), goat anti-rat Alexa Fluor 488 (Molecular Probes), and DyLight 649–labeled goat anti-hamster IgG (Jackson Immunoresearch). Images were acquired on a Leica DM6000 upright confocal microscope using HCX PLAPO ×20 and ×40 oil-immersion objectives at 488, 543, and 633 nm excitation wavelengths. National Institutes of Health Image J was used to adjust brightness and 1-step smoothing on all images in parallel.
Aortic Single Cell Preparations and Flow Cytometry Analysis
Single-cell suspensions from aortas were prepared as described previously.29 Briefly, mice were euthanized by CO2 inhalation, and aortas were perfused with PBS containing heparin (20 U/mL). Aortas were prepared by surgical removal of all adventitial fat under a dissection microscope, cut into small pieces, and incubated for 55 minutes at 37°C with gentle shaking in a mixture of 450 U/mL collagenase type I, 250 U/mL collagenase type XI, 120 U/mL hyaluronidase, and 120 U/mL DNAseI (all enzymes from Sigma Aldrich, St Louis, MO). Cell suspensions were filtered through 70-µm cell strainers and stained with CD45-PerCP (30-F11; BD Biosciences), T cell receptor-β-AF700 (H57-597; BioLegend), CD4-APC (GK1.5; eBioscience), CD11b-eFluor 450 (M1/70; eBioscience), CD11c-APC (N418; eBioscience), CD8α-Cy7APC (53–6.7; BioLegend), and Aqua LIVE/ DEAD fixable dead cell staining kit (Invitrogen, Carlsbad, CA) for flow cytometry (LSRII; Becton-Dickinson, San Jose, CA). Live CD45+ cells were gated and analyzed using FlowJo software (Tree Star Inc, Ashland, OR). For intracellular cytokine staining, cells were restimulated for 6 hours in vitro with phorbol ester and ionomycin in the presence of Brefeldin A, fixed and permeabilized using Cytofix/ Cytoperm kit (BD Biosciences), and stained with IL-17A-PE (BD Biosciences), IFNγ-Cy7PE (XMG 1.2; BD Biosciences), and TNF-APC (MP6-XT22; eBioscience).
Gene Expression
For gene expression analysis, aortas were isolated and snap-frozen in liquid nitrogen. Aortas were homogenized in an RLT buffer (Qiagen), and RNA was isolated using the RNAEasy purification system (Qiagen) and treated with DNase I (Promega) followed by first-strand cDNA synthesis using the Superscript II system, according to the random priming protocol (Invitrogen Life Technologies). Gene expression was analyzed by SYBR green real-time polymerase chain reaction using primers for ribosomal L32, β-actin, T-bet, IFNγ, IL-17, retinoic acid-related orphan receptor-γt, IL-2, IL-10, IL-6, TNF, CCL2, CCL3, CCL5, CCL17, and CCL20. Sequences of the primers were obtained from National Institutes of Health QPrimerDepot (http://mouseprimerdepot.nci.nih.gov). Reactions were performed in triplicate for each gene, and gene expression was normalized to L32 or β-actin expression.
ELISA
Aortas were digested, and cell suspensions were incubated for 48 hours with anti-CD3 and anti-CD28 antibodies in complete RPMI 1640 media containing 10% fetal bovine serum, pen/strep, L-Glu, non-essential amino acids, HEPES, and sodium pyruvate. Supernatants were collected, and cytokine secretion was measured by mouse cytometric bead array (eBioscience). Levels of IL-17A, TNF, IFN-γ, IL-6, and monocyte chemoattractant protein-1 were measured according to the manufacturer’s instructions.
Statistical Analysis
Data were analyzed using Prism software (GraphPad). Student 2-tailed t test and Mann-Whitney U test were used to compare conditions. ANOVA followed by Wilcoxon signed-rank test was used to compare fold induction of gene expression by real-time polymerase chain reaction. Data are expressed as mean±SEM; *P<0.05, ** P<0.01. P<0.05 was considered significant.
Results
IL-27R Deficiency Significantly Increases Atherosclerotic Lesion Development
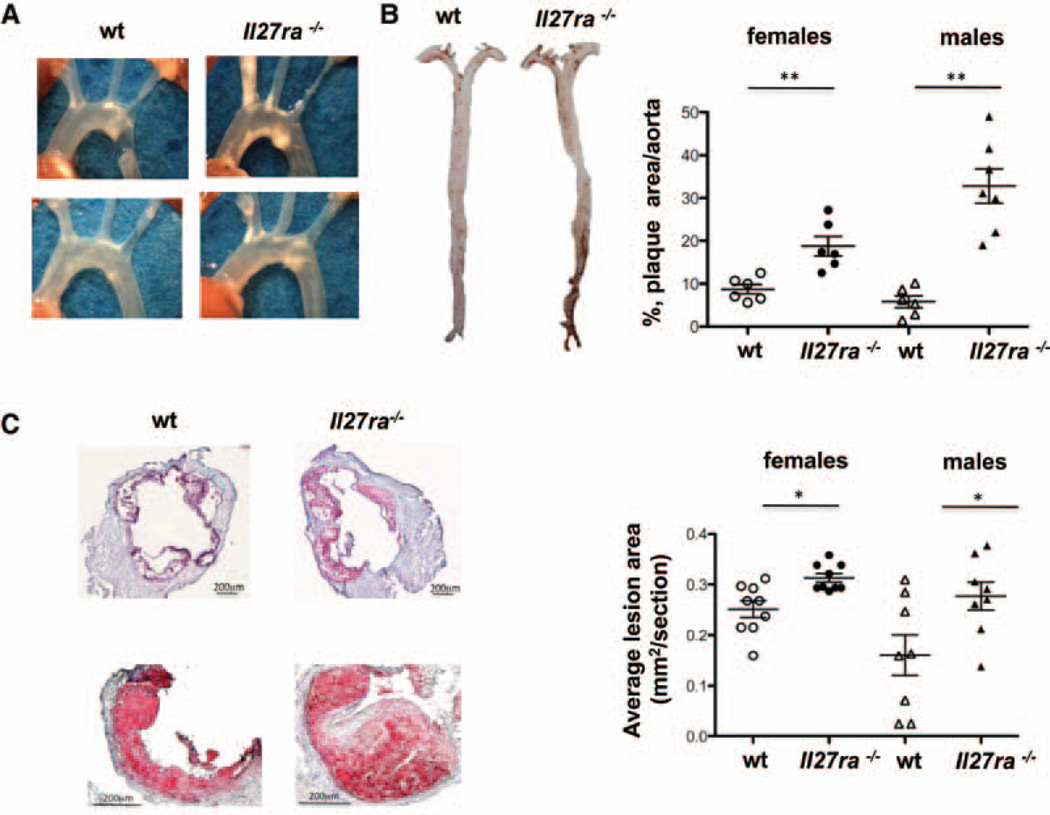
IL-27R can be expressed by various immune and hematopoietic cells but also can be found in the nonhematopoietic compartment.18 In aortic roots, IL-27R expression was found in CD4+ T cells, MOMA-2+ macrophages, and endothelial cells as seen by immunofluorescence (Online Figure I). To investigate the role of IL-27R signaling in hematopoietic cells in the pathogenesis of atherosclerosis, we generated bone marrow chimeras using either wt or Il27ra−/− bone marrow transferred to irradiated atherosclerosis-prone Ldlr−/− recipient mice (Il27ra−/−→Ldlr−/− and wt→Ldlr−/− mice). This resulted in the specific ablation of IL-27R on hematopoietic but not on nonhematopoietic cells. Four weeks after bone marrow transplantation, mice were placed on a high-fat western diet for 16 weeks. Reconstitution efficiency was at least 96±2% for myeloid cells and 93±4% for T lymphocytes (Online Figure II). We did not find a difference in body weight or blood leukocyte count between Il27ra−/−→Ldlr−/− and wt→Ldlr−/− mice (Online Figure IIIA and IIIB). Remarkably, en face analysis of aortas stained with Sudan IV revealed a significant increase of atherosclerotic plaque size in aortas of Il27ra−/−→Ldlr−/− mice compared with wt→Ldlr−/− controls (Figure 1A and 1B). Atherosclerotic plaque size was also increased in aortic roots in the absence of IL-27R (Figure 1C). Interestingly, IL-27R deficiency in hematopoietic cells also led to a dramatic increase in atherosclerotic lesions in the abdominal aorta, which usually is not profoundly affected during atherosclerosis development in Ldlr−/− mice but represents an important site of atherosclerosis in human patients (Figure 1B). Both males and females of Il2 7ra−/−→Ldlr−/− displayed enhanced lesion sizes compared with wt→Ldlr−/− littermate control mice, suggesting that IL-27R controls atherosclerosis regardless of sex differences. Relative aortic collagen contents and composition were essentially unaltered (Online Figure IVA and IVB). Smooth muscle actin was increased in the aortic arches and abdominal aortas of Il27ra−/−→Ldlr−/− mice, most likely as a result of outward remodeling (Online Figure IVC–IVF). Plasma lipoprotein and lipid profiles were not different between wt and IL-27R chimeras, suggesting that the lack of IL-27R signaling does not affect atherosclerosis by interfering with lipoprotein metabolism (Online Figure VA). No differences were found in alanine aminotransferase activity in serum (Online Figure VB). Thus, inactivation of IL-27R in hematopoietic cells significantly enhanced atherosclerosis development in a mouse model of atherosclerosis.
Figure 1. Increased atherosclerotic plaque area inIl27ra−/−bone marrow–transplanted mice.
Ldlr−/− mice were lethally irradiated and reconstituted with 5×106 unfractioned bone marrow cells from C57BL/6 (wild-type [wt]) or Il27ra−/− mice. After 4 weeks of reconstitution, mice were fed with western diet for 16 weeks. A, Images of aortic arch of Ldlr−/− mice receiving Il27ra−/− bone marrow or C57BL/6 (wt) control. B, Representative en face images of Sudan IV–stained whole aortas of Ldlr−/− mice receiving Il27ra−/− or wt bone marrow. B, right, Quantification of plaque area as percentage of aortic surface in Ldlr−/− mice receiving Il27ra−/− or wt bone marrow (n=6–7 females or males per group, respectively). C, left, Representative images of aortic roots (top) and single valve (bottom) of Ldlr−/− mice receiving Il27ra−/− or C57BL/6 (wt) bone marrow. C, right, Aortic root lesions were quantified on frozen sections stained with Oil-red-O in the 300 µm following the aortic valve (wt [n=8–9 males or females] mice and Il27ra−/− [n=8–9 males or females] mice from 3 experiments).
IL-27R Deficiency Leads to Increased Accumulation of T Cells in the Aorta of Ldlr−/− Mice
Given the relevance of IL-27R signaling in hematopoietic cells for atherosclerosis, we next sought to examine the cellular composition of the leukocyte infiltrates in aortas of Il 27ra−/−→Ldlr−/− and wt→Ldlr −/− mice. Flow cytometry revealed a significant increase in CD45+ leukocytes in the aortas of Il27ra−/−→Ldlr−/− mice (Figure 2A). Among CD45+ cells, we particularly found a significant accumulation of Th cells (TCRβ+ CD4+) cells in the aortic wall of Il27ra−/−→Ldlr−/−mice (Figure 2B), whereas no significant difference was found in cytotoxic CD8+ T cells. These observations were further supported by immunofluorescence staining for T cells, where significantly higher numbers of T cells were found in both aortic roots (Figure 2C) and abdominal aortas (Figure 2D) of Il27ra−/−→Ldlr−/− compared with wt controls. We therefore concluded that IL-27R signaling limits accumulation of CD4 T cells in the aortas of atherosclerotic mice and modulates the CD4 Th response during atherosclerosis development.
Figure 2. Leukocyte composition of mouse aorta.
A, Aortas from bone marrow–transplanted mice were made into single-cell suspensions by enzymatic digestion and stained. Live CD45+ leukocytes were counted in aortas of 16-week Western diet–fed Ldlr−/− mice transplanted with Il27ra−/− (n=7) or C57BL/6 (wild-type [wt]; n=7) bone marrow. Left panels show representative dot plots. Right panel shows mean±SEM, *P<0.05. B, left, Live CD45+ cells from aortas were stained for TCRβ and CD4. Numbers indicate percentage in indicated gates. B, right, Absolute number of live CD45+, TCRβ+, CD4+TCRβ+, and CD8+TCRβ+ cells in aortas from Ldlr−/− mice transplanted with Il27ra−/− or wt bone marrow (n=7) each. Mean±SEM *P<0.05. Localization of CD3+ cells in aortic roots (C) and abdominal aortas (D) of Ldlr−/− mice transplanted with Il27ra−/− or C57BL/6 (wt) bone marrow as seen by immunofluorescence. L indicates lumen; P, plaque; A, adventitia. Representative images from 1 of 3 independent experiments. Mean±SEM number of CD3+ cells per section in aortic roots (C, right) and abdominal aortas (D, right) of Ldlr−/− mice transplanted with Il27ra−/− (n=6) or C57BL/6 (wt; n=6) bone marrow are shown.
Ablation of Il27R Signaling in Hematopoietic Cells Causes Dysregulation of Th17 and Th1 Responses in Ldlr−/− Atherosclerotic Mice
To gain insights into potential mechanisms by which IL-27R signaling limits atherosclerosis progression, we first performed real-time polymerase chain reaction analysis on aortic arches, abdominal aortas, paraaortic lymph nodes (LNs), and spleens isolated from Il27ra−/−→Ldlr−/− or wt→Ldlr−/− mice. We found a Th17 signature mRNA expression profile in aortic arches of mice lacking IL-27R signaling, particularly elevated expression of IL-17A and the transcription factor retinoic acid-related orphan receptor-γt mRNAs (Figure 3A). Among other cytokines, mRNA expression of TNF and IL-6 was significantly enhanced in the absence of IL-27R (Figure 3A). Furthermore, we found an upregulation of the Th1 transcription factor T-bet and the Th1 cytokine IFNγ in aortic arches (Figure 3A). TNF and IL-6 expression were also significantly upregulated in the abdominal aortas but not in spleens, suggesting that IL-27R signaling has specific effects at the site of inflammation. We also found a reduction of Foxp3 gene expression in aortic arches and spleens of Il27ra−/−→Ldlr−/−mice, whereas IL-10 expression remained unchanged.
Figure 3. Enhanced production of T-helper 17 cytokines and transcription factors in mice receivingIl27ra−/− bone marrow.
A, Relative gene expression in Il27ra−/−→Ldlr−/− (n=6) mice normalized to β-actin and then normalized to gene expression in wild-type (wt)→Ldlr−/− (n=6) mice in aortic arches, abdominal aortas (Ab. aorta), paraaortic lymph nodes (paLNs), and spleens after 16 weeks of Western diet. B and C, Interleukin (IL)-17A, tumor necrosis factor (TNF), IL-6, and interferon (IFN) γ were measured by bead array in supernatants of aortic (n=8; B) and splenic (n=8; C) cell suspensions, stimulated with anti-CD3/anti-CD28 for 48 h. Mean±SEM; *P<0.05.
To further address the question whether inactivation of IL-27R results in dysregulated cytokine production, we measured cytokines in aortic and splenic supernatants. We found a dramatic increase in IL-17A, IL-6, and TNF in the aortic supernatants of Il27ra−/−→Ldlr−/− mice (Figure 3B). IL-6 and TNF but not IL-17A secretion was also increased in spleens (Figure 3C). Intracellular cytokine staining of CD4+TCRβ+ cells from aortas showed an increased percentage of IL-17A–producing (Th17) T cells in the aortas of Il27ra−/−→Ldlr−/− mice compared with those of wt→Ldlr−/− (Figure 4A). In addition, we observed enhanced TNF expression in CD4+TCRβ+ T cells (Figure 4A). Interestingly, the numbers of IL17+ TNF+ double-positive cells were also upregulated in the aortas but not in spleens or LNs of Il27ra−/−→Ldlr−/− mice (Figure 4A–4C). In spleens or LNs of Il27ra−/−→Ldlr−/− mice, IL-17A- and IFNγ-producing CD4 T cells were reduced or unchanged (Figure 4B and 4C).
Figure 4. Enhanced production of T-helper 17 cytokines in aortas of mice receiving Il27ra−/− bone marrow.
Percent of CD4 T cells expressing tumor necrosis factor (TNF), interleukin (IL)-17A, and interferon (IFN) γ by intracellular staining in aorta (A, left), spleen (B, left), or paraaortic lymph nodes (paLNs) (C, left) of Ldlr−/− mice transplanted with Il27ra−/− (n=6) or wild-type (wt; n=6) bone marrow fed 16 weeks with Western diet. A–C, right, Representative fluorescence-activated cell sorter plot of CD4+TCRβ+ T cells stained for IL-17A and TNF in aortas (A), spleens (B), and paLNs (C) of Ldlr−/− mice transplanted with Il27ra−/− or wt bone marrow fed 16 weeks with Western diet. Mean±SEM; *P<0.05.
To investigate the role of IL-27R signaling in immunosuppressive cells, we analyzed the presence of T regulatory cells (Tregs) and Tr1 cells in the spleen and LN of Il27ra−/−→Ldlr−/−and wt→Ldlr−/− mice. We found a significant decrease in the percentage of Treg in both spleen and LNs of Il27ra−/−→Ld lr−/− (Figure 5A). However, Tr1 (Foxp3− IL10+) cells were decreased only in the spleens of Il27ra−/−→Ldlr−/− (Figure 5B).
Figure 5. Regulatory T cells in spleen and lymph nodes inIl27ra−/− bone marrow–transplanted mice.
A, Percentage of live CD4+TCRβ+Foxp3+ T regulatory cells (Treg) in spleens and paraaortic lymph nodes (paLNs) from Ldlr−/− mice transplanted with Il27ra−/− (n=10) or wild-type (wt) bone marrow (n=10). B, Percentage of live CD4+TCRβ+, Foxp3−IL10+ Tr1 cells in spleens and paLNs of Ldlr−/− mice transplanted with Il27ra−/− (n=10) or wt bone marrow (n=10). Mean±SEM; *P<0.05. LN indicates lymph node.
Taken together, these findings establish that eliminating IL-27R signaling enhances Th17 immune response and production of IL-17A, IL-6, and TNF in the aortas during atherosclerosis. However, ablation of IL-27R in hematopoietic cells leads to decreased accumulation of immunosuppressive Treg and Tr1 cells in peripheral lymphoid organs, which potentially may result in enhanced inflammation.
Absence of IL-27R Enhances Chemokine Expression
IL-17 signaling has many downstream target genes potentially relevant to atherosclerosis, in particular those encoding various chemokines.30,31 Several reports suggest that, during inflammation, IL-17 and TNF can act synergistically, particularly in their ability to induce chemokine production.32 We therefore hypothesized that increased IL-17 production caused by Il27ra deficiency could affect the expression of IL-17−dependent chemokines in atherosclerosis. Although we did not find an upregulation of CXCL1 (KC) (Online Figure VI), we observed a significant increase in CCL20, CCL17, CCL5, CCL3, and CCL2 (monocyte chemoattractant pro-tein-1) mRNA levels in aortic arches and abdominal aortas of Il27ra−/−→Ldlr−/− mice (Figure 6A). CCL2 and CCL20 expression was also enhanced in the spleen, and only CCL20 was upregulated in peripheral LNs of Il27ra-deficient mice (Figure 6A). Enhanced production of CCL2 protein was detected in the aortic culture supernatant of Il27ra−/−→Ldlr−/− mice with or without T-cell stimulation (Figure 6B and 6C). These data suggest that enhanced production of IL-17 and TNF caused by Il27ra deficiency leads to increased expression of various chemokines in mouse aortas, including CCL2.
Figure 6. Analysis of interleukin (IL)-17–dependent chemokine expression in mouse aortas and lymphoid organs.
A, Relative chemokine gene expression in Il27ra−/−→Ldlr−/− (n=6) normalized to β-actin and then normalized to gene expression in wild-type (wt)→Ldlr−/− (n=6) mice in aortic arches, abdominal aortas (Ab. aortas), paraaortic lymph nodes (LNs), and spleens after 16 weeks of Western diet. CCL2 protein in supernatants of aortic cell suspensions unstimulated (n=6; B) or stimulated with anti-CD3/anti-CD28 for 48 h (n=8; C). Mean±SEM; *P<0.05.
Absence of Il27ra Leads to Enhanced Myeloid Cell Recruitment in Mouse Aortas
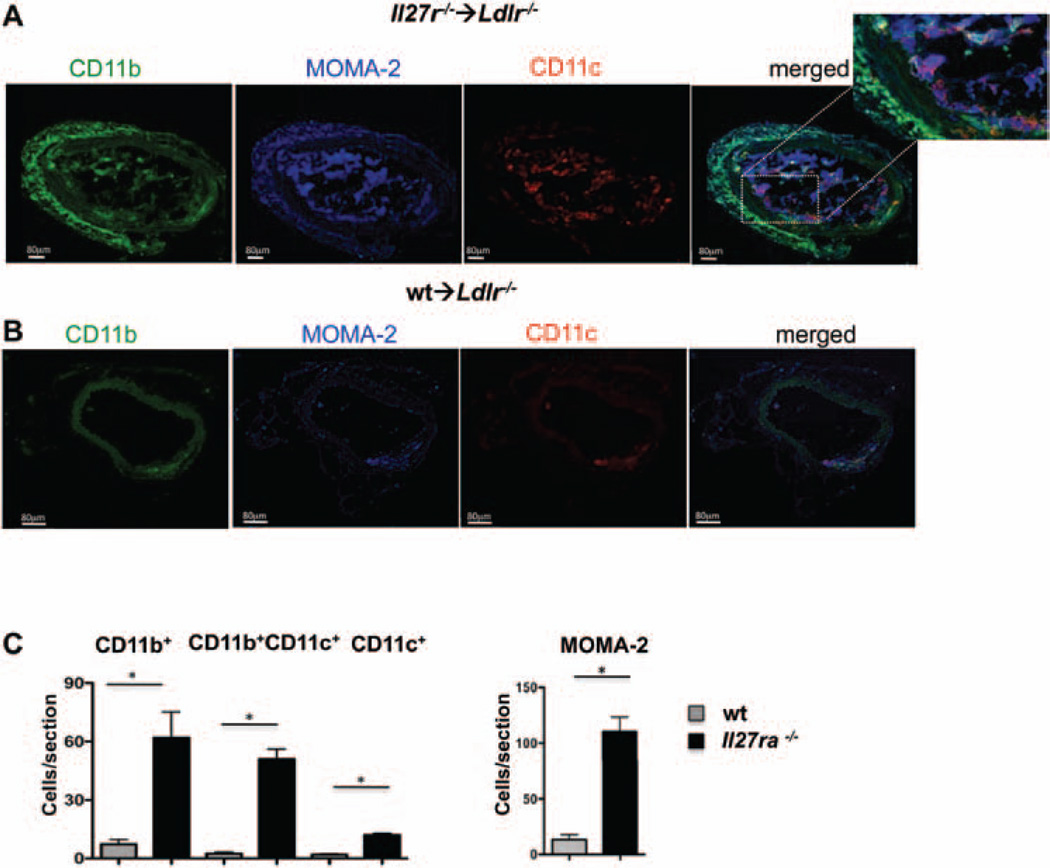
CCL2 has previously been shown to increase recruitment of monocytes to atherosclerotic aortas.33 Enhanced accumulation of these cells in the lesions and arterial wall is associated with acceleration of the disease.3,29 Indeed, the percentage as well as the number of CD11b+ and CD11b+CD11c+ myeloid cells was significantly increased in the Il27ra−/−→Ldlr−/− mice compared with the control wt→Ldlr−/− mice after 16 weeks of Western diet feeding (Figure 7A). To address the localization of these cells in situ, we performed immunofluorescence staining and found enhanced accumulation of CD11b+ and CD11c+ cells in aortic roots (Figure 7B and 7C, and Online Figure VIIB for isotype controls) and abdominal aortas (Figure 8A–8C), supporting the flow cytometry data. Interestingly, most of the CD11b+CD11c+ cells were found in the plaque, whereas the adventitia was dominated by CD11b+CD11c− cells. Most CD11b+CD11c+ and CD11b+CD11c− cells also expressed the pan-macrophage marker MOMA-2 (CD169; Figure 7B and 7C). Overall, our data suggest an inhibitory role of IL-27R signaling that limits the accumulation of myeloid cells in the aorta during atherosclerosis progression.
Figure 7. Interleukin-27 receptor (IL-27R) controls the recruitment of myeloid cells to atherosclerotic aortas.
A, left, Live CD45+ cells from aortas of Ldlr−/− mice transplanted with bone marrow from Il27ra−/− or wild-type (wt) mice fed with Western diet for 16 weeks were stained for CD11b and CD11c. Numbers indicate percentage in each quadrant. A, right, Absolute number of live CD45+, CD11b+CD11c−, CD11b+CD11c+, and CD11b−CD11c+ cells per aorta of Ldlr−/− transplanted with Il27ra−/− (n=8) or wt (n=7) bone marrow was determined based on flow cytometry data. Mean±SEM; *P<0.05. B and C, Localization and abundance of CD11b+CD11c−(green), CD11b+CD11c+ (yellow), CD11b−CD11c+(red), and MOMA-2+ (blue) cells in aortic roots of Ldlr−/− mice transplanted with Il27ra−/− (B) or C57BL/6 (wt; C) bone marrow characterized by immunofluorescence. Dotted white lines indicate border of lamina muscularis. L indicates lumen; P, plaque; A, adventitia. Representative images from 1 of 6 independent experiments. D, Quantification of CD11b+CD11c−, CD11b+CD11c+, CD11b−CD11c+, and MOMA-2+ cells in the aortic roots of Ldlr−/− mice transplanted with Il27ra−/− (n=6) or C57BL/6 (wt) (n=6) bone marrow as in B and C. Mean±SEM; *P<0.05.
Figure 8. Interleukin-27 receptor (IL-27R) controls the recruitment of myeloid cells to abdominal aortas of atherosclerotic mice.
Localization and abundance of CD11b+CD11c−(green), CD11b+CD11c+ (yellow), CD11b−CD11c+(red), and MOMA-2+ (blue) cells in abdominal aortas of Ldlr−/− mice transplanted with Il27ra−/− (A) or C57BL/6 (wild-type [wt]) (B) bone marrow characterized by immunofluorescence. Dotted white lines indicate border of lamina muscularis. L indicates lumen; P, plaque; A, adventitia. Representative images from 1 of 6 independent experiments. C, Quantification of immunofluoresecent staining for CD11b+CD11c−, CD11b+CD11c+, CD11b−CD11c+, and MOMA-2+ cells in the abdominal aortas of Ldlr−/− mice transplanted with Il27ra−/− (n=6) or C57BL/6 (wt; n=6) bone marrow as shown in A and B. Mean±SEM; *P<0.05.
Collectively, our data demonstrate an important inhibitory role of IL-27/IL-27R signaling in hematopoietic cells for controlling Th17 cytokine production and IL-17–dependent chemokine expression. In the absence of IL-27R, IL-17–dependent enhancement of CCL2 expression likely explains increased aortic accumulation of CD11b+CD11c+ inflammatory macrophages and DCs.
Discussion
A large body of evidence demonstrates a crucial role of inflammation in the pathogenesis of atherosclerosis.4 Although the role of various proinflammatory cytokines was extensively studied in atherosclerosis,1 only 2 antiatherogenic cytokines, IL-10 and transforming growth factor-β, were described. Here, we establish IL-27R signaling as a novel and important atheroprotective cytokine pathway. We show that littermate Ldlr−/− mice receiving Il27ra−/− bone marrow had more severe disease manifestation than those receiving wt bone marrow. This effect was consistently observed both in males and females. Interestingly, IL-27R deficiency in hematopoietic cells also led to a dramatic increase in atherosclerotic lesions in the abdominal aorta, which usually is not profoundly affected during atherosclerosis development in Ldlr−/− mice. In humans, the abdominal aorta represents a clinically important site for atherosclerosis associated with aortic aneurysm formation. In the absence of IL-27R, production of IL-17, IL-6, and TNF was increased in aortas, which correlates with the expansion of CD4+ IL-17–producing (Th17) cells and reduction of immunosuppressive Treg and Tr1 cells. Therefore, our data provide collective evidence for an anti-inflammatory and antiatherogenic role of IL-27R signaling in atherosclerosis.
Consistent with the ability of IL-27 to suppress the differentiation of Th17 cells, we found a dramatic increase of IL-17A production in aortas of atherosclerotic mice lacking hematopoietic IL-27R, which positively correlated with enhanced manifestation of the disease. Furthermore, in the absence of IL-27R, we found a significant upregulation of IL-6, which is known to participate in the survival and activation of T cells, in general, and in the regulation of Th17 lineage differentiation, in particular.34 The role of IL-6 in atherosclerosis is controversial. Some studies show that IL-6 promotes athero-sclerosis35; however old Il6−/− mice have also been reported to show enhanced atherosclerosis.36 Despite the extensive study of IL-17A in the pathogenesis of atherosclerosis, its role is still debated, although most studies suggest a proatherogenic function of IL-17. In atherosclerosis-susceptible Apoe−/− mice, the proportion of Th17 (and Th1) cells, the expression of IL-17A, and the Th17 signature transcription factor retinoic acid-related orphan receptor-γt were increased compared with nonatherosclerotic wt littermates.12,13,37–41 Treatment of Apoe−/− mice with neutralizing anti–IL-17A antibody dramatically inhibited the development of atherosclerosis, whereas rIL-17 application significantly promoted the formation of atherosclerotic plaque.37 Double-deficient Il17a−/−Apoe−/− mice had reduced aortic leukocyte and DC infiltration after a high-fat diet, although the lesion burden was not altered in another study41 and increased in a third study.42 Our data support the concept that increased IL-17 production in the absence of IL-27R signaling promotes atherosclerosis.
IL-27R engagement has previously been shown to inhibit Th17 lineage commitment through a Stat-1–dependent pathway.22 IL-27R deficiency in mouse models of multiple sclerosis led to an exacerbated Th17 response, enhanced secretion of IL-17, IL-6, TNF, and GM-CSF, and exacerbated disease.24,43 This was associated with an increased number of IL-17– producing T cells in the central nervous system. In agreement with this, our work demonstrates that IL-17–producing T cells are more numerous in atherosclerotic aortas of Ldlr−/− mice receiving Il27ra−/− bone marrow. This suggests that, in vivo, the loss of the suppressive effect of IL-27R signaling on Th17 cells dominates disease exacerbation in the Ldlr−/− model of atherosclerosis. In vitro, IL-27 also has been reported to enhance Th1 differentiation.44 If indeed that was the main effect of IL-27 in vivo, a reduced Th1 signature would be expected in Il27ra−/− mice. However, in a mouse model of infection with Toxoplasma gondii, Il27ra−/− mice showed the opposite phenotype with increased accumulation and proliferation of CD4 T cells and increased IFNγ production.45,46 We also observe enhancement of IFNγ production in aortas of mice receiving Il27ra−/− bone marrow. Overall, our data suggest that in atherosclerosis IL-27R signaling is critically involved in suppression of Th17 and, to some extent, in suppression of the Th1 aortic immune responses. However, IL-27R signaling can be also important for immunosuppressive Treg and Tr1 cell function during atherosclerosis.
Th17 cells in the aortas of Il27ra−/−→Ldlr−/− mice also likely contribute to the enhanced TNF production, because the number of IL-17+ TNF+ cells in atherosclerotic aortas is increased. The excess of TNF produced in the absence of IL-27R could potentially synergize with IL-17A to induce downstream proatherogenic events. It was previously demonstrated that TNF and IL-17A can upregulate IL-6 production in osteoblasts by activating the transcription factor C/EBPδ.47 In endothelial cells, synergistic effects of TNF and IL-17A on expression of adhesion molecules and chemokines have also been demonstrated. Various chemokines, particularly CXCL1, CXCL2, CXCL5, CCL2, and CCL7, have been reported to be induced by both IL-17A and TNF, with a strong synergistic component when both cytokines were present.32 For example, IL-17A modestly induces expression of CXCL1 in mouse embryonic fibroblasts cells but in combination with TNF has a much stronger effect.48 In aortas from Ldlr−/− mice receiving Il27ra−/− bone marrow, CCL2, CCL3, CCL5, CCL17, and CCL20 were significantly upregulated. Among them, CCL2, CCL3, and CCL5 are known to promote monocyte recruitment, and CCL2 (monocyte chemoattractant protein-1) potentiates the release of monocytes from the bone marrow and their recruitment to the sites of inflammation.49,50 Indeed, mice lacking the Ccl2 gene are protected from atherosclerosis.51 CCL3 (macrophage inflammatory protein-1α) is also an important chemoattractant for monocytes,52 whereas CCL5 (RANTES) is one of the most potent arrest chemokine for monocytes, promoting their transition from rolling to firm adhesion by inducing rapid integrin activation.53 Consistent with the known roles of CCL2, CCL3, and CCL5, we found enhanced recruitment of inflammatory monocytes, macrophages, and DCs (CD11b+, CD11b+CD11c+, and CD11c+) to aortas of Ldlr−/− mice transplanted with Il27ra−/− bone marrow. These myeloid cells have the potential to become foam cells and enhance local inflammation by secreting additional cytokines.3,54 The increased expression of CCL17 may be secondary to the recruitment of these cells. Indeed, accumulation of CCL17-expressing DCs has been reported to exacerbate atherosclerosis.55 IL-17A has also been reported to upregulate CCL20,56 a chemokine that is known to promote recruitment of Th17 cells.57 In aortas of Ldlr−/− mice receiving Il27ra−/− bone marrow, we find increased expression of CCL20, which may provide a positive feedback loop to further enhance Th17 recruitment, IL-17 production, and chemokine expression.
Taken together, our data show an important inhibitory role of IL-27R signaling in controlling inflammation during atherosclerosis. Enhanced production of IL-17 and TNF, as well as decreased number of immunosuppressive T cells caused by inactivation of Il27ra signaling, leads to increased inflammation in the aortic wall and atherosclerotic plaques. Increased expression of IL-17, TNF, and IL-17–dependent and TNF-dependent chemokines, including CCL2, supports the recruitment of myeloid cells, particularly CD11b+CD11c+ inflammatory macrophages and DC, into the growing atherosclerotic plaque.50,58 Therefore, our findings may be of potential clinical relevance, suggesting that IL-27 could be used as an anti-inflammatory cytokine in the treatment or prevention of atherosclerosis.
Supplementary Material
Novelty and Significance.
What Is Known?
Immune and inflammatory response are critical for atherosclerosis development and progression.
Interleukin (IL)-27 receptor (IL-27R) signaling is an important regulator of immune responses.
IL-27R signaling regulates the differentiation of T-helper 1 and T-helper 17 CD4+ T-cell subsets in host defense and mouse models of multiple sclerosis (experimental autoimmune encephalitis) and rheumatoid arthritis.
What New Information Does This Article Contribute?
This article identifies the role of IL-27R in atherosclerosis.
This article demonstrates an important inhibitory role of IL-27R signaling in the regulation of inflammation in atherosclerosis.
The role of proinflammatory cytokines in atherosclerosis was extensively addressed in the past decade. However, little is known about anti-inflammatory cytokines and their ability to potentially suppress atherosclerosis. IL-27, a member of IL-6/IL-12 cytokine superfamily, was shown to suppress immune responses, but the role of IL-27R signaling in the pathogenesis of atherosclerosis has been never addressed. Here, we demonstrate that absence of immune-modulating IL-27R signaling during atherosclerosis development results in dramatic acceleration of the disease. The disease manifestation correlated with enhanced activation of T-helper 17 cells and upregulation of IL-17– and tumor necrosis factor–dependent chemokine expression, particularly CCL2, which leads to accumulation of various myeloid cells and progression of atherosclerotic plaque growth. Our data provide new knowledge in the inflammatory and immune aspects of atherosclerosis. It identifies IL-27 receptor as mediating anti-inflammatory effects in the Ldlr−/− mouse model of atherosclerosis. This mechanism controls inflammation and leukocyte recruitment to the aorta. These findings and new insights into the molecular and cellular mechanisms of anti-inflammatory and antiatherogenic effects of IL-27R may provide a rationale for developing new strategies to inhibit inflammation in atherosclerosis.
Acknowledgments
We thank Hui Ouyang for expert animal husbandry and technical assistance and Dr Ghilardi for antibody to IL-27Ra.
Sources of Funding
This work was supported by National Institutes of Health HL 115232 and HL 55798, project 4 to K.L, AI 89624 to M.K., American Heart Association Fellowship 10POST4160142-01 to E.K.K., and Deutsche Forschungsgemeinschaft Grant to S.v.V.
Non-standard Abbreviations and Acronyms
- DC
dendritic cells
- IL
interleukin
- IL-27R
IL-27 receptor
- IFN
interferon;LN, lymph node
- Th
T helper
- TNF
tumor necrosis factor
- Treg
T regulatory cells
- Tr1
type 1 T regulatory cells
- wt
wild type.
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.112.277525/-/DC1.
References
- 1.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 2.Zernecke A, Weber C. Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc Res. 2010;86:192–201. doi: 10.1093/cvr/cvp391. [DOI] [PubMed] [Google Scholar]
- 3.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 8.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 10.Hauer AD, Uyttenhove C, de Vos P, Stroobant V, Renauld JC, van Berkel TJ, van Snick J, Kuiper J. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation. 2005;112:1054–1062. doi: 10.1161/CIRCULATIONAHA.104.533463. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Niessner A, Nakajima T, Ma-Krupa W, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. Interleukin 12 induces T-cell recruitment into the atherosclerotic plaque. Circ Res. 2006;98:524–531. doi: 10.1161/01.RES.0000204452.46568.57. [DOI] [PubMed] [Google Scholar]
- 12.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Vietinghoff S, Koltsova EK, Mestas J, Diehl CJ, Witztum JL, Ley K. Mycophenolate mofetil decreases atherosclerotic lesion size by depression of aortic T-lymphocyte and interleukin-17-mediated macrophage accumulation. J Am Coll Cardiol. 2011;57:2194–2204. doi: 10.1016/j.jacc.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinderski LJ, Fischbein MP, Subbanagounder G, Fishbein MC, Kubo N, Cheroutre H, Curtiss LK, Berliner JA, Boisvert WA. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 15.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 16.Lutgens E, Daemen MJ. Transforming growth factor-beta: a local or systemic mediator of plaque stability? Circ Res. 2001;89:853–855. [PubMed] [Google Scholar]
- 17.Grainger DJ, Mosedale DE, Metcalfe JC, Böttinger EP. Dietary fat and reduced levels of TGFbeta1 act synergistically to promote activation of the vascular endothelium and formation of lipid lesions. J Cell Sci. 2000;113(Pt 13):2355–2361. doi: 10.1242/jcs.113.13.2355. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida H, Miyazaki Y, Yoshiyuki M. Regulation of immune responses by interleukin-27. Immunol Rev. 2008;226:234–247. doi: 10.1111/j.1600-065X.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 19.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 20.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artis D, Villarino A, Silverman M, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, Yoshimura A, Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177:5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 23.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci USA. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 26.Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, Liew FY. Interleukin 27 attenuates collagen-induced arthritis. Ann Rheum Dis. 2008;67:1474–1479. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu S, Sugiyama N, Masutani K, Sadanaga A, Miyazaki Y, Inoue Y, Akahoshi M, Katafuchi R, Hirakata H, Harada M, Hamano S, Nakashima H, Yoshida H. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1) J Immunol. 2005;175:7185–7192. doi: 10.4049/jimmunol.175.11.7185. [DOI] [PubMed] [Google Scholar]
- 28.Kempe S, Heinz P, Kokai E, Devergne O, Marx N, Wirth T. Epstein-barr virus-induced gene-3 is expressed in human atheroma plaques. Am J Pathol. 2009;175:440–447. doi: 10.2353/ajpath.2009.080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shahrara S, Pickens SR, Mandelin AM2nd, Karpus WJ, Huang Q, Kolls JK, Pope RM. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol. 2010;184:4479–4487. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ, Milos PM. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 35.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 36.Elhage R, Clamens S, Besnard S, Mallat Z, Tedgui A, Arnal J, Maret A, Bayard F. Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17beta-estradiol in apolipoprotein E-deficient mice. Atherosclerosis. 2001;156:315–320. doi: 10.1016/s0021-9150(00)00682-1. [DOI] [PubMed] [Google Scholar]
- 37.Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C, Zhang Y, Chen W, Zhang L. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820–5827. doi: 10.4049/jimmunol.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Es T, van Puijvelde GH, Ramos OH, Segers FM, Joosten LA, van den Berg WB, Michon IM, de Vos P, van Berkel TJ, Kuiper J. Attenuated atherosclerosis upon IL-17R signaling disruption in LDLr deficient mice. Biochem Biophys Res Commun. 2009;388:261–265. doi: 10.1016/j.bbrc.2009.07.152. [DOI] [PubMed] [Google Scholar]
- 39.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Böckler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Shimada K, Zhang W, Huang G, Crother TR, Arditi M. IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol. 2010;185:5619–5627. doi: 10.4049/jimmunol.1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Usui F, Kimura H, Ohshiro T, Tatsumi K, Kawashima A, Nishiyama A, Iwakura YI, Ishibashi S, Takahashi M. Interleukin-17 deficiency reduced vascular inflammation and development of atherosclerosis in western diet-induced apoe-deficient mice. BBRC. 2012;420:72–77. doi: 10.1016/j.bbrc.2012.02.117. [DOI] [PubMed] [Google Scholar]
- 43.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 44.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 45.Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, de Sauvage FJ, Satoskar AR. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. Am J Pathol. 2006;168:158–169 . doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 47.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 48.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 49.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 50.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 51.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 52.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 53.Baltus T, Weber KS, Johnson Z, Proudfoot AE, Weber C. Oligomerization of RANTES is required for CCR1-mediated arrest but not CCR5-mediated transmigration of leukocytes on inflamed endothelium. Blood. 2003;102:1985–1988. doi: 10.1182/blood-2003-04-1175. [DOI] [PubMed] [Google Scholar]
- 54.Soehnlein O, Weber C. Myeloid cells in atherosclerosis: initiators and decision shapers. Semin Immunopathol. 2009;31:35–47. doi: 10.1007/s00281-009-0141-z. [DOI] [PubMed] [Google Scholar]
- 55.Weber C, Meiler S, Döring Y, et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J Clin Invest. 2011;121:2898–2910. doi: 10.1172/JCI44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zrioual S, Toh ML, Tournadre A, Zhou Y, Cazalis MA, Pachot A, Miossec V, Miossec P. IL-17RA and IL-17RC receptors are essential for IL-17A-induced ELR+ CXC chemokine expression in synoviocytes and are overexpressed in rheumatoid blood. J Immunol. 2008;180:655–663. doi: 10.4049/jimmunol.180.1.655. [DOI] [PubMed] [Google Scholar]
- 57.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Surmi BK, Hasty AH. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul Pharmacol. 2010;52:27–36. doi: 10.1016/j.vph.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.