Abstract
Parathyroid carcinoma is a rare malignant endocrine tumor accounting for only 0.5% to 5% of all primary hyperparathyroidism. Among these malignancies, only 10-25% are nonfunctioning. After the review of the literature we could only ascertain a number of 25 cases reported worldwide, since 1929, our case being the 26th, but the first with a very aggressive pathology, treated with chemotherapy scheme usually used for neuroendocrine tumors. Considering these facts, every single case presented is a step forward in defying the clinical presentation, for the awareness of the clinicians, and also in establishing standard adjuvant therapies.
Keywords: Parathyroid, Cancer, Non-functional
Letters to the Editor
Parathyroid carcinoma is one of the very rare malignant endocrine tumors accounting for only 0.5% to 5% of all primary hyperparathyroidism [1]. Among these malignancies, only 10-25% are nonfunctioning [2], with normal values of parathyroid hormone (PTH). The difficulties in diagnosing these tumors rise not only because of the absence of symptoms of hyperparathyroidism, but also because of the hardship of the positive pathologic diagnosis [1-4].
After the review of literature we could only ascertain a number of 25 cases reported worldwide, since 1929 [5-31]. The present case is the 26th, being the first with a very aggressive pathology, treated with chemotherapy scheme usually used in the therapy of neuroendocrine tumors.
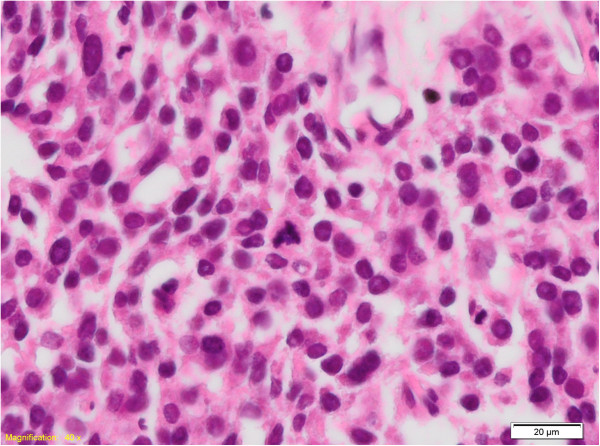
We present the case of a 51-years-woman referred for an indolent cervical mass measuring 2.5 cm in the right supraclavicular area with slight evolution during the last five years. Cervical ultrasound revealed a hypoechogenic nodule located in the lower part of the right thyroid lobe, without any other lesions worth being mentioned. The serum hormones were found in normal range, as follows: thyroid stimulating hormone (TSH) was 1.21 mIU/L (N.V. 0.2-4.2 mIU/L); free-thyroxin (FT4) was 14.4 pmol/L (N.V. 12–22 pmol/L); calcium 9.1 mg/dL (N.V. 8.6-10.2 mg/dL); PTH was 54 ng/L (N.V.15-65 ng/L) and the anti-thyroid peroxidase antibodies (Anti-TPO) were negatives. Chromogranin A (Cg A) was 38 μg/L (N.V. 27–94 μg/L) and 5-hydroxiindolacetic acid (5-HIAA) was 4.2 mg/24 hours (N.V. 2–9 mg/24 hours). We performed the fine needle aspiration biopsy (FNAB) and a 2 ml volume of clear liquid was extracted, the cytology showing only colloid and amorphous material. Three years later, the patient was readmitted with a recurrence of the mass, located in the same area. The computed tomography scan (CT Scan) showed a nodule of 1 cm located in the lower part of the right thyroid lobe and another 2 cm tumor located near the thyroid gland, adherent to it and with close connection with the jugular vein (Figure 1). At this presentation, the serologic parameters were also in normal ranges. It was decided the total thyroidectomy, right selective lymphadenectomy and the radical resection of the tumor, of the jugular vein and of a part of the adjacent muscle. On Hematoxylin and Eosin (H&E) staining the tumor was composed of clear, partially oxyphil cells, showing atypia and nuclear pleomorphism with increased mitotic activity (Figure 2). At the periphery of the tumor a remnant of non-tumoral parathyroid tissue was still visible, with compression changes. The rest of the resected thyroid gland presented the microscopy of a nodular goiter, without any signs of malignancy; all the lymph nodes resected were disease free.
Figure 1.
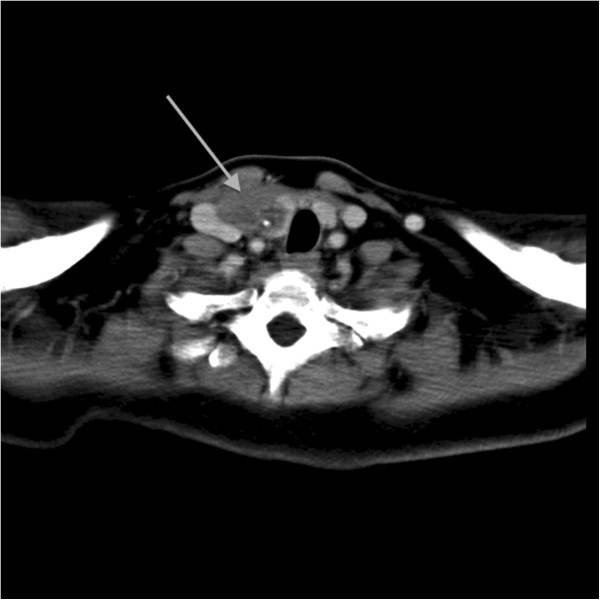
Pre-operative computed tomography of non- functional parathyroid carcinoma (arrowhead) displacing the trachea and the jugular vein, in the right anterior cervical area.
Figure 2.
HE, 400×: Detail of nuclear atipia and multiple mitotic figures, with an atypical one in the center of the image.
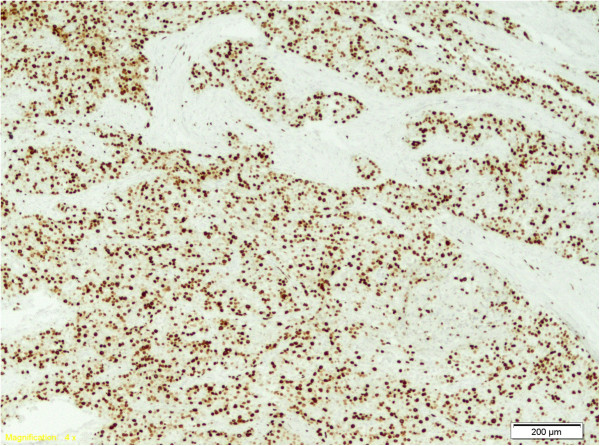
The tumor cells presented the following immunohistochemical (IHC) profile: cytokeratin AE1/AE3 intensely positive; cytokeratin 7 antibody (CK7) focally positive; neuron specific enolase (NSE) intensely positive; Chromogranin A focally positive; gross cystic disease fluid protein (GCDFP15) focally positive in rare, isolated cells; progesterone receptor (PR) weakly positive (1%); estrogen receptor (ER) negative; cytokeratin 20 antibody (CK20) negative; Thyroglobulin negative; Calcitonin negative; Synaptophisin negative; Neural cell adhesion molecules (CD56) negative; Mammaglobin negative; proliferating index (Ki67) staining (Figure 3) showed a high proliferative index (70%).
Figure 3.
Ki67, 100×: Ki67 immunostaining, demonstrating a high proliferation index in the tumor.
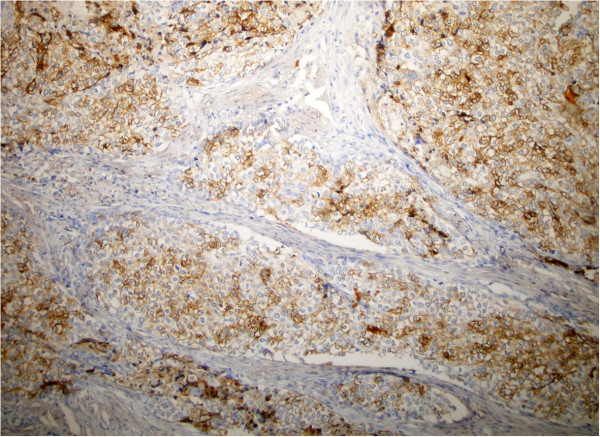
The histopathological diagnosis was probably of right parathyroid carcinoma; the normal levels of PTH and calcium enforced the PTH staining. Due to the impossibility of performing the analysis in the Institute, the accurate diagnosis was not set at that time and the block was sent for a second opinion in a national center, results showing that the PTH was negative. The confusing results misled the clinicians and made them unable to decide the treatment strategy. The capsular invasion and the infiltration of the adjacent muscle represented criteria for radiotherapy as adjuvant therapy; but the primary diagnostic of possible neuroendocrine tumor, with lack of response to external beam radiation and the presence of vascular emboli with high Ki-67 proliferation index (70%) were arguments for the chemotherapy scheme. The clinicians decided to start the chemotherapy cycles as indicated for the neuroendocrine tumors: Etoposide (Vepesid, VP16) 120 mg/sqm day 1–3 and Carboplatin AUC 5 =450 mg/day (VP16 + Carbo). A third histopathological report was demanded to the experts in Athens, Greece. Their findings indicated that PTH was extensively positive (Figure 4) and the final diagnosis was of non-functional parathyroid carcinoma. With this result, after 4 cycles of chemotherapy, the systemic treatment was discontinued. The replacement thyroid hormone therapy with 100 micrograms Levo-thyroxine daily was started and 2 months after surgery the TSH and FT4 were in normal ranges. The follow-up was carried on every 3 months and consisted in determination of the TSH and FT4, PTH, calcium and cervical CT Scan, chest CT Scan and abdominal MRI. All the examinations were constantly normal (Figure 5). The latest check-up was performed at 15 months after surgery and showed no relapse and the highly aggressive pathology did not express an aggressive clinical behavior.
Figure 4.
PTH, 100×: Tumor showing positive PTH immunostaining.
Figure 5.
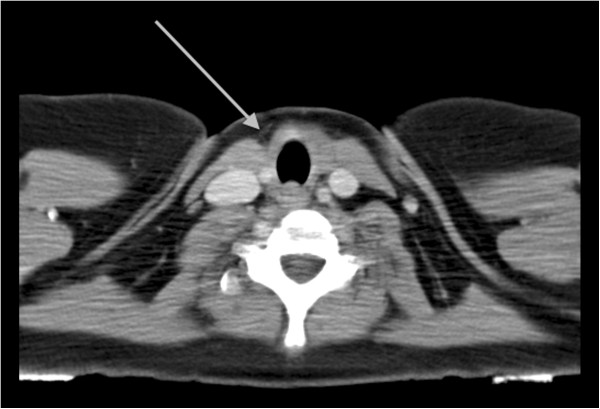
Computed tomography of the neck region, one year after therapy (surgery and chemotherapy); no local recurrence or regional metastases.
In our study, the lack of reference on this pathology leaded the clinicians to establish a chemotherapy protocol (VP16+ Carbo), used for the first time in the context of this condition.
Non –functioning parathyroid carcinoma is an exceptionally rare malignancy, therefore any experience must be known, in order to improve its diagnostic and therapy.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DP has made substantial contributions to conception, acquisition, analysis and interpretation of data. AI performed the surgical procedures and participated in the design of the study. GK carried out the immunohistochemical studies and has been involved in revising the manuscript. AP elaborated the main document of the manuscript. RB carried out the pathological studies, and has given final approval of the version to be published. All authors read and approved the final manuscript.
Contributor Information
Doina Piciu, Email: doina.piciu@gmail.com.
Alexandru Irimie, Email: office@iocn.ro.
George Kontogeorgos, Email: gkonto@med.uoa.gr.
Andra Piciu, Email: piciuandra@gmail.com.
Rares Buiga, Email: raresbuiga@yahoo.fr.
References
- Talat N, Schulte K. Clinical presentation, staging and long-term evolution of parathyroid cancer. Ann Surg Oncol. 2010;17:2156–2174. doi: 10.1245/s10434-010-1003-6. [DOI] [PubMed] [Google Scholar]
- Giessler GA, Beech DJ. Nonfunctional parathyroid carcinoma. JNMA. 2001;93:251–255. [PMC free article] [PubMed] [Google Scholar]
- Wilkins BJ, Lewis J, James S. Non-functional parathyroid carcinoma: a review of the literature and report of a case requiring extensive surgery. Head and Neck Pathol. 2009;3:140–149. doi: 10.1007/s12105-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeson L, Grimelius L, Delellis RA. In: World Health Organisation classification of tumours, pathology and genetics: tumour of endocrine organs. Delellis RA, editor. Lyon: IARC Press; 2004. Parathyroid carcinoma; pp. 124–133. [Google Scholar]
- Guy CC. Tumors of the parathyroid glands. Surg Gynecol Obstet. 1929;149:522–527. [Google Scholar]
- McQuillan AS Parathyroid tumor: report of two cases Ann Surg 1938108464–468. 10.1097/00000658-193809000-0000817857245 [DOI] [Google Scholar]
- Armstrong HG. Primary carcinoma of the parathyroid gland with report of a case. Bull Acad Med Tor. 1938;11:105–110. [Google Scholar]
- Sieracki JC, Horn RC Jr. Nonfunctional carcinoma of the parathyroid. Cancer. 1960;13:502–506. doi: 10.1002/1097-0142(196005/06)13:3<502::AID-CNCR2820130312>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Pachter MR, Lattes R. Uncommon mediastinal tumors. Report of two parathyroid adenomas, one nonfunctional parathyroid carcinoma and one “bronchial-type-adenoma”. Dis Chest. 1963;43:519–528. doi: 10.1378/chest.43.5.519. [DOI] [PubMed] [Google Scholar]
- Altenähr E, Saeger W. Light and electron microscopy of parathyroid carcinoma: report of three cases. Virchows Arch A Pathol Anat. 1973;360:107–122. doi: 10.1007/BF00543222. [DOI] [PubMed] [Google Scholar]
- Chahinian AP, Holland JF, Nieburgs HE. et al. Metastatic nonfunctioning parathyroid carcinoma: ultrastructural evidence of secretory granules and response to chemotherapy. Am J Med Sci. 1981;282:80–84. doi: 10.1097/00000441-198109000-00005. [DOI] [PubMed] [Google Scholar]
- Aldinger KA, Hickey RC, Ibanez ML. et al. Parathyroid carcinoma: a clinical study of seven cases of functioning and two cases of nonfunctioning parathyroid cancer. Cancer. 1982;49:388–397. doi: 10.1002/1097-0142(19820115)49:2<388::AID-CNCR2820490230>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Samaan NA, Vassilopoulou-Sellin R. et al. Parathyroid carcinoma: features and difficulties in diagnosis and management. Surgery. 1983;94:906–915. [PubMed] [Google Scholar]
- Ordoñez NG, Ibanez ML, Samaan NA. et al. Immunoperoxidase study of uncommon parathyroid tumors. Am J Surg Pathol. 1983;7:535–542. doi: 10.1097/00000478-198309000-00004. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Noguchi S, Nakayama I. et al. Light and electron microscopic study of nonfunctioning parathyroid carcinoma: report of a case with a review of the literature. Acta Pathol Jpn. 1984;34:123–132. doi: 10.1111/j.1440-1827.1984.tb02190.x. [DOI] [PubMed] [Google Scholar]
- Merlano M, Conte P, Scarsi P. et al. Non-functioning parathyroid carcinoma: a case report. Tumori. 1985;71:193–196. doi: 10.1177/030089168507100216. [DOI] [PubMed] [Google Scholar]
- Collins FD, Warren MC, Palmer FJ, Rankin DR. Nonfunctioning parathyroid carcinoma: a case history. J Surg Oncol. 1986;31:60–61. doi: 10.1002/jso.2930310114. [DOI] [PubMed] [Google Scholar]
- Murphy MN, Glennon PG, Diocee MS. et al. Nonsecretory parathyroid carcinoma of the mediastinum: light microscopic, immunocytochemical, and ultrastructural features of a case, and review of the literature. Cancer. 1986;58:2468–2476. doi: 10.1002/1097-0142(19861201)58:11<2468::AID-CNCR2820581120>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Klink B, Karulf R, Maimon W, Peoples J. Nonfunctioning parathyroid carcinoma. Am Surg. 1991;57:463–467. [PubMed] [Google Scholar]
- Yamashita H, Noguchi S, Murakami N, Toda M, Adachi M, Daa T. Immunohistological study of nonfunctional parathyroid carcinoma. Report of a case. Acta Pathol Jpn. 1992;42:279–285. doi: 10.1111/j.1440-1827.1992.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Pelizzo MR, Piotto A, Bergamasco A, Rubello D, Casara D. Parathyroid carcinoma. Therapeutic strategies derived from 20 years of experience. Minerva Endocrinol. 2001;26:23–29. [PubMed] [Google Scholar]
- Eurelings M, Frijns CJM, Jeurissen FJF. Painful ophthalmoplegia from metastatic nonproducing parathyroid carcinoma: case study and review of the literature. Neuro Oncol. 2002;4:44–48. doi: 10.1215/15228517-4-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby-Bott J, Lewis P, Harmer CL. et al. One stage treatment of parathyroid carcinoma. Eur J Surg Oncol. 2005;31:78–83. doi: 10.1016/j.ejso.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Ashkenazi D, Elmalah I, Rakover Y, Luboshitzky R. Concurrent nonfunctioning parathyroid carcinoma and parathyroid adenoma. Am J Otolaryngol. 2006;27:204–206. doi: 10.1016/j.amjoto.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ranvier GG, Jensen K, Khanafshar E. Nonfunctioning parathyroid carcinoma: case report and review of the literature. Endocr Pract. 2007;13:750–757. doi: 10.4158/EP.13.7.750. [DOI] [PubMed] [Google Scholar]
- Gao WC, Ruan CP, Zhang JC, Liu HM, Xu XY, Sun YP, Wang Q. Nonfunctional parathyroid carcinoma. J Cancer Res Clin Oncol. 2010;136:969–974. doi: 10.1007/s00432-009-0740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Kataoka H, Sakoda T, Horie Y, Kitano H. Nonfunctional parathyroid carcinoma. Intl J Clin Oncol. 2010;15:500–503. doi: 10.1007/s10147-010-0062-9. [DOI] [PubMed] [Google Scholar]
- Krvavica A, Kovacic M, Baraka I, Rudic M. Non-functioning parathyroid gland carcinoma: case report. Acta Clin Croat. 2011;50:233–237. [PubMed] [Google Scholar]
- Uchida N, Ishiguro K, Suda T, Horie Y, Nishimura M. Metastatic breast tumor due to nonfunctional parathyroid carcinoma. Intl Canc Conf J. 2012;1:47–52. doi: 10.1007/s13691-011-0008-8. [DOI] [Google Scholar]
- Kotromanovic Z, Birtic D, Vceva A. Non-functional parathyroid gland carcinoma. Coll Antropol. 2012;36(Suppl. 2):23–25. [PubMed] [Google Scholar]
- Guo H, Mai R, Liu M, Peng H, Yang X, Wu M, Zhang G. Nonfunctional parathyroid carcinoma after breast carcinoma. J Clin Oncol. 2013. [Epub ahead of print] PubMed PMID: 23341525. [DOI] [PubMed]