Abstract
Chronic treatment of growing pigs with porcine somatotropin (pST) promotes protein synthesis and doubles postprandial levels of insulin, a hormone that stimulates translation initiation. This study aimed to determine whether the pST-induced increase in skeletal muscle protein synthesis was mediated through an insulin-induced stimulation of translation initiation. After 7–10 days of pST (150 μg·kg−1·day−1) or control saline treatment, pancreatic glucose-amino acid clamps were performed in overnight-fasted pigs to reproduce 1) fasted (5 μU/ml), 2) fed control (25 μU/ml), and 3) fed pST-treated (50 μU/ml) insulin levels while glucose and amino acids were maintained at baseline fasting levels. Fractional protein synthesis rates and indexes of translation initiation were examined in skeletal muscle. Effectiveness of pST treatment was confirmed by reduced urea nitrogen and elevated insulin-like growth factor I levels in plasma. Skeletal muscle protein synthesis was independently increased by both insulin and pST. Insulin increased the phosphorylation of protein kinase B and the downstream effectors of the mammalian target of rapamycin, ribosomal protein S6 kinase, and eukaryotic initiation factor (eIF)4E-binding protein-1 (4E-BP1). Furthermore, insulin reduced inactive 4E-BP1·eIF4E complex association and increased active eIF4E·eIF4G complex formation, indicating enhanced eIF4F complex assembly. However, pST treatment did not alter translation initiation factor activation. We conclude that the pST-induced stimulation of skeletal muscle protein synthesis in growing pigs is independent of the insulin-associated activation of translation initiation.
Keywords: translation initiation, mammalian target of rapamycin, growth hormone, eukaryotic initiation factor 4G, protein kinase B
chronic treatment with somatotropin, also known as growth hormone, increases weight gain without raising dietary intake and thereby improves the efficiency with which dietary amino acids are utilized for growth (5, 6, 19). In addition to enhancing protein deposition, somatotropin treatment also decreases the accretion of fat (17, 35). Previously, we demonstrated that 1 wk of porcine somatotropin (pST) treatment increases whole body protein accretion by minimizing protein loss during fasting and maximizing protein gained after feeding (48, 49). Further studies showed tissue-specific differences in the regulation of protein synthesis by pST treatment (2, 4). Chronic treatment with pST increased muscle protein synthesis by enhancing the activation of translation initiation factors that regulate the binding of mRNA and methionyl-tRNA to the ribosomal complex, whereas in the liver pST treatment increased protein synthesis by increasing ribosome number (2). However, the mechanism by which pST treatment is able to elicit these effects has not been identified.
Treatment with pST increases circulating levels of both insulin and glucose (16), and it has been suggested that this is a consequence of insulin resistance to glucose uptake (27). However, the reduced effect of insulin on glucose disposal during pST treatment appears to occur as a result of decreased glucose uptake by adipocytes, whereas the insulin-stimulated glucose uptake in skeletal muscle is not blunted by pST (16, 52). In addition to the promotion of glucose uptake, insulin also stimulates muscle protein synthesis in skeletal muscle of growing animals (10, 40). Studies in rapidly growing untreated pigs have demonstrated that, when insulin is raised to the fed level, protein synthesis in skeletal muscle is enhanced, even when amino acids and glucose are maintained at the fasting level using pancreatic glucose-amino acid clamps (10, 40, 53). Therefore, in the present study we hypothesized that pST stimulates muscle protein synthesis indirectly by promoting increased circulating insulin concentrations.
The binding of insulin to its receptor on the cell surface initiates a cascade of events that lead to the activation of protein kinase B (PKB) and the master protein kinase mammalian target of rapamycin (mTOR) (26, 51). Activation of mTOR induces the phosphorylation of eukaryotic initiation factor (eIF)4E-binding protein-1 (4E-BP1), which in the unphosphorylated state binds to eIF4E, forming an inactive complex (36). Phosphorylation of 4E-BP1 leads to the disassociation of eIF4E from 4E-BP1, allowing eIF4E to associate with eIF4G to form, in conjunction with eIF4A, the active eIF4F complex (36), which mediates the binding of mRNA to the 40S ribosomal subunit (23). In addition, activated mTOR phosphorylates ribosomal protein S6 kinase-1 (S6K1), thereby activating ribosomal protein S6 (23), which had been thought to promote the translation of mRNAs that encode proteins that regulate translation (30). However, recent studies suggest that this activation is not required for regulating ribosomal protein translation (44). The phosphorylation of both 4E-BP1 and S6K1 increases the efficiency of translation initiation and, hence, promotes protein synthesis. In rapidly growing swine, insulin increases muscle protein synthesis by enhancing the activation of translation initiation factors that regulate the binding of mRNA to the ribosomal complex (40). In hepatoma cells in culture, somatotropin activates mTOR via phosphoinostide 3-kinase (PI3K) signaling, leading to an increase in the abundance of the active eIF4E·eIF4G complex (28).
This study aimed to determine whether the increase in skeletal muscle protein synthesis, observed in pigs chronically treated with pST, is a consequence of the pST-induced increase in circulating insulin levels. In addition, we wished to determine whether the mTOR signaling pathway modulates the observed changes. This study was performed in rapidly growing pigs (∼20 kg) treated with pST for 7–10 days and subjected to pancreatic glucose-amino acid clamps, where circulating insulin levels were manipulated to achieve those of fasted and fed control and pST-treated pigs.
MATERIALS AND METHODS
Animals and design.
Thirty-seven 8- to 10-wk-old crossbred (Landrance × Yorkshire × Hampshire × Duroc) female pigs (Agricultural Headquarters, Texas Department of Criminal Justice, Huntsville, TX) weighing 11 ± 0.22 kg were housed in individual cages. Since relatively high protein intakes are required to obtain the maximum promoting effects of pST (7), the animals were fed a 24% protein diet (Producers Cooperative Association, Bryan, TX) at 6% of their body weight and provided water ad libitum. Pigs were adjusted to the diet for 7 days and then randomly assigned to one of two treatment groups, either saline (control) or recombinant pST (gift of Dr. Frank Dunshea) at a rate of 150 μg·kg body wt−1·day−1 for 7–10 days. The dose of saline or pST was divided into two daily injections (75 μg/kg body wt) and was administered in the shoulder region. Body weights were measured daily, and the dietary intake and treatment doses were adjusted accordingly. Pigs treated with pST were offered the diet at 6% of their body weight per day, and control pigs were pair fed to the intake level of the pST-treated pigs to minimize any confounding effect of differences in food intake. Daily feed allowance was divided into two meals to coincide with time of injection.
Three to five days before infusions, the pigs (17.35 ± 0.25 kg) were fasted overnight, and the jugular vein and carotid artery of each pig were catheterized using sterile techniques under general anaesthesia (Aerrane; Anaquest, Madison, WI) as described previously (8). After surgery, pigs were returned to their cages and resumed their regular treatment regimen. The protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
Pancreatic glucose-amino acid clamps.
Control and pST-treated pigs (19.96 ± 0.23 kg) were fasted overnight (16 h) before the infusion studies. On the day of infusion, pigs were administered their daily dose of pST (150 μg/kg body wt) or saline 60 min before infusion. Clamps were performed using techniques similar to those previously described (40, 48, 53) (Fig. 1). Over a 30-min period before the initiation of the clamp, basal blood glucose (YSI 2300 STAT Plus; Yellow Springs Instruments, Yellow Springs, OH) and branched-chain amino acid (BCAA) concentrations were determined to establish the average fasting concentration to be used in the glucose amino acid clamp procedure (53). The clamp was initiated with a primed (10 μg/kg), continuous (40 μg·kg−1·h−1; Bachem, Torrance, CA) somatostatin infusion to suppress endogenous insulin secretion and which concurrently suppresses glucagon secretion. After a 15-min infusion of somatostatin, an infusion of replacement glucagon (150 ng·kg body wt−1·h −1; Eli Lilly, Indianapolis, IN) was initiated and continued to the end the clamp period. Insulin was infused at 18, 90, and 200 ng·kg−0.66·min−1 to reproduce the insulin concentrations normally present in the 1) fasting state of control and pST pigs (5 μU/ml, basal-insulin group), 2) fed state of control pigs (25 μU/ml, low-insulin group), and 3) fed state of pST pigs (50 μU/ml, high-insulin group) (48, 49). Each pig was continuously infused for 2 h; arterial blood samples (0.5 ml) were obtained every 5 min and immediately analyzed for glucose and BCAA concentrations. A 2.5-min enzymatic kinetic assay was used to determine total BCAA concentrations. Dextrose (Baxter Healthcare, Deerfield, IL) solution and a balanced amino acid mixture (10) were infused to maintain glucose and amino acid values at the baseline preclamp fasting level. Additional blood samples (5 ml) were collected at baseline and 120 min for measurement of circulating insulin, plasma urea nitrogen (PUN), IGF-I, somatotropin, and individual amino acid concentrations.
Fig. 1.

Pancreatic glucose-amino acid clamp protocol. Overnight-fasted control and porcine somatotropin (pST)-treated pigs were injected with saline or pST, respectively, 60 min before infusion. BCAA, branched-chain amino acids. Fifteen minutes (t = −15 min) before infusion, a primed, constant infusion of ST was initiated. At t = 0 min, infusions of replacement glucagon and insulin commenced; insulin was infused to reproduce fasting (5 μU/ml), fed control (25 μU/ml), or fed pST-treated (50 μU/ml) levels, while glucose and amino acids were clamped at baseline fasting levels. Fractional rates of protein synthesis were measured using a flooding dose of [3H]phenylalanine. Blood samples were collected at 5-min intervals to clamp glucose and amino acids, with additional samples taken at baseline and 2 h for hormone and substrate analysis.
Tissue protein synthesis in vivo.
The fractional rate of protein synthesis was measured with a flooding dose of l-[4-3H]phenylalanine injected 75 min after the initiation of the clamp procedure (22). Pigs were killed at 2 h, and samples of the longissimus dorsi muscle were collected and rapidly frozen. The specific radioactivity of the protein hydrolysate, homogenate supernatant, and blood supernatant were determined as described previously (9). Previous studies have demonstrated that, after a flooding dose of [3H]phenylalanine is administered, the specific radioactivity of tissue free phenylalanine is in equilibrium with the aminoacyl-tRNA specific radioactivity, and therefore the tissue free phenylalanine is a valid measure of the tissue precursor pool specific radioactivity (12).
Plasma hormones and substrates.
The concentrations of individual amino acids from frozen plasma samples obtained at −15 and 120 min of the insulin infusion were measured with an HPLC method (PICO-TAG reverse-phase column; Waters, Milford, MA) as previously described (13). Plasma radioimmunoreactive insulin and somatotropin concentrations were measured using porcine insulin and growth hormone radioimmunoassay kits (Linco, St. Louis, MO). PUN concentrations were measured by use of an end-point enzyme assay (Roche, Somerville, NJ) to determine the quantity of nitrogen in the urea. Plasma total IGF-I concentrations were measured with a two-site immunoradiometric assay with IGF-I-coated tubes (Diagnostic System Laboratories, Webster, TX).
Protein immunoblot analysis.
Proteins from longissimus dorsi muscle homogenates were separated on polyacrylamide gels (PAGE). For each assay, all samples were run at the same time on triple-wide gels (CBS Scientific C, Del Mar, CA) to eliminate interassay variation. Proteins were electrophoretically transferred to polyvinlidene difluoride transfer membranes (Pall, Pensicola, FL), which were incubated with appropriate primary antibodies, washed, and exposed to an appropriate secondary antibody, as previously described (14).
For normalization, immunoblots preformed with anti-phosphospecific antibodies were stripped in stripping buffer (Pierce Biotechnology, Rockford, IL) and reprobed with the corresponding nonphosphospecific antibodies. Blots were developed using an enhanced chemiluminescence kit (GE Health Sciences, Buckinghamshire, UK), visualized, and analyzed using a ChemiDoc-It Imaging System (UVP, Upland, CA). Primary antibodies that were used in the immunoblotting were PKB (total and Ser473, Cell Signaling Technology, Beverly, MA), 4E-BP1 (total; Bethyl Laboratories, Montgomery, TX, and Thr70, Cell Signaling), and S6K1 (total, Ser235/236, and Ser240/244, Cell Signaling).
Quantification of eIF4E·4E-BP1 and eIF4E·eIF4G complexes.
These complexes were immunoprecipitated using an anti-eIF4E monoclonal antibody (gift of Dr. Leonard Jefferson, Penn State University College of Medicine, Hershey, PA) from aliquots of fresh tissue homogenates (18). Briefly, samples were homogenized in seven volumes of buffer (in mM: 20 HEPES, 2 EGTA, 50 NaF, 100 KCl, and 0.2 EDTA, pH 7.4) containing Sigma P3840 Protease Inhibitor Cocktail (Sigma Chemical, St. Louis, MO) and centrifuged at 10,000 g for 10 min at 4°C. Supernatants were incubated overnight at 4°C with constant rocking with anti-eIF4E antibody. Immunoprecipates were recovered with goat anti-mouse IgG magnetic beads (Polysciences, Warrington, PA), washed, resuspended in sample buffer as described elsewhere (18), and immediately subjected to protein immunoblot analysis using rabbit anti-4EBP1 (Bethyl Laboratories) antibody or rabbit anti-eIF4G (Novus Biologicals, Littleton, CO). Amounts of 4E-BP1 and eIF4G were corrected by the eIF4E recovered from the immunoprecipitate.
Calculations and statistics.
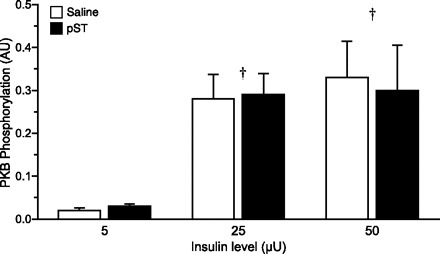
The fractional rate of protein synthesis (Ks, %protein mass synthesized in a day) was calculated as
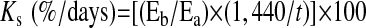 |
where Eb (in dpm/nmol) is the specific radioactivity of the protein-bound phenylalanine, Ea (in dpm/nmol) is the specific radioactivity of the tissue free phenylalanine at the time of tissue collection, t is the time of labeling in minutes, and 1,440 is the minutes-to-day conversion.
ANOVA was carried out using a general linear model to determine main statistical differences, including the interaction term. Between group analysis was performed using a t-test. Probability values of <0.05 were considered significant for all comparisons. Data are presented as means ± SE.
RESULTS
Plasma hormone and substrate concentrations.
The plasma hormone and substrate concentrations shown in Tables 1 and 2 are mean values obtained either at baseline or at the end of the infusion. To determine the effectiveness of pST treatment, circulating PUN, IGF-I, and somatotropin concentrations were measured. Treatment of pigs with pST reduced circulating levels of PUN by ∼30% (P < 0.001) compared with saline-treated controls (Table 1). Compared with saline-treated controls, pST-treated pigs demonstrated 63% (P = 0.001) and 2,600% (P < 0.001) increases in plasma IGF-I and somatotropin levels, respectively (Table 1).
Table 1.
Plasma hormone and substrate concentrations in control and pST-treated pigs at the beginning of the insulin clamp
| Saline | pST | |
|---|---|---|
| PUN, mg/dl | 12.2 ± 0.5 | 8.0 ± 0.4§ |
| IGF-1, ng/ml | 126.6 ± 29.2 | 290.1 ± 32.6§ |
| pST, ng/ml | 4.6 ± 2.8 | 119.6 ± 49.1§ |
Values are means ± SE; n = 11–13/treatment group. PUN, plasma urea nitrogen; IGF-I, insulin-like growth factor I; pST, porcine somatotropin.
P < 0.001 vs. saline-treated pigs. Significance determined by t-test analysis.
Table 2.
Plasma insulin, glucose, and amino acid concentrations in saline and pST-treated pigs during pancreatic-glucose-amino-acid clamps
| Treatment | Preclamp | Target Insulin Levels, μU/ml |
|||||
|---|---|---|---|---|---|---|---|
| 5 | 25 | 50 | |||||
| Insulin | Saline | 2.4 ± 0.5 | 2.7 ± 0.5 | 24.3 ± 2.9† | 42.3 ± 2.6† | ||
| μU/ml | pST | 3.6 ± 0.6 | 4.7 ± 1.0 | 20.4 ± 2.4† | 43.1 ± 2.7† | ||
| Glucose | Saline | 55.2 ± 1.9 | 67.0 ± 6.0† | 52.9 ± 3.8 | 62.4 ± 4.0 | ||
| mg/dl | pST | 64.2 ± 2.6* | 85.4 ± 8.6† | 59.3 ± 5.2 | 72.3 ± 3.7 | ||
| BCAA | Saline | 417.8 ± 22.3 | 414.8 ± 22.3 | 324.0 ± 19.8 | 372.7 ± 21.3 | ||
| nmol/ml | pST | 408.3 ± 19.7 | 362.7 ± 19.7 | 343.2 ± 21.5 | 354.7 ± 25.9 | ||
| EAA | Saline | 940.5 ± 46.7 | 1,067.5 ± 121.4 | 745.9 ± 58.8 | 1,018.7 ± 87.2 | ||
| nmol/ml | pST | 865.6 ± 66.0 | 798.3 ± 99.6 | 869.4 ± 85.0 | 870.7 ± 60.5 | ||
| NEAA | Saline | 2,011.4 ± 98.3 | 2,094.7 ± 191.5 | 2,066.2 ± 104.2 | 2,151.1 ± 66.0 | ||
| nmol/ml | pST | 1,960.9 ± 101.9 | 1,872.7 ± 191.5 | 2,249.5 ± 89.2 | 1,930.2 ± 113.8 | ||
| TAA | Saline | 2,951.9 ± 113.4 | 3,162.2 ± 290.4 | 2,812.0 ± 113.6 | 3,169.8 ± 145.5 | ||
| nmol/ml | pST | 2,826.5 ± 151.3 | 2,671.0 ± 270.4 | 3,119.0 ± 96.5 | 2,800.9 ± 159.7 | ||
Values are means ± SE; n = 5–7/treatment group. BCAA, branched-chain amino acids (Ile, Leu, Val); EAA, essential amino acids (Arg, His, Ile, Leu, Lys, Met, Thr, Trp, Val); NEAA, nonessential amino acids (Ala, Asp, Cit, Glu, Gln, Gly, Orn, Pro, Ser, Tau, Tyr); TAA, total amino acids (all EAA and NEAA). ANOVA indicated that pST treatment raised plasma glucose (P < 0.02), while the insulin infusion period altered glucose and insulin concentrations (P < 0.005). Results from paired t-test:
response in pST-treated pigs different from controls at individual infusion period (P < 0.05);
change during infusion period from preclamp value in either pST or control pigs (P < 0.05).
The effectiveness of the clamp in attaining the desired insulin, glucose, and amino acid concentrations is shown in Table 2. Target insulin levels of 1) fasting control and pST pigs (5 μU/ml), 2) fed levels of control pigs (25 μU/ml), and 3) fed levels of pST-treated pigs (50 μU/ml) during the 2-h clamp were largely achieved in all groups. Plasma glucose levels were for the most part maintained within 10% of fasting, preclamp levels at each insulin level. However, glucose levels in the fasting insulin groups (5 μU/ml) were higher than baseline levels (P < 0.05), even though additional glucose was not provided to these groups. Additionally, pigs treated with pST had higher glucose concentrations than control animals (P < 0.002) in all treatment periods (although this reached significance only in the preclamp phase). BCAA, essential (EAA), nonessential (NEAA), and, hence, total (TAA) amino acid concentrations were successfully maintained over the course of the infusion period by use of the amino acid clamp method (Table 2).
Glucose and amino acid disposal rates.
Net whole body glucose and amino acid disposal rates were calculated from the average infusion rate of dextrose and a balanced amino acid mixture required to maintain circulating levels within 10% of fasting baseline. Glucose disposal rates were altered by circulating insulin levels (P < 0.001); this was driven by the large increase in glucose uptake observed between 5 and 25 μU/ml (P < 0.001) of insulin (Fig. 2A). Additionally, raised plasma insulin levels increased whole body amino acid disposal rates in control and pST-treated animals at fed insulin concentrations (25 and 50 μU/ml), but there was no further increase between 25 and 50 μU/ml of insulin (Fig. 2B).
Fig. 2.
Whole body net glucose (A) and amino acid (B) disposal rates during pancreatic glucose-amino acid clamp in control and pST-treated pigs. Pigs were infused with insulin at 18, 90, and 200 ng·kg−0.66·min−1 to reproduce fasting (5 μU/ml), fed control (25 μU/ml), or fed pST-treated (50 μU/ml) levels, while glucose and amino acids were clamped at baseline fasting levels. Glucose disposal rates were calculated from the average rate of infusion of a dextrose solution over the last hour of infusion. Amino acid disposal rates were calculated from the average rate of infusion of a balanced amino acid solution required for maintenance of circulating BCAA at baseline fasting levels over the last hour of infusion. ANOVA indicated an insulin effect (P < 0.001) for both glucose and amino acid uptakes. Results of paired t-test: †response to insulin dose different from fasting (5 μU/ml, P < 0.05). Values are means ± SE; n = 5–7 per group.
Skeletal muscle protein synthesis.
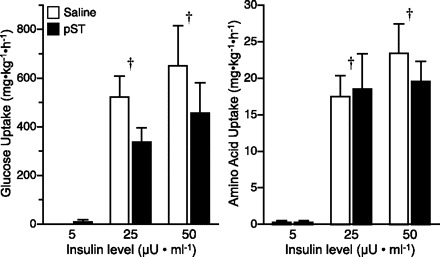
The fractional rate of skeletal muscle protein synthesis was increased by both insulin and pST treatment (P < 0.005; Fig. 3). Increasing circulating insulin concentrations to either 25 or 50 μU/ml increased fractional rates of protein synthesis by 20–25% compared with fasting insulin levels (5 μU/ml, P = 0.001). However, there was no significant difference between the two fed insulin levels (P > 0.15), indicating that maximal rates of protein synthesis may be achieved at or before 25 μU/ml. Pigs treated with pST had higher rates of protein synthesis in the skeletal muscle compared with those treated with saline only, and there was no interaction of the effect of pST and insulin.
Fig. 3.
Fractional rate of protein synthesis (Ks) in skeletal muscle at the end of a pancreatic glucose-amino acid clamp in control and pST-treated pigs. Pigs were infused with insulin at 18, 90, and 200 ng·kg−0.66·min−1 to reproduce fasting (5 μU/ml), fed control (25 μU/ml), or fed pST-treated (50 μU/ml) levels, while glucose and amino acids were clamped at baseline fasting levels. ANOVA indicates an insulin (P < 0.001) and a pST (P < 0.005) effect, but no interaction. Results of paired t-test: *response in pST-treated pigs different from controls at individual insulin dose (P < 0.05); †response to insulin dose different from fasting (5 μU/ml, P < 0.05). Values are means ± SE; n = 5–7 per group.
Translation initiation factors.
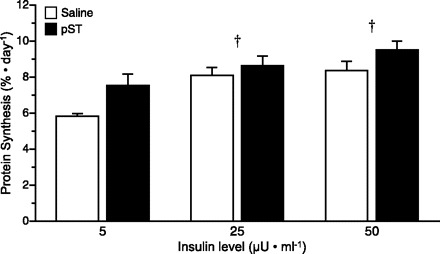
To determine whether chronic pST treatment increased skeletal muscle protein synthesis by promoting insulin-induced translation initiation, Western blot analysis was performed. PKB phosphorylation (Fig. 4) on Ser473 was used as an indicator of the activation of early steps in the insulin-signaling pathway. Raising insulin concentrations from fasting (5 μU/ml) to levels seen in fed animals (25 or 50 μU/ml) increased PKB phosphorylation (P < 0.001). However, doubling the insulin concentration from 25 to 50 μU/ml showed no additional effect on PKB phosphorylation. There was no significant difference on PKB phosphorylation between control and pST-treated animals, suggesting that pST does not stimulate protein synthesis via this pathway.
Fig. 4.
PKB phosphorylation in skeletal muscle at the end of a pancreatic glucose-amino acid clamp in control and pST-treated pigs. Pigs were infused with insulin at 18, 90, and 200 ng·kg−0.66·min−1 to reproduce fasting (5 μU/ml), fed control (25 μU/ml), or fed pST-treated (50 μU/ml) levels, while glucose and amino acids were clamped at baseline fasting levels. ANOVA indicates a significant effect of insulin (P < 0.001). Results of paired t-test: †response to insulin dose different from fasting (5 μU/ml, P < 0.05). Values are means ± SE; n = 5–7 per group.
Phosphorylation of PKB leads to the activation of mTOR, a master kinase, which phosphorylates both S6K1 and 4E-BP1 (26, 51). S6K1 phosphorylation on its Thr389 residue was increased when insulin concentrations were increased from fasting to fed levels (P < 0.001; Fig. 5A), but no additional stimulation was detected when insulin levels were doubled from 25 to 50 μU/ml. Phosphorylation of 4E-BP1 on Thr70 was also increased when the circulating insulin level was increased from fasting to fed levels (P < 0.001; Fig. 5B) with no additional stimulation measured when insulin levels were doubled from 25 to 50 μU/ml. The phosphorylation of 4E-BP1 causes the disassociation of the inactive 4E-BP1·eIF4E complex (36). Measurement of the abundance of the 4E-BP1·eIF4E complex revealed that increasing insulin concentration to fed levels was associated with a reduction in the abundance of this complex (P < 0.001; Fig. 6A). Disassociation of 4E-BP1·eIF4E allows eIF4E to be free to associate with eIF4G and form the active eIF4E·eIF4G complex (36). As predicted, fed levels of insulin showed a greater abundance of the active eIF4E·eIF4G complex compared with pigs maintained at fasting insulin concentrations (P < 0.001; Fig. 6B), with no additional stimulation detected when insulin levels were doubled from 25 to 50 μU/ml. There was no effect of pST treatment on S6K1 phosphorylation, 4E-BP1 phosphorylation, or 4E-BP1·eIF4E and eIF4E·eIF4G complex formation in skeletal muscle.
Fig. 5.
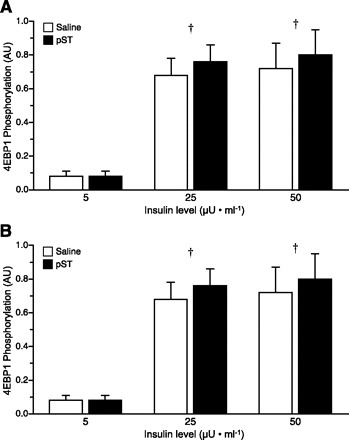
Ribosomal protein S6 kinase 1 (S6K1; A) and eukaryotic initiation factor (eIF)4B-binding protein-1 (4E-BP1; B) phosphorylation in skeletal muscle of pigs at the end of a pancreatic glucose-amino acid clamp in control and pST-treated pigs. Pigs were infused with insulin at 18, 90, and 200 ng·kg−0.66·min−1 to reproduce fasting (5 μU/ml), fed control (25 μU/ml), or fed pST-treated (50 μU/ml) levels, while glucose and amino acids were clamped at baseline fasting levels. ANOVA indicated an effect of insulin (P < 0.01) for both factors. Results of paired t-test: †response to insulin dose different from fasting (5 μU/ml, P < 0.05). Values are means ± SE; n = 5–7 per group.
Fig. 6.
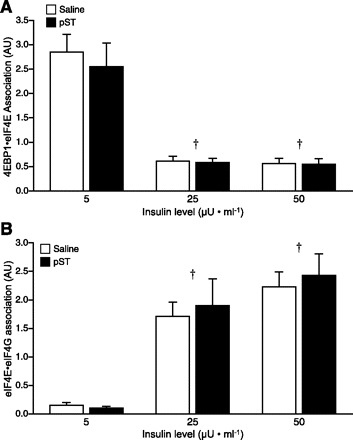
Association of the 4E-BP1·eIF4E (A) and eIF4G·eIF4E (B) complexes in skeletal muscle at the end of a pancreatic glucose-amino acid clamp in control and pST-treated pigs. Pigs were infused with insulin at 18, 90, and 200 ng·kg−0.66·min−1 to reproduce fasting (5 μU/ml), fed control (25 μU/ml), or fed pST-treated (50 μU/ml) levels, while glucose and amino acids were clamped at baseline fasting levels. ANOVA indicated an effect of insulin (P < 0.001) for both complexes. Results of paired t-test: †response to insulin dose different from fasting (5 μU/ml, P < 0.05). Values are means ± SE; n = 5–7 per group.
DISCUSSION
Treatment with pST for one wk increases the rate of skeletal muscle protein synthesis, but the mechanism by which this occurs has not yet been elucidated (2, 4–6). Chronic treatment with pST doubles postprandial circulating levels of insulin (16), a known promoter of protein synthesis in growing animals (10, 41). Insulin activates the insulin-signaling pathway by binding to its receptor, leading to a phosphorylation-signaling cascade that activates mTOR (26, 51). This master protein kinase promotes the formation of the active eIF4E·eIF4G complex and phosphorylation of S6K1, both of which are regulators of translation initiation and, thus, protein synthesis (23, 44). Therefore, the present study aimed to determine whether the pST-induced stimulation of skeletal muscle protein synthesis is a consequence of increased circulating insulin levels and, in turn, whether the elevated insulin promotes protein synthesis via increased translation initiation. This study suggests that the mechanism by which pST increases muscle protein synthesis is independent of the pST-associated increase in circulating insulin levels.
Plasma substrate concentrations.
Chronic treatment with pST is known to alter circulating levels of PUN and IGF-I (21, 49). Previous studies have demonstrated that treatment with pST reduces the activity of urea cycle enzymes (3) and, hence, urea synthesis (49). IGF-I levels have been shown to increase in response to pST treatment (2, 21, 25). The increase of 63% in pST-treated animals in the current study is slightly lower than previously reported (2, 49), the reason for this is not known. Taken together, the reduced PUN and increased IGF-I concentrations confirm the effectiveness of the pST treatment regimen.
Plasma glucose levels are elevated in response to chronic treatment with pST (24, 27, 52), and this rise may be the result of reduced tissue glucose uptake in response to pST-induced insulin resistance (15, 48). In this study, pigs treated with pST did not show a significant reduction in whole body glucose disposal compared with control animals. The lack of effect observed in the current study may be a result of the large variation associated with the measurement of glucose uptake. Alternatively, as the majority of studies investigating the effects of pST treatment are conducted in mature animals near market weight, differences in the body composition between those mature animals and the current rapidly growing pigs may account for the observations. Several studies have shown that insulin resistance in response to long-term pST stimulation occurs in adipose tissue (16, 52, 53), with little or no reported effect on skeletal muscle (17, 52). In pST-treated animals, the decreased response to insulin in adipose tissue may be a consequence of the redistribution of nutrients in pST-treated animals from fat accretion to protein deposition (17, 32). Consequently, mature animals may display a greater insulin resistance to glucose disposal due to higher levels of adipose tissue compared with younger rapidly growing pigs. Importantly, we also found that pigs treated with pST did not show an altered response of whole body amino acid disposal to insulin.
Protein synthesis rates.
In this study, treatment of pigs with pST increased muscle protein synthesis rates by ∼16% compared with saline-treated control animals. It was shown previously that pST treatment increases the efficiency with which nutrients are used for muscle mass gain by redistributing nutrients from fat to muscle (5, 6, 17, 19, 35, 48). Treatment with pST has been shown to increase whole body (24, 47) and skeletal muscle (5, 33, 45) protein synthesis rates; however, the mechanism through which this occurs is unknown. Previous studies conducted in our laboratory have shown that pST increases muscle protein synthesis in the fed state but not in the fasted state (2). Although pigs were fasted overnight in the current study, pST treatment increased the rate of muscle protein synthesis and there was no interactive effect of pST and insulin. Differences between studies may have arisen due to altered periods of pST treatment, i.e., 7–10 days in the current study vs. 7 days in the previous studies. Alternatively, the different measurement times of protein synthesis rates may have influenced the results; i.e., in the current study, pigs were killed and tissues frozen for analysis of protein synthesis rates 3 h after the last pST injection, whereas in the study by Bush et al. (2), tissue sampling occurred 8 h after the last injection. As pST levels peak 1–6 h postinjection (16), the main effect of pST on muscle protein synthesis in the fasting state may have occurred during this time window and declined by 8 h postinjection. However, even 8 h postinjection, pST treatment continued to promote skeletal muscle protein synthesis in animals maintained in the fed state in the previous study (2). Therefore, it appears that a pST-mediated increase in muscle protein synthesis in the fasting state may be transient and that this transient effect could be due to limited substrate availability to maintain increased rates of muscle protein synthesis in the fasted animal.
Postprandial insulin concentrations in pST-treated animals are double that observed in controls (16), and because insulin is a known promoter of skeletal muscle protein synthesis (40), this study investigated whether increased insulin levels alone could account for the rise in protein synthesis in pST-treated animals after feeding. While insulin did increase skeletal muscle protein synthesis in both control and pST-treated animals, pST did not further enhance protein synthesis rates. Furthermore, increasing circulating insulin levels from 25 μU/ml (as seen in fed controls) to 50 μU/ml (as seen in fed pST-treated animals) had no significant effect on protein synthesis rates in the skeletal muscle. This supports studies by Vann et al. (48), wherein raising insulin levels from 25 to 50 μU/ml, as in the current study, did not further enhance whole body protein synthesis. However, that study showed no stimulation of protein synthesis when insulin levels were raised from fasting to 25 μU/ml. Since that study was conducted at the whole body level, tissues such as the liver (2), which do not respond to insulin stimulation and have higher rates of protein synthesis than do muscle, may have obscured the stimulatory effect of insulin in skeletal muscle. Therefore, our results suggest that the pST-induced increase in protein synthesis rates is not a consequence of elevated circulating insulin levels.
Translation initiation.
Translation initiation rates can be altered by several anabolic stimuli, including amino acids, insulin, and IGF-I (11, 18, 20, 31). Anabolic agents have been shown to alter the formation of the active eIF4E·eIF4G complex, which aids the binding of the m7GTP cap to the 5′ end of the mRNA (37–39, 46). Insulin promotes activation of translation initiation via activation of mTOR involved signaling through the PI 3-kinase/PKB pathway (1, 50), which in turn activates the eIF4F-signaling cascade complex. In the current study, the phosphorylation of PKB was increased by insulin but not by pST. Increasing insulin concentrations from fasting to 25 μU/ml caused PKB phosphorylation to rise; however, further raising of insulin levels to 50 μU/ml was unable to further enhance phosphorylation of PKB, indicating that there may be a limit on the ability of insulin to stimulate the insulin-signaling cascade. As PKB is upstream of mTOR, it is not surprising that a similar pattern of phosphorylation was seen for the downstream translation effectors regulated by mTOR, i.e., 4E-BP1 and S6K1 (36, 46). As 4E-BP1 and S6K1 are well known substrates of mTOR activation, the phosphorylation of these two factors were taken as a measure of mTOR activity. Insulin increased 4E-BP1 and S6K1 phosphorylation and formation of the active eIF4E·eIF4G complex between fasting and 25 μU/ml, but raising insulin from 25 to 50 μU/ml insulin did not enhance this effect. This supports our protein synthesis data, which suggests that increased insulin levels in pST-treated animals does not account for the increase in protein synthesis in these animals. We (2) previously showed that pST treatment of growing pigs did not further enhance the effect of feeding on PKB or S6K1. However, in enterally fed growing pigs, pST treatment increased 4E-BP1 phosphorylation, and consequently eIF4E·eIF4G association was increased (2). The ability of feeding, but not insulin, to enhance eIF4E·eIF4G binding in pST-treated pigs suggests that maintaining glucose and amino acids at the fasting rather than the fed level may limit translation initiation. Alternatively, translation initiation and, hence, protein synthesis may be enhanced via an mTOR-independent pathway. Insulin is also known to promote the activity of eIF2B (42), an initiation factor involved in bringing the initiation codon to the ribosome (42). The activity of eIF2B was not measured in this study; however, in our previous work, eIF2B activity was increased by pST treatment but was unaffected by feeding (2).
A study conducted by Hayashi and Proud (28) in cell culture suggested that increased levels of protein synthesis in response to pST treatment are signaled through the mTOR pathway in hepatocytes. In that study, treatment of rat hepatoma cells with either wortmannin (a PI 3-kinase inhibitor) or rapamycin (an inhibitor of mTOR) blocked somatotropin-induced phosphorylation of 4E-BP1 and S6K1, suggesting that somatotropin activates the PI 3-kinase/mTOR pathway in the liver (28). However, the concentration of somatotropin used in that study was not reported. Also, as the study was conducted in hepatic cells, direct comparison to the current study is not possible, because differences in protein synthesis activation have been shown to occur in skeletal muscle and liver (2). However, activation of the PI 3-kinase/PKB pathway in the liver may not indicate a role for insulin in the activation of translation initiation by pST, as somatotropin binding to its own receptor causes phosphorylation of the Janus family tyrosine kinase-2 (34), which can activate PI 3-kinase (29, 43).
Perspectives.
The results of the current study demonstrate that treatment of growing pigs with pST for 7–10 days increases skeletal muscle protein synthesis independently of the pST-induced elevation in circulating insulin levels and the mTOR pathway. In the present study, increasing circulating insulin levels above those normally associated with feeding did not further increase the activation of indexes of translation initiation. Since mTOR is one conversion point for several anabolic and growth-signaling pathways and has been shown to be activated by somatotropin receptor activation in some tissues, a role of mTOR activation under differing nutritional conditions, such as in the presence of fed amino acid levels, deserves further investigation.
GRANTS
This work is a publication of the US Department of Agriculture, Agricultural Research Service (USDA/ARS) Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX. This project was supported by National Research Initiative Competitive Grant no. 2005-35206-15273 from the USDA Cooperative State Research, Education, and Extension Service.
The contents of this publication do not necessarily reflect the views or policies of the US Department of Agriculture, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Acknowledgments
We thank M. Fiorotto for helpful discussions, J. Fleming for technical assistance, D. Miller and J. Stubblefield for care of animals, E. O. Smith for statistical assistance, A. Gillum for graphics, and L. Weiser for secretarial assistance.
Present address of J. S. Jeyapalan: Pediatric Critical Care Medicine, PO Box 100296, Gainesville, FL 32610.
Present address of J. Frank: Dept. of Animal Science, University of Arkansas, Fayetteville, AR 72701.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Avruch J, Hara K, Lin Y, Liu M, Long X, Oritz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene 25: 6361–6372, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bush JA, Kimball SR, O'Connor PMJ, Suryawan A, Orellana RA, Nguyen HV, Jefferson LS, Davis TA. Translational control of protein synthesis in muscle and liver of growth hormone-treated pigs. Endocrinology 144: 1273–1283, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bush JA, Wu G, Suryawan A, Nguyen HV, Davis TA. Somatotropin-induced amino acid conservation in pigs involves differential regulation of liver and gut urea cycle enzyme activity. J Nutr 132: 59–67, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bush JA, Burrin DG, Suryawan A, O'Connor PMJ, Nguyen HV, Reeds PJ, Steele NC, Van Goudoever JB, Davis TA. Somatotropin-induced protein anabolism in hindquarters and portal-drained viscera of growing pigs. Am J Physiol Endocrinol Metab 284: E302–E312, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Caperna TJ, Campbell RG, Ballard MRM, Steele NC. Somatotropin enhances the rate of amino-acid deposition but has minimal impact on amino-acid balance in growing pigs. J Nutr 125: 2104–2113, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Caperna TJ, Komarek DR, Gavelek D, Steele NC. Influence of dietary protein and recombinant porcine somatotropin administration in young pigs: II. Accretion rates of protein, collagen, and fat. J Anim Sci 69: 4019–4029, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Caperna TJ, Steele NC, Komarek DR, McMurtry JP, Rosebrough RW, Solomon MB, Mitchell AD. Influence of dietary protein and recombinant porcine somatotropin administration in young pigs: growth, body composition and hormone status. J Anim Sci 68: 4243–4252, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab 270: E802–E809, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Davis TA, Fiorotto MF, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of sucking rats. Am J Physiol Regul Integr Comp Physiol 257: R1141–R1146, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PMJ. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282: E880–E890, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Davis TA, Fiorotto ML, Burrin DG, Vann RC, Reeds PJ, Nguyen HV, Beckett PR, Bush JA. Acute IGF-I infusion stimulates protein synthesis in skeletal muscle and other tissues of neonatal pigs. Am J Physiol Endocrinol Metab 283: E638–E647, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Davis TA, Fiorotto ML, Nguyen HV, Burrin DG. Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol Endocrinol Metab 277: E103–E109, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol Regul Integr Comp Physiol 265: R334–R340, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab 279: E1226–E1234, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Dominici FP, Turyn D. Growth hormone-induced alterations in the insulin-signaling system. Exp Biol Med 227: 149–157, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Dunshea FR, Bauman DE, Boyd RD, Bell AW. Temporal response of circulating metabolites and hormones during somatotropin treatment of growing pigs. J Anim Sci 70: 123–131, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Dunshea FR, Harris DM, Bauman DE, Boyd RD, Bell AW. Effect of porcine somatotropin on in vivo glucose kinetics and lipogenesis in growing pigs. J Anim Sci 70: 141–151, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab 290: E612–E621, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Etherton TD, Bauman DE. Biology of somatotropin in growth and lactation of domestic animals. Physiol Rev 78: 745–761, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Frank JW, Escobar J, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Dietary protein and lactose increase translation initiation factor activation and tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 290: E225–E233, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Frost RA, Nystrom GJ, Lang CH. Regulation of IGF-1 mRNA and signal transducers and activators of transcription-3 and -5 (STAT-3 and -5) by GH in C2C12 myoblasts. Endocrinology 143: 492–503, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Garlick PJ, Mcnurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J 192: 719–723, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev 15: 807–826, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Harrell RJ, Thomas MJ, Boyd RD, Czerwinski SM, Steele NC, Bauman DE. Effect of porcine somatotropin administration in young pigs during the growth phase from 10 to 25 kilograms. J Anim Sci 73: 3152–3160, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Harrell RJ, Thomas MJ, Boyd RD, Czerwinski SM, Steele NC, Bauman DE. Ontogenic maturation of the somatotropin/insulin-like growth factor axis. J Anim Sci 77: 2934–2941, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci 30: 35–42, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Haverstick LP, Rothkopf MM, Stanislaus G, Suchner U. Growth hormone, its effects on protein metabolism and clinical implications: a review. Top Clin Nutr 7: 52–62, 1991 [Google Scholar]
- 28.Hayashi A, Proud CG. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am J Physiol Endocrinol Metab 292: E1647–E1655, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Herrington J. and Carter-Su C. Signaling pathways activated by the growth hormone receptor. Trends Endocrin Met 12: 252–257, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Jefferies HB, Reinhard C, Kozma SC, Thomas G. Rapamycin selectively represses translation of the ‘polyprimidine tract' mRNA family. Proc Natl Acad Sci USA 91: 4441–4445, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeyapalan AS, Orellana R, Suryawan A, O'Connor PMJ, Nguyen HV, Escobar J, Frank J, Davis TA. Glucose stimulates protein synthesis in skeletal muscle of neonatal pigs through AMPK and mTOR independent process. Am J Physiol Endocrinol Metab 293: E595–E603, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ji S, Guan R, Frank SJ, Messina JL. Insulin inhibits growth hormone signaling via the growth hormone receptor/JAK2/STAT5B pathway. J Biol Chem 274: 13434–13442, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Johnston ME, Nelssen JL, Goodband RD, Kropf DH, Hines RH, Schricker BR. The effects of porcine somatotropin and dietary lysine on growth performance and carcass characteristics of finishing swine fed to 105 or 127 kilograms. J Anim Sci 71: 2986–2995, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Lang CH, Hong-Brown L, Frost RA. Cytokine inhibition of JAK-STAT signaling: a new mechanism of growth hormone resistance. Pediatr Nephrol 20: 306–312, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Lee KC, Azain MJ, Hausman DB, Ramsay TG. Somatotropin and adipose tissue metabolism: Substrate and temporal effects. J Anim Sci 78: 1236–1246, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Mamane Y, Petroulaskis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene 25: 6416, 2006 [DOI] [PubMed] [Google Scholar]
- 37.McKendrick L, Morley SJ, Pain VM, Jagus R, Joshi B. Phosphorylation of eukaryotic initiation 4E (eIF4E) at Ser209 is not required for protein synthesis in vitri and in vivo. Euro J Biochem 268: 5375–5385, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Minich WB, Balasta ML, Goss DJ, Rhodes RE. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic initiation factor eIf-4E; increased cap affinity of the phosphoryaltion form. Proc Natl Acad Sci USA 91277: 7668–7772, 1994. [DOI] [PMC free article] [PubMed]
- 39.Morley SJ, Ray M, Kay JE, Pain VM. Increased phosphorylation of eukaryotic initiation factor 4A during early activation of T lymphocytes correlates with increased initiation factor 4F complex formation. Euro J Biochem 218: 39–48, 1994 [DOI] [PubMed] [Google Scholar]
- 40.O'Connor PMJ, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 284: E110–E119, 2003 [DOI] [PubMed] [Google Scholar]
- 41.O'Connor PMJ, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 285: E40–E53, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans 34: 213–216, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Rosenfeld RG, Belgorosky A, Camacho-Hubner C, Savage MO, Wit JM, Hwa V. Defects in growth hormone receptor signaling. Trends Endocrin Met 18: 134–141, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31: 342–348, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Seve B, Ballevre O, Ganier P, Noblet J, Prugnaud J, Obled C. Recombinant porcine somatotropin and dietary protein enhance protein synthesis in growing pigs. J Nutr 123: 529–540, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Suryawan A, Escobar J, Frank JW, Nguyen HV, Davis TA. Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 291: E849–E859, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Tomas FM, Campbell RG, King RH, Johnson RJ, Chandler CS, Tavemer MR. Growth hormone increases whole-body protein turnover in growing pigs. J Anim Sci 70: 3138–3143, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Vann RC, Nguyen HV, Reeds PJ, Steele NC, Deaver DR, Davis TA. Somatotropin increases protein balance independent of insulin's effects on protein metabolism in growing pigs. Am J Physiol Endocrinol Metab 279: E1–E10, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Vann RC, Nguyen KV, Reeds PJ, Burrin DG, Fiorotto ML, Steele NC, Deaver DR, Davis TA. Somatotropin increases protein balance by lowering body protein degradation in fed, growing pigs. Am J Physiol Endocrinol Metab 278: E477–E483, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Rhodes CJ, Lawrence JC. Activation of mammalian target of rapamycin (mTOR) by insulin is associated with stimulation of 4EBP1 binding to dimeric mTOR complex 1. J Biol Chem 281: 24293–24303, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Wang XM, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology 21: 362–369, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Wray-Cahen D, Bell AW, Boyd RD, Ross DA, Bauman DE, Krick BJ, Harrell RJ. Nutrient uptake by the hindlimb of growing pigs treated with somatotropin and insulin. J Nutr 125: 125–135, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA. Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol Endocrinol Metab 275: E602–E609, 1998 [DOI] [PubMed] [Google Scholar]


