Abstract
Nontypeable Hemophilus influenzae (NTHi) is an important human pathogen in both children and adults. In children, it causes otitis media, the most common childhood infection and the leading cause of conductive hearing loss in the United States. In adults, it causes lower respiratory tract infections in the setting of chronic obstructive pulmonary disease, the fourth leading cause of death in the United States. The molecular mechanisms underlying the pathogenesis of NTHi-induced infections remain undefined, but they may involve activation of NF-κB, a transcriptional activator of multiple host defense genes involved in immune and inflammatory responses. Here, we show that NTHi strongly activates NF-κB in human epithelial cells via two distinct signaling pathways, NF-κB translocation-dependent and -independent pathways. The NF-κB translocation-dependent pathway involves activation of NF-κB inducing kinase (NIK)–IKKα/β complex leading to IκBα phosphorylation and degradation, whereas the NF-κB translocation-independent pathway involves activation of MKK3/6–p38 mitogen-activated protein (MAP) kinase pathway. Bifurcation of NTHi-induced NIK–IKKα/β-IκBα and MKK3/6–p38 MAP kinase pathways may occur at transforming growth factor-β activated kinase 1 (TAK1). Furthermore, we show that toll-like receptor 2 (TLR2) is required for NTHi-induced NF-κB activation. In addition, several key inflammatory mediators including IL-1β, IL-8, and tumor necrosis factor-α are up-regulated by NTHi. Finally, P6, a 16-kDa lipoprotein highly conserved in the outer membrane of all NTHi and H. influenzae type b strains, appears to also activate NF-κB via similar signaling pathways. Taken together, our results demonstrate that NTHi activates NF-κB via TLR2–TAK1-dependent NIK–IKKα/β-IκBα and MKK3/6–p38 MAP kinase signaling pathways. These studies may bring new insights into molecular pathogenesis of NTHi-induced infections and open up new therapeutic targets for these diseases.
The Gram-negative bacterium nontypeable Hemophilus influenzae (NTHi) is an important human pathogen in both children and adults (1). In children, it causes otitis media, the most common childhood infection and the leading cause of conductive hearing loss (2, 3), whereas in adults, it exacerbates chronic obstructive pulmonary diseases (COPD), the fourth leading cause of death in the United States (4, 5). Despite the need for prophylactic measures, development of a vaccine for preventing NTHi infections has been difficult and still remains a great challenge (1). Moreover, inappropriate antibiotic treatment contributes to the worldwide emergence of antibiotic-resistant strains of NTHi. Therefore, development of alternative therapeutic strategies is urgently needed for the treatment of NTHi infections based on understanding the molecular pathogenesis of NTHi infections.
Although much work has been done on identifying the surface molecules of NTHi for vaccine development (1, 2), the molecular mechanisms underlying the pathogenesis of NTHi infections still remain poorly understood. Like most other Gram-negative bacterial infections, both otitis media and chronic obstructive pulmonary diseases are characterized by inflammation, which is mainly mediated by inflammatory cytokines and chemokines such as IL-1β, IL-8, and tumor necrosis factor-α (6–8). Among a variety of transcription regulators, NF-κB has been shown to play a critical role in regulating the expression of large numbers of genes including cytokines, chemokines, and other mediators involved in inflammatory responses (9).
NF-κB is a dimeric transcription factor that is composed of p50 (NF-κB1) and p65 (RelA) subunits (9). In resting cells, NF-κB is retained in the cytoplasm but enters the nucleus in response to various stimuli including bacterial infections. Activation of NF-κB is controlled by an inhibitory subunit, IκB, which retains NF-κB in the cytoplasm. The activation of NF-κB requires sequential phosphorylation, ubiquitination, and degradation of IκB as well as consequent exposure of a nuclear localization signal on the NF-κB molecule. Multiple kinases have been shown to phosphorylate IκB at specific amino-terminal serine residues (10, 11). The most well studied kinases are IκB kinases, IKKα (or IKK-1) and IKKβ (or IKK-2) (9). There is also strong evidence that IKKα and IKKβ are themselves phosphorylated and activated by one or more upstream activating kinases. One such upstream kinase, NF-κB inducing kinase (NIK), was recently identified (9). Phosphorylation of IκB by the IKK pathway will eventually lead to the nuclear translocation of NF-κB, which, in turn, activates expression of target genes in the nucleus.
Based on the essential involvement of NF-κB in inflammatory responses, we hypothesized that NTHi induces inflammatory responses via activation of NF-κB. In the present study, we performed experiments to determine the involvement of NTHi in activation of NF-κB in human epithelial cells. Here, we show that NTHi indeed strongly activates NF-κB via activation of two distinct signaling pathways, NIK–IKKα/β–IκBα and MKK3/6–p38 mitogen-activated protein (MAP) kinase. Toll-like receptor 2 (TLR2)-dependent activation of transforming growth factor-β activated kinase 1 (TAK1) appears to mediate the activation of both signaling pathways. Furthermore, P6, a 16-kDa lipoprotein highly conserved in the outer membrane of all NTHi strains as well as in H. influenzae type b, which causes meningitis in children, also activates NF-κB via similar signaling pathways. These studies may lead to new therapeutic intervention for NTHi-induced infections including otitis media and chronic obstructive pulmonary diseases.
Materials and Methods
Reagents.
Caffeic acid phenethyl ester (CAPE), MG-132, and SB203580 were purchased from Calbiochem.
Bacterial Strains and Culture Conditions.
NTHi strain 12, a clinical isolate, was used in this study. For making NTHi crude extract, NTHi were harvested from a plate of chocolate agar after overnight incubation and incubated in 30 ml of brain heart infusion broth supplemented with nicotinamide adenine dinucleotide (3.5 μg/ml). After overnight incubation, NTHi were centrifuged at 10,000 × g for 10 min, and the supernatant was discarded. The resulting pellet of NTHi was suspended in 10 ml of PBS and sonicated. Subsequently, the lysate was collected and stored at −70°C. NTHi whole cell lysate was used in all of the experiments unless otherwise indicated. NTHi outer membrane protein P6 was purified as described (12).
Cell Culture.
Human cervix epithelial cell line HeLa and lung epithelial cell line A549 (ATCC) were maintained as described (29). Human middle ear epithelial cell line HMEEC-1, derived by human papilloma virus immortalization of primary human middle ear epithelial cells and primary human airway epithelial cells (Clonetics, San Diego), was maintained in bronchial epithelial basal medium (Clonetics) following manufacturer's instruction.
Reverse Transcription–PCR Analysis of TLR2.
Total RNA was isolated from human epithelial cells by using a Qiagen kit (Chatsworth, CA) following manufacturer's instruction. For the reverse transcriptase reaction, the Moloney murine leukemia virus preamplification system (Life Technologies, Rockville, MD) was used. PCR amplification was performed with Taq gold polymerase (Perkin–Elmer) for 32 cycles at 95°C for 60 s, 63°C for 60 s, and 72°C for 60 s (for TLR2) and 32 cycles at 95°C for 60 s, 50°C for 60 s, and 72°C for 60 s (for cyclophilin). The oligonucleotide primers were as follows: TLR2, 5′-GCCAAAGTCTTGATTGATTGG-3′ and 5′-TTGAAGTTCTCCAGCTCCTG-3′; cyclophilin, 5′-CCGTGTTCTTCGACATTGCC-3′ and 5′-ACACCACATGCTTGCCATCC-3′.
Plasmids, Transfections, and Luciferase Assays.
The expression plasmids IκBα, IκBα(S32/36A), NIK(K429/430A), fp38α(AF), fp38β2(AF), MKK3b(A), MKK6b(A), TAK1 mt, hTLR2, hΔTLR2, IKKα(K44M), and IKKβ(K49A) were previously described (14–19). The reporter construct NF-κB luc was generated as described (20). It contains three copies of the NF-κB site from the IL-2 receptor (α) promoter by using the following oligonucleotides: 5′-TCGAGACGGCAGGGGAATCTCCCTCTCCG-3′ and 3′-CTGCCGTCCCCTTAGAGGGAGAGGCAGCT-5′. The reporter construct was sequenced to verify number and orientation of inserted oligonucleotides. All transient transfections were carried out in triplicate by using TransIT-LT1 reagent (Panvera, Madison, WI) following manufacturer's instruction, unless otherwise indicated. In all cotransfections with expression plasmids of signaling molecules, an empty vector was used as a control. Transfected cells were pretreated with or without chemical inhibitors including CAPE, MG-132, and SB203580 for 1 h. NTHi or P6 was then added to the transfected cells 42 h after transfection. After 4 h, the cells were harvested for luciferase assay.
Immunofluorescent Staining.
Cells were cultured on four-chamber microscope slides. After NTHi treatment, the cells were fixed in paraformaldehyde solution (4%), incubated with mouse anti-p65 NF-κB mAb for 1 h (Santa Cruz Biotechnology). Primary antibody was detected with FITC-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology). Samples were viewed and photographed by using a Zeiss Axiophot microscope.
Western Blot Analysis.
Antibodies against phospho-IκBα (Ser-32), IκBα, phospho-p38 (Thr-180/182), p38, and phospho-activating transcription factor-2 were purchased from New England Biolabs. Rabbit polyclonal antiserum and a monoclonal antibody against P6 were developed as described previously (12, 21, 22). Phosphorylation of IκBα, p38, and activating transcription factor-2 was detected as described (14–16).
Assay for p38 Kinase Activity.
p38 kinase assay was carried out as described following the manufacturer's instruction. In brief, cells treated with or without NTHi were harvested in lysis buffer. The cell lysates were immunoprecipitated with anti-phospho-p38 MAP antibody (Thr-180/182), and the p38 MAP kinase activity was measured as described (15, 16).
Results and Discussion
NTHi Strongly Activates NF-κB in Human Epithelial Cells.
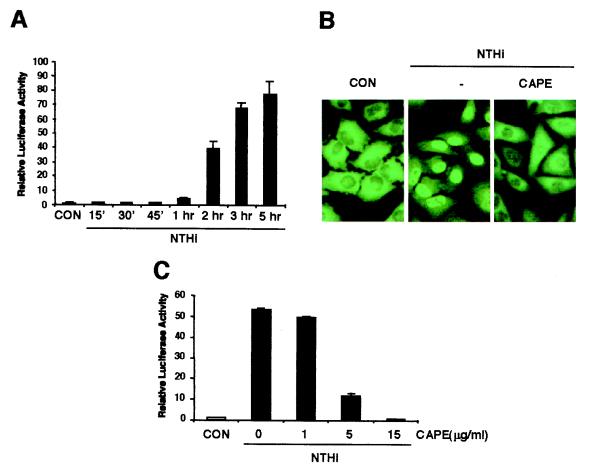
To determine whether NTHi activates NF-κB in human epithelial cells, we first measured NF-κB-dependent promoter activity by using luciferase reporter plasmid in HeLa cells. After stimulation with NTHi, the activity of this NF-κB-dependent promoter was greatly increased in a time-dependent manner (Fig. 1A). Because nuclear translocation is a key step for NF-κB to exert its transcriptional activity (9), we next sought to explore the possibility that NTHi activates NF-κB by inducing its nuclear translocation by using CAPE, a chemical inhibitor that is known to specifically block the translocation of p65 without affecting IκBα degradation (23). As expected, CAPE treatment markedly blocked the nuclear translocation of NF-κB induced by NTHi (Fig. 1B). As shown in Fig. 1C, CAPE indeed abrogated NTHi-induced NF-κB activation in a dose-dependent manner. A complete inhibition was observed with 15 μg/ml CAPE. These results suggest that NTHi activates NF-κB by inducing its nuclear translocation in HeLa cells. Because NTHi is a major pathogen of airway and middle ear infections (1–5), we also assayed A549 (human airway epithelial) and HMEEC-1 (human middle ear epithelial) as well as primary human bronchial epithelial cells. Interestingly, NTHi-induced NF-κB translocation and activation was observed in all of these cells (data not shown).
Figure 1.
NTHi activates NF-κB in human epithelial cells. (A) NTHi activates NF–κB-dependent transcriptional activity. HeLa cells were transiently transfected with an NF-κB-regulated luciferase reporter construct and stimulated with NTHi for various times. Luciferase activity was then assessed in NTHi-treated and untreated cells. (B) CAPE blocks NTHi-induced NF-κB translocation. (C) CAPE inhibits NTHi-induced NF-κB activation. Values are the means ± SD; n = 3. Similar results were also observed in A549, HMEEC-1, and primary human airway epithelial cells.
NIK–IKKα/β-Dependent IκBα Phosphorylation and Degradation Is Involved in NTHi-Induced NF-κB Nuclear Translocation and Activation.
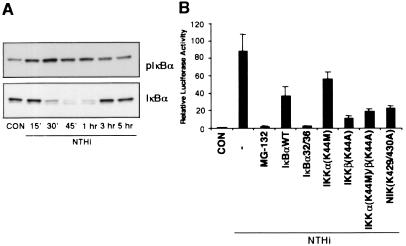
NF-κB is normally present in the cytoplasm in an inactive state and is bound to members of the IκB inhibitor protein family, chiefly IκBα. In this complex, IκBα blocks the nuclear localization signal, thus preventing nuclear translocation. To translocate NF-κB into the nucleus, the cytoplasmic NF-κB:IκBα complex needs to be disrupted (9). A common pathway to achieve disruption of this complex is based on specific phosphorylation of IκBα and the degradation of the phosphorylated IκBα protein by proteasomes. The inhibition of proteasome activity by specific inhibitors such as MG-132 prevents the degradation of IκBα and thus prevents the nuclear translocation of NF-κB (24). To determine the involvement of IκBα phosphorylation and degradation in NTHi-induced NF-κB translocation and activation, we first investigated whether NTHi induces phosphorylation and degradation of IκBα. Phosphorylation and degradation of IκBα was determined by Western blot analysis by using antibodies against phosphorylated and total IκBα, respectively. Fig. 2A shows phosphorylation and degradation of IκBα in HeLa cells treated with NTHi for various times. The IκBα phosphorylation became evident at 15 min, peaked at 30 min, and declined thereafter. Consistent with this, the NTHi-induced degradation of IκBα was observed at 30 min and returned to base line level by 3 h. We next sought to determine the requirement of IκBα degradation by assessing the effect of proteasome inhibitor MG-132 on NTHi-induced NF-κB nuclear translocation and activation. As expected, MG-132 completely blocked NTHi-induced NF-κB translocation (data not shown). Concomitantly, the transcriptional activity of NF-κB is also inhibited by MG-132 (Fig. 2B). To further confirm the involvement of IκBα degradation, we transfected the cells with either a wild-type or a transdominant mutant (S32A, S36A) of IκBα in which two critical serine residues required for inducer-mediated phosphorylation were mutated (19). As seen in Fig. 2B, overexpression of both wild-type and mutant IκBα greatly inhibited NTHi-induced NF-κB activation. These observations implicate the involvement of IκBα phosphorylation and degradation in NTHi-induced NF-κB translocation and activation.
Figure 2.
NIK–IKKα/β-dependent IκBα phosphorylation and degradation is involved in NTHi-induced NF-κB translocation and activation. (A) NTHi induces IκBα phosphorylation and degradation. (B) MG-132, overexpression of wild-type and a transdominant mutant of IκBα (S32A, S36A), and dominant-negative mutants of IKKα (K44M), IKKβ (K44A), and NIK (K429/430A) inhibit NTHi-induced NF-κB activation. Values are the means ± SD; n = 3.
Recently, the IκBα kinases IKKα and IKKβ have been shown to phosphorylate IκB at specific amino-terminal serine residues (11). There is strong evidence that IKKα and IKKβ are phosphorylated and activated by upstream kinase NF-κB inducing kinase (NIK) (9). On the basis of this recent report, we investigated the role of the NIK–IKK pathway in NTHi-induced NF-κB activation. Cotransfection with a dominant-negative mutant form of IKKβ [IKKβ(K44A)] markedly inhibited the NTHi-induced NF-κB activation, whereas overexpression of a dominant-negative mutant of IKKα [IKKα(K44M)] only showed partial inhibition (Fig. 2B). Likewise, overexpression of a dominant-negative mutant form of NIK [NIK(K429/430A)] also inhibited NTHi-induced NF-κB activation (Fig. 2B). Taken together, these findings clearly demonstrate that NTHi activates NF-κB via activation of NIK–IKKα/β-dependent IκBα phosphorylation and degradation, which, in turn, leads to NF-κB nuclear translocation.
Activation of MKK3/6–p38 MAP Kinase Pathway Is Required for NTHi-Induced NF-κB Activation via an NF-κB Translocation-Independent Mechanism.
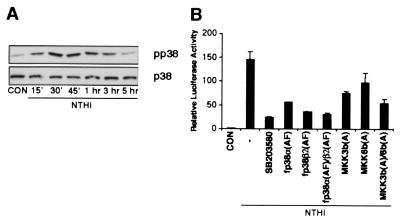
Many cellular stress stimuli can activate both NF-κB and p38 MAP kinase modules (25–29). Because of this overlap, we explored the possibility that activation of p38 is also involved in NTHi-induced NF-κB activation. We first investigated whether NTHi activates p38 MAP kinase. Phosphorylation of p38 MAP kinase was determined by Western blot analysis by using antiphosphorylated p38 MAP kinase. Fig. 3A shows phosphorylation of p38 MAP kinase in HeLa cells treated with NTHi for various times. The highest level of p38 MAP kinase phosphorylation was observed between 30 and 45 min and declined thereafter. The NTHi-induced activation of p38 MAP kinase was further confirmed by performing p38 MAP kinase assay. Activating transcription factor-2, an immediate downstream transcription factor of p38 MAP kinase, was strongly phosphorylated by p38 MAP kinase (data not shown). These results indicate that NTHi also strongly activates p38 MAP kinase in addition to NF-κB. We next sought to determine whether activation of p38 MAP kinase is required for NTHi-induced NF-κB-dependent transcription. As shown in Fig. 3B, the pyridinyl imidazole SB203580, a specific inhibitor for p38 MAP kinase, abrogated NF-κB-dependent gene transcription in response to NTHi (30). Moreover, overexpression of a dominant-negative mutant form of either p38α [fp38α(AF)] or p38β [fp38β2(AF)] also inhibited NTHi-induced NF-κB-dependent transcription, confirming the involvement of p38 MAP kinase in NTHi-induced NF-κB activation (Fig. 3B).
Figure 3.
Activation of MKK3/6–p38 MAP kinase pathway is required for NTHi-induced NF-κB activation by a NF-κB translocation-independent mechanism. (A) NTHi induces p38 MAP kinase phosphorylation. (B) SB203580, overexpression of dominant-negative mutants of p38α (AF), p38β2 (AF), MKK3b (A), and MKK6b (A) inhibit NTHi-induced NF-κB activation. Values are the means ± SD; n = 3.
As upstream activators of p38 MAP kinase, two kinases of the MAP kinase superfamily, MKK3 and MKK6, have been identified. It has been demonstrated that MKK6 is a common activator of p38α and p38β, whereas MKK3 activated only p38α (26). To further investigate whether activation of MKK3/6 is also involved in NTHi-induced NF-κB activation, a dominant-negative mutant form of either MKK3 [MKK3b(A)] or MKK6 [MKK6b(A)] was cotransfected into HeLa cells. The NTHi-induced NF-κB activation was inhibited (Fig. 3B). Collectively, these data demonstrate that activation of MKK3/6–p38α/β MAP kinase pathway is also required for NTHi-induced NF-κB activation.
On the basis of the evidence that nuclear translocation of NF-κB is a key step in stress induced-NF-κB activation and that activation of MKK3/6–p38α/β MAP kinase pathway is required for NTHi-induced NF-κB-dependent transcription, we therefore investigated the possibility that MKK3/6–p38α/β MAP kinase pathway mediates NTHi-induced NF-κB activation via induction of NF-κB nuclear translocation. Surprisingly, neither SB203580 nor overexpression of dominant-negative mutant forms of p38α/β MAP kinases and MKK3/6 blocked NF-κB nuclear translocation, suggesting that MKK3/6–p38α/β MAP kinase pathway mediates NTHi-induced NF-κB activation by NF-κB translocation-independent mechanisms (data not shown). Our results thus raised the possibility that phosphorylated p38 MAP kinase might directly interact with NF-κB in the nucleus. To test this hypothesis, we first investigated whether NTHi induces nuclear translocation of p38 MAP kinase by using rhodamine staining of phosphorylated p38 MAP kinase. When cells were stimulated with NTHi, phosphorylated p38 MAP kinase was indeed translocated to the nucleus (data not shown). We next sought to examine the direct interaction between p38 MAP kinase and NF-κB p65 subunit. Cells were either treated with NTHi or left untreated; lysates were immunoprecipitated with antibody against phosphorylated p38 MAP kinase, and the immunoprecipitated proteins were examined by immunoblot analysis by using antibody against NF-κB p65. Interestingly, NF-κB p65 was not found to be directly associated with phosphorylated p38 MAP kinase in the nucleus (data not shown). Thus, the molecular mechanism linking p38 MAP kinase to NF-κB activation is likely to involve additional, as yet undefined, signaling molecules.
Bifurcation of NTHi-Induced NIK–IKKα/β–IκBα and MKK3/6–p38 MAP Kinase Signaling Pathways Occurs at TAK1.
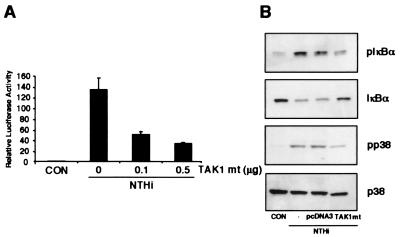
Having identified two independent NIK–IKKα/β–IκBα and MKK3/6–p38 MAP kinase signaling pathways involved in NTHi-induced NF-κB activation, still unknown is which signaling molecule acts upstream of both signaling pathways in NTHi-induced NF-κB activation. Despite extensive analysis of both NIK–IKKα/β–IκBα and MKK3/6–p38 MAP kinase signal transduction pathways, the signaling events upstream of both pathways still remain poorly understood. We took advantage of a recent report that TAK1, a member of the MAP 3-kinase family, can activate both the NIK–IKKα/β–IκBα and the MAP kinase cascade in the IL-1 signaling pathway and therefore investigated whether TAK1 is also involved in NTHi-induced NF-κB activation via similar signaling pathways (18). As shown in Fig. 4A, overexpression of a dominant-negative mutant of TAK1 (TAK1 mt, K63W) inhibited NTHi-induced NF-κB activation in a dose-dependent manner. We next sought to determine whether TAK1 acts upstream of both signaling pathways. Indeed, cotransfecting cells with the same dominant-negative TAK1 mutant also inhibited NTHi-induced phosphorylation of both IκBα and p38 MAP kinase (Fig. 4B). We conclude from these data that bifurcation of NTHi-induced NIK–IKKα/β–IκBα and MKK3/6–p38 MAP kinase signaling pathways occurs at TAK1.
Figure 4.
Bifurcation of NTHi-induced NIK–IKKα/β–IκBα and MKK3/6–p38 MAP kinase signaling pathways occurs at TAK1. (A) Overexpression of a dominant-negative mutant of TAK1 (K63W) abrogates NTHi-induced NF-κB activation. (B) Overexpression of a dominant-negative mutant of TAK1 (K63W) inhibits NTHi-induced phosphorylation of both IκBα and p38 MAP kinase.
Toll-Like Receptor 2 Is Involved in NTHi-Induced NF-κB Activation.
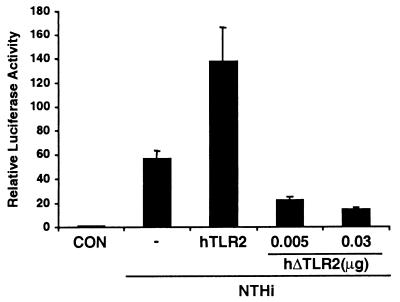
One key issue that has yet to be addressed is which cell surface receptor(s) is involved in transmitting signals from cell surface to the cytoplasm in NTHi-induced NF-κB activation. In review of the host surface receptors involved in bacterial pathogenesis, human toll-like receptors (TLRs) have been suggested to play a critical role in the recognition of various bacterial components, such as lipoprotein, peptidoglycan, lipoteichoic acid, and lipopolysaccharide (31–33). Toll-like receptors are mammalian homologues of the Drosophila toll receptor protein (34). The Drosophila protein Toll was first described as being important for the establishment of dorsoventral polarity in the developing embryo, but in adults Toll is involved in antifungal defenses. Another Drosophila member, 18-Wheeler (18-W), participates in antibacterial responses. Recently, mammalian homologues of Toll have been cloned and designated TLRs. To date, at least nine mammalian TLRs have been described, but only TLR2 and TLR4 have any known function (31–33). Based on the evidence that TLR2 has been implicated in cellular responses to bacterial products and also has engaged in signaling pathways leading to NF-κB activation, we hypothesized that TLR2 is involved in NTHi-induced NF-κB activation (17, 35, 36). We first analyzed the expression of TLR2 in human epithelial cells including HeLa, HMEEC-1, and primary human airway epithelial cells by reverse transcription–PCR. TLR2 appears to be expressed in all three epithelial cells (data not shown). We next sought to determine whether TLR2 is involved in NTHi-induced NF-κB activation. As shown in Fig. 5, cotransfection of HeLa cells with a dominant-negative mutant of TLR2 reduced NTHi-induced NF-κB activation by ≈70–80%. Consistent with this finding, overexpression of wild-type TLR2 enhanced NTHi-induced NF-κB activation by ≈2 to 3-fold. These results suggest that TLR2 is involved in NTHi-induced NF-κB activation in HeLa cells, although our data do not preclude the involvement of other TLRs.
Figure 5.
Toll-like receptor 2 is involved in NTHi-induced NF-κB activation. Cotransfection of HeLa cells with a dominant-negative mutant of TLR2 inhibits NTHi-induced NF-κB activation, whereas overexpression of a wild-type TLR2 enhances NF-κB activation. Values are the means ± SD; n = 3.
P6, a Major Outer Membrane Protein in NTHi, also Induces NF-κB Activation via the Same TLR2–TAK1-Dependent NIK–IKKα/β–IκBα and MKK3/6–p38 MAP Kinase Signaling Pathways.
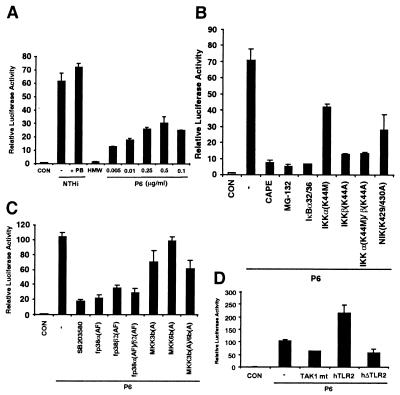
In our studies, whole cell crude extract from sonicated NTHi was used in all of the experiments described above. One unresolved question is which components of NTHi are responsible for the activation of NF-κB. Because our data showed that TLR2 is involved in NTHi-induced NF-κB activation and the best-known ligand for TLR2 is bacterial lipoprotein, it is therefore reasonable to first determine whether NTHi lipoproteins are involved in NF-κB activation (31–33, 35, 36). Among the known NTHi lipoproteins, P6, a 16-kDa outer membrane protein, has been shown to be an important lipoprotein highly conserved on the surface of all strains of NTHi and H. influenzae type b (1, 12, 13). We first performed Western blot analysis by using a monoclonal antibody against NTHi P6 to assure that P6 is present in the NTHi crude extract used in our study. As expected, P6 was indeed found in NTHi crude extract (data not shown). To determine whether P6 induces NF-κB activation, we next measured NF-κB-dependent promoter activity by using luciferase reporter in HeLa cells. On stimulation with purified P6 protein, the NF-κB-dependent transcription greatly increased in a dose-dependent manner (Fig. 6A). The NF-κB activation induced by P6 (0.25 μg/ml) is ≈45–50% of the response induced by NTHi. In contrast to the potent NF-κB inducing activity of P6, the high molecular weight proteins (HMW1 and HMW2) that represent another important group of NTHi outer membrane proteins did not induce NF-κB activation (13). Thus, our results provided evidence that NTHi lipoprotein P6 may be, at least in part, responsible for NTHi-induced NF-κB activation. As lipooligosaccharide (LOS) has been shown to play an important role in inflammatory response and the NTHi crude extract used in our studies does contain LOS, we performed additional experiments to determine the role of LOS in NTHi-induced NF-κB activation. Interestingly, removal of LOS from NTHi crude extract by using polymyxin B did not attenuate NTHi-induced NF-κB activation (Fig. 6A).
Figure 6.
P6 also induces NF-κB activation via the same TLR2–TAK1-dependent NIK–IKKα/β–IκBα and MKK3/6–p38 MAP kinase signaling pathways. (A) P6, but not LOS and HMW, induces NF-κB activation. HeLa cells were stimulated with either NTHi or polymyxin B-pretreated NTHi, or HMW (1.0 μg/ml) or P6 (0.005–0.5 μg/ml). PB, polymyxin B. (B) Inhibition of NIK–IKKα/β–IκBα signaling pathway blocks P6-induced NF-κB activation. (C) Inhibition of MKK3/6–p38 MAP kinase pathway abrogates P6-induced NF-κB activation. (D) Overexpression of a dominant-negative mutant of either TAK1 or TLR2 inhibits P6-induced NF-κB activation, whereas overexpression of a wild type of TLR2 enhances P6-induced NF-κB activation. Values are the means ± SD; n = 3.
We next sought to determine whether P6 activates NF-κB via the same TLR2–TAK1-dependent NIK–IKKα/β–IκBα and MKK3/6–p38 MAP kinase signaling pathways. We first examined the involvement of the NIK–IKKα/β–IκBα pathway by using CAPE, the specific inhibitor for NF-κB translocation, and MG-132, the specific inhibitor for IκBα degradation, or by cotransfecting the cells with a transdominant mutant of IκBα or a dominant-negative mutant of IKKα, or IKKβ or NIK, respectively. As shown in Fig. 6B, P6-induced NF-κB activation was inhibited by all of the treatments. Similarly, P6-induced NF-κB activation was also inhibited either by SB203580, a specific inhibitor for p38 MAP kinase, or by overexpressing a dominant-mutant of p38 MAP kinase α and β, or MKK3/6 (Fig. 6C). Moreover, cotransfecting the cells with a dominant-negative mutant of either TLR2 or TAK1 inhibited P6-induced NF-κB activation (Fig. 6D). Concomitantly, overexpression of wild-type TLR2 greatly enhanced P6-induced NF-κB activation. Together, our data suggest that NTHi lipoprotein P6 activates NF-κB also via TLR2–TAK1-dependent NIK–IKKα/β–IκBα and MKK3/6–p38 MAP kinase signaling pathways, providing additional evidence for the involvement of P6 in NTHi-induced NF-κB activation.
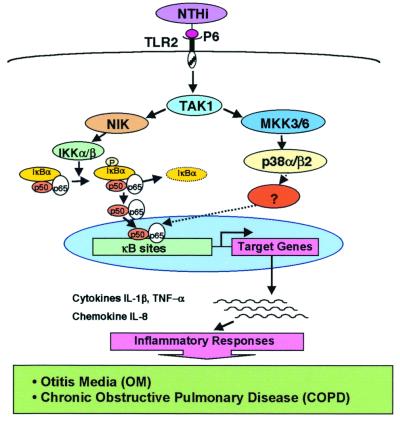
In conclusion, our studies demonstrate that NTHi, a major human pathogen of otitis media and COPD, strongly activates NF-κB in human epithelial cells (Fig. 7). NTHi-induced NF-κB activation is mediated by two distinct signaling pathways, NF-κB translocation-dependent and -independent pathways. The NF-κB translocation-dependent pathway involves NIK–IKKα/β-dependent phosphorylation and degradation of IκBα, whereas the NF-κB translocation-independent pathway involves activation of MKK3/6–p38 MAP kinase pathway. No direct physical interaction was found between phosphorylated p38 MAP kinase and NF-κB p65, although nuclear translocation of phosphorylated p38 MAP kinase was indeed observed, suggesting the involvement of additional, as yet undefined, intermediate signaling events. Overexpression of a dominant-negative mutant of TAK1 blocked not only the NTHi-induced NF-κB activation but also the phosphorylation of both p38 MAP kinase and IκBα, indicating that bifurcation of NTHi-induced NIK–IKKα/β–IκBα and MKK3/6–p38 MAP kinase signaling pathways occurs at TAK1. Moreover, transfecting the cells with a dominant-negative mutant of TLR2 abolished the NTHi-induced NF-κB activation, whereas overexpressing a wild-type TLR2 enhanced NTHi-induced NF-κB activation, providing direct evidence for the involvement of TLR2 in NTHi-induced NF-κB activation. Finally, P6, a 16-kDa outer membrane lipoprotein highly conserved in all NTHi and H. influenzae type b strains, may be partially responsible for NTHi-induced NF-κB activation. It should be noted that NTHi-induced NF-κB activation indeed leads to up-regulation of a variety of key inflammatory mediators including cytokines IL-1β and tumor necrosis factor-α as well as chemokine IL-8 (data not shown).
Figure 7.
Schematic representation of NTHi-induced signal transduction pathways involved in NF-κB activation in human epithelial cells.
Our results may have several important implications. First, understanding the signaling mechanisms involved in NTHi-induced NF-κB activation may bring new insights into the molecular pathogenesis of NTHi infections because the molecular mechanisms by which NTHi causes otitis media and COPD have remained largely unknown (1, 3–6, 37). Second, our finding that Gram-negative bacterium NTHi uses host TLR2 for transmitting signals across a cell membrane should enhance our understanding of bacteria-induced receptor signaling mechanisms in epithelial cells because TLR2 has been shown previously to mediate mainly the host response to Gram-positive bacteria, and little is known about TLR signaling in epithelial cells (20). Finally, our findings may lead to the development of novel therapeutic strategies for NTHi infections including otitis media and COPD (38, 39). In our studies, we have identified TLR2–TAK1-dependent NIK–IKKα/β–IκBα and MKK3/6–p38 MAP kinase signaling pathways to be involved in NTHi-induced NF-κB activation. Possibly of major interest will be the experimental evidence for the involvement of outer membrane protein P6 and TLR2 because of their major, if not exclusive, involvement in the pathogenesis of diseases (1, 5, 31–33).
Acknowledgments
We thank Dr. Ali Andalibi for critically reading this manuscript. This work was supported in part by National Institutes of Health Grants DC04562 (to J.D.L.), AI19641 (to T.F.M.), and AI41637 (to J.H.) and the Henry L. Guenther Foundation (to D.J.L. and J.D.L.).
Abbreviations
- CAPE
caffeic acid phenethyl ester
- COPD
chronic obstructive pulmonary disease
- IKK
IκB kinase
- MAP
mitogen-activated protein
- MKK
MAP kinase kinase
- NIK
NF-κB inducing kinase
- NTHi
nontypeable H. influenzae
- TAK1
transforming growth factor-β activated kinase 1
- TLR2
toll-like receptor 2
References
- 1.Foxwell A R, Kyd J M, Cripps A W. Microbiol Mol Biol Rev. 1998;62:294–308. doi: 10.1128/mmbr.62.2.294-308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy T F. Pediatr Infect Dis J. 2000;19, Suppl. 5:S9–S15. doi: 10.1097/00006454-200005001-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone C D. N Engl J Med. 1982;306:1399–1404. doi: 10.1056/NEJM198206103062305. [DOI] [PubMed] [Google Scholar]
- 4.ATS statement. Am J Respir Crit Care Med. 1995;152, Suppl. 5:78S–121S. [Google Scholar]
- 5.Murphy T F. Semin Respir Infect. 2000;15:41–51. doi: 10.1053/srin.2000.0150041. [DOI] [PubMed] [Google Scholar]
- 6.Bluestone C D. Pediatr Infect Dis J. 2000;19, Suppl. 5:S37–S46. doi: 10.1097/00006454-200005001-00007. [DOI] [PubMed] [Google Scholar]
- 7.Di Mango E, Ratner A J, Bryan R, Tabibi S, Prince A. J Clin Invest. 1998;101:2598–2605. doi: 10.1172/JCI2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melhus A, Ryan A F. Infect Immun. 2000;68:4024–4031. doi: 10.1128/iai.68.7.4024-4031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatada E N, Krappmann D, Scheidereit C. Curr Opin Immunol. 2000;12:52–58. doi: 10.1016/s0952-7915(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 10.Stancovski I, Baltimore D. Cell. 1997;91:290–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 11.Karin M. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 12.Karalus R, Murphy T F. FEMS Immunol Med Microbiol. 1999;26:159–166. doi: 10.1111/j.1574-695X.1999.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 13.Barenkamp S J. J Infect Dis. 1992;165, Suppl. 1:181–184. doi: 10.1093/infdis/165-supplement_1-s181. [DOI] [PubMed] [Google Scholar]
- 14.Lin X, Mu Y, Cunningham E T, Jr, Marcu K B, Geleziunas R, Greene W C. Mol Cell Biol. 1998;18:5899–5907. doi: 10.1128/mcb.18.10.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Li Z, Schwarz E M, Lin A, Guan K, Ulevitch R J, Han J. J Biol Chem. 1997;272:11096–11102. doi: 10.1074/jbc.272.17.11096. [DOI] [PubMed] [Google Scholar]
- 16.Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, Ulevitch R J, Nemerow G R, Han J. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- 17.Yang R B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Nature (London) 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 18.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. Nature (London) 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 19.Beauparlant P, Kwon H, Clarke M, Lin R, Sonenberg N, Wainberg M, Hiscott J. J Virol. 1996;70:5777–5785. doi: 10.1128/jvi.70.9.5777-5785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro V S, Mollenauer M N, Greene W C, Weiss A. J Exp Med. 1996;184:1663–1669. doi: 10.1084/jem.184.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy T F, Nelson M B, Dudas K C, Mylotte J M, Apicella M A. J Infect Dis. 1985;152:1300–1307. doi: 10.1093/infdis/152.6.1300. [DOI] [PubMed] [Google Scholar]
- 22.Nelson M B, Munson R S, Jr, Apicella M A, Sikkema D J, Molleston J P, Murphy T F. Infect Immun. 1991;59:2658–2663. doi: 10.1128/iai.59.8.2658-2663.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natarajan K, Singh S, Burke T R, Jr, Grunberger D, Aggarwal B B. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neish A S, Gewirtz A T, Zeng H, Young A N, Hobert M E, Karmali V, Rao A S, Madara J L. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 25.Schulze-Osthoff K, Ferrari D, Riehemann K, Wesselborg S. Immunobiology. 1997;198:35–49. doi: 10.1016/s0171-2985(97)80025-3. [DOI] [PubMed] [Google Scholar]
- 26.Ono K, Han J. Cell Signalling. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 27.Mercurio F, Manning A M. Oncogene. 1999;18:6163–6171. doi: 10.1038/sj.onc.1203174. [DOI] [PubMed] [Google Scholar]
- 28.Wesselborg S, Bauer M K-A, Vogt M, Schmitz M L, Schulze-Osthoff K. J Biol Chem. 1997;272:12422–12429. doi: 10.1074/jbc.272.19.12422. [DOI] [PubMed] [Google Scholar]
- 29.Li J D, Feng W, Gallup M, Kim J H, Gum J, Kim Y, Basbaum C. Proc Natl Acad Sci USA. 1998;95:5718–5723. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams J L, Lee D. Curr Opin Drug Discovery Dev. 1999;2:96–109. [PubMed] [Google Scholar]
- 31.Aderem A, Ulevitch R J. Nature (London) 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 32.Anderson K V. Curr Opin Immunol. 2000;12:13–19. doi: 10.1016/s0952-7915(99)00045-x. [DOI] [PubMed] [Google Scholar]
- 33.Medzhitov R, Janeway C., Jr N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 34.Medzhitov R, Preston-Hurlburt P, Janeway C A., Jr Nature (London) 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 35.Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G, Finberg R W, Carroll J D, Espevik T, Ingalls R R, Radolf J D, et al. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 36.Underhill D M, Ozinsky A, Smith K D, Aderem A. Proc Natl Acad Sci USA. 1999;96:14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J D, Dohrman A F, Gallup M, Miyata S, Gum J R, Kim Y S, Nadel J A, Prince A, Basbaum C B. Proc Natl Acad Sci USA. 1997;94:967–972. doi: 10.1073/pnas.94.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein J O. Pediatr Infect Dis J. 2000;19, Suppl. 5:S89–S92. doi: 10.1097/00006454-200005001-00013. [DOI] [PubMed] [Google Scholar]
- 39.Berman S. N Engl J Med. 1995;332:1560–1565. doi: 10.1056/NEJM199506083322307. [DOI] [PubMed] [Google Scholar]