Abstract
Background
Studies indicate that physical damage to long-lasting insecticide-treated nets (LLINs) occurs at a surprisingly rapid rate following net distribution. To what extent does such damage affect the impact of LLINs? Can vectors pass a compromised LLIN barrier to bite? Do more resistant vectors enter the insecticide-treated nets (ITNs) through holes?
Methods
The study was carried out in three geo-locations. Two types of LLINs (polyester and polyethylene) with ‘standardized’ physical damage were compared with similarly damaged, but non-insecticidal (control) nets. The proportionate Holes Index (pHI) of each net was 276. Mosquitoes were captured inside the nets, identified taxonomically, and subjected to molecular analysis to estimate Knock-down resistance (Kdr) frequency.
Results
The most commonly observed species was Anopheles gambiae, accounting for approximately 70% (1,076/1,550) of the total mosquitoes collected both in LLINs and non-insecticidal nets. When compared with controls, number of vectors captured in torn LLINs was significantly reduced. Nonetheless in a night, an average of 5 An. gambiae s.l could enter the damaged LLINs to bite. Similar numbers of resistant mosquitoes were collected in both LLINs and non-insecticidal (control) nets (p > 0.05).
Conclusions
At a pHI of 276, man-vector contact was observed in torn LLINs. The insecticide at the surface of LLINs could only reduce the number of vectors. Resistant mosquitoes have opportunity to enter both non-insecticidal (control) nets and LLINs to bite.
Keywords: Long-lasting insecticidal nets, Man-vector contact, Anopheles gambiae
Background
Long-Lasting Insecticide-Treated Nets (LLINs) are widely used in sub-Saharan Africa for malaria vector control [1-3]. This technology is based on the slow release of pyrethroid insecticides, rendering them wash-resistant and extending insecticide residual effectiveness to at least three years without the need of re-treatment [4]. That is why approximately 289 million LLINs were distributed in Africa between 2008–2010 [5]. The initial impact of this intervention is thought to be significant [6-8]. In fact, LLINs can reduce man-vector contact by providing a physical barrier between the human sleeping under the LLIN and the malaria vector mosquito. This protection is enhanced by an insecticide (chemical barrier) that deters, repels, or kills vectors that attempt to bite the sleeper. LLIN that has its physical barrier (mesh) intact will rarely allow mosquitoes to reach the person sleeping under the LLIN [9]. But the critical question about the real operational life (physical integrity and bio-efficacy) of LLINs remains. Current LLIN distributions and replacement needs are based on an assumption that LLINs have an average useful life of 3–5 years [10]. But, there is a significant lack of research in LLIN survival time and variation in performance between LLINs of different textiles. To solve this deficit, the Vector Control Working Group of the Roll Back Malaria (RBM) Partnership called for more field research on LLIN durability [11].
To answer this call, a retrospective study on LLIN durability was conducted in Benin in 2010 [12]. This study showed losses associated with LLIN durability within 12–25 months after a net distribution by the National Malaria Control Program (NMCP) in 2008–2009. A substantial proportion of the LLINs (32%) had finger-sized holes, and/or fist-sized (10%) holes. Other studies conducted in Uganda on physical integrity of mosquito nets showed that 45%-78% of the nets were damaged even within a year of use in operational conditions [13,14]. Recent LLIN assessments in Kenya and Benin, reported a faster-than-anticipated loss of physical (durability) and insecticidal integrity (bio-efficacy), raising concerns about the duration of LLIN effective life [15,16]. In another study [17], repeated treatment of Insecticide Treated Nets (ITNs) with holes protected humans from mosquito bites in a vector susceptible area but failed to do so in a vector resistant area.
Other published studies conducted in Kenya [18,19] on ITNs durability found the majority of ITNs assessed in operational use with holes (78%-99.5%). Using different cut-offs of the proportionate Holes Index (pHI), around half of the torn ITNs (50.5%-61.3%) were classified as ‘effective nets’ and the other one as ‘ineffective nets’.
These observations raise the questions: Can vectors pass a compromised LLIN barrier to bite? Does the insecticidal feature of LLIN do a better job compensating for physical damage than was the case with the ITN? Or do users of torn LLINs remain protected by the insecticidal effect, as suggested in the literature [20]. Finally, do more resistant vectors enter the LLINs through holes?
Methods
Study design
To estimate the presence of malaria vector in the torn LLINs and to assess the potential of insecticidal barrier to reduce mosquito entry rate under such conditions, mosquitoes were caught inside torn LLINs and similarly damaged, but non-insecticidal, nets (controls) at three geo-locations for a period of 5 months (April to August) in 2010. Vector density inside the nets was quantified and a comparison was made between the LLINs with holes and the control (non-insecticidal net in a similar condition).
Study areas and selection of households
There were three study locations each of which had permanent vector breeding sites giving rise to a feature of relatively high vector densities at night, including the nights that vector collections were made. The locations were: Abomey-Takplikpo (6°31′54″N and 2°39′56″E) in Adjarra district, and Bame (7°16′46″N and 2°24′46″E) in Zangnanado district. Abomey-Takplikpo is located in a Guinean-bioclimatic zone and Bame is located in an intermediate bioclimatic zone (tropical Sudano-Guinean climate) (Figure 1). The third location was in the North, in a peri-urban area of Malanville district (11°50′27″N and 3°24′08″E), which has a Sudanian semi-arid bioclimatic zone. The Guinean-bioclimatic zone is located in the south, near the Atlantic coast with two rainy seasons (April–July and September–November) and an average annual rainfall of >1500 mm with degraded tropical forest. The Sudano-Guinean climate zone is located in the center with an average rainfall of 1000 mm per year, characterized as humid savanna. The Sudanian semi-arid bioclimatic zone is located in the north, with only one rainy season from June to October (mean annual rainfall below 900 mm) and characterized by a dry savanna. The three geo-locations are known to have no significant differences in mosquito composition. Resistance levels of vectors to insecticides at these sites were also known. In the Guinean and Sudano-Guinean sites, vectors were resistant, whereas in the Sudanese site, vectors were susceptible to pyrethroid insecticides [17,21,22].
Figure 1.
Study area.
Six random houses whose owners had signed a written consent to participate in the study were selected at each site. The criteria for selection were that each house contains a sleeping room with a close-fitting door and a window. The points of entries for mosquitoes were through open doors or eave gaps between walls and roofs. At the end of the study, participating households received new LLINs.
Mosquito nets
The study evaluated two types of LLINs most commonly distributed and found in the market place in Benin: polyester 75D (PET-75D) (Permanet® 2.0 Vestergaard Frandsen SA, Aarhus, Denmark), and polyethylene 150D (PE-150D) (Olyset®, Sumitomo Chemicals, Osaka, Japan). Results were compared with regard to a non-insecticidal net (manufacturer: Palutech Benin, Thailand). The PET-75D and PE-150D LLINs were blue in color and the control nets, white. The colors are the ones most commonly found in the market place, and communities surrounding the study locations. Mosquito preference for a particular colored net is not known and there was a limitation in this study design of not being able to separate possible confounding factors due to variation in blue colored nets attractiveness from that of white. PET-75D LLINs were treated with deltamethrin (55 mg/m2) and dimensions were 1.95 m long × 1.6 m wide × 2.0 m high. PE-150D nets, had permethrin (2%) incorporated into the net fiber (polyethylene resin) and they were 1.8 m long × 1.9 m wide × 1.5 m high. The non-insecticidal nets had polyester fiber and were 2 m long × 1.6 m wide × 1.9 m high.
To simulate torn nets and assess chemical barrier without need of retreatment by dipping, 12 square holes of 4 cm × 4 cm each (3 holes by side were cut at the same standardized positions except in the roof) in all three types of nets. According to the method described by World Health Organisation [23], the pHI of each net was 276 (Figure 2).
Figure 2.

Photo of a net with holes and collector.
Collectors and mosquito sampling in the torn nets
Comparison testing of the three nets: PET-75D, PE-150D and “Control” occurred concurrently twice a month for five months (April to August 2010) in the three study locations. Two PE-150D, two PET-75D and two non-insecticidal nets were hung (at the habitual sleeping place but not over bed) in the six selected houses at each site. The nets with standardized damage as well as the collectors were randomly rotated between the participating households on successive nights to adjust for any variation in attractiveness to mosquitoes.
Mosquito collectors, stationed under the nets, were selected using three criteria: Each collector (1) was an adult volunteer, (2) signed a written consent to participate in the study, and (3) had access to no other mosquito net. Collectors were trained to capture mosquitoes using an aspirator and a flashlight while inside the nets (Figure 2). They were informed 24 hours before each collection night and slept in the day and tried to satisfy natural call before starting the collection.
The collections were done continuously through the night from 9:00 p.m to 5:00 a.m and volunteers did not exit the nets during the night (to avoid possibility of creating a gap while exiting and re-entering the nets during collections). Mosquitoes were caught as soon as they entered the nets before having possibility to feed on the collectors. Human Biting Rate (HBR) was defined here as: “the number of mosquitoes caught per collector while in the net”.
No other household member was present in the house during the collections (in order not to attract vector from the nets and avoid exposure to vector bite).
Measuring mosquito diversity in the torn nets
All captured mosquitoes were identified to species using taxonomic keys of Gillies & De Meillon [24] and Gillies & Coetzie [25]. We determined the species richness (S) associated with each type of net at each location using the Shannon’s diversity index, H, defined as:
i = 1
Where:
H = the Shannon diversity index
Pi = fraction of the entire population made up of species i
S (Richness) = numbers of species encountered
∑ = sum from species 1 to species S
The H value indicates the number of species, as well as their relative abundance compared with the others in the collections [26].
Frequency of Kdr mutations in An. gambiae s.l collected in the LLINs
Mosquitoes were tested using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analyses to detect the presence of Knock down resistance (Kdr) genes [27]. The mosquitoes tested were selected from the total pool as follow:
–Around 20% of the total of An. gambiae s.l was randomly selected from each site in the resistance area.
–In the susceptible area, 40% of An. gambiae s.l collected was randomly selected to have enough data to compare the presence of resistant and susceptible genotypes in holed nets in this area.
Data analysis
Diversity data were analyzed using PAST 2.07, a diversity software package. Comparison of the Shannon diversities observed for the LLINs versus the non-insecticidal (control) net collections was made using Student’s t test. We assessed the effect of LLINs on mosquito density using the Incidence of Density Ratio (IDR) of LLINs versus non-insecticidal (control). Logistic regression model and odds ratio were used to assess variability of Kdr frequency between LLINs and non-insecticidal nets and to check if more resistant mosquitoes enter LLINs compared to the non-insecticidal nets. Mosquito collections were done concurrently with the three types of nets at each site and the variable “resistance level of the location” was not included in the data analysis.
Significant differences were those with a p-value less than or equal to 0.05. These tests were conducted using R 2.14.1 software.
Ethical considerations
This study was approved by the ethical committee of the Ministry of Health. Community leaders were briefed on the protocol before the study and gave verbal consent before the study began. Written consent was obtained from all participating volunteers, who were vaccinated against yellow fever and provided with malaria prevention and curative treatments according to World Health Organization (WHO) recommended regimen (on the basis of fever and detectable P. falciparum parasitemia).
Results
Diversity and geo-variability of mosquitoes collected in the damaged nets
A total of 10 species belonging to five genera (Culex, Aedes, Coquillettidia, Mansonia and Anopheles) were collected inside the nets with standardized physical damage (Table 1). There were four species of Culex, three species of Anopheles, and one species, each, of Mansonia, Aedes, and Coquillettidia. There was more species in the non-insecticidal net collections (S = 10), than in the LLIN collections, S = 7 for PET-75D collections and S = 6 for PE-150D collections.
Table 1.
Mosquito diversity (S) in collections from damaged bed nets: non-insecticidal (control) nets versus PET-75D and PE-150D LLINs
| Species |
Non-insecticidal nets |
PET-75D |
PE-150D |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ni | pi | (pi) ln (pi) | ni | pi | (ni) ln (ni/N) | ni | pi | (ni) ln (ni/N) | |
|
Anopheles gambiae |
553 |
0.66 |
−0.27 |
234 |
0.62 |
−0.30 |
289 |
0.87 |
−0.12 |
|
Anopheles pharoensis |
36 |
0.04 |
−0.14 |
38 |
0.10 |
−0.23 |
12 |
0.04 |
−0.12 |
|
Anopheles ziemanni |
31 |
0.04 |
−0.12 |
10 |
0.03 |
−0.10 |
4 |
0.01 |
−0.05 |
|
Aedes aegypti |
1 |
0.00 |
−0.01 |
0 |
0 |
NA |
0 |
0 |
NA |
|
Culex. quinquefasciatus |
72 |
0.09 |
−0.21 |
30 |
0.08 |
−0.20 |
12 |
0.04 |
−0.12 |
|
Culex gr decens |
14 |
0.02 |
−0.07 |
18 |
0.05 |
−0.15 |
1 |
0.00 |
−0.02 |
|
Culex nebulosus |
1 |
0.00 |
−0.01 |
3 |
0.01 |
−0.04 |
0 |
0 |
NA |
|
Culex annulioris |
2 |
0.00 |
−0.01 |
0 |
0 |
NA |
0 |
0 |
NA |
|
Coquillettidia Cristata |
1 |
0.00 |
−0.01 |
0 |
0 |
NA |
0 |
0 |
NA |
|
Mansonia africana |
123 |
0.15 |
−0.28 |
44 |
0.12 |
−0.25 |
15 |
0.05 |
−0.14 |
|
Richness (S) |
10 |
7 |
6 |
||||||
|
Shannon-wiener index (H) with 95% CI |
1.13[0.99-1.14] |
1.25[1.14-1.34] |
0.57[0.44-0.67] |
||||||
|
T scores |
NA |
−1.87 |
−7.70 |
||||||
| P-values | NA | 0.06 | 0.000 | ||||||
N total number of individuals, ni number of individuals found in the ith species, pi proportion of individuals found in the ith species, ln the natural (Naperian) logarithms, NA Not Applicable.
There was a highly significant difference in diversity between PE-150D - and non-insecticidal net- collections in the same location (p < 0.001) (Table 1). PET-75D and non-insecticidal nets, showed no difference (p = 0.06). An. gambiae s.l accounted for approximately 70% of the collections (1,076/ 1,550).
The abundance of each mosquito species collected varied by location (Figure 3). There were 234 (57.6%) An. gambiae s.l, 61 (15.0%) other Anopheles species (An. pharoensis and An. ziemanni), 77 (19%) Culex spp, 33 (8.1%) Mansonia africana, and 1 (0.3%) Aedes aegypti collected at the Guinean climatic site. In contrast there were 362 (78.9%) An. gambiae s.l, 2 (0.4%) other Anopheles species (An. pharoensis and An. ziemanni), 15 (3.3%) Culex spp., and 80 (17.4%) Mansonia africana at the Sudano-Guinean site. The greatest number of mosquitoes was recorded at the sudanian site where 480 (70.1%) An. gambiae s.l, 73 (10.6%), other Anopheles species (An. pharoensis and An. ziemanni) 63 (9.2%) Culex spp., and 69 (10.1%) Mansonia africana. There was a high variability of An. gambiae s.l density in the torn nets between the bioclimatic areas (D = 198.447; df = 2; p < 0.001).
Figure 3.

Proportion of mosquitoes collected in the nets at each geo-location.
No feeding rate was recorded in this study because of the unfed status of the collected mosquitoes.
Efficacy of damaged LLINs versus non-insecticidal nets in man-vector contact reduction
Densities of An. gambiae s.l were higher in the non-insecticidal nets than in the LLINs, regardless of the collection areas (Table 2). At Abomey-Takplikpo in the Guinean area, the Incidence Density Ratio (IDR) for An. gambiae s.l was significantly lower in the treated nets than in the non-insecticidal nets (PE-150D: IDR 0.31; 95% CI 0.22-0.43; p < 0.001; PET-75D: IDR 0.36; 95% CI 0.26-0.50; p < 0.001). The overall preventive effect against entry of An. gambiae s.l was 69% (95% CI 57%-78%) for PE-150D nets and 64% (95% CI 50%-74%) for PET-75D. In the Sudano-guinean (Bame) and Sudanian (Malanville) sites, the IDR were also lower in PET-75D and PE-150D nets than in the non-insecticidal nets (Table 2). The overall preventive effect against entry of An. gambiae s.l in the Sudano-Guinean site was 27% (95% CI 07%-42%) for PE-150D nets and 47% (95% CI 31%-59%) for PET-75D nets. In the Sudanian site, the overall preventive effect against entry of An. gambiae s.l was 49% (95% CI 37%-59%) for PE-150D nets and 61% (95% CI 51%-69%) for PET-75D nets. The IDR of PET-75D and PE-150D nets indicated that these LLINs provided similar levels of protection against malaria vectors.
Table 2.
Prevention of mosquito entry into torn LLIN
| Areas | Species | Mosquito nets | Density | IDR (95% CI) | p-value |
|---|---|---|---|---|---|
|
Abomey-Takplikpo (Guinean area) |
An. gambiae |
Non-insecticidal nets |
140 |
1 |
|
| PE-150D |
43 |
0.31 [0.22-0.43] |
<0.001 |
||
| PET-75D |
51 |
0.36 [0.26-0.50] |
<0.001 |
||
| Other culicidae |
Non-insecticidal nets |
78 |
1 |
|
|
| PE-150D |
8 |
0.10 [0.05-0.21] |
<0.001 |
||
| PET-75D |
86 |
1.10 [0.81-1.50] |
0.532 |
||
|
Bame (Sudano-Guinean area) |
An. gambiae |
Non-insecticidal nets |
160 |
1 |
|
| PE-150D |
117 |
0.73 [0.58-0.93] |
<0.001 |
||
| PET-75D |
85 |
0.53 [0.41-0.69] |
<0.001 |
||
| Other culicidae |
Non-insecticidal nets |
77 |
1 |
|
|
| PE-150D |
8 |
0.11 [0.05-0.22] |
<0.001 |
||
| PET-75D |
12 |
0.16 [0.08-0.29] |
<0.001 |
||
| Malanville (Sudanian area) |
An. gambiae |
Non-insecticidal nets |
253 |
1 |
|
| PE-150D |
129 |
0.51 [0.41-0.63] |
<0.001 |
||
| PET-75D |
98 |
0.39 [0.31-0.49] |
<0.001 |
||
| Other culicidae | Non-insecticidal nets |
127 |
1 |
|
|
| PE-150D |
33 |
0.26 [0.18-0.38] |
<0.001 |
||
| PET-75D | 45 | 0.35 [0.25-0.50] | <0.001 |
For other mosquito species, IDR of treated nets were also lower than in the controls. The overall preventive effect against entry of other mosquito species was 90% (95% CI 79%-95%) for PE-150D nets but many other mosquito species were collected in the torn PET-75D nets compared with the non-insecticidal nets at the Guinean site (Table 2). At the Sudano-Guinean site, the overall preventive effect was 89% (95% CI 78%-95%) for PE-150D nets and 84% (95% CI 50%-74%) for PET-75D nets. At the Sudanian site, the overall preventive effect against entry of other mosquitoes was 74% (95% CI 62%-81%) for PE-150D nets and 65% (95% CI 50%-75%) for PET-75D nets. Globally, the two types of LLINs reduced other mosquito species entry at the same level.
In summary, the average Human Biting Rate (HBR) of An. gambiae s.l was 12.65[11.13-14.32] in the non-insecticidal nets versus 6.45[05.38-07.67] for PET-75D LLINs and 4.9[03.97-05.97] for PE-150D LLINs in the Sudanian area (Figure 4). In Sudano-Guinean and Guinean sites, the average human biting rate of An. gambiae s.l was 7.5 (range 7–8) in a non-insecticidal net against 2.15 and 4.25 for PET-75D LLIN and PE-150D LLIN. The same observation was made with other mosquito species (Figure 5). 4–6 other species can bite a man in the non-insecticidal net in a night versus 1–4 for PET-75D and 1–2 for PE-150D LLINs.
Figure 4.
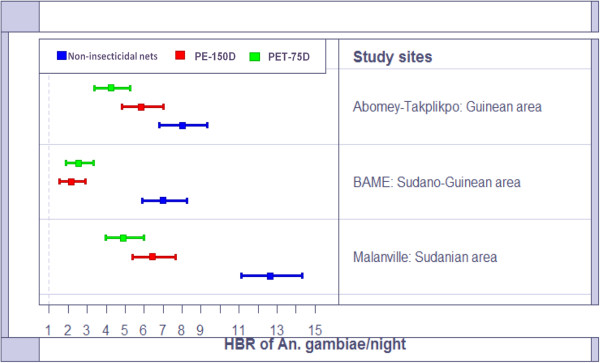
Human biting rate of An. gambiae s.l per night per torn nets.
Figure 5.

Human biting rate of other nuisant mosquito species per night per torn nets.
Kdr genotypic distribution of Anopheles gambiae caught in the torn LLINs
A total of 320 Anopheles gambiae s.l females were tested for Kdr. Kdr frequency was ranged from 0.66 to 0.81 in the females collected from the non-insecticidal (control) nets versus 0.66 to 0.94 in the LLIN collections (Table 3). No significant difference was observed between the Kdr frequencies observed in LLINs versus non-insecticidal nets (p > 0.05). In the susceptible area, no difference was observed between resistant and susceptible vectors collected. The Kdr frequencies recorded were also high in this area and not significantly different from the frequencies observed in the resistance area (Table 3). In the susceptible area, resistant and susceptible mosquitoes can enter the torn nets at similar level (p > 0.05).
Table 3.
Kdr and genotype frequencies of Anopheles gambiae s.l collected in torn nets
| Area | Localities | Mosquito nets | SS | RS | RR | F ( Kdr ) | OR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|
|
Resistance area |
Abomey-Takplikpo (Guinean area) |
Non-insecticidal nets |
2 |
7 |
15 |
0.77 |
1 |
|
| PE-150D |
0 |
3 |
8 |
0.86 |
1.88 [0.47-7.57] |
0.37 |
||
| PET-75D |
0 |
4 |
7 |
0.82 |
1.34 [0.37-4.79] |
0.65 |
||
|
Bame (Sudano-Guinean area) |
Non-insecticidal nets |
0 |
11 |
18 |
0.81 |
1 |
|
|
| PE-150D |
0 |
4 |
23 |
0.92 |
2.93 [0.87-9.83] |
0.08 |
||
| PET-75D |
0 |
3 |
24 |
0.94 |
3.98 [1.05-5.14] |
0.04 |
||
| Susceptible area | Malanville (Sudanian area) | Non-insecticidal nets |
14 |
35 |
45 |
0.66 |
1 |
|
| PE-150D |
6 |
14 |
26 |
0.72 |
1.28 [0.74-2.21] |
0.37 |
||
| PET-75D | 12 | 11 | 28 | 0.66 | 0.96 [0.58-1.60] | 0.89 |
SS homozygous susceptible; RS hybrid resistant and susceptible; RR homozygous resistant.
When we compared the total number of resistant females (RR) in the torn LLINs versus the controls, no significant difference was observed (p > 0.05) (Figure 6). This indicated that resistant mosquitoes can enter both LLINs and non-insecticidal nets at the same level and excluded the possibility to suspect a genetic cost related to the ability of resistant mosquitoes to enter non-insecticidal nets.
Figure 6.
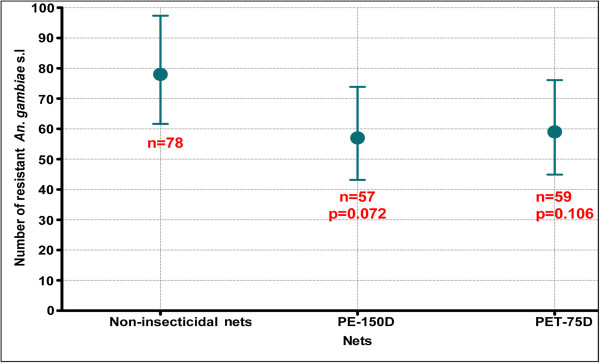
Comparison of resistant An. gambiae s.l. entering LLIN versus non-insecticidal net.
Discussion
Mosquito diversity, observed in torn mosquito nets, could be useful in forecasting infection risk for malaria as well as other vector-borne diseases (chikungunya, filariasis, dengue, etc.). When LLINs were damaged and then tested, ten different mosquito species were caught in the mosquito nets. Four of the species were known as vectors of medical importance in Africa. An. gambiae s.l is the major malaria vector in Benin [21]. Culex quinquefaciatus transmits Bancroftian filariasis and West Nile Virus [28,29]. An. pharoensis and Mansonia africana are key vectors in transmitting Rift Valley Fever virus [30,31]. However, there was a significant difference in term of mosquito diversity between PE-150D LLINs and non-insecticidal (control) nets. No difference in mosquito diversity was observed between PET-75D LLINs and non-insecticidal (control) nets. But difference could be observed with a large sample size. These results suggested that the insecticide effect of LLINs could reduce the number of mosquito species that could enter torn nets.
The relative abundance of each mosquito species in the collections and by geo-location is more difficult to interpret since it may reflect the influence of various factors including the sampling method which targeted only endophilic species. Previous studies on mosquito species composition [32,33] using human landing catch and larvae collection have reported three times more mosquito species (28 species). But those collections were not conducted in the same assessment areas as this study. The few numbers of species collected in this study could not indicate that some species are better in entering torn nets than others because mosquitoes were not collected outside the nets for possible comparison. The abundance of An. gambiae s.l reflected the selection of study locations with high vector potential. Assuming a sporozotic rate compared to other species, the high density of An. gambiae s.l may be due to its high anthropophilic and endophilic behavior [34,35]. An. gambiae s.l density in the torn nets varies between the climatic areas. Changes in temperature may have influenced the variability of mosquito density [36,37]. This study predicts high malaria vectors bite risk with torn nets in areas of permanent vector breeding sites.
We chose to use damaged, but otherwise new, LLINs rather than ordinary household nets. This aimed to measure protection level of chemical barrier provided by torn LLINs against resistant mosquitoes. At a pHI of 276, significant reduction of mosquito density was obtained with torn LLINs regardless of collection area. Due to the presence of chemical barrier, a low number of mosquitoes were able to enter torn LLINs. In another study [38] conducted indoor at the same location (Guinean site), in the same period, using human landing catch, an average of 20 bites /man/night was observed with An. gambiae s.l. These results indicated that chemical barrier of torn LLINs provided additional protection against mosquito’s biting ability in LLINs over that of non-insecticidal nets. While a reduction in vector density was observed when LLINs were compared with non-insecticidal nets, man-vector contact risk cannot be discounted. In fact, an average of 5 (3–7) An. gambiae s.l/man/nights can enter the torn LLINs to bite the sleeper. This show evidence that the sleepers are exposed to bites from malaria vectors when the nets are torn. With large holes (pHI > 276), An. gambiae s.l density could increase in the torn LLINs and protection of humans against mosquito bites could be lost when torn mosquito nets are used. Therefore, categorizing torn ITNs with a pHI over a cut-off of 88 as “ineffective” as done by Mutuku et al.[18] could be useful.
Asidi et al.[17] have also reported loss of protection of torn mosquito nets in southern Benin (resistance area). They treated torn ordinary households nets to show protection loss with resistant An. gambiae and observed, in 2008, low Kdr frequency (0.10) in the susceptible area (Sudanian site). High Kdr frequency (0.66) was observed in 2010 with this study. This may be due to the rapid spread of resistant mosquitoes and confirms the significant increase of Kdr frequency observed in Malanville from October 2008 to June 2010 by Djègbè et al.[39]. No significant reduction was observed between resistant An. gambiae s.l which can enter treated and non-insecticidal nets. The results show the possibility of resistant mosquitoes to penetrate both treated and non-insecticidal mosquito nets. This observation suggests that Kdr may be associated with higher than expected survival across the insecticidal barrier, compromising its protective effect, and rendering LLINs similar to non-insecticidal nets.
In Africa, current malaria control strategy is based on mass distribution of LLINs [40,41]. This technology is developed to reduce man-vector contact [42]. With physical damage of LLINs occurring rapidly [16,18,43], our study emphasizes the potential importance of care and repair of holes in LLINs. Repairing torn LLINs will not have the sole advantage to reduce vector contact but could also increase the operational life of LLINs.
Although the significant findings of this study, it had several limitations. Further assessment would have been possible if intact, old and worn out LLINs were included in the study design. Mosquito collections were restricted to new torn LLINs and limited to the assumption that intact mosquito nets do not allow mosquito entry as shown by Curtis et al.[44], Lines et al.[45] and a study on malaria in children sleeping under mosquito nets which were either intact or torn [46]. It is therefore important to conduct another study whenever possible to provide full details on the influence of holes and washing to the LLINs in preventing mosquito bites effect. Mosquito collections were also limited to environment with high vector breeding sites and the observed results could vary in other environments. In semi-arid or arid environment with low mosquito breeding sites for example, man-vector could be much reduced or prevented because of low vector density.
Conclusions
At a pHI of 276, several mosquito species were able to enter the LLINs through the holes to bite. An. gambiae s.l, the main malaria vector was the most collected mosquito species that entered the torn LLINs to bite. The insecticidal barrier of the LLINs only reduced vector entry into LLINs with holes. Resistant mosquitoes have opportunity to enter both treated and non-insecticidal (control) nets. This study represents an alert for malaria control programs to increase public awareness of LLINs holes repair as a relevant integral part of programs to enhance the effectiveness of the control of malaria.
Competing interests
There are neither any financial competing interests nor any non-financial competing interests (political, personal, religious, ideological, academic, intellectual, commercial or any other) to declare in relation to this manuscript.
Authors’ contributions
VG collected analyzed, interpreted data and wrote the manuscript. RA was responsible for field collection, identification, processing of mosquitoes and helped in drafting the manuscript. RA contributed to the design of the study, helped in drafting the manuscript and revised the manuscript. FO, AS, RO GG and RA helped in data analysis, in reviewing the manuscript and helped with the activities. MCA conceived and designed the study, supervised fields and laboratory procedures, and review the manuscripts. All authors have read and approved the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Virgile Gnanguenon, Email: amerusangel@yahoo.fr.
Roseric Azondekon, Email: roseric_2000@yahoo.fr.
Frederic Oke-Agbo, Email: fredook15@yahoo.fr.
Arthur Sovi, Email: sart52005@yahoo.fr.
Razaki Ossè, Email: ossraz@yahoo.fr.
Gil Padonou, Email: pagergil@yahoo.fr.
Rock Aïkpon, Email: rockypremier@yahoo.fr.
Martin C Akogbeto, Email: akogbetom@yahoo.fr.
Acknowledgements
This work received a financial support from USAID through the President’s Malaria Initiative. The authors would like to thank Dr Raymond Beach (Center of Diseases Control and Prevention) for his technical assistance.
References
- Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev Online. 2004;2:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- Noor AM, Mutheu JJ, Tatem AJ, Hay SI, Snow RW. Insecticide-treated net coverage in Africa: mapping progress in 2000–07. Lancet. 2009;373:58–67. doi: 10.1016/S0140-6736(08)61596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masum H, Shah R, Schroeder K, Daar A, Singer P. Africa’s largest long-lasting insecticide-treated net producer: lessons from A to Z Textiles. Bmc Int Heal Hum Rights. 2010;10(Suppl 1):S6. doi: 10.1186/1472-698X-10-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO | Guidelines for testing. http://www.who.int/whopes/guidelines/en/
- WHO. WHO | World Malaria Report 2010. Geneva: World Health Organization; 2010. xxii. [Google Scholar]
- The Economist African child mortality: The best story in development. http://www.economist.com/node/21555571.
- Alonso PL, Lindsay SW, Armstrong JRM, de Francisco A, Shenton FC, Greenwood BM, Conteh M, Cham K, Hill AG, David PH, Fegan G, Hall AJ. The effect of insecticide-treated bed nets on mortality of Gambian children. Lancet. 1991;337:1499–1502. doi: 10.1016/0140-6736(91)93194-E. [DOI] [PubMed] [Google Scholar]
- Dabiré RK, Diabaté A, Baldet T, Paré-Toé L, Guiguemdé RT, Ouédraogo JB, Skovmand O. Personal protection of long lasting insecticide-treated nets in areas of Anopheles gambiae ss resistance to pyrethroids. Malar J. 2006;5:12. doi: 10.1186/1475-2875-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovmand O. Insecticidal bednets for the fight against malaria – present time and near future. Open Biol J. 2010;3:92–96. doi: 10.2174/1874196701003030092. [DOI] [Google Scholar]
- WHO | Long-lasting insecticidal nets for malaria prevention (archived) http://www.who.int/malaria/publications/atoz/insecticidal_nets_malaria/en/index.html. [DOI] [PMC free article] [PubMed]
- Roll Back Malaria Partnership |Aims for 2010–11. Vector Control Working Group http://www.rollbackmalaria.org/mechanisms/winwg.html23946726
- Martin A, Azondekon R, Gueye S, Green M, Beach R. Tracking Long-lasting Insecticidal (mosquito) Nets (LLINs) Distributed via National Campaign: Assessing LLIN Loss, Physical Deterioration, and Insecticidal Decay in Benin: Retrospective studyTracking Long-lasting Insecticidal (mosquito) Nets (LLINs) Distributed via National Campaign: Assessing LLIN Loss, Physical Deterioration, and Insecticidal Decay in Benin: Retrospective Study. Benin: PMI/USAID/CREC; 2011. pp. 10–28. [Google Scholar]
- Kilian A, Byamukama W, Pigeon O, Gimnig J, Atieli F, Koekemoer L, Protopopoff N. Evidence for a useful life of more than three years for a polyester-based long-lasting insecticidal mosquito net in Western Uganda. Malar J. 2011;10:299. doi: 10.1186/1475-2875-10-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian A, Byamukama W, Pigeon O, Atieli F, Duchon S, Phan C. Long-term field performance of a polyester-based long-lasting insecticidal mosquito net in rural Uganda. Malar J. 2008;7:49. doi: 10.1186/1475-2875-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githinji S, Herbst S, Kistemann T, Noor AM. Mosquito nets in a rural area of Western Kenya: ownership, use and quality. Malar J. 2010;9:250. doi: 10.1186/1475-2875-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azondekon R, Gnanguenon V, Oké-Agbo F, Houevoessa S, Green M, Beach R, Akogbeto M. Tracking a long-lasting insecticidal (mosquito) net intervention: coverage, durability and bio-efficacy following a 2011 national distribution in Benin. Malar J. 2013. In press. [DOI] [PMC free article] [PubMed]
- Asidi A, N’Guessan R, Akogbeto M, Curtis C, Rowland M. Loss of household protection from use of insecticide-treated nets against Pyrethroid-Resistant Mosquitoes, Benin. Emerg Infect Dis. 2012;18:1101–1106. doi: 10.3201/eid1807.120218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutuku FM, Khambira M, Bisanzio D, Mungai P, Mwanzo I, Muchiri EM, King CH, Kitron U. Physical condition and maintenance of mosquito bed nets in Kwale County, coastal Kenya. Malar J. 2013;12:46. doi: 10.1186/1475-2875-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia P, Teklehaimanot H, Tesfaye Y, Teklehaimanot A. Physical condition of Olyset(R) nets after five years of utilization in rural western Kenya. Malar J. 2013;12:158. doi: 10.1186/1475-2875-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale P, Bitsindou P, Diomandé L, Robert V. Insecticide impregnation can restore the efficacy of torn bed nets and reduce man-vector contact in malaria endemic areas. Trans R Soc Trop Med Hyg. 1992;86:362–364. doi: 10.1016/0035-9203(92)90219-3. [DOI] [PubMed] [Google Scholar]
- Djogbénou L, Pasteur N, Bio-Bangana S, Baldet T, Irish SR, Akogbeto M, Weill M, Chandre F. Malaria vectors in the Republic of Benin: distribution of species and molecular forms of the Anopheles gambiae complex. Acta Trop. 2010;114:116–122. doi: 10.1016/j.actatropica.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Ossè R, Gnanguenon V, Sèzonlin M, Aikpon R, Padonou G, Yadouleton A, Akogbeto M. Relationship between the presence of kdr and Ace-1 mutations and the infection with Plasmodium falciparum in Anopheles gambiae s.s. in Benin. J. November 2012. J Parasitol Vector Biol. 2012;4:3139. [Google Scholar]
- Guidelines for monitoring the durability of long-lasting insecticidal mosquito nets under operational conditions. http://apps.who.int/iris/handle/10665/44610.
- Gillies MT, de Meillon B. The anophelinae of Africa South of the Sahara. South Afr Inst Med Res. 1968;54:343. [Google Scholar]
- A supplement to the anophelinae of Africa south of the Sahara (Afrotropical region) (Open Library) http://openlibrary.org/books/OL15097319M/A_supplement_to_the_anophelinae_of_Africa_south_of_the_Sahara_(Afrotropical_region)
- Diversity Index: Shannon Index/Shannon-Weaver Index (H) http://microbeatic.wordpress.com/2012/01/26/diversity-index-shannon-indexshannon-weaver-index-h/
- Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, Guillet P, Pasteur N, Pauron D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P, Bryant B, Chapman SB, Dong Y, Erickson SM, Karunaratne SHPP, Kokoza V, Kodira CD, Pignatelli P, Shin SW, Vanlandingham DL, Atkinson PW, Birren B, Christophides GK, Clem RJ, Hemingway J, Higgs S, Megy K, Ranson H, Zdobnov EM, Raikhel AS. et al. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;330:88–90. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil H, Al-Ali AAE-B. A study on Culex species and Culex transmitted diseases in Al-Madinah Al-Munawarah, Saudi Arabia. Parasitol United J Puj. 2008;1:101–108. [Google Scholar]
- Gordon SW, Tammariello RF, Linthicum KJ, Dohm DJ, Digoutte JP, Calvo-Wilson MA. Arbovirus isolations from Mosquitoes collected during 1988 in the Senegal River Basin. Am J Trop Med Hyg. 1992;47:742–748. doi: 10.4269/ajtmh.1992.47.742. [DOI] [PubMed] [Google Scholar]
- Grobbelaar AA, Weyer J, Leman PA, Kemp A, Paweska JT, Swanepoel R. Molecular epidemiology of Rift valley fever virus. Emerg Infect Dis. 2011;17:2270–2276. doi: 10.3201/eid1712.111035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djènontin A, Bio-Bangana S, Moiroux N, Henry M-C, Bousari O, Chabi J, Ossè R, Koudénoukpo S, Corbel V, Akogbéto M, Chandre F. Culicidae diversity, malaria transmission and insecticide resistance alleles in malaria vectors in Ouidah-Kpomasse-Tori district from Benin (West Africa): a pre-intervention study. Parasit Vectors. 2010;3:83. doi: 10.1186/1756-3305-3-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingenfelser A, Rydzanicz K, Kaiser A, Becker N. Mosquito fauna and perspectives for integrated control of urban Vector-mosquito populations in southern benin (west africa) Ann Agric Environ Med. 2010;17:49–57. [PubMed] [Google Scholar]
- Dekker T, Steib B, Cardé RT, Geier M. L-lactic acid: a human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Med Vet Entomol. 2002;16:91–98. doi: 10.1046/j.0269-283x.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- Service M. Medical Entomology for Students. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Yé Y, Louis V, Simboro S, Sauerborn R. Effect of meteorological factors on clinical malaria risk among children: an assessment using village-based meteorological stations and community-based parasitological survey. BMC Publ Health. 2007;7:101. doi: 10.1186/1471-2458-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks GD, Hay SI, Stern DI, Biomndo K, Snow RW. Meteorologic influences on plasmodium falciparum malaria in the Highland Tea Estates of Kericho, Western Kenya. Emerg Infect Dis. 2002;8:1404–1408. doi: 10.3201/eid0812.020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossè R, Aikpon R, Padonou GG, Oussou O, Yadouléton A, Akogbéto M. Evaluation of the efficacy of bendiocarb in indoor residual spraying against pyrethroid resistant malaria vectors in Benin: results of the third campaign. Parasit Vectors. 2012;5:163. doi: 10.1186/1756-3305-5-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djègbè I, Boussari O, Sidick A, Martin T, Ranson H, Chandre F, Akogbéto M, Corbel V. Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malar J. 2011;10:261. doi: 10.1186/1475-2875-10-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudou B, Ghattas H, Essé C, Nsanzabana C, Rohner F, Utzinger J, Faragher B, Tschannen A. The use of insecticide-treated nets for reducing malaria morbidity among children aged 6–59 months, in an area of high malaria transmission in central Côte d’Ivoire. Parasit Vectors. 2010;3:91. doi: 10.1186/1756-3305-3-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll Back Malaria Progress and Impact Series - Defeating Malaria in the Americas, Europe, the Middle East and the Pacific. http://www.rollbackmalaria.org/ProgressImpactSeries/report12.html.
- Darriet F. Moustiquaires Imprégnées et Résistance Des Moustiques Aux Insecticides. Paris: Institut de Recherche pour le Développement; 2007. [Google Scholar]
- Allan R, O’Reilly L, Gilbos V, Kilian A. An observational study of material durability of three World Health Organization–recommended long-lasting insecticidal Nets in Eastern Chad. Am J Trop Med Hyg. 2012;87:407–411. doi: 10.4269/ajtmh.2012.11-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CF, Myamba J, Wilkes TJ. Comparison of different insecticides and fabrics for anti-mosquito bednets and curtains. Med Vet Entomol. 1996;10:1–11. doi: 10.1111/j.1365-2915.1996.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Lines JD, Myamba J, Curtis CF. Experimental hut trials of permethrin-impregnated mosquito nets and eave curtains against malaria vectors in Tanzania. Med Vet Entomol. 1987;1:37–51. doi: 10.1111/j.1365-2915.1987.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Mwangi TW, Ross A, Marsh K, Snow RW. The effects of untreated bednets on malaria infection and morbidity on the Kenyan coast. Trans R Soc Trop Med Hyg. 2003;97:369–372. doi: 10.1016/S0035-9203(03)90056-3. [DOI] [PubMed] [Google Scholar]



