Abstract
Airway mucus presents a first line of defense against inhaled materials. It also, however, is a significant pathological contributor to chronic lung diseases such as asthma, cystic fibrosis, and chronic obstructive pulmonary disease. Thus, gaining a better understanding of the mechanisms of mucus production and secretion is an important goal for improving respiratory health. Mucins, the chief glycoprotein components of airway mucus, are very large polymeric glycoproteins, and measuring their production and secretion in experimental animals present significant technical challenges. Over the past several years, we have developed assays for accurately quantifying mucin production and secretion using histological and biochemical assays. These methods are described here.
Keywords: airways, asthma, cystic fibrosis, chronic obstructive pulmonary disease, goblet cell, lungs, mouse, mucin, mucous, mucus
1. Introduction
The amount of mucin present within secretory cells of the airway epithelium reflects a balance between two tightly regulated processes - mucin production and mucin secretion (1–3). The predominant secreted mucins produced in mouse airways are Muc5ac and Muc5b (4). Muc5b is produced constitutively, but its rate of production may be further increased during lung inflammation (4–7). Muc5ac is scarcely produced at baseline, but its production is greatly increased during inflammation (4,7,8).
In the healthy respiratory tract, mucins are continuously secreted at a low basal rate, but the rate of secretion can be greatly stimulated by an increase in the concentration of extracellular secretagogues (9). Basal secretion reflects tonic activity of a regulated secretory machinery, as indicated by the spontaneous accumulation of intracellular mucin when a key regulatory protein, Munc13-2, is genetically deleted (6). The rate of basal secretion is sufficiently high that very little mucin accumulates intracellularly when mucins are produced at basal rates. Indeed, intracellular mucin content under these conditions is so low that mucins are not detected by histochemical stains such as alcian blue-periodic acid Schiff’s (AB-PAS) and periodic acid fluorescent Schiff’s (PAFS) (9), even though significant amounts of Muc5b are detected by more sensitive enzyme-linked immunolabeling probes (6,7).
Increased mucin production results in histochemically visible intracellular mucin accumulation traditionally termed “mucous metaplasia”. The most efficacious stimuli for mucous metaplasia are agents that induce IL-13 predominant allergic inflammation[m1] (3). The principal endogenous ligand regulating mucin secretion, both basal and stimulated, appears to be ATP (10).
Despite the fact that intracellular mucin content levels depend upon two variables, the rate of production and the rate of secretion, the measurement of intracellular mucin content can be useful to interrogate the rate of just one of these if the rate of the other is held at steady state. For example, increased intracellular mucin content indicates increased production if the rate of secretion is not altered (9). Conversely, increased intracellular mucin content indicates decreased secretion if the rate of production is not altered (6,9,11,12).
Assessment of intracellular mucin content has the following three advantages:
For measuring mucin production, it is simple to measure and to correlate with other phenotypic parameters in pathophysiological models (9).
For measuring basal secretory rate, small differences are magnified by progressive intracellular mucin accumulation, which makes these differences detectable (6).
For measuring stimulated secretion, fractional release can be readily determined (see 3.7, below), thereby relating stimulated secretory function to initial intracellular content to avoid artifacts from changes in intracellular pool size[m2] (9,12).
Disadvantages of the measurement of intracellular mucin content to assess mucin production or secretion are the lack of strict linearity of image-based techniques, and the lack of sensitivity of lectin-based detection on blots following electrophoresis when measuring basal rates of mucin production and secretion.
2. Materials
Because mice are very frequently used for experimental respiratory research, many details below (e.g, identifying anatomical structures during dissection) focus on them[m3]. While mice differ from humans and other large animals anatomically (e.g. airway sizes and gland presence) and morphologically (e.g. epithelial shape and stratification), the applications for detection of mucin production and secretion described below can be applied across species. We do not specify a particular disease model or exposure system because these vary widely. Indeed, mucous metaplasia is a phenotype common to allergen exposed, viral infected, and cytokine treated mice (2). Details for establishing these models are contained in the following references: (9,13–19). The materials and methods suggested below are suitable for use in numerous animal species, and they can also be applied to human pathological, segmental challenge, or primary cell culture studies.
2.1 Histology
Neutral buffered formalin: Histological grade formalin (37% formaldehyde content) diluted 1:10 (v/v) in 0.1 M phosphate buffer or phosphate buffered saline (pH 7.4). Store at room temperature.
Paraffin-embedded tissue sections can be prepared by the investigator or by an institutional histopathology core. Tissue sections should be collected on positively charged glass slides to allow for strong adhesion should heated antigen retrieval be necessary in downstream immunohistochemical labeling experiments.
Periodic acid solution: Prepare 1% (w/v) fresh for each usage by dissolving electrophoresis grade periodic acid in ddH2O.
Fluorescent Schiff’s reagent: Prepare at least 48 h prior to use by dissolving acriflavine hydrochloride in an appropriate volume of ddH2O to obtain a final concentration 0.5% (w/v) in the final product. Once acriflavine is dissolved, concentrated HCl (~10 N) is added to obtain a final concentration of 1% (v/v), and sodium metabisulfite is added to obtain a final concentration of 1% (w/v). The solution is mixed well, stoppered tightly, and stored at room temperature in the dark for at least 48 h. Fluorescent Schiff’s reagent can then be used or stored at 4° C and is good for at least 1 month. (see Note 1)
Acid alcohol solution: Dilute concentrated HCl to 1% (v/v) with 70% ethanol.
Mounting medium: Make a 1:1 v/v mixture of Canada balsam (Fisher Scientific, Pittsburgh, PA) and methyl salicylate (Fisher). Store at room temperature.
2.2. Mucin Extraction and Specimen Preparation
Guanidinium buffer: dissolve 6 M guanidinium chloride (Sigma), 0.1 M Tris-HCl, pH 8.0, 5 mM ethylenediamine tetraacetic acid (EDTA) in ddH2O. Store at 4°C.
Protease inhibitor buffer: Prepare fresh by dissolving 1 Complete Protease Inhibitor Cocktail Tablet (Roche Applied Science, Indianapolis, IN) in 7 mL of guanidinium buffer.
Urea buffer: dissolve 6 M urea (Sigma), 0.1 M Tris-HCl, pH 8.0, 5 mM EDTA in ddH2O. Store at 4°C.
Loading buffer (10X): dissolve 77 mg dithiothreitol (DTT) and 25 mg bromophenol blue (Fisher Scientific, Fair Lawn, NJ) in 5 ml of 50% (v/v) glycerol and 1% (w/v) sodium dodecyl sulfate (SDS) in urea buffer. Aliquot and store at −20°C.
Alkylation buffer (10X): dissolve 250 mM iodoacetamide in water. Prepare fresh.
2.3. SDS-Agarose Gel Electrophoresis
Tris-acetate-EDTA (TAE) buffer (50X): dissolve 242 g Tris base in 750 mL deionized water, 57.1 mL glacial acetic acid and 100 mL of 0.5 M EDTA (pH 8.0) and adjust the solution to a final volume of 1 L (final pH 8.5). Store at room temperature. Dilute to 1X with deionized water.
Electrophoresis buffer: 0.1% (w/v) SDS in TAE buffer. Store at room temperature.
2.4. Vacuum Blotting
Saline-sodium citrate (SSC) buffer 20X: 3.0 M NaCl and 0.3 M Na citrate in ddH2O. Adjust to pH 7.0 with HCl. Store at 4°C.
Transfer buffer: 0.2% (w/v) SDS in 4X (SSC) buffer. Store at 4°C.
Reducing transfer buffer: Dissolve DTT in transfer buffer to a final concentration of 10 mM. Prepare fresh.
Immobilon-NC 0.45 µm nitrocellulose membrane (Millipore, Billerica, MA) and blotting paper (Bio-Rad Laboratories, Hercules, CA).
Phosphate buffered saline (PBS) (10X): 1.37 M NaCl, 27 mM KCl, 80 mM Na2HPO4, and 20 mM KH2PO4 in ddH2O. Sterilize by autoclaving. Adjust the final volume to 1 L. Store at room temperature. Dilute to 1X with ddH2O (final pH 7.4).
0.3% (v/v) Tween 20 (Bio-Rad Laboratories, Hercules, CA) in 0.01 M Tris-HCl, pH 6.8, and 0.1% (v/v) Tween 20 in 0.01 M Tris-HCl, pH 6.8.
Blocking solution and antibody dilution buffer: 5% (w/v) Blotting Grade Non-Fat Dry Milk (Bio-Rad Laboratories, Hercules, CA) and 0.1% (v/v) Tween 20 in PBS (1X). Prepare fresh.
Reagents for enhanced chemiluminescence (ECL) detection: SuperSignal® West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) and Bio-Max Light Film (Kodak, Rochester, NY). Films are developed in a Konica Film Processor.
3. Methods
The two methods for measuring mucin production in vivo that will be described below utilize microscopic imaging and biochemical assays.
Histological specimens provide a useful tool to determine the localization and degrees of mucin production and secretion. For measuring intracellular mucin content, a technique such as transmission electron microscopy is exquisitely sensitive. However, instrument expense and sample preparation time can be prohibitive when large numbers of samples are assessed. For these reasons, we have relied more heavily on light microscopy and the use of inexpensive technologies to measure mucin production and secretion. Images obtained at relatively low magnification (e.g. using a 40 x specimen objective), can be analyzed and compared at different anatomical locations in the same slide, provide adequate sample sizes (10’s to 100’s of cells per image), and display sufficient detail to determine whether there is heterogeneity among cells. Numerous image analysis software tools make quantitation of staining simple and inexpensive.
Immunoblotting is also an efficacious approach for measuring the airway mucin content. Due to particular biochemical properties of polymeric mucins, treatment with a chaotropic agent such as guanidinium chloride is necessary for breaking non-covalent bonds and solubilization (20). Polymeric mucins are also held together by disulfide bonds. Therefore, it is important to reduce these with agents such as dithiothreitol prior to electrophoresis and transfer (21). These procedures will permit resolution of single bands of monomeric mucins. Mucins can then be blotted and detected using selective probes. Their heavy glycosylation has made use of specific antibodies difficult in some instances (22), but this same property makes mucins suitable for detection with lectins, a class of highly specific sugar binding proteins. Results can be compared relative to each other within blots or across different vacuum blots when a standard curve and appropriate internal controls are also applied.
3.1 Animal and tissue preparation
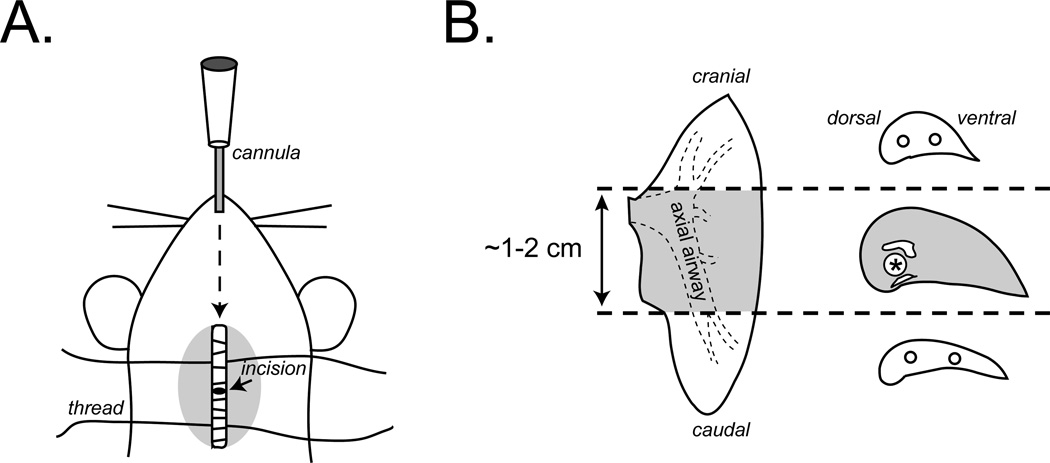
To preserve airspace morphology, the lungs are preserved via intracheal instillation of fixative. Simple immersion of lungs in fixative results in alveolar and airway collapse, tissue folding, and accumulation airway debris. For surgical preparation[DT4], small laboratory animals are deeply anesthetized and tracheostomized by removing a patch of skin from the neck surface[m5]. The trachea is isolated by removing connective, glandular and infrahyoid muscle tissues. Using rounded forceps, the trachea is then separated from the esophagus, and a 30 cm length of surgical thread is folded in half, passed underneath the trachea and pulled through such that half of the double length thread is on either side of the trachea. The thread is then cut at the center point releasing two 15 cm pieces that will be used to secure and stabilize the tracheal cannula. A partial incision is made in ventral half of the trachea using micro dissecting spring scissors, and the animal is cannulated. For mice, a blunt syringe catheter (18–20 G) is suggested, and for larger animals (e.g. rats and guinea pigs) 2–3 mm O.D. polyethylene tubing is suggested. Once inserted the cannula is secured by tying the threads above and below the insertion site.
Once cannulated, animals are opened at the abdomen, the descending aorta is cut, and the animals are euthanized by exsanguination.
Another incision is made longitudinally along the sternum, and then the diaphragm is separated from the thorax by cutting along its rib attachment edges.
To clear blood from the pulmonary vasculature[m6], saline solution is slowly injected through the right heart circulation by placing a needle into the right cardiac ventricle. Upon successful exsanguination, the lungs will turn from pink to white. For mice, 3–10 mL are required. This is optional, and is primarily done to eliminate excessive blood from samples, which in many cases, can interfere with downsteam immunohistochemical stains. Use of fixative for perfusion is possible, but not required for optimal airway fixation.
The lungs are fixed in situ by instilling 4% neutral buffered formalin intratracheally. For consistent results, the fixative should be instilled by siphoning from a large vessel held at a specific height above the mouse to keep the lungs inflated while maintaining constant pressure during fixation. For volume estimation, this is essential, and 5–30 cm H2O is physiologically relevant. Importantly, once a set pressure is chosen, this should be kept constant across experiments.
After 30 min, the lungs should be removed and kept in fixative overnight at 4°C.
To prepare tissues for paraffin embedding, the right and left lungs should be separated from the trachea at the main stem bronchi. In mice, the left lung is a single lobe, and it is the largest. Therefore, it is commonly used for histology. Once separated, it is first divided into 3 pieces by making cuts in cross-section with a razor or scalpel blade. As shown in Fig. 1, the first cut is made ~1 mm cranial to the root of the lung (the entry point of the lobar bronchus), and the second cut is made immediately cranial to the diaphragmatic curvature. The central portion contains the axial bronchus, which is the predominant location of mucin production in mouse lungs (6,9,23). This can be carefully dissected into ~2 mm thick “filets” that can be embedded together in the same paraffin blocks to provide adequate sampling of mucin producing cells along the proximal-distal axis of the bronchus in a slide specimen.
Dissected tissues should be embedded in paraffin, cut into 5 µm thick sections, and collected on positively charged glass slides according to standard microtome procedures.
Figure 1.
Surgical preparation, lung dissection, and isolation of mucin-producing airways. Anesthetized mice are tracheostomized by dissecting superficial tissues, isolating the trachea, and inserting cannula through a small partial incision made in the trachea (A). For histology, the left lung is isolated and sectioned into three large pieces at the areas depicted by the dashed lines using a razor blade (B). The middle piece containing the main axial airway (gray) is further sectioned into 2–3 mm thick “filets.” This region contains an easily identifiable airway situated between the pulmonary artery and vein and running along the dorsal aspect of the lung. This is the axial airway. Filets should be embedded in paraffin while keeping this airway positioned for cross-sectioning onto microscope slides. Non-mucin producing regions (white) can be discarded or embedded separately and used for other staining procedures to fit investigators’ needs.
3.2 PAFS Staining (all procedures in this section take place at room temperature)
Slides are dewaxed in an organic solvent such as xylene, toluene, or a non-toxic substitute such as Histo-Clear® by incubating 2 × 10 min.
Slides are then rehydrated through graded ethanol solutions - 100% (2 × 2 min), 95% (2 × 2 min), 90% 1 × 1 min; 70% 1 × 1 min – and then submerged in ddH2O until staining. Once rehydrated, do not let the tissues dry until staining is complete (see Note 2).
Oxidize tissues for 10 min in freshly prepared 1% w/v periodic acid dissolved in ddH2O.
Rinse 3 × 5 min in ddH2O.
Treat with fluorescent Schiff’s reagent for 20 min (see Note 3).
Rinse 3 × 5 min in ddH2O and 2 × 5 min in acid alcohol (0.1 N HCl in 70% ethanol).
Air dry in a dark dust-free place.
Attach a cover slip with Canada balsam mounting medium. Keep slides on benchtop 2 h to overnight in the dark prior to imaging. Slides will be ready to image after setting overnight. (see Note 4)
3.3 Fluorescence microscopy
Place a slide on the microscope stage and obtain a focused image of a bronchial airway section at low magnification. Position the airway and use a computerized random number generator (e.g., http://www.random.org/), or a dodecahedral die, to identify a number between 1 and 12. This number will be used to re-position the specimen and center it to the corresponding hour graduations on an analog clock.
For observation of intracellular mucin and other glycoconjugates use Texas red excitation-emission. In our experience, this works best with a FITC/Texas red filter set (No. 51006, Chroma, Bellows Falls, VT). Samples are obtained under dual 500/573 nm peak excitation with image acquisition using dual emission with peaks at 531 nm (green) and 628 nm (red). Acriflavine Schiff’s reagent binds covalently to the aldehydes formed during periodate-mediated oxidation, and when excited over a broad range (380–580 nm) this label fluoresces red (600–650 nm emission). In this staining procedure, acriflavine also intercalates within nucleic acids as an acridine agent, and this fluoresces green.
Two images are acquired. The first is a green and red two-color image that demonstrates all cells and structures. This is used to make a boundary length measurement – in this case the length of the basement membrane – as described in the morphometry section below, see 3.4.
The second image is acquired as a red-only image. This can be attained by manually inserting a long pass (600-∞ nm) or a band pass (600–680 nm) filter or by digitally altering camera acquisition settings. Since the green emission due to intercalated acriflavine binding to nucleic acids overlies the cytosolic and nuclear compartments almost completely, the remaining signal in the epithelium is predominantly labeled glycoconjugates in cytoplasmic organelles, especially secretory granules. It is critical to obtain images that are suitable for image analysis below (see 3.4). The acquisition settings should be determined such that a largely non-mucin producing airway [m7](e.g. a naïve mouse bronchus, or a terminal bronchiole) has few or no visible red pixels.
For image analysis, files are best saved in formats such as a raw (.raw), bitmap (.bmp), or tagged image file format (.tif). “Lossy[DT8]” compression files such as JPEG (.jpg) can be used, but care should be taken to ensure that data-loss is minimized by not re-saving JPEG files.
3.4 Morphometry
Choose appropriate software. For morphometric analyses, there are numerous software packages available. A popular choice is NIH ImageJ. This freeware is Java based, so it can be used on multiple computing platforms. It is available for download at http://rsbweb.nih.gov/ij/. ImagePro (Media Cybernetics, Bethesda, MD) and other software packages for morphometry are commercially available. Similar functions to those described below are applicable to most software packages, but care should be taken in determining which is most suitable for the individual user.
Set image analysis parameters. Because pixel density, image intensity, and software/video signal amplification will be highly variable among labs, it is critical that steps be taken to set consistent image acquisition standards. This is done by establishing a working range wherein background signals from non-mucin producing airways are as low as possible during initial image acquisition. Using a terminal bronchiole, for example, the area of red pixel staining is assessed at all pixel intensity levels, and the lower limit of the pixel intensity histogram is raised above 0 to the highest value in which no pixels are detected on the image. In ImagePro, the lowest pixel value is usually set to 45 on a 0–255 scale. The precision of this is then assessed in separate non-mucin containing samples. Afterwards, a section containing abundant mucin is measured in order to ensure that all observable contents are detected using exactly the same settings (see Note 5).
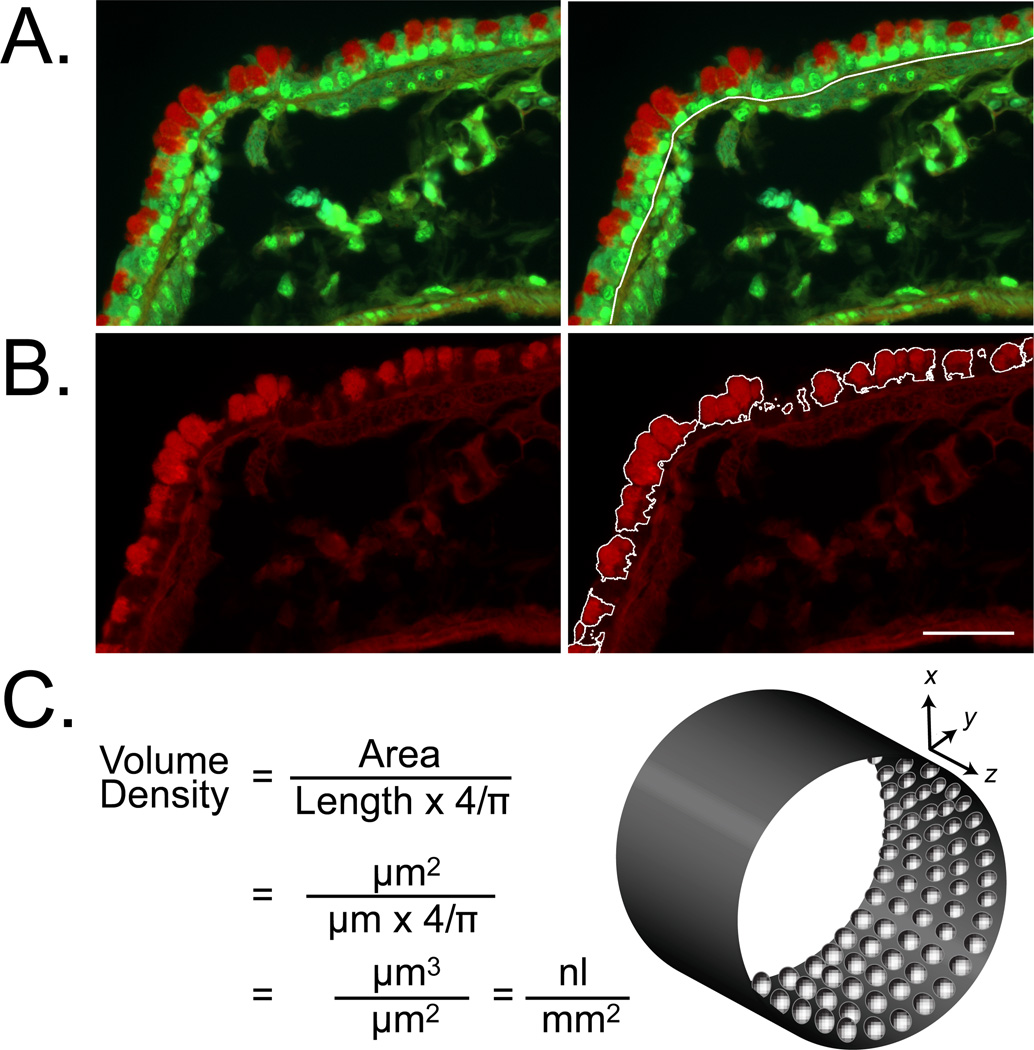
Measure the sample boundary length (Fig. 2A). Open a two-color image, and ensure that the correct spatial calibration setting is selected for the objective used to acquire the image. Select the appropriate length measurement tool in the software being used, and measure and record the length of the basal lamina.
Measure the area of mucin staining in the sample (Fig.ure 2B). Open the red-only one-color image corresponding to that used in Step 2 above. Measure the area of cytoplasmic red (number of red pixels) in the conducting airway epithelium. Stained fibers in the submucosa will also be highlighted. These need to be omitted from the final count. Record the remaining area of red staining.
Calculate mucin volume density (Fig. 2C). Divide the area of mucin staining by the product of the boundary length and 4/π. The resulting value is a volume density with units of volume of epithelial mucin per area of basement membrane. For a typical image measured on a µm scale, this value will have units of nL/mm2 (9) (see Note 6).
Figure 2.
Image analysis and quantitation of intracellular mucin content in PAFS stained airways. Tissues from antigen challenged mice were stained with PAFS and imaged using an Olympus BX-61 upright microscope under FITC/Texas Red dual fluorescence. In two color images (A) the full length of the airway region to be analyzed is measured (white line along basal lamina). In red-only images (B) the area of red in PAFS stained epithelium is identified and measured digitally. The area and length of staining are applied to the formula for volume density calculation (C) for conversion into a three-dimensional estimate of mucin volume per square millimeter of airway epithelium. Scale bar is 20 µm in A and B.
3.5. Extracted Mucins Specimen Preparation, Electrophoresis and Transfer
Perform surgical preparations as described above in Section 3.1, Steps 1–3. Using a syringe, inflate lungs with guanidium buffer containing protease inhibitors (0.035 mL buffer/g of body weight when inflating both lungs) (see Note 7).
Remove thoracic organs, and carefully remove the heart and thymus.
Homogenize the lungs in 1 mL of guanidium buffer and incubate at 4°C overnight mixing gently. Centrifuge at 16,000 × g for 30 min at 4°C, and collect supernatants.
Dialyze the supernatants against urea buffer using Slide-A-Lyzer Mini Dialysis Units, 2–10K MWCO, (Thermo Scientific, Rockford, IL) overnight at 4°C with gentle stirring.
Measure protein concentration by bicinchoninic acid (BCA) protein [m9]assay[MACC10] (Thermo) (see Note 8).
Prepare loading buffer. To reduce disulfide bonds and denature mucins, add 8 volumes of sample to 1 volume of 10X loading buffer. Incubate at 95°C for 20 min.
Alkylate free thiol moieties. Add 1 volume of alkylation buffer and incubate for 30 min at room temperature in the dark. Keep at 4°C until agarose gel electrophoresis.
Prepare 1% agarose (GenePure LE, ISC Bioexpress, Kaysville, UT) in TAE buffer and submerge in electrophoresis buffer (see Note 9). Load specimens and run at 90 V for 90 min or until the blue dye front has run ~90% of the gel length (see Note 10).
Wash the gel in 4X SSC for 5 min and then incubate for 20 min at room temperature in 4X SSC containing 10 mM DTT.
Transfer proteins to nitrocellulose membrane with a Vacuum Blotter (Model 785, Bio-Rad) at a pressure of 10 in Hg for 4 h at room temperature in 4x SSC buffer.
3.6. Detection of Blotted Mucins with Antibodies and Lectins
After transfer, wash the membrane two times in PBS and then block with the appropriate protein and/or detergent solution for the chosen detection system. When using antibodies, 5% (w/v) non-fat milk or bovine serum albumin in PBS is usually effective for blocking. However, when using lectins, these can cause nonspecific signal in the background. When using the Ulex europaeus agglutinin UEA-1 to detect fucosylated mucins, pre-incubation with a solution of 0.3% (v/v) Tween 20 in PBS is adequate.
Incubate with primary antibody or lectin either for 2 h at room temperature or overnight at 4°C.
Wash 3 × 10 min with 0.1% (v/v) Tween 20 in PBS.
Incubate with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. Alternatively, incubate in the dark with a fluorochrome-conjugated secondary antibody (see Note 11).
Wash 3 × 10 min with 0.1% (v/v) Tween 20 in PBS.
Detect and analyze signals. Perform enhanced chemiluminescence detection using commercially available kits per manufacturer’s instructions (see Materials). Scan films and perform densitometry using image analysis software (e.g., ImageJ). Alternatively, chemiluminescent or fluorescent signals may be directly detected using an advanced imaging system (e.g., Odyssey system (LI-COR Biosciences) to image and measure the signal intensities.
3.7. Interpretation of Data for Assessment of Mucin Secretion
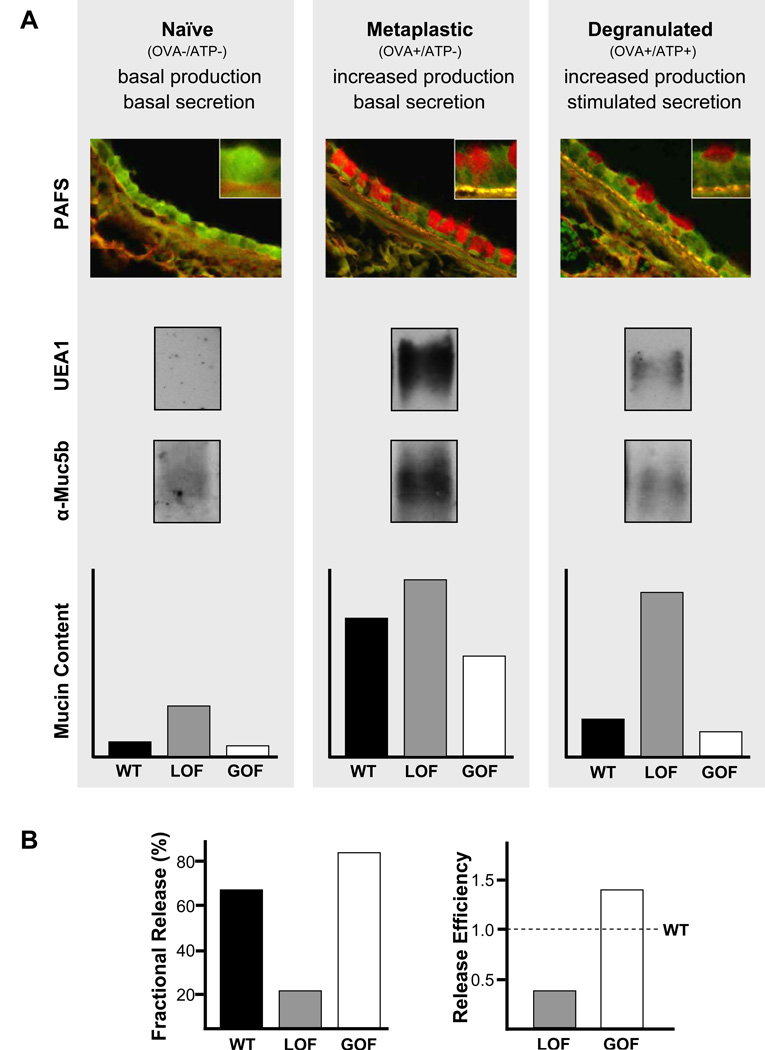
For assessment of a change in basal secretion rate, the most straightforward method is to compare intracellular mucin content (measured either by PAFS image analysis or probing of vacuum blots) between control and experimental groups. This will yield fold-increases of intracellular mucin content for a loss-of-function phenotype (see Note 12), and fractional decreases for a gain-of-function phenotype (Fig. 3A). An additional comparator should be provided for loss-of-function experiments by including a positive control specimen with robust mucous metaplasia (e.g. antigen challenged wild type mouse samples). Thus, the additional statement can be made that intracellular mucin accumulation due to the loss of secretory function results in a value equal to some fraction of that measured in mucous metaplasia.
For assessment of a change in stimulated secretory function, the most straightforward method is to first induce mucous metaplasia in control and experimental groups, then to compare intracellular mucin content between control and experimental groups after treatment with a maximal (100 mM) aerosolized ATP stimulus (Fig. 3A). In antigen challenged wild type mice, 60–75% of intracellular mucin is acutely released on average (see Note 13). . A loss-of-function phenotype is indicated by a reduced fractional release compared to control (Fig. 3B, left), and a gain-of-function phenotype is indicated by an increased fractional release (see Note 14). Data may be further analyzed as “release efficiency”, calculated as the fractional release of the experimental group divided by the fractional release of the control group. This yields numbers less than 1 for a loss-of-function and greater than 1 for a gain-of-function (Fig. 3B, right). Expressing data this way can be helpful when integrating multiple experiments in which there is variation in fractional release by control animals.
Since intracellular mucin content is low in naïve mice, an increase in basal secretion rate may be difficult to measure. Therefore, a gain in secretory function can also be measured by first inducing mucous metaplasia, then comparing intracellular mucin content between control and experimental groups after 7–14 days. In this case, a gain-of-function basal secretory phenotype is indicated by less intracellular mucin (see Note 15).
Figure 3.
Assessment of mucin secretion in vivo by measurement of intracellular mucin content. (A) Airway[DT11] epithelial mucin content is measured in three conditions – naïve, metaplastic, and [m12][MACC13]degranulated. These three conditions can be induced by treatment or not with ovalbumin immunization and airway challenge (OVA), followed or not by airway challenge with ATP. Treatment with OVA results in increased mucin production, and treatment with ATP induces acute mucin secretion. Intracellular mucin content after treatment can be measured by image analysis of tissue sections stained with PAFS, or lung tissue extracted with guanidinium hydrochloride, electrophoresed through 1% agarose, then transferred to nitrocellulose and probed with UEA1 lectin to detect Muc5ac or antibodies against Muc5b (all shown for wild-type mice only). Intracellular mucin content measured in each of these conditions (naïve, metaplastic, degranulated) by any of these methods is illustrated for wild-type (WT) mice (black bars), and for mice with a loss-of-secretory-function (LOF, grey bars) or gain-of-secretory-function (GOF, white bars) due to genetic mutation or pharmacologic treatment . (B) For further comparison of differences in stimulated secretory function between WT and LOF or GOF animals, “fractional release” may be calculated as mucin content in degranulated animals as a percentage of mucin content in metaplastic animals (left), or “release efficiency” may be calculated as fractional release by LOF and GOF animals divided by fractional release by WT animals (right).
Acknowledgements
This work was supported by NIH grants HL080396 (C.M.E.), HL094848 (B.F.D.) and HL097000 (B.F.D.), American Heart Association Grant 10GRNT4200070 (C.M.E.), and Cystic Fibrosis Foundation grant 08GO (B.F.D.). The authors thank C. William Davis for instruction in the performance of vacuum blotting of mucins.
Notes
Use acriflavine hydrochloride, not pure acriflavine, as the latter does not dissolve well in aqueous solvents.
It is important to use highly purified water for incubation steps and to thoroughly rinse glassware throughout the staining procedures, as impurities in tap water interfere with staining.
If a caustic sulfite smell does not emanate from the bottle upon opening and swirling, the reagent has deteriorated and should not be used.
Full “drying” takes approximately 2 weeks, and slides should be kept upright during that period to maintain cover slip centering. For long term storage and analysis of slides, aqueous mounting media commonly used for immunofluorescence are not recommended here. Use of these will result in excess diffusion of acriflavine from nucleic acids. They can be useful, however, for short-term colocalization experiments using immunofluorescence in combination with PAFS.
Video output varies among monitors and is not necessarily a reliable gauge of actual pixel values. If separate workstations are being used for image acquisition and analysis, it is highly recommended that users determine analysis parameters for the appropriate workstation.
This formula is a simplified version of that described by Weibel (24) and derived by Harkema and colleagues. (25).
It is possible to use one lung for PAFS staining and the other for vacuum blotting, or one lung for measurement of intracellular mucin by either technique and the other for other assays such as measurement of transcript levels or bronchoalveolar lavage fluid cell counts and cytokines. To do so, apply a clamp to the main bronchi connected to the lung to be preserved before starting to inflate. Alternatively, a thread can be used to make a knot to block guanidinium buffer flow to one of the lungs.
At high concentrations, urea can interfere with the BCA assay. Therefore it is recommended to dilute protein standards (usually ovalbumin) with the same buffer in which the specimens to be read are dissolved. For example, if a small portion of the specimen is diluted 1:5 in water before the BCA assay, it is suggested to use a 1:5 diluted urea buffer to prepare the standard. It is also important to keep in mind that excessive dilution in water can trigger mucin aggregation.
It is suggested to fill a 20 × 20 cm tray with 100 mL of 1% agarose and to utilize two combs to have two rows of 12 or 20 wells.
A working range of 50–100 µg of protein per specimen to be subjected to electrophoresis is suggested.
It is recommended to utilize a high molecular weight internal loading control to minimize loading errors. One example of a suitable housekeeping protein is APC (adenomatous polyposis coli) which is 312 kDa, and antibodies to APC can be purchased conjugated to horseradish peroxidase (Santa Cruz Biotechnology).
Muc5ac production is low in naïve mice, and UEA1 lectin staining of blotted proteins is usually not sufficiently sensitive to detect Muc5ac protein in these mice, complicating the quantitation of mucin accumulation.
If less than 50% of intracellular mucin is released in control animals, it may be best to discard the experiment, since this suggests a problem with aerosol delivery of intact secretagogue to the bronchial airways.
Since stimulated secretion is so high in wild type mice, a gain-of-function may be best uncovered using submaximal stimulation with a 10 mM ATP aerosol.
This technique may also be used to measure a subtle loss-of-function phenotype as increased intracellular mucin retention.
References
- 1.Davis CW, Dickey BF. Annu. Rev. Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. 487–512. [DOI] [PubMed] [Google Scholar]
- 2.Evans CM, Koo JS. Pharmacol. Ther. 2009;121:332–348. doi: 10.1016/j.pharmthera.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahy JV, Dickey BF. N. Engl. J. Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young HW, Williams OW, Chandra D, Bellinghausen LK, Perez G, Suarez A, Tuvim MJ, Roy MG, Alexander SN, Moghaddam SJ, Adachi R, Blackburn MR, Dickey BF, Evans CM. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Zhao YH, Wu R. Am. J Respir. Crit Care Med. 2001;164:1059–1066. doi: 10.1164/ajrccm.164.6.2012114. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, Dickey BF, Davis CW. J Physiol. 2008;586:1977–1992. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen LP, Omoluabi O, Parra S, Frieske JM, Clement C, mmar-Aouchiche Z, Ho SB, Ehre C, Kesimer M, Knoll BJ, Tuvim MJ, Dickey BF, Bond RA. Am J Respir Cell Mol. Biol. 2008;38:256–262. doi: 10.1165/rcmb.2007-0279RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zudhi Alimam M, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Am. J. Respir. Cell Mol. Biol. 2000;22:253–260. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]
- 9.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, Stripp BR, Dickey BF. Am J. Respir. Cell Mol. Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis CW, Lazarowski E. Respir. Physiol Neurobiol. 2008;163:208–213. doi: 10.1016/j.resp.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. Nat. Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 12.Tuvim MJ, Mospan AR, Burns KA, Chua M, Mohler PJ, Melicoff E, Adachi R, mmar-Aouchiche Z, Davis CW, Dickey BF. J Biol. Chem. 2009;284:9781–9787. doi: 10.1074/jbc.M807849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Am J Respir Cell Mol. Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 15.Tomkinson A, Cieslewicz G, Duez C, Larson KA, Lee JJ, Gelfand EW. Am J Respir Crit Care Med. 2001;163:721–730. doi: 10.1164/ajrccm.163.3.2005010. [DOI] [PubMed] [Google Scholar]
- 16.Trifilieff A, Ahmed E, Bertrand C. Am. J. Physiol Lung Cell Mol. Physiol. 2000;279:L1120–L1128. doi: 10.1152/ajplung.2000.279.6.L1120. [DOI] [PubMed] [Google Scholar]
- 17.Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. J. Clin. Invest. 2002;110:165–175. doi: 10.1172/JCI14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 19.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ, Chapman HA, Shapiro SD, Elias JA. J. Clin. Invest. 2000;106:1081–1093. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlstedt I, Lindgren H, Sheehan JK, Ulmsten U, Wingerup L. Biochem. J. 1983;211:13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aksoy N, Thornton DJ, Corfield A, Paraskeva C, Sheehan JK. Glycobiology. 1999;9:739–746. doi: 10.1093/glycob/9.7.739. [DOI] [PubMed] [Google Scholar]
- 22.Thornton DJ, Rousseau K, McGuckin MA. Annu. Rev Physiol. 2007 doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 23.Reader JR, Tepper JS, Schelegle ES, Aldrich MC, Putney LF, Pfeiffer JW, Hyde DM. Am. J. Pathol. 2003;162:2069–2078. doi: 10.1016/S0002-9440(10)64338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weibel ER. Sterological Methods. London: Academic Press Inc. Ltd; 1979. [Google Scholar]
- 25.Harkema JR, Plopper CG, Hyde DM, St George JA. J. Histochem. Cytochem. 1987;35:279–286. doi: 10.1177/35.3.2434556. [DOI] [PubMed] [Google Scholar]