Abstract
BACKGROUND & AIMS
Endoplasmic reticulum (ER) stress has been associated with development of inflammatory bowel disease. We examined the effects of ER stress–induced chaperone response and the orally active chemical chaperones tauroursodeoxycholate (TUDCA) and 4-phenylbutyrate (PBA), which facilitate protein folding and reduce ER stress, in mice with colitis.
METHODS
We used dextran sulfate sodium (DSS) to induce colitis in mice that do not express the transcription factor ATF6α or the protein chaperone P58IPK. We examined the effects of TUDCA and PBA in cultured intestinal epithelial cells (IECs); in wild-type, P58IPK−/−, and Atf6α−/− mice with colitis; and in Il10−/− mice.
RESULTS
P58IPK−/− and Atf6α−/− mice developed more severe colitis following administration of DSS than wild-type mice. IECs from P58IPK−/− mice had excessive ER stress, and apoptotic signaling was activated in IECs from Atf6α−/− mice. Inflammatory stimuli induced ER stress signals in cultured IECs, which were reduced by incubation with TUDCA or PBA. Oral administration of either PBA or TUDCA reduced features of DSS-induced acute and chronic colitis in wild-type mice, the colitis that develops in Il10−/− mice, and DSS-induced colitis in P58IPK−/− and Atf6α−/− mice. Reduced signs of colonic inflammation in these mice were associated with significantly decreased ER stress in colonic epithelial cells.
CONCLUSIONS
The unfolded protein response induces expression of genes that encode chaperones involved in ER protein folding; these factors prevent induction of colitis in mice. Chemical chaperones such as TUDCA and PBA alleviate different forms of colitis in mice and might be developed for treatment of inflammatory bowel diseases.
Keywords: IBD, Mouse Model, Ulcerative Colitis, Therapeutic Agent
The processes of protein folding, modification, and maturation in the endoplasmic reticulum (ER) are sensitive to environmental changes and multiple cellular disturbances, including ER Ca2+ depletion, defective glycosylation, metabolic stimuli, altered redox status, energy deprivation, inflammatory stimuli, and increased protein secretion. When ER protein folding is perturbed or when cells are stimulated to secrete large amounts of protein, unfolded/misfolded proteins accumulate in the ER lumen, a condition called ER stress.1 To restore ER function and improve protein-folding homeostasis (proteostasis), eukaryotes evolved the unfolded protein response (UPR). In mammalian cells, the UPR is initiated by 3 ER-localized transmembrane protein sensors: activating transcription factor 6α (ATF6α), inositol-requiring kinase 1α (IRE1α), and PKR-like ER kinase.2–4 UPR signaling can lead to either adaptation or apoptosis. In the adaptive UPR, ER protein folding is remodeled through transactivation of genes encoding ER chaperones, ER trafficking machinery and ER-associated protein degradation, and eIF2α phosphorylation-mediated global translation attenuation.5–8 Alternatively, prolonged and/or severe ER stress leads to the activation of the proapoptotic UPR, including the transcription factor CHOP and the IRE1α-activated c-Jun-N-terminal kinase (JNK) pathway.9 Moreover, chronic ER stress impairs cellular homeostasis through energy depletion, leakage of ER Ca2+, mitochondrial damage, oxidative stress, and activation of caspases.10 Therefore, persistent protein misfolding in response to chronic environmental stress and/or ineffective adaptive UPR signaling can compromise cell function and homeostasis and induce apoptosis.11
Recent studies link ER stress to the pathogenesis of inflammatory bowel disease (IBD). For example, patients with active Crohn’s disease and ulcerative colitis exhibit signs of ER stress in their ileal and/or colonic epithelium.12–15 In addition, human genetic studies of IBD have identified primary genetic abnormalities in several genes, including XBP1, AGR2, and ORMDL3, that encode proteins associated with ER stress.15–19 Previous studies have indicated that cells with a high load of protein folding and secretion are sensitive to altered ER homeostasis and this can induce inflammatory response gene expression.5,20,21 Intestinal microbiota and their molecules stimulate intestinal epithelial cells (IECs) to increase secretion of mucins and antimicrobial peptides that can overwhelm their protein secretory capacity. On the other hand, exposure to inflammatory stimuli can cause ER stress, although the precise mechanism is not well understood.21 On exposure to high levels of exogenous antigens and inflammatory cytokines in the intestinal lumen, IECs may require efficient UPR-mediated ER chaperone induction to survive the heavy burden of protein folding and secretion.
In this study, we show that protein misfolding in the ER caused by deletion of the ER cochaperone gene P58IPK/Dnajc3 exacerbates experimental colitis in mice. ATF6α is a potent transcriptional activator for a number of ER chaperone genes, including BiP, Grp94, and P58IPK in many cell types.2–4 Although whole body deletion of Atf6α does not generate an obvious phenotype under normal conditions, it is required for cells to survive chemical-induced ER stress.8 We found that in the absence of P58IPK or ATF6α, mice are sensitive to colitis and exhibit reduced induction of ER chaperone genes and hyperactivation of proapoptotic UPR signaling in colonic IECs. The chemical chaperones tauroursodeoxycholate (TUDCA) and 4-phenylbutyrate (PBA) are Food and Drug Administration–approved bioactive small molecules that function to facilitate protein folding and reduce ER stress both in vitro and in vivo by stabilizing protein-folding intermediates and preventing protein aggregation.22–28 In this study, we show that oral delivery of either TUDCA or PBA dramatically decreases the clinical, histologic, and biochemical signs of inflammation in both innate immunity– and T cell–dependent colitis through reducing ER stress signaling in colonic IECs.
Materials and Methods
Mice
Atf6α−/− and P58IPK−/− mice (C57BL/6J background) were described previously.8,29 Wild-type C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All animal care and procedures were conducted according to the protocols and guidelines approved by the University of Michigan University Committee on Use and Care of Animals and the Sanford-Burnham Medical Research Institute Institutional Animal Care and Use Committee.
Cell Culture
IEC-6 cells (passages 8–10) were kindly provided by Dr Linda Samuelson (Department of Physiology, University of Michigan Medical Center, Ann Arbor, MI). The cells were maintained in Dulbecco’s modified Eagle medium containing 4.5 mg/mL glucose and supplemented with 2 mmol/L l-glutamine, 10 mmol/L HEPES, 100 μg/mL streptomycin, and 100 U/mL penicillin. Cells were seeded 5 × 105 cells/well in 12-well plates, and all experiments were performed 1 day after cultures reached confluence. Cells were treated with 100 ng/mL rat tumor necrosis factor (TNF)-α (R&D Systems, Minneapolis, MN), 100 ng/mL rat MCP-1 (R&D Systems), 25 ng/mL rat interleukin (IL)-1β (R&D Systems), and 5 mmol/L PBA (Scandinavian Formulas, Inc, Sellersville, PA) or TUDCA (EMD Chemicals, Billerica, MA).
Results
Dextran Sodium Sulfate–Induced Colitis Induces ER Stress in Colonic Epithelium
To characterize the role of ER stress and the UPR in IECs during intestinal inflammation, we used the dextran sodium sulfate (DSS)-induced colitis murine model. C57BL/6J mice at 8 weeks of age were fed 3% DSS in their drinking water for 3 or 5 days to develop colitis in the large intestine. The purity of isolated colonic epithelial cells was greater than 95%, measured by flow cytometry using an antibody against murine epithelial cell adhesion molecule (Supplementary Figure 1A). Analysis of UPR markers, including the ER chaperone BiP, phosphorylation of the ER stress sensor PKR-like ER kinase, and the UPR transcription factors ATF4, CHOP, and spliced XBP1 showed a time-dependent induction in colonic epithelium that coincided with progression of DSS-induced colitis. A similar messenger RNA induction pattern of UPR genes was observed by quantitative reverse-transcription polymerase chain reaction analysis and immunohistochemistry (IHC) staining (Supplementary Figure 1C and D). The ER stress induction in the DSS-induced colitis model is similar to that observed in intestinal tissues from patients with active IBD,12–15 suggesting that this experimental colitis model is valid for studies of ER stress and the UPR in IECs on intestinal inflammation.
The ER Cochaperone P58IPK Protects From DSS-Induced Colitis
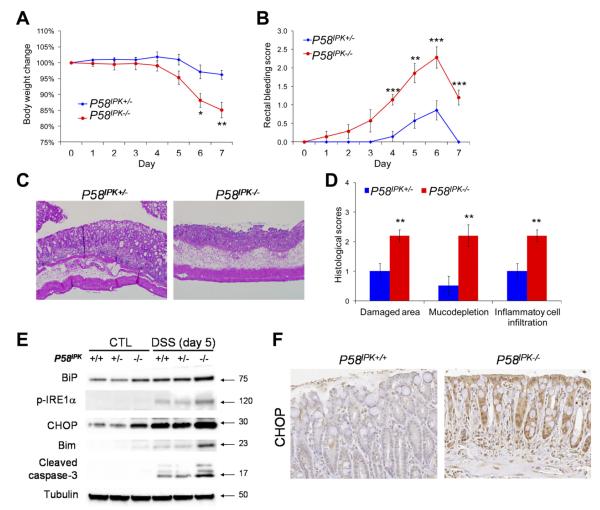
To determine the requirement for proper protein folding in the ER for IEC function, we analyzed mice with deletion in P58IPK. P58IPK is a heat-shock 40-kilodalton protein that belongs to the DNAJ chaperone family and resides in the ER lumen in association with the ER chaperone BiP and promotes proper protein folding.30–32 The UPR induces transcription of P58IPK, while cells and mice deleted in P58IPK display slight protein misfolding and are sensitive to ER stress.29,30 The role of P58IPK in colonic epithelia was studied using bone marrow chimeras of P58IPK+/− and P58IPK−/− mice to exclude the effect of P58IPK deletion in hematopoietic cells. On challenge with DSS, P58IPK−/− mice displayed severe body weight loss, rectal bleeding, and shortening of the large intestine (Figure 1A and B and Supplementary Figure 2A). Consistently, the P58IPK−/− mice showed significantly more severe mucosal damage, loss of goblet cells, and inflammatory cell infiltration in the colon compared with their heterozygous littermates (Figure 1C and Supplementary Figure 2B). After DSS challenge, immunoblots showed up-regulation of ER stress markers BiP and phosphor-IRE1α in isolated colonic IECs from P58IPK−/− mice compared with both P58IPK+/+ and P58IPK+/− mice. The proapoptotic transcription factor CHOP was also induced in P58IPK−/− colonic IECs before and after treatment with DSS (Figure 1E). Consistently, IHC indicated increased expression of CHOP in P58IPK−/− colonic epithelium with DSS-induced colitis (Figure 1F). Bim, a BH3-only member of the Bcl-2 family that is transactivated by CHOP, plays a critical role in ER stress–induced apoptosis.33 On DSS challenge, Bim and cleaved caspase-3 were highly induced in the colonic epithelium of P58IPK−/− mice compared with both P58IPK+/+ and P58IPK+/− mice (Figure 1E). These data suggest that the elevated susceptibility of P58IPK−/− mice to DSS-induced colitis is due to a hyperactivated ER stress response and proapoptotic UPR signaling, including CHOP and Bim, in colonic IECs during inflammation.
Figure 1.
Loss of P58IPK exacerbates DSS-induced colitis in mice due to a hyperactivated proapoptotic UPR. P58IPK+/− and P58IPK−/− littermates with wild-type bone marrow cells were fed 2.5% DSS in drinking water for 5 days, followed by 2 days of fresh water. (A) Body weight and (B) rectal bleeding were measured over 7 days. (C) After administration of DSS, the colons were isolated and fixed for H&E staining. Representative images are shown (original magnification 100×). (D) Histologic scores were measured in mice with DSS-induced colitis. (E) The proapoptotic transcription factor CHOP was induced whereas antiapoptotic Bcl2 was reduced in P58IPK−/− IECs. (F) IHC shows CHOP is induced in P58IPK−/− colonic epithelium with DSS-induced colitis. n = 7 for each group. *P < .05, **P < .01, ***P < .001.
ATF6α-Mediated ER Chaperone Induction Protects Against DSS-Induced Colitis
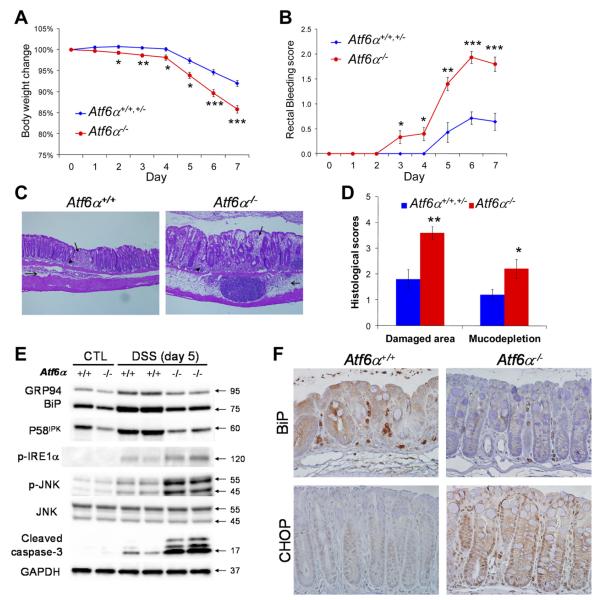
To further elucidate the role of ER chaperones induced during intestinal inflammation, we studied mice with a deletion in Atf6α, the master regulator of ER chaperone gene expression. The colon of Atf6α−/− mice is indistinguishable from wild-type, as indicated by H&E and periodic acid–Schiff staining (Supplementary Figure 3A). Age- and sex-matched Atf6α+/+, Atf6α+/−, and Atf6α−/− littermate mice were reconstituted with wild-type bone marrow cells. The genetic ablation of Atf6α exacerbated symptoms of DSS-induced colitis, including severe body weight loss, rectal bleeding (Figure 2A and B), and significantly greater mucosal damage, goblet cell loss, and macrophage infiltration in the colon (Figure 2C and D and Supplementary Figure 3B). Atf6α−/− mice displayed reduced expression of ER chaperone genes, including BiP, Grp94, and P58IPK, in both protein and messenger RNA levels (Figure 2E and Supplementary Figure 3C), indicating that the adaptive UPR signaling in colonic IECs of Atf6α−/− mice is compromised in response to intestinal inflammation. In contrast, the proapoptotic IRE1α-JNK pathway was induced in inflamed colonic epithelium of Atf6α−/− mice (Figure 2E). After induction of colitis, the apoptotic markers cleaved caspase-3 and DNA fragmentation (by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling [TUNEL] staining) were also increased in colonic epithelium of Atf6α−/− mice compared with Atf6α+/+ and Atf6α+/− mice (Figure 2E and Supplementary Figure 3D and E). Consistently, IHC showed reduced expression of the ER chaperone BiP and increased expression of the proapoptotic transcription factor CHOP in Atf6α−/− colonic epithelium (Figure 2F). These data are consistent with the notion that ATF6α is an important transactivator of ER chaperone genes in colonic IECs during colitis. The impaired ER chaperone induction in Atf6α−/− mice leads to unresolved ER stress, which induces proapoptotic UPR signaling in colonic IECs and exacerbates DSS-induced colitis.
Figure 2.
Loss of ATF6α exacerbates DSS-induced colitis in mice due to defective ER chaperone induction and a hyperactivated proapoptotic UPR. Atf6α+/+, Atf6α+/−, and Atf6α−/− littermates with wild-type bone marrow cells were fed 3% DSS in drinking water for 5 days, followed by 2 days of fresh water. (A) Body weight and (B) rectal bleeding were measured over 7 days. After administration of DSS, the colons were isolated and fixed for H&E staining. Representative images are shown (C; original magnification 100×). (D) Histologic scores were measured in mice with DSS-induced colitis. (E) The expression of ER chaperones BiP, GRP94, and P58IPK is reduced whereas the proapoptotic IRE1α-JNK pathway and caspase-3 are activated in Atf6α−/− IECs with DSS-induced colitis. (F) IHC shows that expression of the ER chaperone BiP is impaired whereas the proapoptotic transcription factor CHOP is increased in Atf6α−/− colonic epithelium with DSS-induced colitis. n = 14 or 15. *P < .05, **P < .01, ***P < .001.
TUDCA and PBA Alleviate Inflammation-Induced ER Stress in an IEC Line
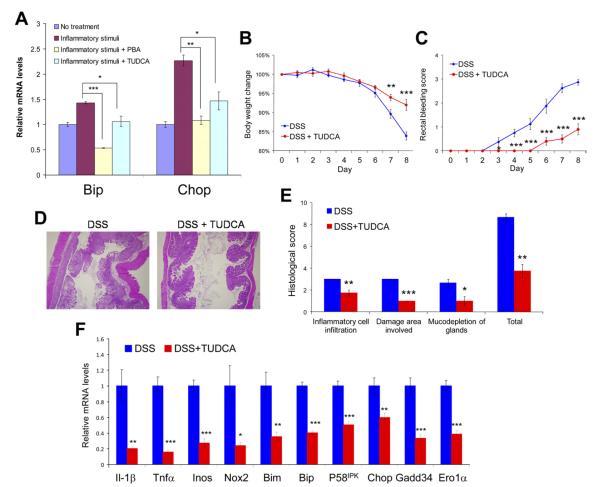
Given the protective role of the ER chaperone response in IECs against intestinal inflammation, we tested whether the chemical chaperones TUDCA and PBA can reduce ER stress in IECs and alleviate colitis in mice. We first analyzed the effect of PBA and TUDCA in the nontransformed rat IEC line IEC-6 that was treated with physiologically relevant stimuli to cause ER stress. The inflammatory cytokines TNF-α, MCP-1, and IL-1β are highly up-regulated in animal models of enterocolitis and patients with IBD. We found that a combined cocktail of TNF-α, MCP-1, and IL-1β induces ER stress in IEC-6 cells, as monitored by the up-regulation of ER stress markers BiP and CHOP (Figure 3A). Prior treatment and cotreatment of IEC-6 cells with either PBA or TUDCA reduced CHOP and BiP expression in response to the inflammatory stimuli (Figure 3A). These data indicate that proinflammatory cytokines induce ER stress in IECs in vitro, and this cellular stress can be mitigated by treatment with either PBA or TUDCA.
Figure 3.
TUDCA and PBA alleviate inflammatory stimuli-induced ER stress in IECs in vitro; TUDCA ameliorates DSS-induced colitis by reducing ER stress in colonic epithelium in vivo. (A) IEC-6 cells were treated with a combination of inflammatory cytokines (TNF-α, MCP-1, and IL-1β) for 8 hours or pretreated with 5 mmol/L TUDCA or PBA for 4 hours, followed by treatment with the same inflammatory signals with 5 mmol/L TUDCA or PBA for 8 hours. The cells were then collected for RNA extraction and quantitative reverse-transcription polymerase chain reaction. The messenger RNA levels were normalized to the expression of 18S ribosomal RNA. Wild-type mice were fed 2.5% DSS in drinking water and received 500 mg/kg body wt TUDCA or the same amount of phosphate-buffered saline (PBS) without TUDCA daily by gavage (n = 8 or 10 per group). (B) Body weight and (C) rectal bleeding were measured over 8 days. (D) After administration of DSS, the colons were isolated and fixed for H&E staining. Representative images are shown (original magnification 40×). (E) Histologic scores are shown from TUDCA-treated and control mice with DSS-induced colitis. (F) Expression of genes associated with inflammation, oxidative stress, and apoptosis in colonic mucosa as well as ER stress markers in colonic IECs is shown (normalized to the expression of Gapdh). n = 8 for each group; *P < .05, **P < .01, ***P < .001.
TUDCA and PBA Alleviate Signs of DSS-Induced Colitis by Reducing ER Stress Signaling in Colonic Epithelial Cells
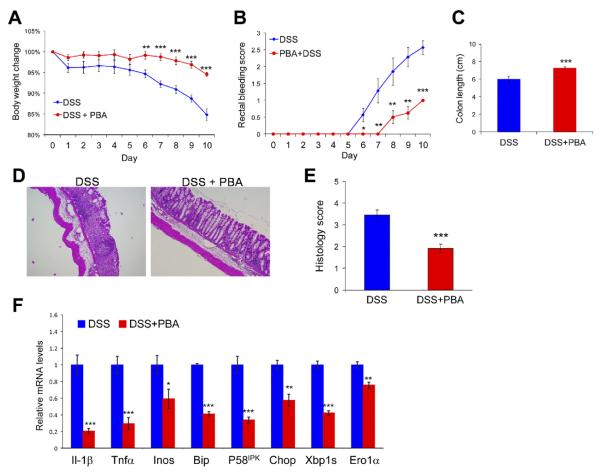
The therapeutic potential of chemical chaperones was tested in a prevention paradigm in the DSS-induced colitis murine model. C57BL/6J mice were fed 2.5% DSS in their drinking water for 8 days. TUDCA was administrated orally by gavage at 500 mg/kg body wt per day (single dose) throughout the whole period. Compared with mice subjected to DSS challenge only, feeding of TUDCA significantly ameliorated the symptoms of DSS-induced colitis, as indicated by the lower clinical scores (Figure 3B and C and Supplementary Figure 4). TUDCA dramatically reduced the histologic manifestations of DSS-induced colitis (Figure 3D and E). In parallel, the expression of proinflammatory cytokines IL-1β and TNF-α in the colon was significantly reduced by feeding of TUDCA (Figure 3F). Additionally, the induction of oxidative/nitrosative stress and apoptotic signaling was inhibited by treatment with TUDCA, as indicated by diminished expression of genes encoding iNOS, NOX2, and Bim (Figure 3F). As expected, the induction of ER stress markers BiP, P58IPK, CHOP, GADD34, and ERO1α in colonic IECs was considerably reduced in mice fed with TUDCA during the induction of DSS colitis (Figure 3F), which is consistent with the observations in IEC-6 cells treated with inflammatory signals and TUDCA. Similarly, feeding of PBA dramatically ameliorated the symptoms of DSS-induced colitis (Figure 4A–E). Induction of ER stress in colonic epithelium was significantly reduced on administration of PBA during the induction of DSS colitis (Figure 4F).
Figure 4.
PBA alleviates DSS-induced colitis by reducing ER stress in colonic epithelium. Wild-type mice were fed 2% DSS in drinking water and received 500 mg/kg body wt PBA or the same amount of PBS without PBA daily by gavage. (A) Body weight and (B) rectal bleeding were measured over 10 days. (C) Colon lengths were measured after induction of DSS colitis. (D) After administration of DSS, the colons were isolated and fixed for H&E staining. Representative images are shown (original magnification 40×). (E) Histologic scores are shown from control and PBA-treated mice with DSS-induced colitis. (F) Expression of genes associated with inflammation, oxidative stress, and ER stress in colonic mucosa as well as ER stress markers in colonic IECs is shown (normalized to the expression of Gapdh). n = 8 for each group; *P < .05, **P < .01, ***P < .001.
TUDCA and PBA Reverse DSS-Induced Chronic Colitis
We then examined whether the chemical chaperones can reverse the symptoms of chronic colitis. The mice were fed with 3 cycles of 2% DSS in drinking water and then received 300 mg/kg body wt TUDCA daily (double dose, 150 mg/kg body wt per dose) by oral administration for 10 days. Dramatically, the histologic scores of the large intestine, including damaged area involved, ulceration, mucodepletion of glands, and inflammatory cell infiltration, were significantly reduced after the administration of TUDCA compared with the controls (Figure 5A and B). For delivery of PBA, C57BL/6J mice with chronic colitis were fed 500 mg/kg body wt PBA daily (double dose, 250 mg/kg body wt per dose) for 10 days. PBA-treated mice also showed a similar recovery from chronic colitis (Figure 5C and D).
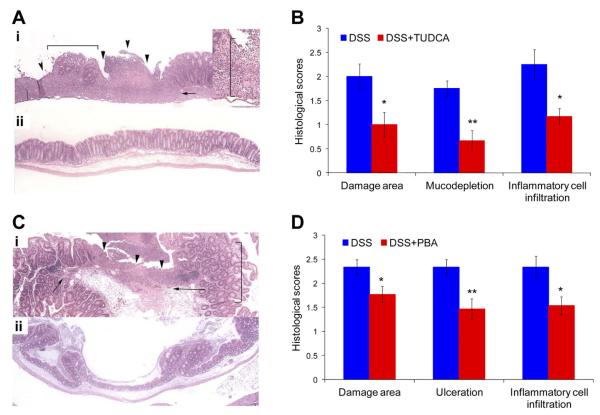
Figure 5.
Either TUDCA or PBA mitigates inflammation in mice with chronic colitis. (A) Wild-type mice with established chronic DSS-induced colitis received 300 mg/kg body wt TUDCA or the same amount of PBS without TUDCA (control) daily by gavage for 10 days. (a) Colon from a mouse with chronic colitis fed with PBS alone (control), showing severe epithelial ulceration (arrowheads), loss of goblet cell morphology (mucodepletion; bracket), and transmural inflammatory infiltrate (arrow). Inset shows transmural inflammatory infiltrate at the edge of an ulcer (bracket). (b) Mice with chronic colitis treated with TUDCA display reduced mucosal damage and inflammation (original magnification 40×; 100× for inset). (B) Histologic scores, including damaged area involved, mucodepletion of glands, and inflammatory cell infiltration, are shown from TUDCA-treated and control mice with chronic DSS-induced colitis. n = 6 or 8 for each group. (C) Wild-type mice with established chronic DSS-induced colitis received 500 mg/kg body wt PBA or the same amount of PBS without PBA (control) daily by gavage for 10 days. (a) Cecum from a mouse with chronic colitis fed with PBS alone (control) showing severe ulceration (arrowheads), inflammatory infiltrates (arrows), and loss of goblet cell morphology (bracket). (b) Cecum from a mouse with chronic colitis treated with PBA showing reduced mucosal damage and inflammation (original magnification 40×). (D) Histologic scores are shown from PBA-treated and control mice with chronic DSS-induced colitis. n = 9 or 13 for each group. *P < .05, **P < .01.
TUDCA and PBA Complement the Requirement for P58IPKand ATF6α in Preventing DSS-Induced Colitis
To further explore the molecular mechanisms of how chemical chaperones function in alleviating intestinal inflammation, we tested the 2 compounds on DSS-induced colitis in the P58IPK or Atf6α mice. Where P58IPK−/− and Atf6α−/− mice were more susceptible to DSS-induced colitis, as shown in Figures 1 and 2, feeding of TUDCA or PBA reduced the clinical and histologic scores of P58IPK−/− and Atf6α−/− mice to levels similar to those of their littermate controls (Figure 6A–F). These data indicate that the chemical chaperones can complement the requirement for molecular chaperones, correct the protein folding defects in animals with an impaired ER chaperone response, and protect mice against intestinal inflammation.
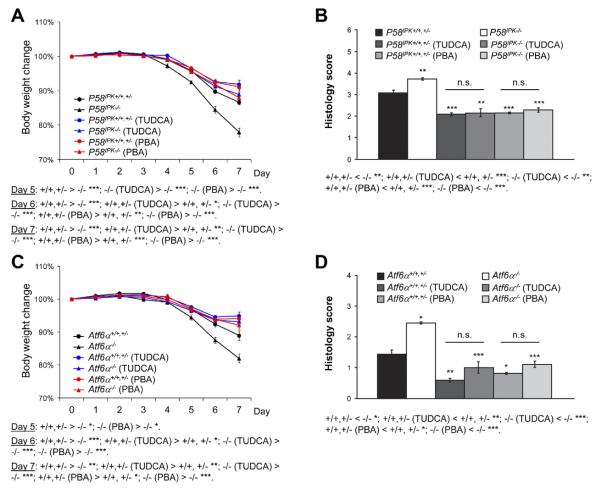
Figure 6.
Feeding of TUDCA or PBA corrects the defects of P58IPK−/− and Atf6α−/− mice in response to DSS-induced colitis. P58IPK+/+, P58IPK+/−, and P58IPK−/− mice and Atf6α+/+, Atf6α+/−, and Atf6α−/− mice were reconstituted with wild-type bone marrow cells and fed 3% DSS in drinking water for 5 days, followed by 2 days of fresh water. During the same period, the animals received 500 mg/kg body wt TUDCA, PBA, or the same amount of PBS without TUDCA/PBA daily by gavage. (A and C) Body weight was measured over the 7-day period. (B and D) After treatment, the colons were isolated and fixed for H&E staining and histologic scoring. n = 7–14 for each group; *P < .05, **P < .01, ***P < .001.
TUDCA and PBA Dramatically Mitigate Colitis in Il10−/− Mice
Because TUDCA and PBA can alleviate both acute and chronic colitis induced by DSS, we then examined whether the 2 chemical chaperones can ameliorate nonsteroidal anti-inflammatory drug–induced colitis in Il10−/− mice, a T cell–dependent IBD model. Il10−/− mice with established colitis received either TUDCA or PBA in the drinking water at a concentration of 2 mg/mL (5.2 mg/mouse per day) for 3 weeks. Dramatically, the histologic scores of the large intestine were dramatically reduced after the administration of either TUDCA or PBA (Figure 7A [upper and middle panels], B, and C). Furthermore, trichrome staining indicated that fibrosis in the large intestine was significantly reduced by the feeding of TUDCA or PBA (Figure 7A [lower panel] and D). In the colonic epithelia of Il10−/− mice with colitis, the induction of ER stress markers including BiP, phospho-eIF2α, and CHOP, mitochondrial UPR marker HSP60, as well as apoptotic markers including cleaved caspase-3/12 were considerably reduced after treatment with TUDCA or PBA (Figure 7E). These data show that the chemical chaperones have potent anti-inflammatory and antifibrotic effects in the ll10−/− colitis model through the suppression of ER stress and cell death in colonic epithelium.
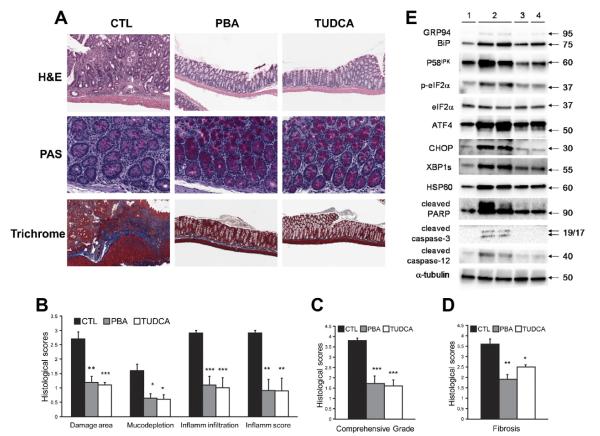
Figure 7.
Either TUDCA or PBA alleviates chronic colitis in Il10−/− mice. Il10−/− mice with piroxicam-induced colitis received 2 mg/mL PBA or TUDCA in the drinking water for 3 weeks. (A) Feeding of TUDCA or PBA reduces signs of chronic colitis in Il10−/− mice. Periodic acid–Schiff (PAS) staining shows mucin in goblet cells, and trichrome staining indicates collagen deposition in the colon. (B) Histologic scores are shown using the standard of Otuska et al.49 (C) Histologic scores are shown using the standard of Berg et al.50 (D) Histologic scores are shown for colonic fibrosis. n = 10 –11 for each group. (E) The mice were killed after the experiment, and the colonic IECs were isolated for protein extraction and Western blotting. Representative immunoblots are shown. 1, no piroxicam-induced colitis; 2, colitis; 3, colitis → TUDCA; 4, colitis → PBA. *P < .01, **P < .001, ***P < .0001.
Discussion
Recent studies indicate that inflammatory conditions in the gastrointestinal tract can activate the UPR in IECs.12–15 However, it is unknown whether these pathway(s) function to disrupt cellular homeostasis and induce apoptosis or to restore ER function and prevent cell death. Previous studies showed that P58IPK binds to newly synthesized secretory proteins in the ER and promotes protein folding/maturation in cells.30–32 Consistent with a role for P58IPK in reducing protein misfolding in the cell, we showed that P58IPK prevents dysfunction of IECs and progression of DSS-induced colitis. During the development of colitis, P58IPK−/− mice display increased expression of the proapoptotic factor CHOP and reduced expression of the prosurvival protein Bcl2 in IECs due to an unresolved/prolonged ER stress. CHOP is a major cell death–inducing factor during the UPR9,15 and has been shown to exacerbate colitis in mice.34 Previous studies have shown that a hypomorphic mutation in the gene encoding S1P in mice enhances the sensitivity to DSS-induced colitis.35 However, given that S1P targets several ER stress–induced bZIP transcription factors, including Luman, OASIS, and CREBH, as well as the SREBPs,36 it was not clear whether the increased susceptibility is attributed to reduced activation of ATF6 or other transcription factors. In this study, we showed that colonic IECs from Atf6α−/− mice have reduced adaptive ER chaperone expression and increased proapoptotic UPR signaling, including the IRE1α-JNK pathway.9,15 In mice with an IEC-specific deletion of Xbp1, hyperphosphorylated IRE1α activates JNK and induces spontaneous inflammation in the ileum.15,37 Therefore, compromised ER chaperone expression, by loss of either an ER chaperone itself or the upstream transactivator, leads to unresolved ER stress and activation of proapoptotic signaling and therefore impairs cell function and exacerbates inflammation. In these experiments, we used bone marrow chimeras to exclude the effect of P58IPK or Atf6α deletion in hematopoietic cells, including macrophages, neutrophils, T cells, and B cells, during intestinal inflammation. However, bone marrow reconstitution is not able to replace lamina propria fibroblasts and smooth muscle cells in the gut, which may still contribute to colitis. Villin-Cre–directed conditional deletion models, if available, would be ideal for this study.
Consistent with the protective role of ER chaperones in IECs for intestinal homeostasis, we showed that the chemical chaperones TUDCA and PBA, which promote ER homeostasis and increase ER folding capacity, reduce ER stress signaling in IECs and alleviate colitis in mice. Furthermore, we showed that feeding of TUDCA or PBA corrected the defects in P58IPK−/− and Atf6α−/− mice with impaired ER chaperone induction during DSS-induced colitis, suggesting that TUDCA and PBA resolve intestinal inflammation due to their function in promoting protein folding and alleviating ER stress.
Recent studies showed that TUDCA inhibits the expression of UPR genes in an intestinal epithelial cell line induced by the widely used ER stressor tunicamycin.38 In our study, we showed that either TUDCA or PBA reduces ER stress gene expression in IECs induced by inflammatory stimuli that are physiologically relevant to IBD. More strikingly, the feeding of either TUDCA or PBA dramatically protected the intestinal mucosa in both a DSS-induced model and a T cell–dependent genetic model of colitis. These data suggest that TUDCA and PBA exert an epithelial-protective effect during the intestinal inflammation that is predominated by either the innate or adaptive immune response in the mucosa.
The chemical chaperones TUDCA and PBA are Food and Drug Administration–approved drugs that have outstanding safety profiles in humans. TUDCA is safely used as a hepatoprotective drug for the treatment of primary biliary cirrhosis.39 PBA is approved for clinical use in urea cycle disorders.40 Both compounds are in clinical trials for the treatment of a number of diseases that are associated with protein misfolding in the ER, including cystic fibrosis, amyotrophic lateral sclerosis, spinal muscular atrophy, Huntington’s disease, and type 2 diabetes (http://clinicaltrialsfeeds.org/clinical-trials/results/term=TUDCA; http://clinicaltrialsfeeds.org/clinical-trials/results/intr=4-phenylbutyric+acid).2,41–44 Current medications used to treat IBD, including corticosteroids, immunosuppressants, and biologics, have significant risks and adverse effects.45–47 If efficacy can be shown in patients with IBD, given the safety profile and potential for oral delivery of TUDCA and PBA, this therapy could fill an important gap in our current therapeutic armamentarium. Ursodeoxycholate, the unconjugated bile salt of TUDCA, is a promising drug for chemoprevention of colorectal cancer.48 Although TUDCA can inhibit inflammation-induced ER stress in nontransformed IEC-6 cells, it exacerbates ER stress in some colon cancer cell lines (unpublished results May, 2012.) It would be worthwhile to determine whether TUDCA is able to suppress the growth of carcinogenic colonocytes while protecting normal colonic epithelial cells against ER stress in patients with ulcerative colitis. Based on our findings in multiple murine models of colitis, the chemical chaperones TUDCA and PBA may warrant clinical investigation as a novel treatment for IBD.
Supplementary Material
Supplementary Figure 1. DSS colitis induces ER stress in colonic epithelial cells. (a) The purity of isolated colonic epithelial cells was determined by flow cytometry using an antibody against murine EpCAM. (b) Wild-type mice were fed 3% DSS in the drinking water for 0, 3 and 5 days. After DSS administration for the indicated days, the mice were euthanized and the colonic IECs were isolated for protein extraction and western blotting. Representative immunoblots of colon tissues from mice are shown. (c) Wild-type mice were fed 3% DSS in the drinking water for 0, 2, 4 and 6 days. After DSS administration for the indicated days, the mice were euthanized and the colonic IECs were isolated for RNA extraction and Q-RT-PCR. The mRNA levels were normalized to the expression of Gapdh. (d) Wild-type mice were fed 3% DSS in the drinking water for 6 days, then the mice were euthanized and the colons were removed, fixed and paraffin embedded for immunohistochemical staining of BiP, CHOP and XBP1. Representative immunohistochemical images are shown; each group contained a minimum of 6 individual mice.
Supplementary Figure 2. P58IPK−/− mice exhibit shorter colon lengths (a) and higher levels of macrophage marker F4/80 in colonic mucosa (b) upon DSS colitis.
Supplementary Figure 3. (a) Atf6α−/− mice display normal colon morphology under normal conditions. Colons were isolated from two-month old Atf6α+/+ and Atf6α−/− littermates mice for H&E and PAS staining. (b) Atf6α−/− mice showed higher levels of macrophage marker F4/80 in colonic mucosa upon DSS colitis. (c) Atf6α−/− mice exhibit an impaired induction of ER chaperone genes including Bip, Grp94, P58IPK and Pdi in colonic IECs upon DSS colitis. (d) Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-staining of apoptotic epithelial cells in the colon sections from Atf6α+/+ and Atf6α−/− mice with DSS colitis. (e) The apoptotic indices were calculated as the number of TUNEL-positive epithelial cells per 100 randomly selected crypts in the colon sections. 4 mice per group; * p < 0.05.
Supplementary Figure 4. Shortening of colon lengths upon DSS colitis was significantly mitigated by the feeding of TUDCA.
Supplementary Table 1. Sequence of primers used in this
Acknowledgments
The authors thank Dr Michael Katze (Department of Comparative Medicine, University of Washington, Seattle, WA) for providing P58IPK−/− mice, Dr Linda Samuelson (University of Michigan Medical Center, Ann Arbor, MI) for providing IEC-6 cells, University of Michigan Comprehensive Cancer Center Tissue Core and Histopathology Core at Sanford Burnham Medical Research Institute (SBMRI; La Jolla, CA) for histologic analysis, Jian Xing of SBMRI Gene Analysis Facility for technical support of quantitative reverse-transcription polymerase chain reaction, Yoav Altman of SBMRI Flow Cytometry Core for flow cytometry analysis, Jenna Rousseau and Joseph Burzynski at University of Michigan, and Francis Lee and Kinh-Vy Nguyen at SBMRI for general technical support.
Funding Supported by National Institutes of Health grants P01 HL057346, R37 DK042394, R01 DK088227, and R01 HL052173 and a Crohn’s and Colitis Foundation of America Senior Research Award 3800 (to R.J.K.).
Abbreviations used in this paper
- ATF6α
activating transcription factor 6α
- DSS
dextran sulfate sodium
- ER
endoplasmic reticulum
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cell
- IHC
immunohistochemistry
- IL
interleukin
- IRE1α
inositol-requiring kinase 1α
- JNK
c-Jun-N-terminal kinase
- PBA
4-phenylbutyrate
- PBS
phosphate-buffered saline
- TNF
tumor necrosis factor
- TUDCA
tauroursode-oxycholate
- UPR
unfolded protein response
Footnotes
Supplementary Material Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2013.01.023.
Author names in bold designate shared co-first authorship.
Conflicts of interest The authors disclose no conflicts.
References
- 1.Dorner AJ, Wasley LC, Kaufman RJ. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J Biol Chem. 1989;264:20602–20607. [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutkowski DT, Arnold SM, Miller CN, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao SS, Kaufman RJ. Unfolded protein response. Curr Biol. 2012;22:R622–R626. doi: 10.1016/j.cub.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Rutkowski DT, Dubois M, et al. ATF6alpha optimizes longterm endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhotra JD, Miao H, Zhang K, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 12.Shkoda A, Ruiz PA, Daniel H, et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Hu S, Ciancio MJ, Fujiya M, et al. Translational inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology. 2007;133:1893–1904. doi: 10.1053/j.gastro.2007.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heazlewood CK, Cook MC, Eri R, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaser A, Lee AH, Franke A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGovern DP, Gardet A, Torkvist L, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42:332–337. doi: 10.1038/ng.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng W, Rosenstiel P, Huse K, et al. Evaluation of AGR2 and AGR3 as candidate genes for inflammatory bowel disease. Genes Immun. 2006;7:11–18. doi: 10.1038/sj.gene.6364263. [DOI] [PubMed] [Google Scholar]
- 18.Park SW, Zhen G, Verhaeghe C, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci U S A. 2009;106:6950–6955. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao F, Edwards R, Dizon D, et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev Biol. 2010;338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertolotti A, Wang X, Novoa I, et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107:585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang K, Shen X, Wu J, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 22.Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci U S A. 2000;97:1796–1801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh OV, Pollard HB, Zeitlin PL. Chemical rescue of deltaF508-CFTR mimics genetic repair in cystic fibrosis bronchial epithelial cells. Mol Cell Proteomics. 2008;7:1099–1110. doi: 10.1074/mcp.M700303-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powers ET, Morimoto RI, Dillin A, et al. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 25.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Xiao C, Giacca A, Lewis GF. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes. 2011;60:918–924. doi: 10.2337/db10-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–1905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladiges WC, Knoblaugh SE, Morton JF, et al. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005;54:1074–1081. doi: 10.2337/diabetes.54.4.1074. [DOI] [PubMed] [Google Scholar]
- 30.Rutkowski DT, Kang SW, Goodman AG, et al. The role of p58IPK in protecting the stressed endoplasmic reticulum. Mol Biol Cell. 2007;18:3681–3691. doi: 10.1091/mbc.E07-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrova K, Oyadomari S, Hendershot LM, et al. Regulated association of misfolded endoplasmic reticulum lumenal proteins with P58/DNAJc3. EMBO J. 2008;27:2862–2872. doi: 10.1038/emboj.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao J, Petrova K, Ron D, et al. Crystal structure of P58(IPK) TPR fragment reveals the mechanism for its molecular chaperone activity in UPR. J Mol Biol. 2010;397:1307–1315. doi: 10.1016/j.jmb.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puthalakath H, O’Reilly LA, Gunn P, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 34.Namba T, Tanaka K, Ito Y, et al. Positive role of CCAAT/enhancer-binding protein homologous protein, a transcription factor involved in the endoplasmic reticulum stress response in the development of colitis. Am J Pathol. 2009;174:1786–1798. doi: 10.2353/ajpath.2009.080864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandl K, Rutschmann S, Li X, et al. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc Natl Acad Sci U S A. 2009;106:3300–3305. doi: 10.1073/pnas.0813036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 37.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and Inflammation in the intestine. Cell. 2010;140:859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger E, Haller D. Structure-function analysis of the tertiary bile acid TUDCA for the resolution of endoplasmic reticulum stress in intestinal epithelial cells. Biochem Biophys Res Commun. 2011;409:610–615. doi: 10.1016/j.bbrc.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 40.Rottner M, Freyssinet JM, Martínez MC. Mechanisms of the noxious inflammatory cycle in cystic fibrosis. Respir Res. 2009;10:23. doi: 10.1186/1465-9921-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribeiro CM, Boucher RC. Role of endoplasmic reticulum stress in cystic fibrosis-related airway inflammatory responses. Proc Am Thorac Soc. 2010;7:387–394. doi: 10.1513/pats.201001-017AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nassif M, Matus S, Castillo K, et al. Amyotrophic lateral sclerosis pathogenesis: a journey through the secretory pathway. Antioxid Redox Signal. 2010;13:1955–1989. doi: 10.1089/ars.2009.2991. [DOI] [PubMed] [Google Scholar]
- 43.Gkogkas C, Middleton S, Kremer AM, et al. VAPB interacts with and modulates the activity of ATF6. Hum Mol Genet. 2008;17:1517–1526. doi: 10.1093/hmg/ddn040. [DOI] [PubMed] [Google Scholar]
- 44.Vidal R, Caballero B, Couve A, et al. Converging pathways in the occurrence of endoplasmic reticulum (ER) stress in Huntington’s disease. Curr Mol Med. 2011;11:1–12. doi: 10.2174/156652411794474419. [DOI] [PubMed] [Google Scholar]
- 45.Rogler G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract Res Clin Gastroenterol. 2010;24:157–165. doi: 10.1016/j.bpg.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Coffin CS, Fraser HF, Panaccione R, et al. Liver diseases associated with anti-tumor necrosis factor-alpha (TNF-α) use for inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:479–484. doi: 10.1002/ibd.21336. [DOI] [PubMed] [Google Scholar]
- 47.Dahan A, Amidon GL, Zimmermann EM. Drug targeting strategies for the treatment of inflammatory bowel disease: a mechanistic update. Expert Rev Clin Immunol. 2010;6:543–550. doi: 10.1586/eci.10.30. [DOI] [PubMed] [Google Scholar]
- 48.Solimando R, Bazzoli F, Ricciardiello L. Chemoprevention of colorectal cancer: a role for ursodeoxycholic acid, folate and hormone replacement treatment? Best Pract Res Clin Gastroenterol. 2011;25:555–568. doi: 10.1016/j.bpg.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Otsuka M, Kang YJ, Ren J, et al. Distinct effects of p38alpha deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterology. 2010;138:1255–1265. doi: 10.1053/j.gastro.2010.01.005. 1265.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berg DJ, Zhang J, Weinstock JV, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. DSS colitis induces ER stress in colonic epithelial cells. (a) The purity of isolated colonic epithelial cells was determined by flow cytometry using an antibody against murine EpCAM. (b) Wild-type mice were fed 3% DSS in the drinking water for 0, 3 and 5 days. After DSS administration for the indicated days, the mice were euthanized and the colonic IECs were isolated for protein extraction and western blotting. Representative immunoblots of colon tissues from mice are shown. (c) Wild-type mice were fed 3% DSS in the drinking water for 0, 2, 4 and 6 days. After DSS administration for the indicated days, the mice were euthanized and the colonic IECs were isolated for RNA extraction and Q-RT-PCR. The mRNA levels were normalized to the expression of Gapdh. (d) Wild-type mice were fed 3% DSS in the drinking water for 6 days, then the mice were euthanized and the colons were removed, fixed and paraffin embedded for immunohistochemical staining of BiP, CHOP and XBP1. Representative immunohistochemical images are shown; each group contained a minimum of 6 individual mice.
Supplementary Figure 2. P58IPK−/− mice exhibit shorter colon lengths (a) and higher levels of macrophage marker F4/80 in colonic mucosa (b) upon DSS colitis.
Supplementary Figure 3. (a) Atf6α−/− mice display normal colon morphology under normal conditions. Colons were isolated from two-month old Atf6α+/+ and Atf6α−/− littermates mice for H&E and PAS staining. (b) Atf6α−/− mice showed higher levels of macrophage marker F4/80 in colonic mucosa upon DSS colitis. (c) Atf6α−/− mice exhibit an impaired induction of ER chaperone genes including Bip, Grp94, P58IPK and Pdi in colonic IECs upon DSS colitis. (d) Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-staining of apoptotic epithelial cells in the colon sections from Atf6α+/+ and Atf6α−/− mice with DSS colitis. (e) The apoptotic indices were calculated as the number of TUNEL-positive epithelial cells per 100 randomly selected crypts in the colon sections. 4 mice per group; * p < 0.05.
Supplementary Figure 4. Shortening of colon lengths upon DSS colitis was significantly mitigated by the feeding of TUDCA.
Supplementary Table 1. Sequence of primers used in this