Abstract
A substantial body of research exists to support the idea that cells of the immune system produce growth hormone (GH). However, the structure and mechanism of action of lymphocyte-derived GH continues to remain largely unknown. Here we present the results of Western analysis of whole cell extracts showing that different molecular weight isoforms of GH of approximately 100 kDa, 65 kDa, and 48 kDa can be detected in primary mouse cells of the immune system and in the mouse EL4 cell line. The identity of the 65 kDa and 48 kDa isoforms of GH were confirmed by mass spectrometry. The various isoforms were detected in both enriched T and B spleen cell populations. The large molecular weight isoform appears to reside primarily in the cytoplasm whereas the lower molecular weight 65 kDa and 48 kDa isoforms were detected primarily in the nucleus. These results also suggest that GH isoforms are induced by oxidative stress. In EL4 cells overexpressing GH, the expression of luciferase controlled by a promoter containing the antioxidant response element is increased almost three-fold above control. The data suggest that the induction of isoforms of the GH molecule in cells of the immune system may be an important mechanism of adaptation and/or protection of lymphoid cells under conditions of oxidative stress.
Keywords: Growth hormone, Lymphocytes, Oxidative stress, Molecular weight isoforms
1. Introduction
Expression of the growth hormone (GH) gene, once thought to be exclusive to the pituitary somatotrope, has now been found to occur in other tissues. Such sites include neuronal cells within the central nervous system [1], endothelial cells of blood vessels [2], fibroblasts [3], epithelial cells of the mammary gland [4], thymic epithelial cells [1], and cells of the immune system, including T cells, B cells, natural killer cells, and macrophages [5]. A potential role for lymphocyte-derived GH in immunoregulation has been suggested for lymphocyte growth, survival, and cytokine production [6–15]. Lymphocyte GH appears to stimulate IFNγ production with a small positive effect on IL-10 production [15]. Both norepinephrine and cortisol inhibit lymphocyte GH production and the studies suggest that lymphocyte GH may be an important mediator of cellular immune function mediated by the TH-1 pathway [15]. In an early study, we showed that treatment of rat lymphocytes with a specific GH antisense oligodeoxynucleotide decreased the amount of lymphocyte GH synthesized and at the same time reduced lymphocyte proliferation [6]. In studies with neutralizing antibodies (Abs) to GH, we measured a 2-fold decrease in the number of cells positive for insulin-like growth factor I (IGF-I), strongly supporting an important role for endogenously produced GH in the induction of lymphocyte-derived IGF-I [7]. The small amounts of GH synthesized and secreted by cells of the immune system [16], along with data showing that the same cells that synthesize GH also produce IGF-1 [17], suggests that GH may be classified as an intracrine hormone, acting primarily intracellularly [18]. More recently, we have studied the function of lymphocyte-derived GH by examining the consequences of its overexpression in a lymphoid cell line devoid of the GH receptor. In this model, EL4 T lymphoma cells overexpressing GH decrease the production of superoxide, increase production of nitric oxide and the expression of IGF-1 and the IGF-1R resulting in protection from apoptosis by a mechanism most likely involving an increase in the production of BcL-2 [8–11]. In addition, we have observed upregulation of the IGF-2R, transforming growth factor β1 (TGF-β1), and the inhibitor of differentiation/DNA binding type 2 (Id2) protein in cells overexpressing GH [12;13;19]. Taken together, the results suggest that a complex intracrine/autocrine regulatory circuit may be important for the production and function of leukocyte-derived GH and IGF-1 within the immune system. The circuit could fulfill local tissue needs for these hormones independent of the pituitary or liver and at the same time not disrupt homeostasis of other organ systems.
A well established feature of GH is that it exists as a family of molecular isoforms. Almost 20 years ago, GH was postulated to exist in more than 100 forms in circulation [20]. The main 22 kDa isoform of GH represents approximately 43% of all circulating GH found in high and low affinity complexes, as well as free GH [21]. Other GH isoforms include glycosylated hGHs with molecular weights of 24 kDa and 12 kDa [22;23], a 20 kDa hGH, deamidated hGHs, phosphorylated hGHs, a 35 kDa hGH, oligomeric hGHs, and cleaved hGHs with molecular weights of 17 kDa and 5 kDa [21;24;25]. Large molecular weight variant oligomers may exist as covalent and noncovalent bonded complexes of homodimers, heterodimers, and binding protein. The structure of an unusually stable mercaptoethanol-resistant (MER) 45 kDa GH has been reported that is a homodimer of 22 kDa GH monomers held together by interchain disulfide bonds [26]. The MER-45 kDa GH was reported to bind to GH receptors of IM9 lymphocytes and stimulate the proliferation of Nb2 lymphoma cells [27]. The data suggest that oligomeric GH may possess different biological and immunoreactive abilities when compared with the monomeric GH [21]. The effects of exercise, both resistance and aerobic, have been shown to increase the proportion of non-22 kDa GH isoforms and thereby potentially enhance a diabetogenic effect and prevent postexercise hypoglycemia [21]. More recently, it has been shown that acute resistance may lead to the appearance of disulfide-linked GH aggregates for which the physiological significance remains uncertain [28].
In the few studies where the size of lymphocyte GH was reported, including our initial report, the predominant size, derived from conditioned and concentrated extracellular fluids, was around 22 kDa along with a variety of other size variants [29–34]. Two previous studies in humans that examined the structure of intracellular GH in cell extracts reported immunoreactive proteins with higher molecular weights (37 kDa from PBL and 44 kDa from HL60 cells) than pituitary GH [30;32]. We postulate that it is important to consider the molecular heterogeneity of GH in order to better understand its potential biological functions in cells of the immune system. In this work, we report our results of studies on the structure and location of intracellular lymphocyte GH and the role of the redox status of the cell in the formation of GH isoforms. The data show that higher molecular weight isoforms of GH exist in cells of the immune system that were increased after oxidative stress.
2. Materials and methods
2.1 Cell culture conditions
The mouse EL4 T lymphoma cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in RPMI medium supplemented with 10% fetal calf serum and penicillin, streptomycin, and mycostatin (100 U/ml). Cell viability was monitored by trypan blue exclusion. Adult male Balb/C mice were obtained from Jackson Laboratories. Following sacrifice, spleens were removed and teased into single cell suspensions at 107 cells per ml in RPMI supplemented with 10% fetal bovine serum plus penicillin, streptomycin and mycostatin (100 U/ml). Nylon adherent (B cells) and nonadherent (T cells), lymphocytes were obtained by passage through nylon wool columns as previously described [35]. The cells were treated and cultured for approximately 16 h prior to centrifugation and preparation of cell extracts for Western blot analysis. All animal manipulations were conducted according to the guidelines and requirements of the University of Alabama at Birmingham Animal Welfare Committee.
2.2 Chemicals and reagents
Goat GH antiserum (T-20, SC-10365) for detection of mouse and human GH was purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Monoclonal anti-β-actin Ab (A5441) was purchased from Sigma-Aldrich Corporation (St. Louis, MO) and proliferating cell nuclear antigen (C-20, SC-9857) from Santa Cruz Biotechnology. All other chemicals were obtained at the highest grade from Sigma-Aldrich Corporation (St. Louis, MO). The control (Ti/con-Luc) and antioxidant response element (ARE) luciferase promoter constructs were kindly provided by Dr. William Fahl (McArdle Laboratory for Cancer Research at The University of Wisconsin, Madison, WI).
2.3 Western blot analysis
Human peripheral blood lymphocytes, mouse spleen cells or EL4 cells were pelleted, resuspended with Tris/Triton-X lysis buffer (1mM Tris containing 0.1% Triton-X, 10 µg/mL leupeptin, 2 µg/mL of aprotinin, 1 mg/mL PMSF). The cell lysate mixture was incubated on ice for 45 min and then centrifuged for 15 min at 13,000 × g at 4°C. Protein concentration was determined with the Bio-Rad protein assay reagent. The lysate was snap frozen and stored at −70 C until analyzed by Western blotting. Extracts were thawed on ice and immediately denatured by boiling for 5 min in Laemmli SDS sample loading buffer, followed by SDS-PAGE with 8% polyacrylamide gels and transferred to Immunoblot nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). Nonspecific binding sites were blocked by incubating the membranes in PBS (pH 7.4) with 0.1% Tween-20 and 10% skim milk for 1 h at 25°C. A polyclonal Ab specific for the detection of mouse and human GH (T-20, SC-10365 from Santa Cruz Biotechnology, Santa Cruz, CA) was added according to the manufacturer’s instructions and the membrane incubated with the antisera overnight at 4°C and washed in PBS containing 0.1% Tween-20. The membrane was then incubated 3 h with a 1:2000 dilution of affinity-purified rabbit anti-goat antisera, horseradish peroxidase conjugated (BioRad Laboratories) and washed twice in PBS containing 0.1% Tween-20 and once in dH2O. Immunoreactive proteins were visualized using the ECL Western blotting analysis system (Amersham Pharmacia Biotech, Inc., Sunnyvale, CA). Gels were scanned and analyzed using Scion Image Software (Scion Corp., Frederick, MD).
2.4 Cytoplasmic and nuclear extract preparation
Cytoplasmic and nuclear extracts were prepared by a method previously described [36]. Briefly, lymphocytes were harvested by centrifugation after treatment and washed with PBS, harvested and pelleted. Cells were resuspended in five packed cell volumes (PCV) of buffer A (10 mM Hepes, pH 7.2, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT and 0.5 mM PMSF), incubated on ice for 10 min and centrifuged at 1000 ×g for ten minutes. The cell pellet was resuspended in buffer B (buffer A containing 0.05% NP-40) and homogenized with 40 strokes in a Dounce homogenizer, type B pestle. The mixture was centrifuged at 1000 × g for 10 minutes and the supernatant harvested. The supernatant was further centrifuged at 10,000 × g for 10 min, and the supernatant designated the cytoplasmic fraction and the pellet designated the cytoplasmic membrane fraction. The 1,000 × g pellet from above containing the nuclei was resuspended and homogenized in 1 ml of buffer C (20 mM Hepes, pH 7.2, 1.5 mM MgCl2, 420 mM NaCl, 25% glycerol, 0.2 mM EDTA, 0.5 mM DTT and 0.5 mM PMSF), and gently shaken for 30 min at 4°C. Nuclei were pelleted at 10,000 × g for 30 min, and the supernatant harvested as the nuclear fraction. The pellet was resuspended in tris/triton-X lysis buffer and was designated the nuclear membrane fraction. The success of the nuclear and cytoplasmic isolation procedure was confirmed by the Western blotting of actin for the cytoplasmic and fraction and proliferating cell nuclear antigen (PCNA) for the nuclear fraction [37].
2.5 In-gel digestion
Western blot analyses confirming the areas of interest on a corresponding SDS gel was used to determine gel excisions for mass spectrometry. Bands were excised and excess stain was removed by an overnight wash of 50% 100 mM ammonium bicarbonate/50% acetonitrile. After destaining, the disulfide bonds were reduced by treatment with 25 mM dithiothreitol at 50°C for 60 min. Alkylation of the free thiols groups was carried out with 55 mM iodoacetamide for 60 min in complete darkness. The excess alkylating agent was removed and the gel pieces were washed twice with a 100 mM ammonium bicarbonate solution for 30 min. The gel pieces were evaporated to dryness in a SpeedVac (Savant) before the addition of the enzyme. A 12.5 ng/µl concentration of trypsin (Promega Gold) was added to each gel sample and incubated overnight at 37°C. Peptides were extracted from the gel pieces using a 50%, 5% formic acid 50% acetonitrile solution twice for 15 min. Extractions were pooled and evaporated to dryness. The samples were then resuspended in 30 µl of 0.1% formic acid prior to use for mass spectrometry analysis.
2.6 NanoLC-tandem mass spectrometry
An aliquot (5–10 µl) of each digest was loaded onto a 5 mm × 100 µm i.d. C18 reverse-phase cartridge at 2 µl/min using a PAL robot (Leap Technologies, Carrboro, NC). After washing the cartridge for 5 min with 0.1% formic acid in ddH2O, the bound peptides were flushed onto a 22 cm × 100 µm i.d. C18 reverse-phase pulled tip analytical column with a 25 min linear 5–50% acetonitrile gradient in 0.1% formic acid at 500 nL/min using a Shimadzu nanopump. The column was washed with 90% acetonitrile-0.1% formic acid for 15 min and then re-equilibrated with 5% acetonitrile-0.1% formic acid for 24 min. The eluted peptides were passed directly from the tip into a modified MicroIonSpray interface of an Applied Biosystems-MDS-Sciex (Concorde, Ontario, Canada) 4000 Qtrap mass spectrometer. The interface was rebuilt in order to apply the electrospray voltage through a liquid-liquid junction at the top of the column rather than at the end of the column. This arrangement resulted in very high chromatographic resolution by elimination of the post-column dead volume. The IonSpray voltage was 2500 V and the declustering potential was 60 V. Ionspray and curtain gases were set at 10 psi and 15 psi, respectively. The interface heater temperature was 160°C.
2.7 Determination and multiple reaction monitoring (MRM)
Possible Mouse Growth Hormone (x02891) sequence for multiple reaction monitoring scans were determined by using an in silico digestion software (Protein Prospector, UCSF) for possible parent/daughter transitions. The Multiple Reaction Monitoring (MRM) technique was used as a diagnostic tool for further sample analyses. This technique utilizes the triple quadrupole detection platform on the ABI 4000 Qtrap Mass Spectrometer. The first of the three quadrupoles selects for the parent mass (parent ion) of the desired analyte. The second quadrupole dissociates the parent ions by collision with an inert gas (N2) into daughter ions (b and y ions). The third quadrupole selects for one of the daughter ions. The parent/daughter ion combination allows for a highly specific and sensitive diagnostic tool for detection and quantification for specific protein(s) in complex solutions.
2.8 Transfection and luciferase/β-galactosidase (β-Gal) assays
Cells were subdivided three days before transfection. After harvesting, the cell pellets were resuspended at 30 × 106 cells/ml in RPMI 1640 (no serum) + 10 mM dextrose, 0.1 mM dithiothreitol (DTT) containing 20 µg of ARE luciferase promoter construct plasmid and 20 µg of pON249 β-Gal plasmid DNA. A pulse of 400 mV and 960 µF was delivered to the cells in a 0.4 cm-cuvette using the Bio-Rad Gene Pulser (Bio-Rad Laboratories, Hercules, CA). After the pulse, the cells were maintained in growth medium. Twenty-four hours after transfection, the cells were washed two times with cold phosphate-buffered saline (PBS) and lysed in 0.4 ml of lysis buffer [0.1 M KPO4 (pH 7.9), 0.5% Triton X-100, and 1 mM DTT)] on ice for 15 min. Luciferase activity was determined as follows: A 75 × 12-mm polystyrene tube containing 100 µl of cellular extract was placed in an Optocomp I luminometer (MGM Instruments, Hamden, CT), 200 µl of assay buffer [100 mM tricine, 10 mM MgSO4, 2 mM ethylenediaminetetraacetic acid, 1 mM DTT, 2 mM ATP, and 0.1 mM luciferin (pH 7.8)] was injected, and peak luminescence was measured over a 10-s window after a l–2 delay. β-Gal activity was used to normalize for variations in transfection efficiency and was determined by incubating 100 µl of cellular extract with 60 mM β-mercaptoethanol, and 1 mg/ml O-nitro-phenyl-β-D-galactopyranoside in 0.1 M Na2HPO4 (pH 7.3) (total volume=300 µl) at 37°C for 15 min. The reaction was stopped by the addition of 700 µl of 0.1 M Na2CO3; absorbance at 410 nm was measured on a spectrophotometer. The luciferase activity of a particular construct was divided by that of the control β-Gal activity, and the quotient was expressed as relative luciferase activity.
2.9 Data analysis
Each experiment was repeated at least three times, and data are reported as mean ± standard error of the mean (SEM). Significant differences between various experimental treatment groups were determined by analysis of variance (ANOVA) and Student’s t-test. Densitometric analysis of the scanned images of Western blots was done using Scion Image Software (Scion Corporation, Frederick, MD). Use of * in figures designates p ≤ 0.05.
3. Results
3.1 High molecular weight forms of intracellular lymphocyte GH
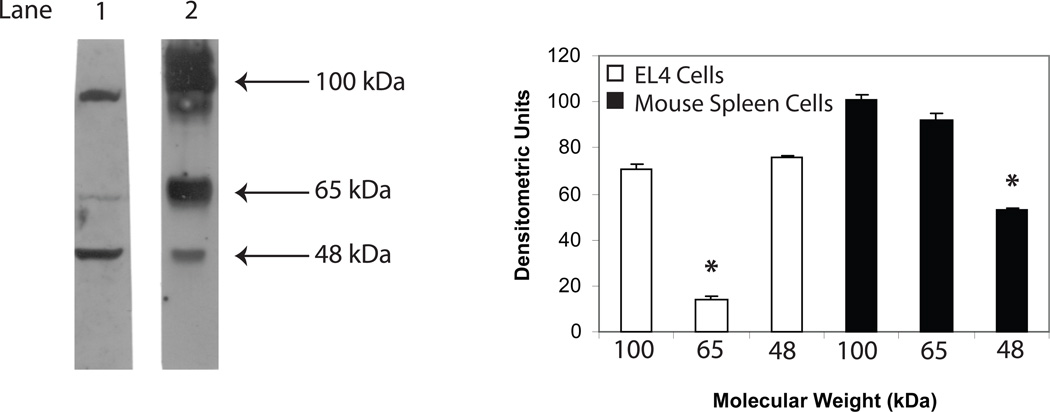
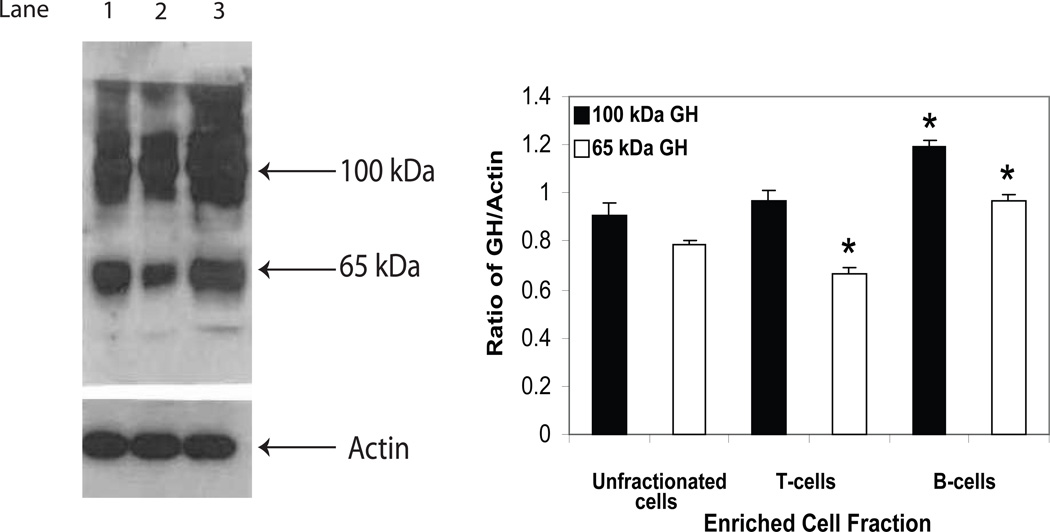
For some time, we have hypothesized that the function of lymphocyte-derived GH was exerted primarily intracellularly and that the hormone may function in an intracrine manner [38]. This was based in part on the small amounts of hormone produced or detected in cells of the immune system, the percentage of GH-producing cells detected by immunofluorescence compared to the cells secreting GH (2% vs. 0.1%) and the fact that the same cells producing GH also produce IGF-1 [16;17]. Despite the proposed intracrine model of action for lymphocyte GH, we had not examined the endogenous nature of intracellular GH. Therefore, using Western blot analysis, we investigated the nature of intracellular lymphocyte GH. Surprisingly, typical results from proteins obtained from whole cell extracts prepared from EL4 cells and primary mouse spleen cells reveal two major bands in EL4 cells (lane 1, ~100 kDa and ~48 kDa) and two major bands in primary mouse spleen cells (lane 2, ~100 kDa, ~65 kDa) (Fig. 1). The 65 kDa band in the EL4 cell line and the 48 kDa band from primary spleen cells were significantly less intense and on some occasions not even observed. A single slightly larger molecular weight GH species (~54,000 kDa), which may reflect variant glycosylation, was obtained in preliminary studies from cell extracts prepared from human primary peripheral blood lymphocytes (data not shown). A polypeptide of approximately 22 kDa was not detected in mouse or human primary lymphoid cells or from the mouse EL4 cell line. We also separated mouse spleen cells into T cell and B cell enriched cell fractions by nylon chromatography and examined whole cell extracts for GH isoforms (Fig. 2). The data show that although the same isoforms of GH were observed in both T and B cell fractions, significantly less 65 kDa GH isoform was present in the T cell enriched fraction whereas greater levels of both the 100 kDa GH and 65 kDa GH were detected in B cell enriched cell fractions compared to unfractionated cells.
Fig. 1.
GH protein expression in EL4 cells and primary mouse spleen cells. Whole cell extracts were prepared from EL4 cells (lane 1) and primary mouse spleen cells (lane 2) as described in the methods. After SDS-PAGE (8%) and transfer to PVDF membranes, Western blot analysis was performed using commercial Ab to GH (Santa Cruz) and bands visualized using a chemiluminescence substrate for HRP (GE Healthcare). The approximate molecular weight for isoforms of GH are shown with arrows on the right. The results shown above are typical of an experiment repeated 5 times (*p<0.05).
Fig. 2.
GH protein expression in primary mouse spleen, mouse T, and mouse B cells. Whole cell extracts were prepared from unfractionated spleen cells and nylon column purified T and B cells as described in the methods. After SDS-PAGE (8%) and transfer to PVDF membranes, Western blot analysis was performed using commercial Ab to GH (Santa Cruz) and bands visualized using a chemiluminenscence substrate for HRP (GE Healthcare). The approximate molecular weight for isoforms of GH are shown with arrows on the right. Key: Lane 1 (unfractionated spleen cells); lane 2 (T cells); lane 3 (B cells). The results shown above are typical of an experiment repeated 5 times (*p<0.05).
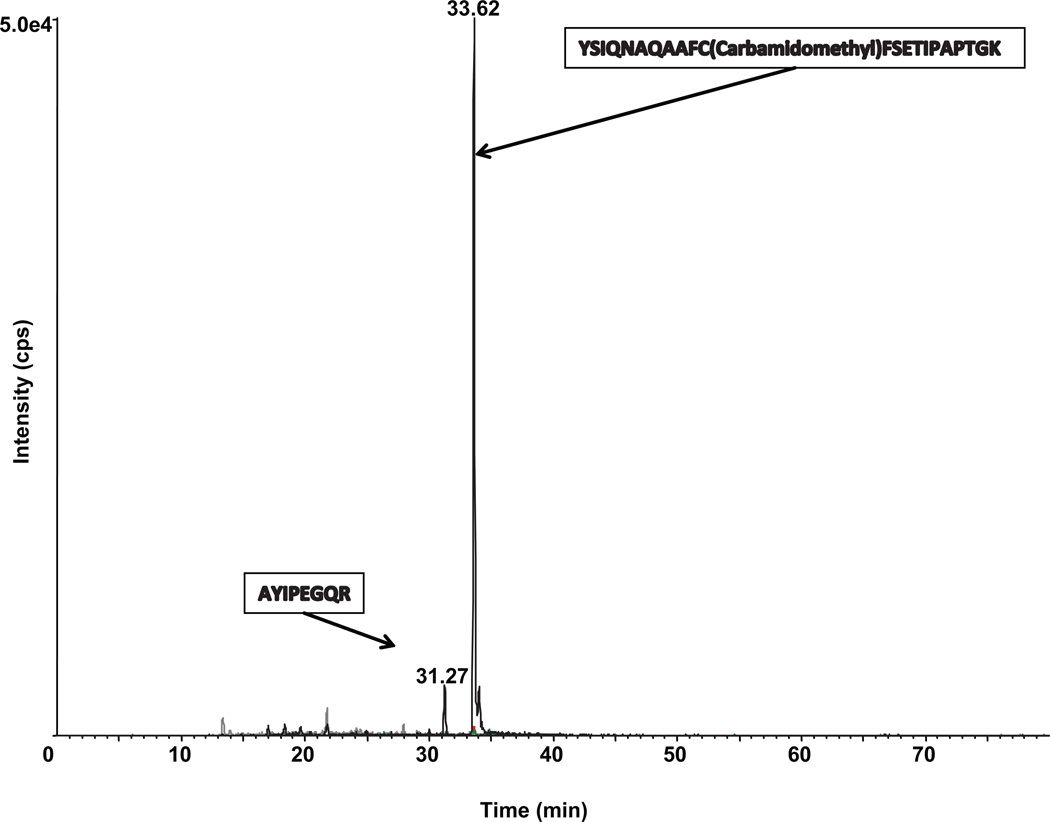
To confirm that the bands positive by Western blot analysis were GH, we analyzed the specific polyacrylamide gel slices by mass spectrometry (MS). The protein bands at approximately 48 kDa and 65 kDa were excised, destained, dried, and then rehydrated and digested in-gel with trypsin overnight at 37°C. Aliquots of the trypsin digest were then analyzed by nanoLC-tandem mass spectrometry. The polypeptide was identified as GH by comparing the profile of tryptic peptide masses generated by the mass spectrometer with predicted tryptic peptides expected from pituitary-derived GH using ProteinProspector software. The spectra generated by multiple reaction monitoring detected two peptides of mouse GH corresponding to the 48 kDa protein positive for GH on Western blot analysis (Fig. 3). The peptides detected were AYIPEGQR corresponding to amino acids numbered 60–67 in the GH protein and YSIQNAQAAFCFSETIPAPTGK corresponding to amino acids numbered 68–89 in the GH protein. The AYIPEGQR peptide was also detected in the 65 kDa protein which was also positive for GH by Western blot analysis (data not shown). The data confirm that the higher molecular weight proteins we have detected by Western blot analysis in cells of the immune system are indeed GH proteins.
Fig. 3.
NanoLC-tandem mass spectra of lymphocyte mouse GH.
3.2 Distribution of GH proteins in EL4 cells and mouse spleen cells
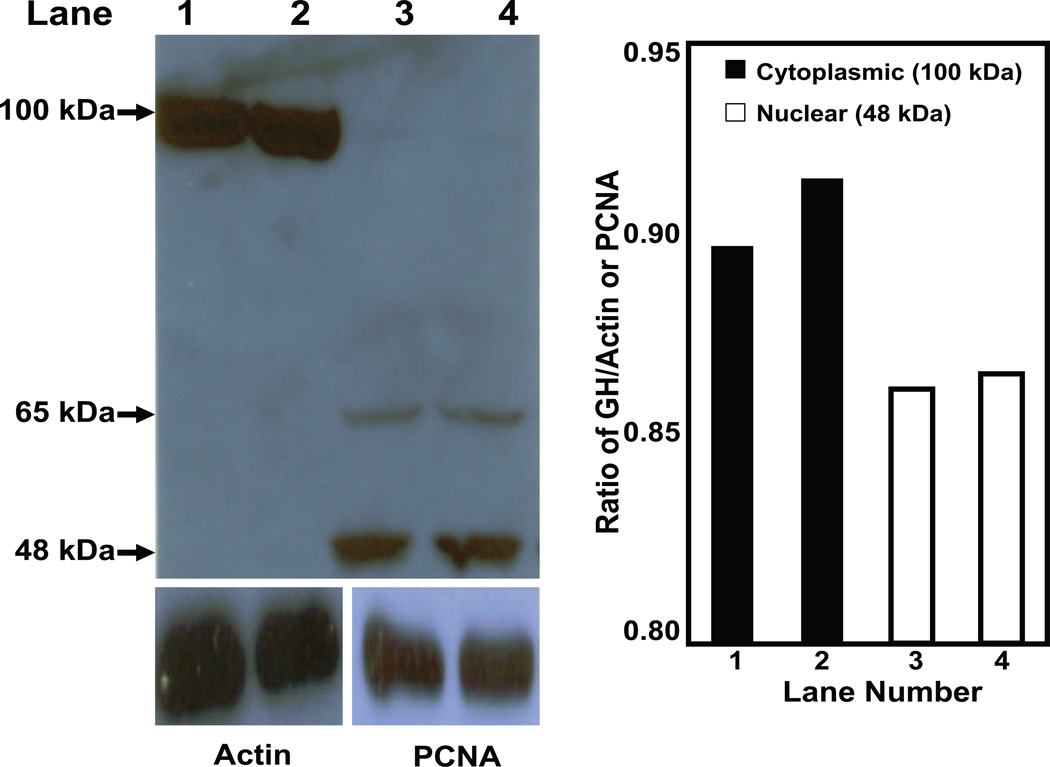
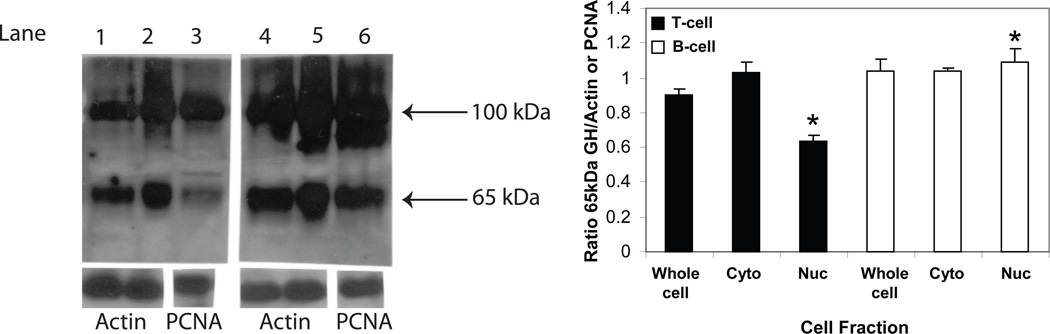
Since the GH molecule in lymphocytes was composed of a mixture of different sizes, we considered the possibility of partitioning lymphocyte GH into different subcellular compartments. To study this, EL4 cells were fractionated into crude cytoplasmic, cytoplasmic membrane, nuclear, and nuclear membrane enriched components as described in the Materials and Methods. Each fraction was analyzed for the presence of GH protein(s) by Western blot analysis (Fig. 4). The data show that the higher molecular weight isoform was primarily located in the cytoplasm and cytoplasmic membrane preparations, whereas the lower molecular weight isoform was principally found in the nucleus and nuclear membrane preparations. Densitometric analysis suggests that approximately 75% of GH in EL4 cells is cytoplasmic under normal in vitro growth conditions. We performed a similar analysis to identify the location of GH isoforms in isolated cytoplasmic and nuclear enriched subcellular fractions from both spleen T cell and B cell enriched cell types. The data (Fig. 5) show significantly less GH (65 kDa) in the T cell nucleus compared to the T cell cytoplasm. In B cell enriched cells, there appear to be similar levels of GH in the cytoplasm and the nucleus. The data show significantly more GH in the B cell nuclear fraction, however, compared to the T cell nuclear fraction.
Fig. 4.
Lymphocyte GH in enriched cytoplasmic and nuclear fractions of EL4 cells. Subcellular fractions were prepared as described in the methods. After SDS-PAGE (8%) and transfer to PVDF membrane, Western blot analysis was performed using commercial Ab to GH (Santa Cruz) and bands visualized using a chemiluminescence substrate for HRP. Blots were stripped and reprobed with specific Abs to actin or proliferating cell nuclear antigen (PCNA). Key: Lane 1 (cytoplasm); lane 2 (cytoplasmic membrane), lane 3 (nucleus); lane 4 (nuclear membrane). Approximate molecular weight for isoforms of GH are shown with arrows on the left. The results shown above are typical of an experiment repeated 5 times.
Fig. 5.
Lymphocyte GH in enriched cytoplasmic and nuclear fractions of mouse spleen cells. Subcellular fractions were prepared as described in the methods. After SDS-PAGE (8%) and transfer to PVDF membrane, Western blot analysis was performed using commercial Ab to GH (Santa Cruz) and bands visualized using a chemiluminescence substrate for HRP. Blots shown below were stripped and reprobed with specific Abs to actin (lanes 1,2,4,5) or PCNA (lanes 3,6). The approximate molecular weight for isoforms of GH are shown with arrows on the right. Key; Lane 1 (T cells, whole cell); lane 2 (T cells, cytoplasm), lane 3 (T cells, nucleus); lane 4 (B cells, whole cell); lane 5 (B cells, cytoplasm); lane 6 (B cells, nucleus). Asterisks (*) denote a significant difference (p<0.05) in the T cell nuclear fraction to the whole T cell fraction and between the B cell nuclear fraction to the T cell nuclear fraction. The results shown above are typical of an experiment repeated 5 times.
3.3 Effect of pyrrolidine dithiocarbamate (PDTC) and diethyl maleate (DEM) on the synthesis of GH in mouse spleen cells
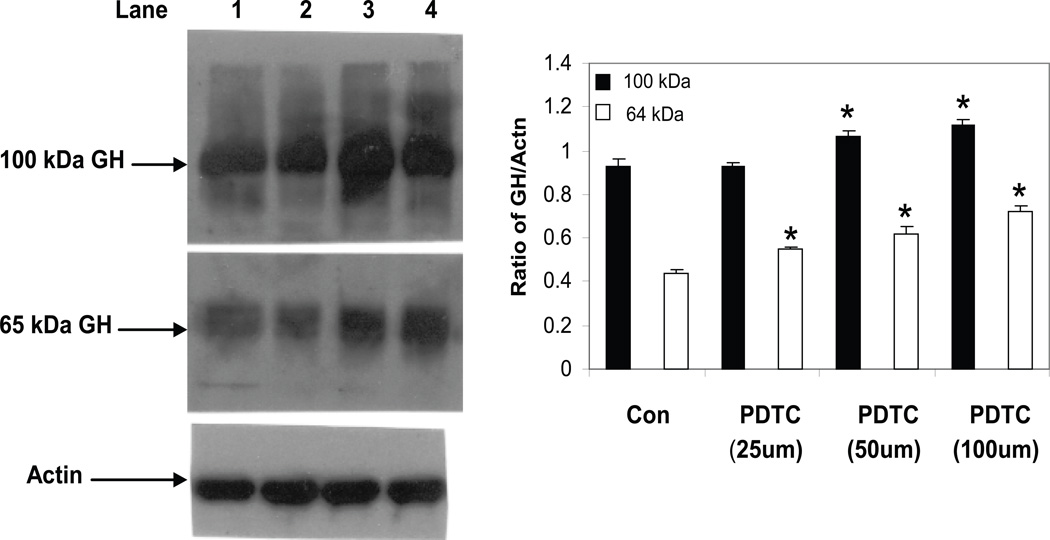
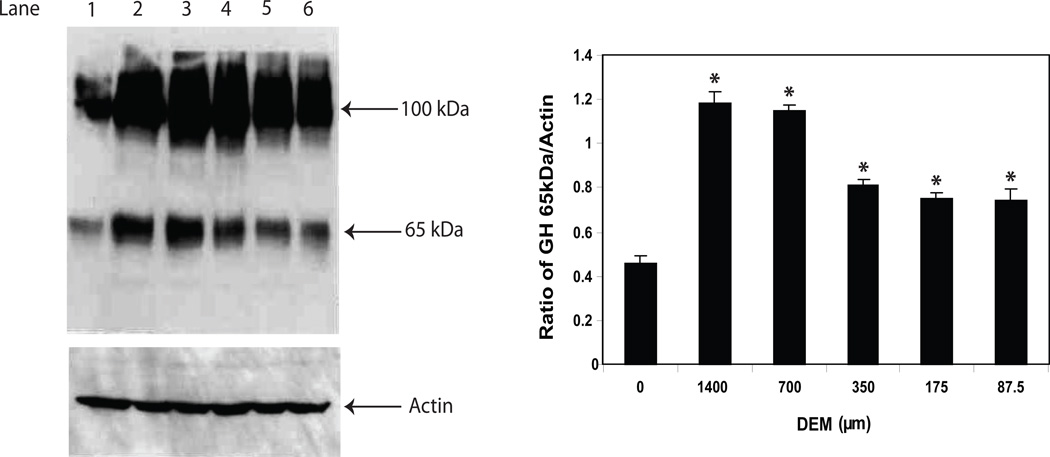
One of the biological functions of GH in cells of the immune system is the protection of lymphocytes from apoptosis induced by an alkylating agent [10]. The mechanism appears to involve the production of nitric oxide (NO) and increased levels of Bcl2 [9]. In a follow-up study, we showed that GH inhibited cyp2E1 activity reducing O2− production and thereby potentially reducing toxicity by peroxynitrite in the face of increasing NO [8]. These results, coupled with findings by others highlighting a role for redox reactions, in protein compartmentation [39;40], prompted us to examine redox-dependent mechanisms on the synthesis and location of GH. To this end, we initially examined the effect of treatment with pyrrolidinedithiocarbamate (PDTC) on the levels of lymphocyte GH in mouse spleen cells. PDTC, known to be a stable antioxidant or pro-oxidant in different cell situations, has been widely used to inhibit the activation of NF-κB [41]. Following a 16 h treatment with different concentrations of PDTC, whole cell extracts were prepared from control and treated cultures and the isoforms of GH measured by Western blot analysis (Fig. 6). Although no significant change was detected in the 48 kDa isoform species (data not shown), the results showed a dose-dependent increase in the levels of both the 65 kDa and 100 kDa isoforms in mouse spleen cells. In another study, we examined the levels of GH in primary spleen cells following exposure to an oxidizing agent, diethyl maleate (DEM). Treatment with DEM has been used by numerous investigators and shown to cause a rapid and sustained depletion of GSH levels by direct conjugation for example in HepG2 cells [42]. Treatment of mouse spleen cells with various concentrations of DEM from 87.5 µM to 1400 µM significantly increased the level of the 65 kDa isoform of GH (Fig. 7). Although the Western blot analysis of the 100 kDa isoform of GH follows a pattern similar to that observed for the 65 kDa isoform after DEM treatment, the densitometric analysis is not shown since it failed to reach statistical significance (Fig. 7).
Fig. 6.
GH protein expression in mouse spleen cells treated with PDTC. Cells were treated for 16 hrs with different doses of PDTC after which whole cell extracts were prepared as described in the methods. After SDS-PAGE (8%) and transfer to PVDF membranes, Western blot analysis was performed using commercial Ab to GH (Santa Cruz) and bands visualized using a chemiluminence substrate for HRP. Blots were stripped and reprobed with specific Abs to actin. Asterisks (*) denote a significant difference (p<0.05) from control GH isoforms. The results shown are typical of an experiment repeated 3 times. Key: Lane 1 (nontreated control); lane 2 (PDTC, 25 µM); lane 3 (PDTC, 50 µM); lane 4 (PDTC, 100 µM).
Fig. 7.
GH protein expression in mouse spleen cells treated with DEM. Cells were treated for 16 hrs with different doses of DEM after which whole cell extracts were prepared as described in the methods. After SDS-PAGE (8%) and transfer to PVDF membranes, Western blot analysis was performed using commercial Ab to GH (Santa Cruz) and bands visualized using a chemiluminence substrate for HRP. Blots were stripped and reprobed with specific Abs to actin. Asterisks (*) denote a significant difference (p<0.05) from control. The approximate molecular weights for isoforms of GH are shown with arrows on the right. The results shown are typical of an experiment repeated 3 times. Key: Lane 1 (nontreated control); lane 2 (DEM, 1400 µM); lane 3 (DEM, 700 µM); lane 4 (DEM, 350 µM); lane 5 (DEM, 175 µM); lane 6 (DEM, 87.5 µM).
3.4 Antioxidant response element (ARE) promoter activity in control and GHo cells
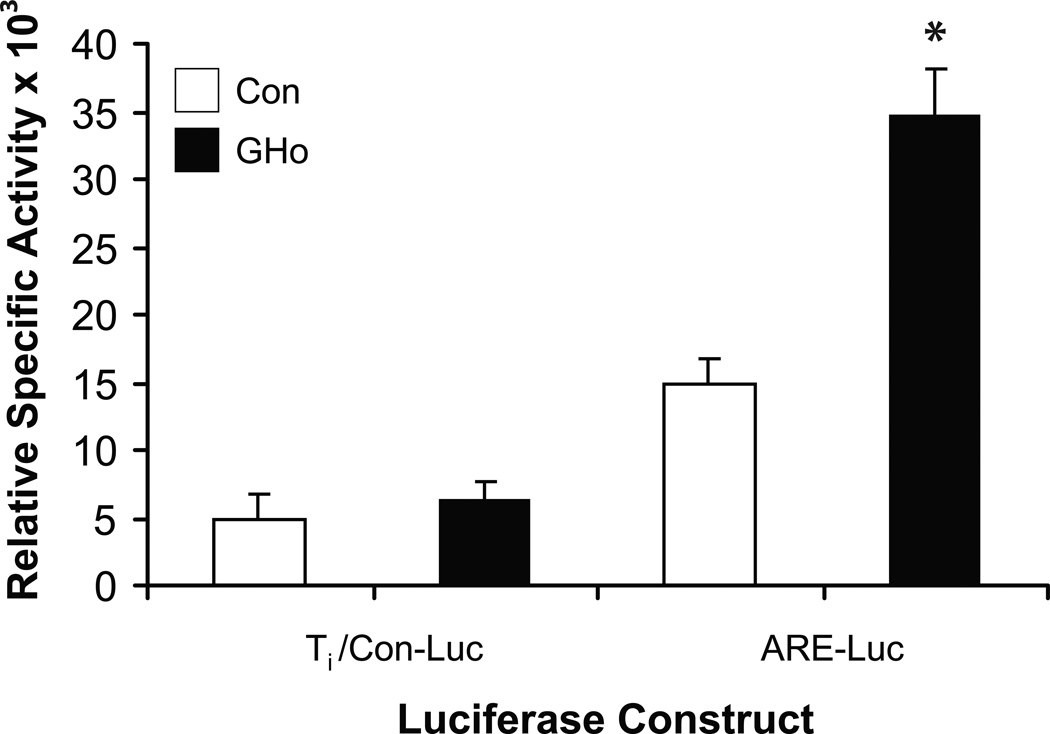
To begin to understand a role for GH in the antioxidant response, we studied the promoter activity of an ARE luciferase construct in control EL4 cells and EL4 cells overexpressing GH (GHo) (Fig. 8). The ARE is a cis-acting element, activated by redox-cycling chemicals that regulates inducible and/or constitutive gene expression, including the phase II detoxification enzymes [43]. Thus, Ti/Luc control and ARE Luc constructs were transiently transfected into vector alone control and GHo EL4 cells as previously described. Cell extracts were prepared 24 h later for the determination of luciferase and β-galactosidase enzyme activities. The results show a significant increase in (2.9 fold) luciferase activity from the ARE luciferase construct in GHo cells compared to the vector alone control EL4 cells (Fig. 8).
Fig. 8.
Antioxidant promoter activity in vector alone control and GHo cells. EL4 cells were transfected by electroporation as described in the methods section with control Ti/Lµc, ARE Luc, and β-Gal reporter constructs. Twenty-four hours later, cells were harvested and the expression of luciferase measured in cell extracts and normalized by β-Gal. The results represent means ± SEM from three separate experiments (* p<0.05).
4. Discussion
The present investigation provides evidence that high molecular weight forms of GH are detected in cells of the immune system. The use of specific Ab to GH in the Western blot indicated the expression of approximately 100 kDa, 65 kDa, and 48 kDa isoforms. Monomer-size GH was not detected. Secreted forms of GH were also not detected in concentrated (20×) supernatant medium (data not shown). Most importantly, the 48 and 65 kDa protein bands from electrophoresis of whole cell mouse spleen extracts could be excised, trypsinized, analyzed by nanoLC-tandem mass spectrometry and identified as GH. Two previous studies examining intracellular GH by Western analysis detected higher molecular weight forms of GH (37–44 kDa) either with the 22 kDa isoform [30], or in the absence of the 22 kDa [32]. Neither study confirmed the identity of GH by mass spectrometry. As a step toward understanding GH function in cells of the immune system, we also attempted to establish the subcellular localization of GH. Our studies (Figs. 4,5) suggest that under normal culture conditions, lymphocyte GH exists in the cytoplasm primarily as an isoform of approximately 100 kDa and in the nucleus as an isoform of approximately 48 kDa (EL4 cells) or 65 kDa (primary spleen cells). Although GH protein isoforms appear to reside in different compartments, the actual site(s) at which it exerts its function remain unknown. The physiological significance or importance of molecular weight isoforms of GH remains obscure, however, it is likely to represent an important regulatory function in mediating intracellular GH activity. This may be supported by the fact that higher levels of the cytoplasmic 100 kDa isoform and particularly the nuclear 65 kDa isoforms of GH were found in B cells compared to T cells (Fig. 5). From previous work by others [26;34], it appears that the 44–48 kDa isoform of GH can be very stable in that it resists conversion to the 22 kDa monomer form under reducing conditions. In one study, it was demonstrated that the exceptionally stable 45 kDa hGH exists as an homodimer of 22 kDa hGH monomers held together by interchain disulfide bonds and not by divalent metal cation bridges [26]. GH-binding proteins and variants of the GH-receptor (GHR) have also been described [44]. The difference between the presence of the 48 kDa species in EL4 cells and the 65 kDa GH isoform in spleen cells may be a consequence of EL4 cells lacking the GHR. It should be noted that receptor-mediated nuclear translocation of GH [45] and the GH receptor have also been reported [46]. Interestingly, the translocation of GH appears to occur independent of the GHBP, which is also found in the nucleus, and temporarily distinct from the nuclear translocation of the GH receptor [46]. Though the previous work on nuclear translocation and/or localization of GH, GHBP, and the GHR was done in rat liver, the data suggest the possibility in cells of the immune system that the GH receptor and/or the GHBP may serve particular roles in the intracrine actions of lymphocyte-derived GH. Future investigations are necessary to clarify the function and mechanisms of action of the high molecular weight forms of GH in cells of the immune system. We are currently examining the higher molecular weight forms of GH for the presence of GH-receptor/GHBP signature protein sequences by mass spectrometry.
Our study also provides the first evidence that expression of lymphocyte GH is enhanced in primary spleen cells exposed to oxidative stress. The results show that primary spleen cells respond to a challenge with PDTC or DEM by augmenting the synthesis of lymphocyte GH. The increase could be seen in both the 100 kDa and 65 kDa isoforms of GH (Figs. 6,7). Enhanced levels of the 100 kDa and 65 kDa isoforms of GH were also observed in the cytoplasmic and nuclear fractions, respectively (data not shown). We hypothesize that the 100 kDa higher molecular weight cytoplasmic isoform/complex of GH may consist of GH, GHR and/or GHBP, and that during oxidation, lower molecular weight isoforms of GH are released as a dimer; either a GH homodimer (~48 kDa) or a GH-GHBP heterodimer (~65 kDa). Our data at the present time do not permit us, however, to conclude that the 100 kDa isoform of GH under oxidizing conditions is converted to the 65 kDa isoform of GH which is free to migrate into the nucleus. The concept of altering the location of proteins by means of redox conditions or changing the oligomerization state, suggested here for the first time for GH, has been previously shown for ATP-independent heat shock proteins [39;40].
Previous work by others has identified a core sequence, commonly known as ARE, comprised of TGACNNNGC in their 5’ flanking region [47]. Although our results do not yet permit us to identify the detailed mechanism of induction of GH in cells of the immune system, a consensus ARE-like sequence containing four nucleotides instead of three in the TGACNNNGC consensus core motif was identified at position −233bp relative to the ATG initiator site. Functional ARE-like elements with this same sequence motif have been previously described for the Vanin-1 gene promoter in a thymic epithelial cell line [48]. It should also be noted that SP-1 binding may also increase after stress and confer resistance to oxidative stress [49]. In a previous report, we showed that SP transcription factors bind to the region at −138/−133 bp containing a GGGAGG motif in the GH promoter and appeared to inhibit GH promoter luciferase activity in the P-388 monocyte cell line [50]. Although this result may initially appear puzzling, the two studies were done in different cell lines and under different experimental conditions.
Lymphoid cells have the potential to increase the expression of several classes of proteins in response to inflammation or oxidative stress [51]. This may involve endogenous heat shock proteins, enzymatic antioxidants, including superoxide dismutase, catalase and glutathione peroxidase and Phase I and II detoxication reactions [47]. The results from our study here provide the first evidence that lymphocyte GH may be involved in the antioxidant response. Transfection of EL4 cells overexpressing GH with an ARE promoter luciferase construct resulted in a significant increase (2.9-fold) in luciferase expression compared to control (Fig. 8). The ARE is a cis-acting regulatory element in promoter regions of several genes encoding phase II detoxification enzymes and antioxidant proteins [47]. The potential regulation of these genes in lymphoid cells by GH could therefore be important for understanding how lymphoid cells are protected from oxidative stress. GH may bind to the ARE core nuclear sequence or interact as a cofactor and function as a critical transcription factor [47] stimulating higher levels of antioxidant proteins. In addition, three major signaling pathways including MAP kinases, p13 kinases and PKC, have been implicated in the regulation of the ARE transcriptional response [47], and it is conceivable that lymphocyte GH may alter phosphorylation of a transcriptional protein(s) during oxidative stress; however, much more work needs to be done to support this kind of conjecture. We hypothesize that the lower molecular weight form(s) of GH that are seen in the nucleus may function to stimulate and/or inhibit specific gene expression and facilitate adaptation and survival and defend the cell against oxidative damage.
A large number of peptide growth factors, including hormones and cytokines, that act at receptors on the surface of cells have also been shown to act as targets within cells [52]. This area of study, termed intracrine action, though not yet widely appreciated, is slowly emerging as playing roles in differentiation, hormonal responsiveness, memory, tumor biology, stem cell biology, and senescence [18]. Our working hypothesis for some time now [29] has been that lymphocyte GH functions primarily within cells of the immune system and can be classified as an intracrine hormone. This idea is supported by our findings that only small amounts of GH can be detected in cells of the immune system, whereas GH is not routinely detected in the surrounding medium. A large body of our previous work was done in the EL4 cell line overexpressing GH which lacks the GH receptor. This work, in part, showed that lymphocyte GH promotes the synthesis of NO [9] and exerts inhibitory effects on O2− generation via inhibition of cyp2E1 [8;10]. The induction of the ARE, shown in the present work in cells overexpressing GH in the absence of the GH receptor, along with the data showing an increase in the 48 and 65 kDa GH isoforms by stress agents strongly suggests a role for lymphocyte GH in the stress response. A better understanding of the molecular mechanism and the role of GH in oxidative stress may be important for the future development of approaches to protect cells of the immune system from redox imbalance and the stresses of pathological states (i.e., cancer, autoimmunity) and inflammation as well as the aging process.
Many years ago (1984), it was proposed [53] that the immune system, because of shared ligands and receptors with the nervous system, could be considered a sensory organ (sixth sense) detecting noncognitive stimuli (bacteria, virus, antigen) not recognized by the central nervous system. Although a large number of hypothalamic and pituitary peptide hormones have been shown to be produced by cells of the immune system, the function of the peptides in cells of the immune system are still being investigated [54]. In our model, we propose that cells of the immune system recognize the association of bacteria, virus, and tumors as an oxidative stress event and signal the release and transport of a lower molecular weight isoform of GH into the nucleus. Once in the nucleus, hypothetically, GH would be free to influence transcriptional responses to the stress event and to defend the cell against oxidative damage. Our results support the concept that changes in the cellular redox status influence the intracellular levels of lymphocyte GH which may exert effects on elements mediating the oxidative stress response. Further studies are clearly needed, however, to define a mechanism for GH in sensing and adapting to a stressful event [34;49;55;56].
Highlights.
Cells of the immune system produce growth hormone. > The growth hormone exists in high molecular isoforms of 100 kDa, 65 kDa, and 48 kDa. > Lymphocyte growth hormone isoforms are induced by oxidative stress. > Lymphocyte growth hormone activates the antioxidant response element. > Growth hormone may protect cells to oxidative stress.
Acknowledgements
This work was supported by grants from the National Institutes of Health. The author is grateful to Landon Wilson and the Mass Spectrometry Core Facility at the University of Alabama at Birmingham (P50 AT00477; U54 CA100949; P30 AR050948; P30 DK079337; and the UAB Lung Health Center), Diane Weigent for manuscript preparation, and Dr. Matthew Hardison for reading the manuscript and help in preparing the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Maggiano N, Piantelli M, Ricci R, Larocca LM, Capelli A, Ranelletti O. Detection of growth hormone-producing cells in human thymus by immunohistochemistry and non-radioactive in situ hybridization. J Histochem Cytochem. 1994;42:1349–1354. doi: 10.1177/42.10.7930517. [DOI] [PubMed] [Google Scholar]
- 2.Wu H, Devi R, Malarkey WB. Localization of the growth hormone messenger ribonucleic acid in the human immune system--a clinical research center study. J Clin Endocrinol Metab. 1996;81:1278–1282. doi: 10.1210/jcem.81.3.8772612. [DOI] [PubMed] [Google Scholar]
- 3.Palmetshofer A, Zechner D, Luger TA, Barta A. Splicing variants of the human growth hormone mRNA: detection in pituitary, mononuclear cells and dermal fibroblasts. Mol Cell Endocrinol. 1995;113:225–234. doi: 10.1016/0303-7207(95)03633-i. [DOI] [PubMed] [Google Scholar]
- 4.Mol JA, VanGardesen E, Seman PJ, Wolswinkel J, Rijinberk A, Rutterman GR. Growth hormone mRNA in mammary gland tumours of dogs and cats. J Clin Invest. 1995;95:2028–2034. doi: 10.1172/JCI117888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weigent DA, Blalock JE. The production of growth hormone by subpopulations of rat mononuclear leukocytes. Cell Immunol. 1991;135:55–65. doi: 10.1016/0008-8749(91)90253-8. [DOI] [PubMed] [Google Scholar]
- 6.Weigent DA, Blalock JE, LeBoeuf RD. An antisense oligodeoxynucleotide to growth hormone messenger ribonucleic acid inhibits lymphocyte proliferation. Endocrinology. 1991;128:2053–2057. doi: 10.1210/endo-128-4-2053. [DOI] [PubMed] [Google Scholar]
- 7.Baxter JB, Blalock JE, Weigent DA. Characterization of immunoreactive insulin-like growth factor-I from leukocytes and its regulation by growth hormone. Endocrinology. 1991;129:1727–1734. doi: 10.1210/endo-129-4-1727. [DOI] [PubMed] [Google Scholar]
- 8.Arnold RE, Weigent DA. The inhibition of superoxide production in EL4 lymphoma cells overexpressing growth hormone. Immunopharm Immunotoxicol. 2003;25:159–177. doi: 10.1081/iph-120020467. [DOI] [PubMed] [Google Scholar]
- 9.Arnold RE, Weigent DA. The production of nitric oxide in EL4 lymphoma cells overexpressing growth hormone. J Neuroimmunol. 2003;134:82–94. doi: 10.1016/s0165-5728(02)00420-4. [DOI] [PubMed] [Google Scholar]
- 10.Arnold RE, Weigent DA. The inhibition of apoptosis in EL4 lymphoma cells overexpressing growth hormone. Neuroimmunomodulation. 2004;11:149–159. doi: 10.1159/000076764. [DOI] [PubMed] [Google Scholar]
- 11.Weigent DA, Arnold RE. Expression of insulin-like growth factor-1 and insulin-like growth factor-1 receptors in EL4 lymphoma cells overexpressing growth hormone. Cell Immunol. 2005;234:54–66. doi: 10.1016/j.cellimm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Farmer JT, Weigent DA. Expression of insulin-like growth factor-2 receptors on EL4 cells overexpressing growth hormone. Brain Behav Immun. 2007;21:79–85. doi: 10.1016/j.bbi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Farmer JT, Weigent DA. TGF-beta-1 expression in EL4 lymphoma cells overexpressing growth hormone. Cell Immunol. 2006;240:22–30. doi: 10.1016/j.cellimm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Weigent DA. Regulation of Id2 expression in EL4 T lymphoma cells overexpressing growth hormone. Cell Immunol. 2009;255:46–54. doi: 10.1016/j.cellimm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Malarkey WB, Wang J, Cheney C, Glaser R, Nagaraja H. Human lymphocyte growth hormone stimulates interferon gamma production and is inhibited by cortisol and norepinephrine. J Neuroimmunol. 2002;123:180–187. doi: 10.1016/s0165-5728(01)00489-1. [DOI] [PubMed] [Google Scholar]
- 16.Kao TL, Harbour DV, Meyer WJ., 3d Immunoreactive growth hormone production by cultured lymphocytes. Ann NY Acad Sci. 1992;650:179–181. doi: 10.1111/j.1749-6632.1992.tb49117.x. [DOI] [PubMed] [Google Scholar]
- 17.Weigent DA, Baxter JB, Blalock JE. The production of growth hormone and insulin-like growth factor-I by the same subpopulation of rat mononuclear leukocytes. Brain Behav Immun. 1992;6:365–376. doi: 10.1016/0889-1591(92)90035-m. [DOI] [PubMed] [Google Scholar]
- 18.Re RN, Cook JL. Senescence, apoptosis, and stem cell biology: the rationale for an expanded view of intracrine action. Am J Physiol Heart Circ Physiology. 2009;297:H893–H901. doi: 10.1152/ajpheart.00414.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weigent DA. Regulation of Id2 expression in EL4 T lymphoma cells overexpressing growth hormone. Cell Immunol. 2009;255:46–54. doi: 10.1016/j.cellimm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Baumann G. Growth hormone heterogeneity: genes, isohormones, variants, and binding proteins. Endocrine Rev. 1991;12:424–449. doi: 10.1210/edrv-12-4-424. [DOI] [PubMed] [Google Scholar]
- 21.Nindl BC, Kramer WJ, Marx JO, Tuckow AP, Hymer WC. Growth hormone molecular heterogeneity and exercise. Exerc Sport Sci Rev. 2003;31:161–166. doi: 10.1097/00003677-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Diaz MJ, Dominguez F, Haro LS, Ling N, Devesa J. A 12-kilodalton N-glycosylated growth hormone-related peptide is present in human pituitary extracts. J Clin Endocrin Metab. 1993;77:134–138. doi: 10.1210/jcem.77.1.8325936. [DOI] [PubMed] [Google Scholar]
- 23.Haro LS, Lewis UJ, Garcia M, Bustamante J, Martinez AO, Ling NC. Glycosylated human growth hormone (hGH): A novel 24 kDa hGH-N variant. Biochem Biophys Res Commun. 1996;228:549–556. doi: 10.1006/bbrc.1996.1697. [DOI] [PubMed] [Google Scholar]
- 24.Baumman G. Growth hormone heterogeneity: genes, isohormones, variants, and binding proteins. Endocr Rev. 1991;12:424–449. doi: 10.1210/edrv-12-4-424. [DOI] [PubMed] [Google Scholar]
- 25.Lewis UJ, Sinha YN, Lewis GP. Structure and properties of members of the hGH family: A review. Endocrin J. 2000;47:S1–S8. doi: 10.1507/endocrj.47.supplmarch_s1. [DOI] [PubMed] [Google Scholar]
- 26.Grigorian AL, Bustamante JJ, Hernandez P, Martinez AO, Haro LS. Extraordinarily stable disulfide-linked homodimer of human growth hormone. Protein Sci. 2005;14:902–913. doi: 10.1110/ps.041048805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grigorian AL, Bustamante J, Aguilar RM, Munoz J, Maratinez AO, Haro LS. Characterization of 45 kDa mercaptoethanol-resistant human growth hormone by radioimmunoassay and bioassays. 2003:381. [Google Scholar]
- 28.Pierce JR, Tuckow AP, Alemany JA, Rarick KR, Staab JS, Harman EA, Nindl BC. Effects of acute and chronic exercise on disulfide-linked growth hormone variants. Med Sci Sports Exer. 2009;41:581–587. doi: 10.1249/MSS.0b013e31818c6d93. [DOI] [PubMed] [Google Scholar]
- 29.Weigent DA, Baxter JB, Wear WE, Smith LR, Bost KL, Blalock JE. Production of immunoreactive growth hormone by mononuclear leukocytes. FASEB J. 1988;2:2812–2818. doi: 10.1096/fasebj.2.12.3044906. [DOI] [PubMed] [Google Scholar]
- 30.Costoya JA, Vidal A, Garcia-Barros M, Arce V, Devesa J. Expression of the human growth hormone normal gene (hGH-N) in proliferating and differentiated HL-60 cells. Exper Cell Res. 1996;228:164–167. doi: 10.1006/excr.1996.0312. [DOI] [PubMed] [Google Scholar]
- 31.Sabharwal P, Varma S. Growth hormone synthesized and secreted by human thymocytes acts via insulin-like growth factor I as an autocrine and paracrine growth factor. J Clin Endo Metab. 1996;81:2663–2669. doi: 10.1210/jcem.81.7.8675594. [DOI] [PubMed] [Google Scholar]
- 32.Kooijman R, Gerlo S, Coppens A, Hooghe-Peters EL. Growth hormone and prolactin expression in the immune system. Ann NY Acad Sci. 2006;917:534–540. doi: 10.1111/j.1749-6632.2000.tb05418.x. [DOI] [PubMed] [Google Scholar]
- 33.Lytras A, Quan N, Vrontakis ME, Shaw JE, Cattini PA, Friesen HG. Growth hormone expression in human Burkitt lymphoma serum-free Ramos cell line. Endocrinology. 1993;132:620–628. doi: 10.1210/endo.132.2.7678796. [DOI] [PubMed] [Google Scholar]
- 34.Baglia LA, Cruz D, Shaw JE. Production of immunoreactive forms of growth hormone by the Burkitt tumor serum-free cell line sfRamos. Endocrinology. 1992;130:2446–2454. doi: 10.1210/endo.130.5.1572277. [DOI] [PubMed] [Google Scholar]
- 35.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 36.Dignam JD. Preparation of extracts from higher eukaryotes. Methods Enzymol. 1990;182:194–203. doi: 10.1016/0076-6879(90)82017-v. [DOI] [PubMed] [Google Scholar]
- 37.Leonardi E, Girlando S, Serio G, Mauri FA, Perrone G, Scampini S, Dalla Palma P, Barbareschi M. PCNA and Ki 67 expression in breast carcinoma: Correlations with clinical and biological variables. J Clin Pathol. 1992;45:416–419. doi: 10.1136/jcp.45.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weigent DA, Blalock JE. Production of peptide hormones and neurotransmitters by the immune system. In: Blalock JE, editor. Neuroimmunoendocrinology. Basel: Karger; 1997. pp. 1–30. [DOI] [PubMed] [Google Scholar]
- 39.Hansen J, Watson W, Jones O. Compartmentation of Nrf-2 redox control: Regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin-1. Toxicol Sci. 2007;82:308–317. doi: 10.1093/toxsci/kfh231. [DOI] [PubMed] [Google Scholar]
- 40.Shearwin-Whyatt LM, Harvey NL, Kumar S. Subcellular localization and CARD-dependent oligomerization of the death adaptor RAIDD. Cell Death Differ. 2000;7:155–165. doi: 10.1038/sj.cdd.4400632. [DOI] [PubMed] [Google Scholar]
- 41.Wild AC, Mulcahy RT. Pyrrolidine dithiocarbamate up-regulates the expression of the genes encoding the catalytic and regulatory subunits of gamma-glutamycysteine synthetase and increases intracellular glutathione levels. Biochem J. 1999;338:659–665. [PMC free article] [PubMed] [Google Scholar]
- 42.Casey W, Anderson S, Fox T, Dold K, Colton H, Morgan K. Transcriptional and physiological responses of HepG2 cells exposed to diethyl maleate: time course analysis. Physiol Genomics. 2002;8:115–122. doi: 10.1152/physiolgenomics.00064.2001. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Lee J-M, Johnson DA, Johnson JA. Antioxidant responsive element activation by Quinones: Antioxidant responsive element target genes, role of PI3 kinase in activation. Methods in Enzymology. 2001;378:238–258. doi: 10.1016/S0076-6879(04)78019-2. [DOI] [PubMed] [Google Scholar]
- 44.Edens A, Talamantes F. Alternative processing of growth hormone receptor transcripts. Endo Rev. 1998;19:559–582. doi: 10.1210/edrv.19.5.0347. [DOI] [PubMed] [Google Scholar]
- 45.Lobie PE, Mertani H, Morel G, Morales-Bustos O, Norstedt G, Waters MJ. Receptor-mediated nuclear translocation of growth hormone. J Biol Chem. 1994;269:21330–21339. [PubMed] [Google Scholar]
- 46.Lobie PE, Wood TJJ, Chen CM, Waters MJ, Norstedt G. Nuclear translocation and anchorage of the growth hormone receptor. J Biol Chem. 1994;269:31735–31746. [PubMed] [Google Scholar]
- 47.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 48.Berruyer C, Martin FM, Castellano R, Macone A, Malergue F, Garrido-Urbani S, Millet V, Imbert J, Dupre S, Pitari G, Naquet P, Galland F. Vanin-1 −/− mice exhibit a glutathione-mediated tissue resistance to oxidative stress. Mol Cell Biol. 2004;24:7214–7224. doi: 10.1128/MCB.24.16.7214-7224.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schafer G, Cramer T, Suske G, Kemmner W, Wiedenmann B, Hocker M. Oxidative stress regulates vascular endothelial growth factor-A gene transcription through Sp1- and Sp3-dependent activation of two proximal GC-rich promoter elements. J Biol Chem. 2003;278:8190–8198. doi: 10.1074/jbc.M211999200. [DOI] [PubMed] [Google Scholar]
- 50.Vines CR, Weigent DA. Identification of SP3 as a negative regulatory transcription factor in the monocyte expression of growth hormone. Endocrinology. 2000;141:938–946. doi: 10.1210/endo.141.3.7381. [DOI] [PubMed] [Google Scholar]
- 51.Polla BS, Bachelet M, Elia G, Santoro MG. Stress proteins in inflammation. Ann NY Acad Sci. 1998;851:75–85. doi: 10.1111/j.1749-6632.1998.tb08979.x. [DOI] [PubMed] [Google Scholar]
- 52.Re RN, Cook JL. The basis of an intracrine pharmacology. J Clin Pharmacol. 2008;48:344–350. doi: 10.1177/0091270007312155. [DOI] [PubMed] [Google Scholar]
- 53.Blalock JE. The immune system as a sensory organ. J Immunol. 1984;132:1067–1070. [PubMed] [Google Scholar]
- 54.Weigent DA, Blalock JE. Associations between the neuroendocrine and immune systems. J Leukocyte Biol. 1995;58:137–150. doi: 10.1002/jlb.58.2.137. [DOI] [PubMed] [Google Scholar]
- 55.Miyamoto N, Izumi H, Miyamoto R, Bin H, Kondo H, Tawara A, Sasaguri Y, Kohno K. Transcriptional regulation of activating transcription factor 4 under oxidative stress in retinal pigment epithelial ARPE-19/HPV-16 cells. Invest Ophthalmol Vis Sci. 2011;52:1226–1234. doi: 10.1167/iovs.10-5775. [DOI] [PubMed] [Google Scholar]
- 56.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKG1a enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]