Abstract
After traumatic brain injury (TBI), both primary and secondary injury cascades are initiated, leading to neuronal death and cognitive dysfunction. We have previously shown that the combinational bioflavonoid, Pycnogenol® (PYC), alters some secondary injury cascades and protects synaptic proteins when administered immediately following trauma. The purpose of the present study was to explore further the beneficial effects of PYC and to test whether it can be used in a more clinically relevant fashion. Young adult male Sprague-Dawley rats were subjected to a unilateral moderate/severe cortical contusion. Subjects received a single intravenous (i.v.) injection of PYC (1, 5, or 10 mg/kg) or vehicle, with treatment initiated at 15 min, 2 h, or 4 h post injury. All rats were killed at 96 h post TBI. Both the cortex and hippocampus ipsilateral and contralateral to the injury were evaluated for possible changes in oxidative stress (thiobarbituric acid reactive species; TBARS) and both pre- and post-synaptic proteins (synapsin-I, synaptophysin, drebrin, post synaptic density protein-95, and synapse associated protein-97). Following TBI, TBARS were significantly increased in both the injured cortex and ipsilateral hippocampus. Regardless of the dose and delay in treatment, PYC treatment significantly lowered TBARS. PYC treatment significantly protected both the cortex and hippocampus from injury-related declines in pre- and post-synaptic proteins. These results demonstrate that a single i.v. treatment of PYC is neuroprotective after TBI with a therapeutic window of at least 4 h post trauma. The natural bioflavonoid PYC may provide a possible therapeutic intervention in neurotrauma.
Key words: bioflavonoids, natural compounds, neurotrauma, oxidative stress, pycnogenol, synaptic proteins, traumatic brain injury
Introduction
It is estimated that approximately 1.7 million individuals in the United States will suffer traumatic brain injury (TBI) this year, with a large percentage requiring hospitalization.1 Multiple primary and secondary injury cascades are involved in TBI, which result in delayed neuronal dysfunction, synapse loss, and cell death.2–9 These secondary injury cascades can occur very rapidly, within minutes or hours after the trauma and last for days or weeks. Although there are many different factors, most researchers believe that pharmacologic intervention following trauma can disrupt these cascades and result in a more positive outcome. It is now clear that therapeutic intervention needs to be a multifaceted approach to target several components in a complimentary way.10 Such a pharmacologic therapeutic regime would have to take into consideration drug compatibility and timing issues.
As part of the secondary injury cascade, there are large transient increases in excitatory neurotransmitter efflux11 that result in excitotoxicity, ATP depletion, ionic imbalance, proteolysis, and oxidative stress. Due to its high content of polyunsaturated fatty acids, the brain is extremely sensitive to stress and particularly vulnerable to free radical attacks and lipid peroxidation.3 A close relationship exists between the degree of oxidative stress and the pathogenesis of TBI.12 Oxidative stress-related cascades resulting from TBI have been implicated in cytoskeletal damage, mitochondrial dysfunction,13 and altered signal transduction.14,15 Therapeutic intervention following TBI would have to disrupt the secondary injury cascades, such as oxidative stress, in order to promote a positive outcome.
Increased oxidative stress can affect the function and transportation of mitochondria to synapses, that might associate with the loss of synaptic function16–18 and neurodegeneration after brain injury.19–21 We have previously shown that following TBI there is a significant loss of synaptic proteins in both the cortex and hippocampus.22,23 This synaptic change occurs much later than the increase in levels of oxidative stress, which is a very early and long-lasting event. This early increase in oxidative stress and related cascades may play important role in synaptic loss following TBI and be responsible for behavioral dysfunction as a consequence of the injury. Therapeutic interventions must be able to afford protection of synaptic homeostasis.
Initiation of therapy immediately following the trauma would provide the greatest protection, provided that all cascades had the identical time course but this may not be the case. For a potential therapy to be clinically relevant, it must be able to alter secondary injury cascades when administered at extended times post trauma. In addition, since the injury cascades are complex, a rationale therapy would have to be able to alter multiple secondary injury cascades. A previous study from our laboratory showed neuroprotective effects of Pycnogenol® (PYC) in a rodent model of TBI.24 PYC is a patented combination of bioflavonoids extracted from the bark of French maritime pine tree (Pinus maritima) and has a high capacity to scavenge free radicals and promote cellular health. Bioflavonoids are natural compounds that are known as neuroprotective agents, due in part to their antioxidant and anti-inflammatory properties25 and their ability to modulate intracellular signaling.26 In our previous study, animals were treated with multiple high intraperitoneal doses of PYC immediately after the trauma. The neuroprotective effects of PYC shown in that study were not limited to only antioxidant and anti-inflammatory actions, but also spared synaptic proteins after neurotrauma, suggesting an agent that addresses multiple aspects of the pathophysiology following trauma. In the present series of studies, we investigated whether a single intravenous (i.v.) treatment of PYC given at delayed times post injury would also afford neuroprotection and alter levels of oxidative stress.
Methods
Chemicals
Mouse monoclonal anti β-actin, goat polyclonal anti PSD-95, and anti-synapse associated protein-97 (SAP-97) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal anti synapsin-I and anti synaptophysin were purchased from Millipore (Millipore Inc. MA, USA). Rabbit polyclonal anti-drebrin, alkaline phosphatase conjugated anti-mouse, anti-goat, anti-rabbit secondary antibodies, and all other chemicals and reagents used were purchased from Sigma (St Louis, MO, USA). Electrophoresis materials and chemicals were purchased from Bio-Rad (Hercules, CA, USA) unless stated otherwise. PYC was gifted from Horphag Research Inc. USA.
Animals and surgical procedure
Adult male Sprague-Dawley rats (n=74, 250–275 g; Harlan Laboratories, Indianapolis, IN) were housed in group cages (2 per cage) on a 12 h light/dark cycle with free access to food and water. All experimental protocols and procedures involving animals were approved by the Animal Care and Use Committee of the University of Kentucky. The animals were randomly assigned to one of twelve groups: Vehicle or PYC (1 mg/kg, 5 mg/kg, 10 mg/kg) with i.v. treatment beginning at 15 min, 2 h, or 4 h post trauma. All injections were administered via the tail vein with the total volume of each injection 100 μL. For injections occurring at 2 h and 4 h post injury, animals were anesthetized with 2% isoflurane. Unilateral cortical contusions were carried out under isoflurane anesthesia (2%) as previously described.22,23 All injuries were completed using an electronic controlled pneumatic impact device (TBI 0310, Precision Systems & Instrumentation, Fairfax Station, VA) with a hard stop Bimba cylinder (Bimba Manufacturing, Monee, IL) and a beveled 5-mm impactor tip. The depth of impact was 2.0 mm from the cortical surface with an impact velocity of 3.5 m/sec and a dwell time of 500 msec. The body temperature of each rat was monitored and maintained at 37°C. The animals were allowed to survive for 96 h post trauma for biochemical investigations.
Tissue processing
Sample preparation for the analysis was carried out as previously described.22,23 Briefly, animals were euthanized with Fatal Plus® (Vortech, Dearborn, MI), rapidly killed and the brain removed. An 8-mm punch was used to sample the ipsilateral (IP) site of cortical impact and the underlying hippocampus was isolated. The corresponding contralateral (CON) cortex and hippocampus were also sampled. At the time of dissection, none of the samples were compromised by the presence of surface blood products. These samples were immediately frozen in liquid nitrogen and stored at −80°C until used for analysis. Tissues were lysed using an ultrasonic cell disruptor (Microson Farmingdale, NY) on ice in 2.0 mL 0.1M, PBS (pH 7.4) containing 10 mM HEPES, 2.0 mM EDTA, 2.0 mM EGTA, 0.6 mM MgSO4, 4.6 mM KCl, and protease inhibitors cocktail (Millipore Inc.). Samples were centrifuged at 1000 g for 10 min/4°C to remove cell debris, and the collected supernatant was centrifuged at 15,000 g for 10 min/4°C. Supernatants were used for the analyses. Biochemical assays were completed in 96-well plates and analyzed with a SpectraMax® microplate reader (Molecular Devices, Sunnyvale, CA). Total protein concentrations were determined using the Pierce BCA method (Sigma).
Thiobarbituric acid reactive substance (TBARS)
A marker of total oxidative damage was measured as previously described.22,23 Briefly, two sets of samples (0.2 mL) were simultaneously incubated at 37±1°C and 0°C for 1 h. After 1 h of incubation, 0.2 mL of 10% trichloroacitic acid and 0.4 mL of 0.67% TBA were added to both sets of samples (i.e., 0°C and 37°C). The reaction mixture was vortexed and centrifuged at 3500 g for 15 min. The supernatant was then transferred to another tube and placed in a boiling water bath for 10 min. The samples were cooled to room temperature for 30 min, absorbance was recorded at 535 nm, and values were calculated by using a molar extinction coefficient of 1.56×105 M−1 cm−1.
Pre- and post-synaptic proteins
Synaptic marker proteins (synapsin-I, synaptophysin, drebrin, SAP-97, and PSD-95) were evaluated by Western blot as previously described.22,23,27 Briefly, samples were normalized for 50 μg protein in 25 μL of loading buffer and loaded with the appropriate marker on a gradient gel (4–20% Tris-HCl), followed by transfer to nitrocellulose membrane using a semi-dry transfer system (Bio-Rad) for 2 h at 15 volt. The membrane was blocked with 5% milk or bovine serum albumin (BSA) in Tris buffer/saline Tween-20 (TBST). Following the application of the primary antibody at the manufacturer's recommended concentrations, membranes were incubated overnight at 4°C. Beta actin was also simultaneously probed as a loading control. The blots were then washed three times in TBST and incubated for 1 h with alkaline phosphatase conjugated secondary antibodies in a 1:8000 dilution. The blots were developed in Sigma Fast™ BCIP/NBT tablets (Sigma), dried, scanned with Adobe Photoshop, and quantified with Scion Image (PC version of Macintosh-compatible NIH Image). Percent changes in levels of synaptic proteins were determined by comparing the IP hemisphere to the CON hemisphere, thus using each animal as its own control.
Statistical analysis
Dose and time-dependent differences in oxidative stress marker TBARS and synaptic marker protein levels are reported as mean±standard deviation. Differences between the group means were evaluated with a two-way analysis of variance (ANOVA) (Time by Dose) coupled with a Fisher's PLSD post hoc test when warranted (StatView 5.0, SAS Institute). For significance, alpha was set at 0.05.
Results
Oxidative damage (TBARS): Cortex
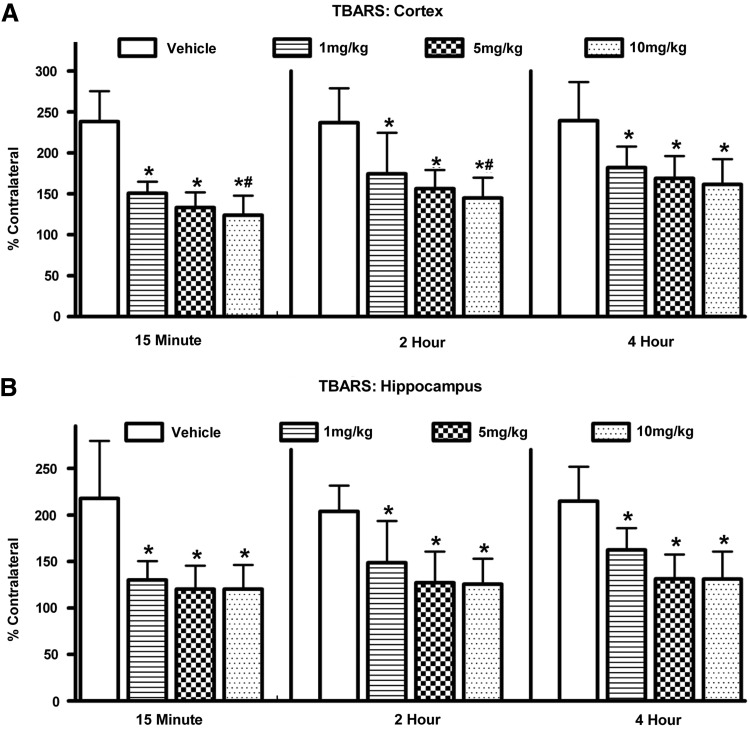
Levels of TBARS were elevated after injury regardless of treatment. A two-way ANOVA (Time by Dose) revealed a significant main effect for Time [F(2,62)=4.552; p<0.05] indicating that as the delay increased between trauma and initiation of therapy the levels of TBARS increased (Fig. 1A). Post hoc comparison revealed that levels were significantly lower at 15 min compared to both 2 h and 4 h (p<0.05). There was no difference between 2 h and 4 h (p>0.1). There was a significant main effect for Dose [F(3,62)=32.116; p<0.0001], indicating that the drug was significantly better at reducing oxidative stress than vehicle alone. Post hoc testing revealed that although the 10 mg/kg group reduced oxidative stress to a greater degree than 1 mg/kg (p<0.05), it was not significantly better than the 5 mg/kg treatment. There was no significant interaction between Time and Dose [F(6,62)=0.474; p>0.1].
FIG. 1.
Changes in the level of oxidative stress (TBARS) in both the cortex (A) and hippocampus (B) at 96 h after a moderate unilateral cortical contusion. Levels of TBARS were significantly increased in both brain regions following the brain injury. Vehicle-treated animals showed the greatest increases (200%). Animals treated with PYC showed significantly lower levels of oxidative stress whether treatment was delayed for 15 min, 2 h, or 4 h. Each bar represents the group mean±SD. *p<0.001 compared to vehicle; #p<0.05 compared to the lower 1 mg/kg dose.
Oxidative damage (TBARS): Hippocampus
Levels of TBARS in the hippocampus were evaluated using a two-way ANOVA. This analysis revealed a significant main effect for Dose [F(3,62)=26.024; p<0.0001], indicating that the drug significantly altered the levels of oxidative damage (Fig. 1B). Post hoc testing revealed that levels of TBARS were significantly higher in the vehicle-treated group compared to all doses of PYC-treated subjects (p<0.0001). The analysis failed to reveal a significant main effect for Time [F(2, 62) 0.956; p>0.1] or any interaction [F(6,62)=0.380; p>0.1]. Increasing the time between the trauma and initiation of PYC therapy did not appear to alter the level of TBARS, indicating that the reduction observed at 4 h with PYC was equivalent to that at 15 min.
Synaptic proteins: Cortex
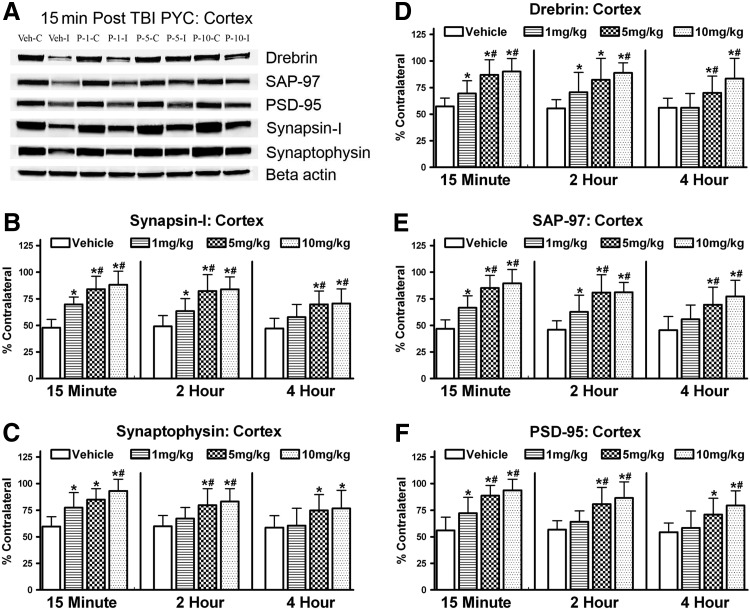
Five different synaptic proteins were evaluated at 96 h following the trauma. As can be seen in Figure 2A, all five were affected by the PYC therapeutic intervention. Two different pre-synaptic proteins (synapsin-I; synaptophysin) were evaluated. A two-way ANOVA (Time by Dose) for synapsin-I revealed a significant main effect for both Time [F(2,62)=6.267; p<0.005] and Dose [F(3,62)=30.616; p<0.0001] (Fig. 2B). As for the timing of the initiation of therapy, post hoc evaluation revealed that the 15 min and 2 h times were significantly better than 4 h delay (p<0.05), and 15 min and 2 h were not significantly different (p>0.05). Post hoc evaluation of the dose data showed that in all cases the PYC-treated animals were significantly different than the vehicle-treated animals, showing increased levels of synapsin-I (p<0.0001). In addition, animals treated with either 5 mg/kg or 10 mg/kg were significantly better than the 1 mg/kg PYC group (p<0.0005). The two higher doses were not significantly different. There was no significant interaction between Time and Dose [F(6,62)=0.695; p>0.1].
FIG. 2.
Changes in the levels of synaptic proteins in the ipsilateral cortex following a cortical contusion injury at 96 h. Both pre (synapsin-I (B); synaptophysin (C)) and post (drebrin (D), SAP-97 (E), PSD-95 (F)) synaptic proteins were significantly decreased in vehicle-treated animals, while PYC treatment afforded significant protection. (A) Typical immunoblot showing densities of the bands used in analysis. Time indicates the delay following injury when treatment was initiated. Each bar represents the group mean±SD. *p<0.001 compared to vehicle; #p<0.05 compared to the lower 1 mg/kg dose.
The synaptophysin data mimicked the synapsin-I results (Fig. 2C). A two-way ANOVA (Time by Dose) revealed significant main effects for both Time [F(2,62)=4.451; p<0.05] and Dose [F(3,62)=12.873; p<0.0001]. Therapy at 15 min post trauma was significantly better than therapy initiated at 4 h (p<0.005). In all cases, the PYC-treated animals demonstrated significantly greater levels of synaptophysin compared to vehicle-treated controls (p<0.005). The post hoc comparisons also revealed that the higher doses (5 mg/kg; 10 mg/kg) were significantly better (p<0.05; p<0.001) than the 1 mg/kg cohort. There was no significant interaction between Time and Dose [F(6,62)=0.483; p>0.1].
Three different important postsynaptic proteins (drebrin, PSD-95, SAP-97) were also evaluated. Possible changes in drebrin were evaluated with a two-way ANOVA (Time by Dose) and, while there was a significant main effect for Dose [F(3,62)=17.320; p<0.0001], there was no significant effect for Time [F(2,62)=2.836; p>0.05] indicating that delaying the therapy was not responsible for a significant portion of the variance (Fig. 2D). Post hoc testing did reveal that the PYC treatment significantly increased levels of drebrin compared to vehicle treated subjects (p<0.01) and that the 5 mg and 10 mg/kg groups were significantly better than the 1 mg/kg cohort (p<0.005). There was no significant interaction between Time and Dose [F(6,62)=0.441; p>0.1].
Evaluation of the SAP-97 results showed a significant main effect for Time [F(2,62)=3.884] and Dose [F(3,62)=29,782; p<0.0001] (Fig. 2E). Animals treated at 15 min post trauma demonstrated significantly higher levels of SAP-97 compared to the 4 h delay group (p<0.01) but were not significantly different from the 2 h cohort (p>0.1). As with the other synaptic proteins, treatment with any dose of PYC significantly elevated levels of SAP-97 (p<0.0005). Both the 5 mg/kg and 10 g/kg dosing were significantly better in elevating levels of SAP-97 compared to the lower 1 mg/kg dose. There was no significant interaction between Time and Dose [F(6,62)=0.391; p>0.1].
Possible changes in PSD-95 were also evaluated with a two-way ANOVA (Time by Dose) and demonstrated a significant main effect for both Time [F(2,62)=5.518; p<0.01] and Dose [F(3,62)=21.763; p<0.0001] (Fig 2F). Post hoc testing revealed that the 15 min initiation of therapy was significantly better than the 4 h delay (p<0.005) but not significantly different from 2 h (p>0.05). The PYC treatment, regardless of dose, significantly increased PSD-95 levels compared to vehicle-treated cohorts (p<0.001). The 5 mg/kg and 10 mg/kg groups were not significantly different from each other (p>0.1) but were significantly better than the 1 mg/kg treated animals (p<0.0005). There was no significant interaction between Time and Dose [F(6,62)=0.461; p>0.1].
Synaptic proteins: Hippocampus
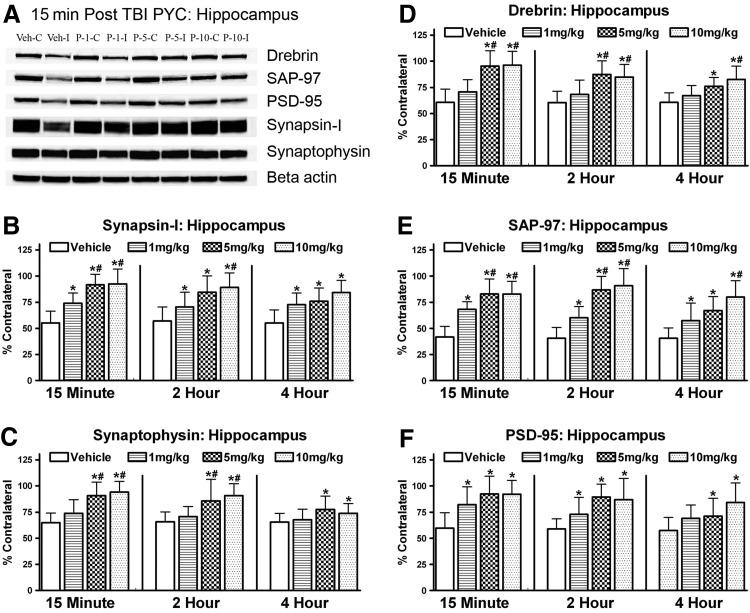
Hippocampal synaptic proteins were altered in a manner similar to the cortex following the cortical contusion (Fig. 3A). Western blot analysis revealed a decline in both pre- and post-synaptic proteins. A two-way ANOVA (Time by Dose) for synapsin-I revealed a significant main effect for Dose [F(3,62)=24.029; p<0.001] but not for Time [F(2,62)=1.590; p>0.1] (Fig. 3B). Post hoc testing revealed that PYC, regardless of dose, significantly spared this synaptic marker compared to the vehicle-treated animals (p<0.0005). The two higher doses were significantly better at protecting synapsin-I compared to the 1 mg/kg dose (p<0.005) but were not different from each other (p>0.1). There was no significant interaction between Time and Dose [F(6,62)=0.575; p>0.1].
FIG. 3.
Changes in the levels of synaptic proteins in the ipsilateral hippocampus after a cortical contusion injury at 96 h. Both pre (synapsin I (B); synaptophysin (C)) and post (drebrin (D), SAP-97 (E), PSD-95 (F)) synaptic proteins were significantly decreased in vehicle-treated animals. Subjects treated with PYC were afforded significant neuroprotection. Panel (A) shows typical immunoblotting bands used in the analysis. Time indicates the delay following injury when treatment was initiated. Each bar represents the group mean±SD. *p<0.001 compared to vehicle; #p<0.05 compared to the lower 1 mg/kg dose.
Analysis of the synaptophysin data revealed a significant main effect for both Time [F(2,62)=4.386; p<0.05] and Dose [F(3,62)=13.456; p<0.0001] (Fig. 3C). Therapy at 15 min was significantly better at protecting this synaptic protein compared to a delay of 4 h (p<0.005). but was not different from the 2 h delay (p>0.1). The two higher doses of PYC showed significantly higher levels of synaptophysin compared to vehicle (p<0.0001) and the lower PYC dose (p<0.001). but were not different from each other (p>0.1). There was no significant interaction between Time and Dose [F(6,62)=0.995; p>0.1].
Changes in the post synaptic protein, drebrin, showed a Time [F(2,62)=3.758; p<0.05] and Dose [F(3,62)=22.334; p<0.0001] change in levels with PYC therapy (Fig. 3D). As with the pre synaptic proteins, delaying therapy for 4 h significantly affected levels compared to 15 min (p<0.01). All groups treated with PYC were significantly better at protecting drebrin levels. The two higher doses provided the greatest protection (p<0.0001) and were also significantly better compared to the1 mg/kg group (p<0.0001). There was no significant interaction between Time and Dose [F(6,62)=0.924; p>0.1].
A two-way analysis of the SAP-97 data showed a robust dose effect [F(3,62)=44.177; p<0.0001], but no main effect for Time [F(2,62)=3.090; p>0.05] (Fig. 3E). Post hoc testing revealed that PYC, regardless of dose, was significantly better at protecting the SAP-97 protein (p<0.0001) compared to vehicle. In addition, the two higher doses of PYC (5 mg/kg; 10 mg/kg) showed significantly greater levels (p<0.005) than the 1 mg/kg group. There was no significant interaction between Time and Dose [F(6,62)=0.924; p>0.1].
Analysis of the PSD-95 results revealed changes similar to the other post synaptic proteins (Fig. 3F). There was a significant main effect for Dose [F(3,62)=11.993; p<0.0001]. but no significant main effect for Time [F(2,62)=2.986; p>0.05]. PYC treatment, regardless of dose showed significantly greater protection of PSD-95 compared to the vehicle treated groups (p<0.005). The 10 mg/kg group showed the greatest sparing and was significantly greater than the 1 mg/kg cohort (p<0.05) but not the 5 mg/kg group (p>0.1). There was no significant interaction between Time and Dose [F(6,62)=0.560; p>0.1].
Discussion
This is the first study evaluating possible neuroprotective aspects of PYC therapy in a clinically relevant fashion in a well-established animal model of TBI. Animals treated i.v. post injury with PYC showed significant neuroprotective effects. PYC treatment reduced oxidative stress and spared both pre- and post-synaptic proteins in both the cortex and hippocampus. The levels of neuroprotection were affected by the different doses of PYC administered. In addition, we showed that the therapeutic window can be extended up to at least 4 h post trauma.
Oxidative stress is a key component of the secondary injury cascades following TBI. The brain is highly sensitive to oxidative damage because of its high content of polyunsaturated fatty acids, which are vulnerable to free radical attacks and lipid peroxidation.3 Significant increases can be observed as early as 30 min post trauma.28 Levels of oxidative stress peak around 24 to 48 h after injury.22,23 We previously reported that when PYC (100 mg/kg) was administered in multiple i.p. injections, there was a significant reduction in oxidative stress following a moderate/severe TBI.24 The present experiments used a more clinically relevant paradigm by treating animals with a single i.v. injection of PYC. Compared to the i.p. administration, animals receiving the single i.v. injection were afforded greater reduction in oxidative stress. However, even with early i.v. treatment (i.e., 15 min), the levels of oxidative stress were elevated compared with the uninjured hemisphere. These results support the idea that secondary injury cascades occur very rapidly following TBI but can be manipulated with therapeutic intervention including bioflavonoids.
The neuroprotective effects of PYC are not confined to a single cell type or region of the brain. As in our previous study,24 PYC showed significant effects in both the injured cortex and hippocampus. Both regions showed a dose-dependent effect in regards to both the levels of oxidative stress and the sparing of synaptic proteins. Even the lowest dose evaluated (1 mg/kg) showed significant protection when compared to vehicle-treated animals, while the highest levels (10 mg/kg) afforded the greatest neuroprotection. Differences between 5 mg/kg and 10 mg/kg were only discernible in relatively few comparisons. These neuroprotective effects occurred in both regions of interest. The sparing of synaptic proteins in both brain regions is very important because it indicates a possible sparing of synaptic contacts that are known to be disrupted following TBI.8
For a therapeutic compound to be considered clinically relevant, it must provide a significant effect when the therapy onset is delayed for an extended time post trauma. To explore the therapeutic window, onset of PYC treatment was initiated at different times post trauma. There was a significant time effect in the injured cortex. Animals treated with PYC at 15 min demonstrated significantly lower levels of oxidative stress compared to a 2 or 4 h delay following injury. There was no significant difference observed between 2 and 4 h treatment of PYC. The hippocampus failed to show a significant time effect, indicating that the significant reduction in oxidative stress was just as effective at 4 h as it was when initiated at 15 min. One possible explanation might be because the level of oxidative stress was substantially lower in the hippocampus compared with the ipsilateral cortex. This might indicate that PYC treatment may show its maximum benefit under conditions of mild TBI.
There were limited effects of therapy delay in both the cortex and hippocampus in regards to the protection of synaptic proteins. In the cortex, there were significant differences between 15 min and 4 h for SAP-97, synapsin-I, synaptophysin, and PSD-95. In the hippocampus, these two times differed for synaptophysin and drebrin. However, these differences were not very robust. There were no differences between 15 min versus 2 h delay or between the 2 h and 4 h delay. In all cases, PYC treatment significantly spared synaptic proteins in both regions, regardless of the dose or time of treatment onset. This was an expected outcome considering the decline in synaptic proteins is known to be delayed following trauma and i.p. treatment of PYC previously demonstrated strong protective effects.24 Maximum changes in the synaptic protein levels have previously been reported at 96 h following an identical injury with no significant changes occurring within the first 6 to 12 hours.22,23 These results support the idea that i.v. injection of PYC has a reasonable therapeutic window. It has yet to be determined whether neuroprotective timing can be extended even further.
Bioflavonoids are unique compounds, present ubiquitously in nature and found in high concentrations in many fruits, vegetables, tea, coca, and wine.29 Previous studies have shown that rodents can tolerate extremely high doses of bioflavonoids without any significant detrimental effects.30 A human clinical study reported that elderly individuals tolerated 150 mg/day of PYC for 3 months without any significant side effects.31 PYC has very low acute and chronic toxicity with mild unwanted effects occurring in a small percentage of human subjects.32 The beneficial effects of PYC24 indicate it can cross a compromised blood-brain barrier, which is breached soon after injury.33
Various studies indicate that bioflavonoids may have neuroprotective effects in TBI.21,34–37 Bioflavonoids have the ability not only to reduce inflammation, brain edema, and neuronal death,29,38–43 but can improve both motor and cognitive function in rodents after TBI.35,36,40,41,44,45 PYC has the unique characteristic of providing a multifaceted approach of neuroprotection, demonstrated by the fact it reduced inflammation, decreased oxidative damage, and spared synaptic proteins in the brain following TBI.24,46 The neuroprotective effects of PYC are likely more than free radical quenching. Bioflavonoids can interact with various cell signaling cascades.47–50 Among those cascades, some of the enzymes and related mechanisms have been well investigated in neurotrauma.51–56 The interaction of the bioflavonoids with these cascades can inhibit apoptotic processes and improve cell survival.57–62
In conclusion, this study demonstrates the substantial neuroprotective effects of PYC following moderate to severe TBI in rats by reducing oxidative stress and sparing synaptic proteins. The results show a relatively long therapeutic window (up to 4 h) for PYC treatment following neurotrauma, with the same protective effects in both the cortex and the hippocampus. For i.v. treatment, 5–10 mg/kg PYC is a sufficient dose to use for protection without toxicity. It has yet to be determined what specific mechanisms may be responsible for the neuroprotective effects of PYC. It will be important to determine if specific cell signaling cascades or molecular changes in the transcriptional factors may be influenced. Additional studies are needed to determine whether these same neuroprotective effects of PYC will be observed in other TBI models.
Abbreviations Used
- ATP
adenosine triphosphate
- BSA
bovine serum albumin
- CON
contralateral
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IP
ipsilateral
- PSD-95
post synaptic density 95
- PYC
pycnogenol
- SAP-97
synapse associated protein 97
- TBARS
thiobarbituric acid reactive substance
- TBI
traumatic brain injury
- TBST
Tris buffer/saline-Tween-20
Acknowledgments
This work was supported by National Institute of Health Grant R21NS66117 and Kentucky Spinal Cord Brain Injury Trust 12-16A. Pycnogenol® was a very generous gift from Horphag Research Inc., Hoboken, NJ.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M. Xu L. Wald M. Coronado V. Atlanta, GA: Centers for Disease control and Prevention National Center for Injury Prevention and Control; 2010. Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006. [Google Scholar]
- 2.Baldwin SA. Gibson T. Callihan CT. Sullivan PG. Palmer E. Scheff SW. Neuronal cell loss in the CA3 subfield of the hippocampus following cortical contusion utilizing the optical disector method for cell counting. J Neurotrauma. 1997;14:385–398. doi: 10.1089/neu.1997.14.385. [DOI] [PubMed] [Google Scholar]
- 3.Hall ED. Vaishnav RA. Mustafa AG. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hovda DA. Yoshino A. Kawamata T. Katayama Y. Becker DP. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: A cytochrome oxidase histochemistry study. Brain Res. 1991;567:1–10. doi: 10.1016/0006-8993(91)91429-5. [DOI] [PubMed] [Google Scholar]
- 5.Kawamata T. Katayama Y. Hovda DA. Yoshino A. Becker DP. Lactate accumulation following concussive brain injury: The role of ionic fluxes induced by excitatory amino acids. Brain Res. 1995;674:196–204. doi: 10.1016/0006-8993(94)01444-m. [DOI] [PubMed] [Google Scholar]
- 6.Lenzlinger PM. Morganti-Kossmann MC. Laurer HL. McIntosh TK. The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol. 2001;24:169–181. doi: 10.1385/MN:24:1-3:169. [DOI] [PubMed] [Google Scholar]
- 7.McIntosh TK. Smith DH. Meaney DF. Kotapka MJ. Gennarelli TA. Graham DI. Neuropathological sequelae of traumatic brain injury: Relationship to neurochemical and biomechanical mechanisms. Lab Invest. 1996;74:315–342. [PubMed] [Google Scholar]
- 8.Scheff SW. Price DA. Hicks RR. Baldwin SA. Robinson S. Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J Neurotrauma. 2005;22:719–732. doi: 10.1089/neu.2005.22.719. [DOI] [PubMed] [Google Scholar]
- 9.Xiong Y. Gu Q. Peterson PL. Muizelaar JP. Lee CP. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- 10.Margulies S. Hicks R. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biegon A. Fry PA. Paden CM. Alexandrovich A. Tsenter J. Shohami E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci USA. 2004;101:5117–5122. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao C. Roberts KN. Markesbery WR. Scheff SW. Lovell MA. Oxidative stress in head trauma in aging. Free Radic Biol Med. 2006;41:77–85. doi: 10.1016/j.freeradbiomed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Singh IN. Sullivan PG. Hall ED. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J Neurosci Res. 2007;85:2216–2223. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- 14.Bayir H. Kagan VE. Clark RS. Janesko-Feldman K. Rafikov R. Huang Z. Zhang X. Vagni V. Billiar TR. Kochanek PM. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem. 2007;101:168–181. doi: 10.1111/j.1471-4159.2006.04353.x. [DOI] [PubMed] [Google Scholar]
- 15.Hall ED. Detloff MR. Johnson K. Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- 16.Cayabyab FS. Khanna R. Jones OT. Schlichter LC. Suppression of the rat microglia Kv1.3 current by src-family tyrosine kinases and oxygen/glucose deprivation. Eur J Neurosci. 2000;12:1949–1960. doi: 10.1046/j.1460-9568.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- 17.de la Monte SM. Neely TR. Cannon J. Wands JR. Oxidative stress and hypoxia-like injury cause Alzheimer-type molecular abnormalities in central nervous system neurons. Cell Mol Life Sci. 2000;57:1471–1481. doi: 10.1007/PL00000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matzilevich DA. Rall JM. Moore AN. Grill RJ. Dash PK. High-density microarray analysis of hippocampal gene expression following experimental brain injury. J Neurosci Res. 2002;67:646–663. doi: 10.1002/jnr.10157. [DOI] [PubMed] [Google Scholar]
- 19.Gilmer LK. Ansari MA. Roberts KN. Scheff SW. Age-related mitochondrial changes after traumatic brain injury. J Neurotrauma. 2010;27:939–950. doi: 10.1089/neu.2009.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotulska K. LePecheur M. Marcol W. Lewin-Kowalik J. Larysz-Brysz M. Paly E. Matuszek I. London J. Overexpression of copper/zinc-superoxide dismutase in transgenic mice markedly impairs regeneration and increases development of neuropathic pain after sciatic nerve injury. J Neurosci Res. 2006;84:1091–1097. doi: 10.1002/jnr.21000. [DOI] [PubMed] [Google Scholar]
- 21.Wu A. Ying Z. Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Ansari MA. Roberts KN. Scheff SW. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J Neurotrauma. 2008;25:513–526. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- 23.Ansari MA. Roberts KN. Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheff SW. Ansari MA. Roberts KN. Neuroprotective effect of Pycnogenol(R) following traumatic brain injury. Exp Neurol. 2013;239:183–191. doi: 10.1016/j.expneurol.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yen TL. Hsu CK. Lu WJ. Hsieh CY. Hsiao G. Chou DS. Wu GJ. Sheu JR. Neuroprotective effects of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus), in ischemic stroke of rats. J Agricull Food Chem. 2012;60:1937–1944. doi: 10.1021/jf204909p. [DOI] [PubMed] [Google Scholar]
- 26.Mercer LD. Kelly BL. Horne MK. Beart PM. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: Investigations in primary rat mesencephalic cultures. Biochem Pharmacol. 2005;69:339–345. doi: 10.1016/j.bcp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Ansari MA. Keller JN. Scheff SW. Protective effect of Pycnogenol in human neuroblastoma SH-SY5Y cells following acrolein-induced cytotoxicity. Free Radic Biol Med. 2008;45:1510–1519. doi: 10.1016/j.freeradbiomed.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh IN. Sullivan PG. Deng Y. Mbye LH. Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: Implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- 29.Tunon MJ. Garcia-Mediavilla MV. Sanchez-Campos S. Gonzalez-Gallego J. Potential of flavonoids as anti-inflammatory agents: Modulation of pro-inflammatory gene expression and signal transduction pathways. Curr Drug Metab. 2009;10:256–271. doi: 10.2174/138920009787846369. [DOI] [PubMed] [Google Scholar]
- 30.Ince I. Yesil-Celiktas O. Karabay-Yavasoglu NU. Elgin G. Effects of Pinus brutia bark extract and Pycnogenol in a rat model of carrageenan induced inflammation. Phytomedicine. 2009;16:1101–1104. doi: 10.1016/j.phymed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Ryan J. Croft K. Mori T. Wesnes K. Spong J. Downey L. Kure C. Lloyd J. Stough C. An examination of the effects of the antioxidant Pycnogenol on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J Psychopharmacol. 2008;22:553–562. doi: 10.1177/0269881108091584. [DOI] [PubMed] [Google Scholar]
- 32.Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Intl J Clin Pharmacol Therapeut. 2002;40:158–168. doi: 10.5414/cpp40158. [DOI] [PubMed] [Google Scholar]
- 33.Baldwin SA. Fugaccia I. Brown DR. Brown LV. Scheff SW. Blood-brain barrier breach following cortical contusion in the rat. J Neurosurg. 1996;85:476–481. doi: 10.3171/jns.1996.85.3.0476. [DOI] [PubMed] [Google Scholar]
- 34.Bistrian BR. Askew W. Erdman JW. Oria MP. Nutrition and traumatic brain injury: A perspective from the Institute of Medicine Report. J Parent Enter Nutrit. 2011;35:556–559. doi: 10.1177/0148607111416122. [DOI] [PubMed] [Google Scholar]
- 35.Itoh T. Imano M. Nishida S. Tsubaki M. Hashimoto S. Ito A. Satou T. (-)-Epigallocatechin-3-gallate protects against neuronal cell death and improves cerebral function after traumatic brain injury in rats. Neuromol Med. 2011;13:300–309. doi: 10.1007/s12017-011-8162-x. [DOI] [PubMed] [Google Scholar]
- 36.Schultke E. Kamencic H. Zhao M. Tian GF. Baker AJ. Griebel RW. Juurlink BH. Neuroprotection following fluid percussion brain trauma: A pilot study using quercetin. J Neurotrauma. 2005;22:1475–1484. doi: 10.1089/neu.2005.22.1475. [DOI] [PubMed] [Google Scholar]
- 37.Sharma S. Zhuang Y. Ying Z. Wu A. Gomez-Pinilla F. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161:1037–1044. doi: 10.1016/j.neuroscience.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ates O. Cayli S. Altinoz E. Gurses I. Yucel N. Sener M. Kocak A. Yologlu S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem. 2007;294:137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- 39.Chen CC. Hung TH. Wang YH. Lin CW. Wang PY. Lee CY. Chen SF. Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-kappaB signaling after experimental traumatic brain injury. PLoS One. 2012;7:e30294. doi: 10.1371/journal.pone.0030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen SF. Hsu CW. Huang WH. Wang JY. Post-injury baicalein improves histological and functional outcomes and reduces inflammatory cytokines after experimental traumatic brain injury. Br J Pharmacol. 2008;155:1279–1296. doi: 10.1038/bjp.2008.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laird MD. Sukumari-Ramesh S. Swift AE. Meiler SE. Vender JR. Dhandapani KM. Curcumin attenuates cerebral edema following traumatic brain injury in mice: A possible role for aquaporin-4? J Neurochem. 2010;113:637–648. doi: 10.1111/j.1471-4159.2010.06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HF. Lee TS. Kou YR. Anti-inflammatory and neuroprotective effects of triptolide on traumatic brain injury in rats. Respir Physiol Neurobiol. 2012;182:1–8. doi: 10.1016/j.resp.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Morales DM. Marklund N. Lebold D. Thompson HJ. Pitkanen A. Maxwell WL. Longhi L. Laurer H. Maegele M. Neugebauer E. Graham DI. Stocchetti N. McIntosh TK. Experimental models of traumatic brain injury: Do we really need to build a better mousetrap? Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 44.Di Giovanni S. Movsesyan V. Ahmed F. Cernak I. Schinelli S. Stoica B. Faden AI. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci USA. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singleton RH. Yan HQ. Fellows-Mayle W. Dixon CE. Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. J Neurotrauma. 2010;27:1091–1099. doi: 10.1089/neu.2010.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho KJ. Yun CH. Packer L. Chung AS. Inhibition mechanisms of bioflavonoids extracted from the bark of Pinus maritima on the expression of proinflammatory cytokines. Ann NY Acad Sci. 2001;928:141–156. doi: 10.1111/j.1749-6632.2001.tb05644.x. [DOI] [PubMed] [Google Scholar]
- 47.Gamet-Payrastre L. Manenti S. Gratacap MP. Tulliez J. Chap H. Payrastre B. Flavonoids and the inhibition of PKC and PI 3-kinase. Gen Pharmacol. 1999;32:279–286. doi: 10.1016/s0306-3623(98)00220-1. [DOI] [PubMed] [Google Scholar]
- 48.Schroeter H. Spencer JP. Rice-Evans C. Williams RJ. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J. 2001;358:547–557. doi: 10.1042/0264-6021:3580547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spencer JP. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007;2:257–273. doi: 10.1007/s12263-007-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams RJ. Spencer JP. Rice-Evans C. Flavonoids: Antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Carloni S. Girelli S. Scopa C. Buonocore G. Longini M. Balduini W. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy. 2010;6:366–377. doi: 10.4161/auto.6.3.11261. [DOI] [PubMed] [Google Scholar]
- 52.Endo H. Nito C. Kamada H. Nishi T. Chan PH. Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1479–1489. doi: 10.1038/sj.jcbfm.9600303. [DOI] [PubMed] [Google Scholar]
- 53.Hu LY. Sun ZG. Wen YM. Cheng GZ. Wang SL. Zhao HB. Zhang XR. ATP-mediated protein kinase B Akt/mammalian target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase signaling pathway activation promotes improvement of locomotor function after spinal cord injury in rats. Neuroscience. 2010;169:1046–1062. doi: 10.1016/j.neuroscience.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 54.Noshita N. Lewen A. Sugawara T. Chan PH. Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol Dis. 2002;9:294–304. doi: 10.1006/nbdi.2002.0482. [DOI] [PubMed] [Google Scholar]
- 55.Shapira M. Licht A. Milman A. Pick CG. Shohami E. Eldar-Finkelman H. Role of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injury. Mol Cell Neurosci. 2007;34:571–577. doi: 10.1016/j.mcn.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Shein NA. Tsenter J. Alexandrovich AG. Horowitz M. Shohami E. Akt phosphorylation is required for heat acclimation-induced neuroprotection. J Neurochem. 2007;103:1523–1529. doi: 10.1111/j.1471-4159.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- 57.Seger R. Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 58.Azevedo MF. Camsari C. Sa CM. Lima CF. Fernandes-Ferreira M. Pereira-Wilson C. Ursolic acid and luteolin-7-glucoside improve lipid profiles and increase liver glycogen content through glycogen synthase kinase-3. Phytother Res. 2010;24:S220–224. doi: 10.1002/ptr.3118. [DOI] [PubMed] [Google Scholar]
- 59.de la Torre AV. Junyent F. Folch J. Pelegri C. Vilaplana J. Auladell C. Beas-Zarate C. Pallas M. Verdaguer E. Camins A. GSK3beta inhibition is involved in the neuroprotective effects of cyclin-dependent kinase inhibitors in neurons. Pharmacol Res. 2012;65:66–73. doi: 10.1016/j.phrs.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Johnson JL. Rupasinghe SG. Stefani F. Schuler MA. Gonzalez de Mejia E. Citrus flavonoids luteolin, apigenin, and quercetin inhibit glycogen synthase kinase-3beta enzymatic activity by lowering the interaction energy within the binding cavity. J Med Food. 2011;14:325–333. doi: 10.1089/jmf.2010.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parajuli P. Joshee N. Chinni SR. Rimando AM. Mittal S. Sethi S. Yadav AK. Delayed growth of glioma by Scutellaria flavonoids involve inhibition of Akt, GSK-3 and NF-kappaB signaling. J Neurooncol. 2011;101:15–24. doi: 10.1007/s11060-010-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vauzour D. Vafeiadou K. Rice-Evans C. Williams RJ. Spencer JP. Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J Neurochem. 2007;103:1355–1367. doi: 10.1111/j.1471-4159.2007.04841.x. [DOI] [PubMed] [Google Scholar]