Abstract
The high water content of hydrogels allows these materials to closely mimic the native biological extracellular conditions, but it also makes difficult the histological preparation of hydrogel-based bioengineered tissue. Paraffin-embedding techniques require dehydration of hydrogels, resulting in substantial collapse and deformation, whereas cryosectioning is hampered by the formation of ice crystals within the hydrogel material. Here, we sought to develop a method to obtain good-quality cryosections for the microscopic evaluation of hydrogel-based tissue-engineered constructs, using polyethylene glycol (PEG) as a test hydrogel. Conventional sucrose solutions, which dehydrate cells while leaving extracellular water in place, produce a hydrogel block that is brittle and difficult to section. We therefore replaced sucrose with multiple protein-based and nonprotein-based solutions as cryoprotectants. Our analysis demonstrated that overnight incubation in bovine serum albumin (BSA), fetal bovine serum (FBS), polyvinyl alcohol (PVA), optimum cutting temperature (OCT®) compound, and Fisher HistoPrep frozen tissue-embedding media work well to improve the cryosectioning of hydrogels. The protein-based solutions give background staining with routine hematoxylin and eosin, but the use of nonprotein-based solutions PVA and OCT reduces this background by 50%. These methods preserve the tissue architecture and cellular details with both in vitro PEG constructs and in constructs that have been implanted in vivo. This simple hydrogel cryosectioning technique improves the methodology for creation of good-quality histological sections from hydrogels in multiple applications.
Introduction
Hydrogel materials are commonly used in tissue engineering and drug delivery. Due to their rich water content and polymeric structure, hydrogels can mimic the physiological environments to support long-term survival of numerous cell types1–4 and can provide three-dimensional frameworks to direct cellular behaviors and interactions.5–7 Polyethylene glycol (PEG) is a synthetic bioinert polymer that has no innate cell adhesiveness and biological activity; however, PEG can be easily functionalized with bioactive peptide sequences or proteins, such as RGD or BMP-2, to present biological cues and stimulate cell behaviors.8,9 Inclusion of cell process-catalyzed degradable crosslinking molecules, such as matrix metalloprotease (MMP)-sensitive peptides, can further promote the remodeling of the basic PEG polymeric structures and mediate native cellular matrix deposition. Previous studies have demonstrated that PEG hydrogels can be formed via a gentle, cell-compatible photopolymerization reaction using long-wave ultraviolet (UV) light.10,11 Cells encapsulated in PEG hydrogels using this method maintain both their survival and differentiation capabilities. For example, mesenchymal stem cells (MSCs) seeded into a PEG hydrogel matrix were able to differentiate toward osteogenic, chondrogenic, and adipogenic fates12,13; similarly, valvular interstitial cell (VIC) migration, proliferation, and differentiation within PEG hydrogels have been tuned using the polymer network density and the attached functional ligands.14 Functionalized PEG matrices even show utility in wound repair, as a cell-degradable PEG hydrogel implanted in the areas of skull defect has been shown to quickly resorb while substantially stimulating bone formation.15 These results demonstrate the utility and versatility of the PEG hydrogel for biocompatible, synthetic matrix-based tissue engineering.
Optimal visualization of the cell–matrix interactions and cell growth patterns is crucial for evaluating the success of tissue engineering strategies. Histological sections provide important information on cell compatibility, distribution, implant degradation, and host response. However, due to their water-rich and polymeric natures, hydrogels are very sensitive to routine tissue histology procedures. Standard paraffin processing of polylactide sponges has been shown to cause distortion of the scaffold structure.16 This process is performed at a high temperature (60°C) and involves replacement of tissue water, initially with ethanol and then with a nonpolar solvent. This process generally causes collapse of the hydrogel structure, yielding specimens that bear little resemblance to their original state. Additionally, the hydrophobic properties of paraffin may cause incomplete infiltration of the sample, making sectioning difficult. Cryosectioning is another common histological technique used on tissues and scaffolds to preserve epitopes sensitive to paraffin processing, but the quality of hydrogel cryosections tends to be poor due to the mismatch in mechanical properties between hydrogels and typical embedding media at sectioning temperatures (i.e., below freezing). In routine cryohistology, tissues are infiltrated with 30% sucrose solution for cryoprotection before sectioning. Sucrose osmotically dehydrates the cells, and since the majority of tissue water resides intracellularly in vivo, this reduces the formation of ice crystals and makes the tissues more sectionable. In hydrogels, however, there is substantially more extracellular water, and frozen blocks from sucrose-treated hydrogels remain too brittle to be cut with standard instruments.
In this study, an optimized cryosection method for PEG hydrogels was established. The main objective was to generate complete and undistorted sections and minimize background during routine histological staining. For these purposes, sucrose was replaced in the traditional role of a cryoprotectant, and several other infiltrating solutions were examined for more optimal sectionability and hematoxylin and eosin (H&E) staining with PEG hydrogels.
Materials and Methods
Reagents
Sucrose and Fisher HistoPrep frozen tissue-embedding media (Fisher cryogel) were purchased from Fisher Scientific. Ficoll®400, bovine serum albumin (BSA), PEG 400, and polyvinyl alcohol (PVA, MW 146,000–186,000; 99+% hydrolyzed) were purchased from Sigma-Aldrich Chemical Co, and Tissue-Tek Optimal Cutting Temperature® Compound from Sakura-Finetek (OCT®; Sakura-Finetek). Normal goat serum (NGS) was obtained from Jackson ImmunoResearch, and fetal bovine serum (FBS, Central America, USDA origin) was from Biosera. Carnation® nonfat dry milk came from Nestle. To prepare 1% PVA solution, PVA was dissolved in boiling phosphate-buffered saline (PBS). All the other infiltration solutions were prepared with PBS at room temperature.
3% PEG hydrogel preparation
4-arm 20-kDa PEG–norbornene (PEG-NB) was synthesized as described previously.12 Briefly, a 12% aqueous PEG-NB solution was prepared in Dulbecco's phosphate-buffered saline (DPBS; GIBCO®-BRL). To make 3% hydrogels, a stoichiometrically balanced (1 -ene to 1 thiol) polymer solution was made with the 12% PEGNB solution, 0.05 wt% lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP photoinitiator),10 1 mM CGRGDS peptides, and MMP-degradable crosslinkers. About 30 μL of the polymer solution was loaded into a sterile 1-mL syringe (BD Biosciences) with its tip cut off. Photopolymerization of the above mixture was initiated by placing the loaded syringe under long-wavelength ultraviolet light (365 nm, UVP, LLC) for 2 min at room temperature. After polymerization, each disk-shaped construct was removed from the syringe and stored in DPBS at 4°C for further processing.
Cell culture and encapsulation
Human mesenchymal stem cells (MSCs) of passage 6 were maintained on gelatin-coated plates in MSCGM (Lonza). Before encapsulation, cells were trypsinized from the culture plates and pelleted in a centrifuge. The concentration of cells was adjusted to 2 million cells per 100 μL of the polymer solution; 30 μL of the cell–polymer mixture was then placed into a modified syringe and photopolymerized as described above. The constructs were then placed back into MSCGM for 2 days.
Cryosections
The procedure of cryosectioning is showed in Table 1. Constructs were removed from PBS or the culture medium, placed into a 24-well plate (Corning), and fixed with 4% paraformaldehyde for 30 min. Constructs were then incubated with an infiltration solution overnight at 4°C. In addition to 30% sucrose, five different protein-based solutions (100% NGS, nonfat milk, 100% FBS, and 10% and 30% BSA) and eight nonprotein-based solutions (1% PVA, 100% PEG400, 100% DMSO, 30% glycerol, OCT, Fisher cryogel, and 10% and 30% Ficoll 400) were used as the infiltration solution. Constructs were then removed from the infiltration solutions, rinsed with OCT, and transferred to cryomolds with one flat plane facedown. Each cryomold was then filled with OCT and frozen by floating in an ethanol/dry ice slurry or cold isopentane with liquid nitrogen. After solidification, cryomolds were removed from the slurry, dried with Kimwipes®, and stored at −80°C until cryosectioning. Routine cryosectioning was performed on the frozen blocks using a cryostat (Leica CM 1850) at −21°C. For each block, 10-μm cross-sections along the flat plane of the construct were collected on SuperFrost® Plus Gold slides (Fisher Scientific). The slides were air-dried for several hours before H&E staining (both from Sigma).
Table 1.
Cryosection Protocol for Polyethylene Glycol Constructs
| Step | # | Description | Volume | Time |
|---|---|---|---|---|
| Fixation | 1 | 4% paraformaldehyde | 2 mL | 30 min |
| Cryoprotection | 2 | Incubate with infiltration media at 4°C. | 2 mL | Overnight |
| Cryoembedding | 3 | Rinse construct with OCT®. | ||
| 4 | Place in cryomolds and cover with OCT. | |||
| 5 | Freeze cryomolds using an ethanol/dry ice slurry (−80°C). | |||
| Cryosectioning | 6 | Section on the cryostat. |
OCT, optimum cutting temperature.
Modified H&E staining
Because routine H&E staining results in high background staining of the peptide-containing hydrogels, the destaining (with acid alcohol) and blueing (Scott's blue) steps were prolonged, and the incubation time of slides in eosin was reduced. Briefly, the sample slides were rehydrated and stained with hematoxylin for 5 min and dipped into acid alcohol 15 times. Slides were then transferred to Scott's blue for another 5 min and dipped in distilled water before a 20-s eosin staining. After staining, the slides were dehydrated and coverslipped with a Permount® mounting medium (Fisher Scientific).
Image acquisition
The microscope (Nikon eclipse 80i) and camera (Qcolor3, Olympus) were switched on at least 20 min before the start of photography to establish a stable apparatus temperature. The camera was set to manual mode, so that all shots were taken with exactly the same exposure time. A sample slide was used to set appropriate light density and exposure time. First, a dark-field image was captured by blocking the light path when taking the image. Then, the sample slide was focused under the microscope. A region without construct was first imaged for use as the bright-field image for blank field correction. Then, the slide was moved to the construct region for imaging. One image was captured from each construct, and 3 constructs were quantitatively analyzed for each condition (n=3). To study the hydrogel morphology under different freezing methods, six sections from random regions of constructs frozen with either dry ice/ethanol slurry (n=2) or liquid nitrogen (n=2) were imaged for pore-size analysis.
Quantitative analysis of microscopic imaging
ImageJ was used to evaluate the photomicrographs. To eliminate uneven background illumination and achieve a uniform background, a blank-field correction (image division) was used as previously described (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea).17 Briefly, the dark-field-corrected sample images (sample image minus dark field image) were normalized to the dark-field-corrected bright-field images (bright-field image minus dark-field image). A threshold pixel value from the nonconstruct region was used to subtract the background in the blank-field-corrected image. After background subtraction, pore sizes were evaluated using the particle analysis tool from ImageJ, and the background intensity was calculated from the total pixel intensity of the resultant image divided by the number of nonzero pixels. In the background intensity analysis, one-way ANOVA and a post hoc test (the Bonferroni method with 95% confident interval) were used to determine the differences between experimental groups. Ten groups of comparisons were tested with a Student's t-inverse cumulative distribution function (t*) of 3.58.
Cell viability assay
The LIVE/DEAD® Viability/Cytotoxicity Kit (Invitrogen) was employed to verify the viability of encapsulated MSCs, and conducted according to the manufacturer's protocol. In this assay, live cells hydrolyze the acetoxy methyl ester of calcein and retain the green fluorophore in their cytoplasm, while dead cells permit the entry of ethidium through membrane holes and have red-fluorescent nuclei. The whole construct was removed from the culture medium 18 h after cell encapsulation and incubated in PBS containing 10 μM calcein AM and 10 μM ethidium homodimer (EthD-1). After 1-h incubation at room temperature, the whole construct was transferred to a FluoroDish™ cell culture dish (World Precision Instruments) with PBS and covered with a coverslip, before viewing with a confocal microscope (Nikon A1 Confocal).
In vivo implantation and cryohistology
Using a 1-cm biopsy punch, full-thickness wounds were created in the backs of 35–45-kg pigs. Each wound was filled with a monomer solution, which was polymerized in situ with a hand-held 385-nm LED light. Wounds were covered with Tegaderm and allowed to heal for 1 week. At this time, each wound was resected and placed into in 10% neutral buffered formalin (NBF; Fisher Scientific) for a minimum of 24 h before gross dissection. Full skin thickness, representative cross-sections were dissected at 2–3 mm and fixed in NBF for an additional 24 h. One set of tissues with engrafted constructs was incubated in 30% sucrose solutions for 48 h. The other sets of tissues were impregnated in 3 mL 30% BSA, OCT, or 1% PVA for 72 h at 4°C. Each tissue section was blotted dry and placed into a standard cryomold (Electron Microscopy Sciences), filled with OCT (EMS), and frozen on a precooled stainless steel bar that was mostly submerged in liquid nitrogen. Frozen sections were obtained at 6–10-μm thick using a Tissue Tek Cryostat (−15°C to −20°C; Sakura) and mounted on plus charged slides (Fisher). The slides were then air-dried for 1 h at room temperature and postfixed in methanol (Fisher) for 10 min. Slides were subjected to H&E staining.
Results
PEG hydrogels as tissue engineering constructs
Cell encapsulation within the PEG hydrogel matrix demonstrated high cell viability and adequate cell spreading after 24 h, as demonstrated by whole-construct confocal microscopy using a viability and cytotoxicity dual assay (Supplementary Fig. S2). The incidence of dead cells was correspondingly low (<10%).
A new approach for infiltration and sectioning
Routine paraffin-embedding histology protocols include dehydration steps during processing, which resulted in severe deformation of the PEG constructs. Further, the collapsed constructs were enmeshed into the surrounding padding used to support the construct during processing and were unable to be extricated for embedding and sectioning. In contrast, sucrose-infiltrated PEG hydrogels maintained shape without significant structural distortion after freezing; however, they shattered during cryosectioning. In short, two routine histological techniques, paraffin embedding and cryosectioning, failed to generate slides with intact cross-sections of PEG-based constructs.
To identify an optimal method for routine histological characterization of PEG hydrogel scaffolds, we tested multiple dehydration solutions that would preserve tissue architecture, in the context of a modified cryohistology protocol (Table 1). A 30-m PF fixation did not cause substantial volumetric change for cell-free constructs. However, noticeable construct shrinkage occurred with incubations in 30% Ficoll 100% DMSO and 100% PEG 400; each resulted in approximately a 50% reduction in the construct diameter. Constructs infiltrated with protein-based solutions became yellowish, while constructs infiltrated with nonprotein-based solutions remained transparent and colorless. After overnight incubations in each potential cryoprotectant solution, constructs were rinsed with OCT and placed into a cryomold with one plane face downward to obtain complete cross-sections. As summarized in Table 2, among the infiltration solutions tested, only five yielded a complete circular cross-section upon block sectioning. The sectionability of constructs incubated in different solutions was graded based on the integrity of the cross-section by gross examination and the reproducibility of the results. The shattered construct cross-section result from 30% sucrose and 30% glycerol infiltration were set at the bottom end of the scale (—) with constructs shattering during sectioning, and no slide generated for further characterization; 100% DMSO and 100% PEG 400 also yielded unsuitable results and were graded as (—). Constructs shrank significantly and became elastic after incubation with 100% PEG 400 and DMSO overnight, and after embedding in OCT, the apparent stiffness mismatch between construct and the surrounding block resulted in separation of the construct from the rest of the block during sectioning, such that no slides were attainable. Constructs in five solutions (100% NGS, nonfat dry milk, 10% BSA, 10% Ficoll, and 30% Ficoll) were able to be sectioned in complete cross-sections, but the construct center regions were relatively fragile and often tore during the sectioning. Due to the poor integrity of the cross-sections, these conditions were also not considered favorable and were graded as “-”. Nonhomogeneity of sectioning may indicate incomplete penetration of the cryoprotectant solution into the construct's core. In contrast, five conditions were able to generate complete construct cross-sections with reproducible sectionability (marked as “+” in Table 2). Two of these were protein-based solutions (30% BSA and 100% FBS), and three were nonprotein-based (1% PVA, OCT, and Fisher cryogel). On the other hand, the change of freezing technique from dry ice/ethanol slurry to liquid nitrogen, although it froze the constructs faster, did not change the pore size or distribution of PEG compared to standard freezing in dry ice/ethanol slurry (Supplementary Fig. S3).
Table 2.
Summary of Hydrogel Cryosection and Hematoxylin and Eosin Staining Results from Different Infiltration Solutions
| Reagent | Sectionability | Background Intensity |
|---|---|---|
| 30% sucrose | — | |
| Protein-based | ||
| 100% NGS | − | |
| Nonfat Milk | − | |
| 100% FBS | + | 1.75±0.16a |
| 10% BSA | − | |
| 30% BSA | + | 5.63±0.92 |
| Nonprotein-based | ||
| 10% Ficoll® 400 | − | |
| 30% Ficoll 400 | − | |
| 1% PVA | + | 2.12±0.19a |
| 100% PEG 400 | — | |
| 100% DMSO | — | |
| 30% glycerol | — | |
| OCT® | + | 2.43±0.14a |
| Fisher cryogel | + | 3.74±0.29 |
Construct sectionability was graded based on the integrity of cross-sections and the reproducibility of results. Five conditions (100% FBS, 30% BSA, 1%PVA, OCT, and Fisher Cryogel) were able to markedly improve PEG hydrogel cryosectioning results. A “+” indicates sectionable (complete cross-section and repeatable results), a “-” indicates partially sectionable (complete cross-section; occasional void formation), and a “—” indicates not sectionable (constructs shattering during sectioning).
Constructs from three conditions (100% FBS, 1% PVA, and OCT) showed statistically significant lower background (t=6.113, 5.53, and 5.042, respectively; t> t*=3.58) compared to constructs in 30% BSA.
NGS, normal goat serum; FBS, fetal bovine serum; BSA, bovine serum albumin; PVA, polyvinyl alcohol.
H&E staining
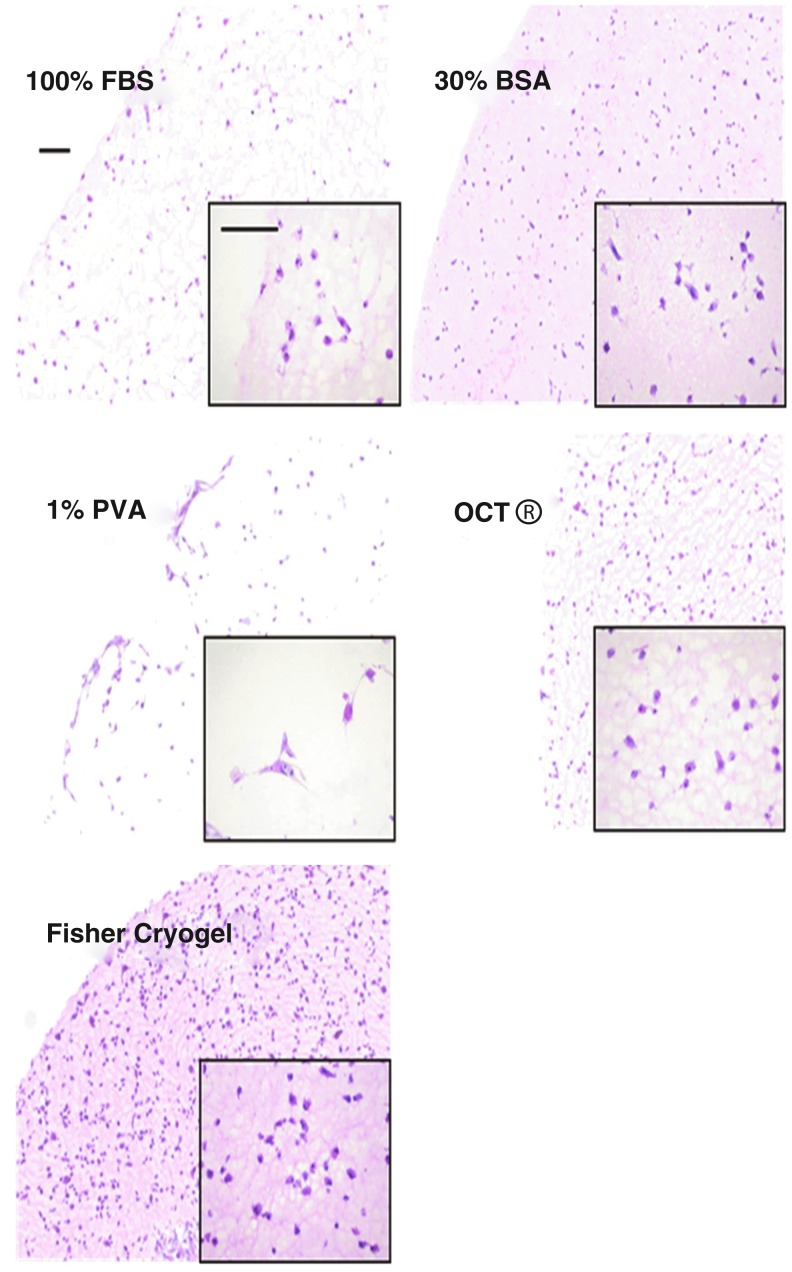
H&E staining is commonly applied in histology to provide basic structural visualization of tissues. The hematoxylin stains the negatively charged DNA of nuclei blue, and the eosin stains positively charged elements such as proteins red-orange. Since the composition of our constructs included eosinophilic peptides (RGD adhesion sequences and MMP-degradable crosslinkers), routine H&E staining resulted in a slightly red background. Infiltration with protein-based cryoprotectant solutions may further enhance such reddish background and obscure cellular structures within the constructs. To delineate the effect of cryoprotectant on staining background, slides from the various sectionable cryoprotectant groups were subjected to a modified H&E staining protocol, and representative images of these stained constructs are shown in Figure 1. The background intensity in these constructs was calculated using ImageJ, such that a low intensity delineates low background noise in the sample constructs. Constructs in three sectionable conditions, 100% FBS, 1% PVA, and 100% OCT, showed more than two-fold lower background (statistically significant, t=6.113, 5.53, and 5.042, respectively; t>t*=3.58) compared to constructs in 30% BSA (Table 2). Interestingly, the 100% FBS solution demonstrated the lowest background among all conditions, while 30% BSA had the highest background, although both solutions are BSA-based (100% FBS has ∼10% BSA). Although no enhanced background was observed in 100% FBS, some unwanted background could be detected around the edges of the construct cross-sections if the sample was not rinsed thoroughly in OCT before cryoembedding. For MSC-encapsulated constructs, both 30% BSA and Fisher cryogel markedly increased the reddish background such that the cytoplasm cannot be discerned from the hydrogel (Fig. 1). In contrast, cytoplasmic processes of the spread cells were clearly visible in FBS, 1% PVA, and OCT. Thus, these three infiltration solutions provided the best results among those tested.
FIG. 1.
Bright-field microscopic images of mesenchymal stem cell (MSC)-encapsulated construct cross-sections after hematoxylin and eosin (H&E) staining (10× objective and 40× objective, lower right corner). The imaging parameters were kept constant during the photography. MSCs can be observed clearly in fetal bovine serum (FBS)-, polyvinyl alcohol (PVA)-, and optimum cutting temperature (OCT®)-infiltrated constructs, whereas the high background from bovine serum albumin (BSA) and Fisher cryogel markedly decreased visualization of the cellular structures. The scale bars are 100 μm.
Cryosectioning from in vivo samples
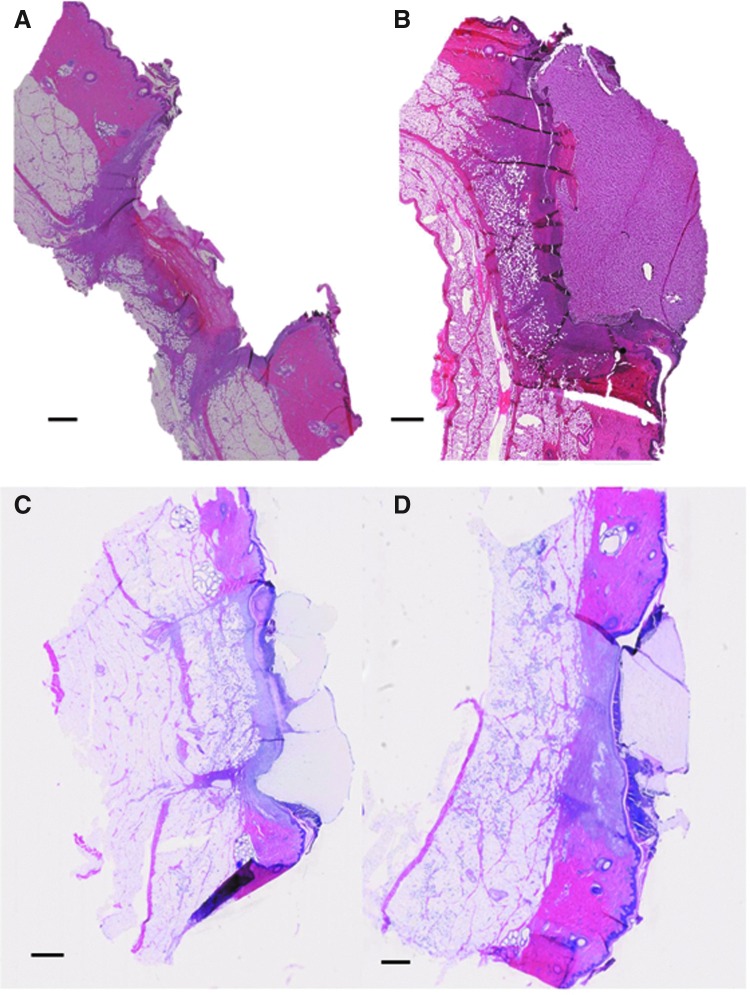
We tested the histological sections prepared from in vivo cutaneous hydrogel implants after infiltrating them with 30% sucrose, 30% BSA, OCT, and 1% PVA. Even though the dermal tissue around the implant could be sectioned well with sucrose infiltration, the region containing the hydrogel construct was ripped from the section as demonstrated in Figure 2A. The subcutaneous hydrogels and surrounding tissue yielded intact sections with no tearing at the tissue–biomaterial interface after infiltrating with 30% BSA, OCT, and 1% PVA (Fig. 2B–D). It should be noted that a thick sample (>3 mm) might not be as easily penetrated by viscous solutions such as OCT and 1% PVA. Inadequate penetration may result in inability to section the tissue around the construct. In addition, the sample infiltrated with 30% BSA showed higher background in the PEG region than those infiltrated with nonprotein-based reagents. Cellular infiltration into the construct was readily observed. These results demonstrate that the biomaterial–tissue interaction can be examined using this modified cryoprotection method.
FIG. 2.
Polyethylene glycol (PEG) hydrogel-engrafted skin cryosection infiltrated with 30% sucrose (A), 30% BSA (B), 1% PVA (C), and OCT (D). When infiltrated with 30% sucrose solution, the hydrogel implant easily ripped from the host tissue. In contrast, the hydrogel graft remained intact, and the host–graft interaction zones could be clearly observed when the sucrose solution was replaced with BSA solution, 1% PVA, or OCT. Lower hydrogel background is apparent with the usage of the nonprotein-based solutions PVA and OCT (C and D). Scale bar, 1 mm.
Discussion
The relative fragility of PEG hydrogels with cryosectioning depends primarily on the dynamics of ice crystal formation within the hydrogels. The highly ordered tetrahedral structure of water molecules in large ice crystals results in sample brittleness, as observed with incompletely infiltrated hydrogels. Formation of ice crystals in cryosection freezing generally depends on two variables: 1, the rate of freezing related to the freezing temperature and the method used; and 2, the composition of aqueous solution surrounding the sample. Control of both variables should be able to minimize the size and density of ice crystals within frozen samples. Rapid cooling of samples has previously been shown to be an efficient way to reduce the ice crystal size.18 The ethanol/dry ice slurry used in this study maintains freezing temperatures around −70°C. Samples in standard cryomolds (25×20×5 mm) generally take 2 to 3 min to become fully frozen, which is not ideal for avoiding large-sized ice crystal formation. Although the usage of isopentane with liquid nitrogen took less time (about 1 min) to fully freeze the block and did not affect the sectionability or background of the constructs, there is no obvious pore size change as shown in Supplementary Figure S3. Access to a more highly specialized freezing system might be needed to further control the morphology change of PEG. However, the methods to rapidly adjust the freezing rate are not accessible to every laboratory. Instead, use of a different infiltration solution provides a more widely applicable method to enhance the cryosection quality. Infiltration with an appropriate amount of chemically inert and hydrophilic solutes, such as sucrose, may disturb hydrogen bond formation during the ice crystallization and control the friability of frozen samples.19 However, cryoprotection with 30% sucrose is frequently not satisfactory for such water-rich and soft tissue samples as the brain,20 or certain hydrogels. Different tissues may require different sucrose concentrations for a similar cryosectioning quality. A higher sucrose concentration may enhance the quality of cryosection in water-rich tissues, such as spinach leaves, which contain large vacuolar spaces.19 It should also be noted that higher sucrose concentration means higher solution viscosity, which may potentially affect the infiltration rate of sample, such that longer incubation times may be required for viscous solutions to fully infiltrate such samples. For water-rich materials such as hydrogels, high sucrose concentrations and concurrent long incubation times for complete sample infiltration may make the cryoprotection step inefficient.
Many studies have concentrated on optimizing cryosectioning or histological processing for different hydrogels, focusing especially on the cryoprotection step. Yang et al. showed that adapted infiltration with an ascending ratio of freezing media:sucrose can enhance the cryosection quality of both collagen and alginate scaffolds.21 For polylactide and alginate scaffolds, gradual glycol methacrylate (GMA) preinfiltration before embedding has been shown to preserve the hydrogel morphology and minimize sectioning artifacts in alginate hydrogels.16,22,23 These techniques are not universally applied for all kinds of hydrogel systems; chemically different hydrogels may require optimization to different histological approaches based on the unique molecular structures of each. Taken as a whole, useful histological techniques for examining hydrogels should enable optimal morphological evaluation and involve minimal time and cost.
Protein-based solutions were the first type of infiltration solutions we examined for improving cryosectioning of hydrogel materials. Previous studies have shown that highly hydrated tissue regions are generally more difficult for sectioning than protein-rich regions.19 Studies have also demonstrated successful embedding of tissues in BSA-based solutions for thin electron microscopic section preparation.24,25 All of the protein-based solutions we examined here facilitated to some extent cryosectioning of the PEG constructs, but only 30% BSA and 100% FBS were able to reproducibly retain the construct integrity. The formation of voids within cross-sections suggests that incomplete construct infiltration was a common problem, which might depend on the category and the concentration of solutes in the infiltration solutions, as well as the duration of incubation. Albumin is the dominant component of the protein-based solutions we tested here, either in isolation or as a component of serum. Although FBS or 30% BSA solutions yielded good histological results, samples incubated in NGS appeared to be incompletely infiltrated. We do not know the basis for the differences in sectioning between the goat serum-based and bovine serum-based conditions, but this could relate to differences in protein content, serum lipids, or other species-specific properties in the solutions. Interestingly, nonfat milk protein (∼80% casein in cow milk; lesser concentrations of globulins and albumin) did not work as a cryoprotectant. Casein is in a class of phosphoproteins that exist in a micelle form, and it is less soluble than albumin. Lower concentrations of protein-based solutions were generally not favorable for cryosection; 10% BSA and milk both generate construct cross-sections with voids and cracks in the center.
Although certain protein-based solutions tested here can provide infiltration environments that improve cryosectioning, their eosinophilic properties also provide a high background for H&E staining. Moreover, the use of serum or protein-based solutions for cryoprotection may severely limit the usage of antibodies with cross-reactivity to those species. For example, bovine antibodies cannot be utilized for analysis of the 100% FBS- and 30% BSA-infiltrated constructs, which otherwise cryosectioned suitably. For both of these reasons, a nonprotein-based infiltration solution is preferable.
Seven different nonprotein solutions were examined in our studies. Ficoll is a carbohydrate-based chemical formed by copolymerization of sucrose with epichlorohydrin. This compound is rich in hydroxyl groups and has been used in cell and macromolecule storage as a cryoprotectant due to its relatively lower osmotic pressure and hydrophilicity.26 However, the performance of this chemical as a cryoprotectant in these studies was only slightly better than sucrose; voids and cracks in the center regions of the construct cross-sections suggested incomplete penetration of the solution. The tests with permeable solutions, such as glycerol and DMSO, also showed negative results for improving the sectionability for PEG hydrogels despite their common usage in cryopreservation of cells and tissues. On the other hand, both OCT and Fisher cryogel incubations resulted in reproducibly sectionable construct blocks. According to the manufacture's information, OCT contains 10.24% PVA, 4.26% PEG, and 85.5% nonreactive ingredients, which presumably is mostly water, whereas Fisher cryogel consists of <80% water, 20% PVA, <10% 2-phenoxyethanol, and <1% polyoxypropylene–polyoxyethylene copolymer. Although the molecular weight and other specific details of these ingredients are not revealed, it indicates that PVA and PEG might be the potential factors necessary for successful hydrogel cryosectioning. Use of PVA and PEG individually, however, told us different stories. Constructs in 1% PVA did not show obvious shrinkage and were able to be sectioned smoothly without cracks or formation of voids. Each entire cross-section was robust enough for direct contact with brushes or gentle touches without an observable damage. In contrast, constructs in PEG 400 demonstrated considerable shrinkage. Even after the constructs were frozen, there were noticeable color and stiffness differences between the construct area and the surrounding OCT block. Unlike the brittle sucrose-infiltrated constructs, which fractured during cutting, PEG-infiltrated constructs remained more elastic than the surrounding OCT. Thus, during cryosection, the apparent stiffness difference between constructs and surrounding block resulted in the compression of constructs by the blade. This situation would then result in the entire construct squeezing out of the solidified OCT block.
Previous OCT studies demonstrated that aqueous PVA solutions played a major role in cryomicrotomy, and PEG 400 was only used as a plasticizer to prevent fracture between samples and the embedding area.27 Here, noticeable fracturing was not apparent in our PVA-infiltrated samples, even though no PEG 400 was used in the PVA solution. Additional uses for PVA solution have been found in external stabilization of the cryosection before each sectioning stroke.28 Coating the block face with the PVA solution has been reported to enable preparation of thinner cryosections (down to 3 μm), by enhancing section sturdiness to maintain the section integrity.28 Compared to protein-based solutions, the nonprotein-based solutions tested here generally give a less-intense eosin background and have less potential cross-reactivity with secondary antibodies used in immunostaining, as well as have more defined ingredients. Each of these aspects makes 1% PVA or OCT better alternative infiltration solutions, although even protein-based solutions are superior for sectioning than the current cryohistology standard of sucrose solution.
Conclusions
Successful cryosectioning of PEG hydrogels may be attained by prior infiltration of the hydrogel sample with 1% PVA or OCT overnight before cryoembedding. These nonprotein-based solutions improve the cryosection quality by (1) retaining section integrity upon sectioning and (2) maintaining low background in standard H&E staining. This cryosectioning protocol can be further used for the study of the cell–matrix interactions and cellular behaviors within these cytocompatible PEG-based matrices and hydrogel-based tissue engineering approaches generally.
Supplementary Material
Acknowledgments
We thank Dr. Ron Seifert and the Lynn and Mike Garvey Imaging Core at the University of Washington for kind assistance with confocal imaging. This work was supported by the following National Institutes of Health Grants: R01HL64387, R01HL084642, P01HL094374, U01HL100405, and P01GM81619 (to CEM), T32HL007312-Experimental Pathology of Cardiovascular Disease training grant, and T32GM007266- MSTP (to NLT).
Disclosure Statement
No competing financial interests exist.
References
- 1.Gerecht S. Burdick J.A. Ferreira L.S. Townsend S.A. Langer R. Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thonhoff J.R. Lou D.I. Jordan P.M. Zhao X. Wu P. Compatibility of human fetal neural stem cells with hydrogel biomaterials in vitro. Brain Res. 2008;1187:42. doi: 10.1016/j.brainres.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie X. Tang Z. Chen J. Yang J. Zeng W. Liu N. Liu Y. Neurogenesis of adipose-derived stem cells in hydrogel. J Huazhong Univ Sci Technol Med Sci. 2011;31:174. doi: 10.1007/s11596-011-0246-1. [DOI] [PubMed] [Google Scholar]
- 4.Kraehenbuehl T.P. Ferreira L.S. Zammaretti P. Hubbell J.A. Langer R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials. 2009;30:4318. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman A.S. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 6.Loessner D. Stok K.S. Lutolf M.P. Hutmacher D.W. Clements J.A. Rizzi S.C. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 2010;31:8494. doi: 10.1016/j.biomaterials.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee K. Lin-Gibson S. Wallace W.E. Parekh S.H. Lee Y.J. Cicerone M.T. Young M.F. Simon C.G. The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials. 2010;31:5051. doi: 10.1016/j.biomaterials.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nuttelman C.R. Tripodi M.C. Anseth K.S. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24:208. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Benoit D.S.W. Collins S.D. Anseth K.S. Multifunctional hydrogels that promote osteogenic hMSC differentiation through stimulation and sequestering of BMP2. Adv Funct Mater. 2007;17:2085. doi: 10.1002/adfm.200700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairbanks B.D. Schwartz M.P. Bowman C.N. Anseth K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2, 4, 6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 2009;30:6702. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairbanks B.D. Schwartz M.P. Halevi A.E. Nuttelman C.R. Bowman C.N. Anseth K.S. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mater. 2009;21:5005. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson S.B. Lin C.-C. Kuntzler D.V. Anseth K.S. The performance of human mesenchymal stem cells encapsulated in cell-degradable polymer-peptide hydrogels. Biomaterials. 2011;32:3564. doi: 10.1016/j.biomaterials.2011.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuttelman C.R. Tripodi M.C. Anseth K.S. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res A. 2004;68:773. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 14.Benton J.A. Fairbanks B.D. Anseth K.S. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials. 2009;30:6593. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terella A. Mariner P. Brown N. Anseth K. Streubel S.-O. Repair of a calvarial defect with biofactor and stem cell-embedded polyethylene glycol scaffold. Arch Facial Plast Surg. 2011;12:166. doi: 10.1001/archfacial.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loebsack A.B. Halberstadt C.R. Holder W.D. Culberson C.R. Beiler R.J. Greene K.G. Roland W.D. Burg K.J. The development of an embedding technique for polylactide sponges. J Biomed Mater Res. 1999;48:504. doi: 10.1002/(sici)1097-4636(1999)48:4<504::aid-jbm16>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Marty G. Blank-field correction for achieving a uniform white background in brightfield digital photomicrographs. BioTechniques. 2007;42:716. doi: 10.2144/000112488. [DOI] [PubMed] [Google Scholar]
- 18.Schwabe K.G. Terracio L. Ultrastructural and thermocouple evaluation of rapid freezing technique. Cryobiology. 1980;17:571. doi: 10.1016/0011-2240(80)90072-3. [DOI] [PubMed] [Google Scholar]
- 19.Tokuyasu K.T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973;57:551. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosene D.L. Roy N.J. Davis B.J. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem. 1986;34:1301. doi: 10.1177/34.10.3745909. [DOI] [PubMed] [Google Scholar]
- 21.Yang C. Jenkins L. Burg K.J.L. Adapted cryosectioning method for hydrogels used in regenerative medicine. J Histotechnol. 2007;30:185. [Google Scholar]
- 22.Webster S.S. Jenkins L. Burg K.J.L. Histological techniques for porous absorbable polymeric scaffolds used in tissue engineering. J Histotechnol. 2003;26:57. [Google Scholar]
- 23.James R. Jenkins L. Ellis S.E. Burg K.J.L. Histological processing of hydrogel scaffolds for tissue-engineering applications. J Histotechnol. 2004;27:133. [Google Scholar]
- 24.McLean J.D. Singer S.J. A general method for the specific staining of intracellular antigens with ferritin-antibody conjugates. Proc Natl Acad Sci U S A. 1970;65:122. doi: 10.1073/pnas.65.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopwood D. The reactions between formaldehyde, glutaraldehyde and osmium tetroxide, and their fixation effects on bovine serum albumin and on tissue blocks. Histochem Cell Biol. 1970;24:50. doi: 10.1007/BF00310003. [DOI] [PubMed] [Google Scholar]
- 26.Kasai M. Komi J.H. Takakamo A. Tsudera H. Sakurai T. Machida T. A simple method for mouse embryo cryopreservation in a low toxicity vitrification solution, without appreciable loss of viability. J Reprod Fertil. 1990;89:91. doi: 10.1530/jrf.0.0890091. [DOI] [PubMed] [Google Scholar]
- 27.Cocco C. Melis G.V. Ferri G.-L. Embedding media for cryomicrotomy. Appl Immunohistochem Mol Morphol. 2003;11:274. doi: 10.1097/00129039-200309000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Fink S. A solvent-free coating-procedure for the improved preparation of cryostat sections in light microscope histochemistry. Histochemie. 1992;97:243. doi: 10.1007/BF00267634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.