Abstract
A wide range of plant foods and dietary supplements are able to modify the functioning of the central nervous system. In the present study, we observed that oral administration of ginsenoside Rh2 (10 mg/mL) for 3 weeks significantly improved spatial learning and memory. Spatial memory and learning was evaluated in mice by hippocampus-dependent tasks (Morris water maze test) and immunohistochemical marker of cell genesis bromodeoxyuridine. Ginsenoside Rh2 treatment (30 days) promoted cell survival and genesis. Further, ginsenoside Rh2 treatment in enriched condition had no significant effects on cell survival compared with standard condition exposure. These results revealed that ginsenoside Rh2-mediated spatial learning and memory improvement was associated with cell genesis and survival and may be parallel to the mechanism of environmental enrichment. Therefore, ginsenoside Rh2 may have efficacy as a dietary supplement for spatial learning and memory improvement.
Key Words: cell genesis, cell survival, ginsenoside Rh2, hippocampus, learning and memory, supplement
The dentate gyrus (DG) in the hippocampus is one of two brain regions with lifelong neurogenesis in mammals from rodents to humans.1–3 Neurogenesis may have a function in memory improvement and brain repair as demonstrated in exposure to enriched environments4 and running exercise.5 In addition, new neurons are generated in hippocampus after stroke6 and seizures,7 indicating that neurogenesis is important for recovery from brain injury. On the other hand, stress8 and low dose irradiation9,10 have been related to the decrease of hippocampal neurogenesis and memory impairment.
Ginseng usually refers to the dried root of several species in the plant genus Panax (Araliaceae family). The most widely used ginseng is indigenous to China and Korea. Ginsenosides, the major bioactive compounds in ginseng, have a dammarane skeleton with sugars attached to the skeleton at only two positions, and it can be mainly divided into two types protopanaxadiol-based and protopanaxatriol-based (PPT) ginsenosides. In addition, sixty analogues of ginsenosides have been identified; hence, various individual ginsenosides may have different potentials for therapy. Examples of neuroprotective effects include a demonstration with 5 min forebrain ischemia, which demonstrated both neuroprotective properties and prevention of learning disability.11 Similarly, ginsenoside Rg1 increased ischemia-induced cell proliferation and survival in the DG of adult gerbils.12 Neuroprotective effects of ginsenoside Rg3 against homocysteine-induced excitotoxicity in rat hippocampus have been shown both in vitro and vivo.13 Orally administered ginsenoside Rg3 may be metabolized to ginsenoside Rh2 by intestinal bacteria before intestinal absorption.14 Therefore, this study focused on the effect of ginsenoside Rh2 on memory and learning. Whether ginsenoside Rh2 supplement can affect learning and memory ability has a number of practical implications for future research and dietary supplement development. The present study therefore includes the consideration of whether and how ginsenoside Rh2 supplement influence memory and learning by evaluating hippocampal-dependent Morris water maze task and the cell survival and proliferation of dentate granule.
Control and Rh2 groups were comprised of six to eight male ICR mice (8 weeks of age). Mice were housed with a 12 h light/dark cycle with lights on from 7:00 am to 7:00 pm and had access to food and water ad libitum. Prior to experiments, the mice were left undisturbed for 4 days and were randomly assigned to one of four experimental conditions: Control groups (saline, n=8 for behavior test; n=6 for cell genesis test; n=6 for cell survival test; and n=6 for enrichment condition test) and ginsenoside Rh2 groups (n=8 for behavior test; n=6 for cell genesis test; n=6 for cell survival test; and n=6 for enrichment condition test). To avoid the confounding influence of behavioral training, only the mice without water maze test were used for determining the level of cell genesis and survival. Mice in the control and ginsenoside Rh2 groups were housed in standard cages, whereas mice of the two enriched groups were placed in a comprehensive set of enrichment environment for 30 days. This enriched environment consisted of toys, wooden block, channels, climbing platform, and small houses. All experimental procedures were performed in strict accordance with the recommendations of the National Institutes of Health and Chungnam National University.
Animals from control group and ginsenoside Rh2 groups were orally administered saline and ginsenoside Rh2 (10 mg/kg) respectively. To evaluate hippocampal cell genesis and cell survival, all mice were injected with bromodeoxyuridine (BrdU, 100 mg/kg, intraperitoneal; Sigma) twice per day for three consecutive days on the designated days (Fig. 1). The number of new cells in the hippocampus increases between 2 h and 1 week after DNA synthesis and then dramatically declines by the 2-week time point. Hence, cell genesis and survival could be detected by BrdU (a pyrimidine analogue that is incorporated into DNA-synthesizing nuclei) injected at the beginning or at the end of the supplementation period.
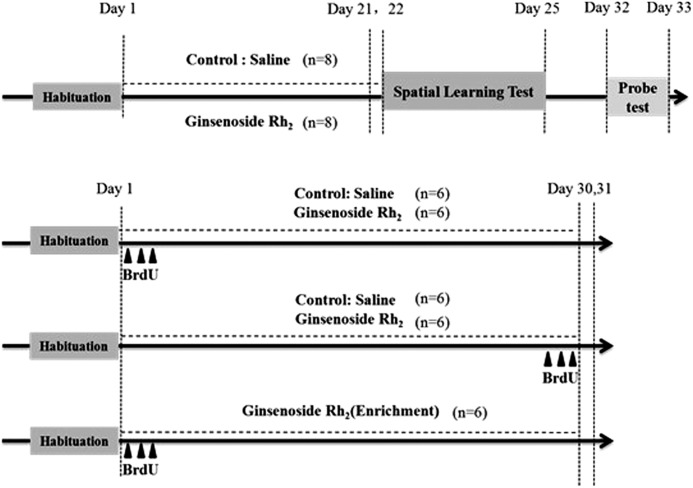
FIG. 1.
Schematic representation of the experimental design and time course of the protocol. Saline and ginsenoside Rh2 was administrated daily during the period, day 1–21 or day 1–30. Bromodeoxyuridine (BrdU) was injected on days 1, 2, and 3 (arrowheads, neurogenesis test) or days 28, 29, and 30 (arrowheads, cell survival test). For spatial learning task, animals were tested on days 22–25 and the probe test was carried out after 1 week habituation.
Hippocampal-dependent spatial learning ability was tested by the Morris water maze.15 Mice were placed in a circular pool (90 cm in diameter and 45 cm in height), with a submerged platform contained in its southeast quadrant. The water was made opaque with nontoxic black paint. The racks of rat cages and posters were placed as spatial cues with which the mice could learn the location of the hidden platform. Before the acquisition period, the mice were habituated to the water maze for 60 s in the absence of the platform. Mice were allowed to stay on the platform for 15 s once they found it, or if after 60 s a mouse failed to find the platform it was placed on the platform by the experimenter. Mice were trained for 4 days, with six trials per day and an intertrial interval of 5 min. With equal frequency, mice were randomly placed at each of the four start positions initially facing the wall of the pool. One week after training mice were returned to the pool for a probe trial. The hidden platform was removed and mice were allowed to swim freely for 60 s. The amount of time spent in the quadrant where the platform was previously located (target) relative to the other three quadrants was an index of long-term memory capacity of the mice.
Mice were sacrificed and the brains were removed 30 days or 2 h after the last BrdU injection for cell genesis or cell survival test. The brains were fixed with 4% phosphate-buffered paraformaldehyde for 12 h. The tissues were embedded in paraffin and cut into sections. Sections were mounted on glass slides and were kept overnight at 42°C. After deparaffinization with xylene and rehydration in analytical grade ethanol, the sections were rinsed in 0.01 M phosphate-buffered solution (PBS), and hydrolyzed with 2 N HCl in PBS at 37°C for 15 min. The sections were then stained using the Invitrogen kit (Invitrogen). The sections were incubated in serum blocking solution, at 1:50 dilution of a mouse monoclonal antibody against BrdU (Santa Cruz Biotechnology) overnight at 4°C, in biotinylated secondary antibody at room temperature for 30 min, and finally in streptavidin-peroxidase conjugate at room temperature for 20 min. After each step, the sections were rinsed with PBS. The sections were then incubated in 3,3′-diaminobenzidine (DAB) solution. After that, sections were incubated in 1% ferric chloride solution at room temperature for 5 min. BrdU-positive nuclei exhibited deposits of black-colored precipitates. The sections were counterstained with hematoxylin and cover slipped under a histomount. Exhaustive counting of the stained cells, assuming 10 sections per animal and six animals per experimental group, was performed.
Data were expressed as the mean±standard error of the mean. Statistical differences were evaluated by using Student's t-test. The level for a statistically significant difference was set at P<.05.
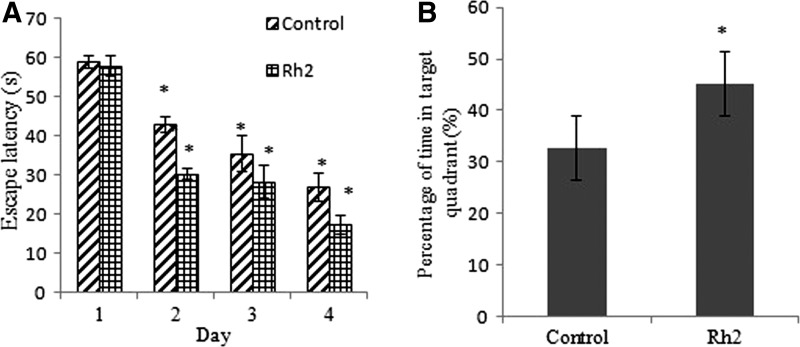
To test the effects of ginsenoside Rh2 on memory, mice were trained in the water maze and after 4 days memory recognition was assessed by the probe test. During the acquisition time, the escape latency of both control and ginsenoside Rh2 groups to find the submerged platform declined every day. On day 1 there was no significant difference between groups in the trials, indicating equal baselines. The escape latency in ginsenoside Rh2 group was significantly shorter than that of control group and the time to reach the platform (latency) reached the significance level on day 2 (Fig. 2A). In addition, ginsenoside Rh2 showed remarkable effect of shortening the escape latency, with significant shorter swim path, and at all time points, there were no differences in the average swim speed (data not shown). In the probe test, time of ginsenoside Rh2 group spent in target quadrant was significantly longer than that of control group (Fig. 2B).
FIG. 2.
Ginsenoside Rh2 decreases the escape latency (A) in the water maze test and increases the percentage of time spent in the target quadrant (B). Values were presented as mean±standard error of the mean (SEM). Statistical comparisons were made using Student t-test. *P<.05 compared with control group.
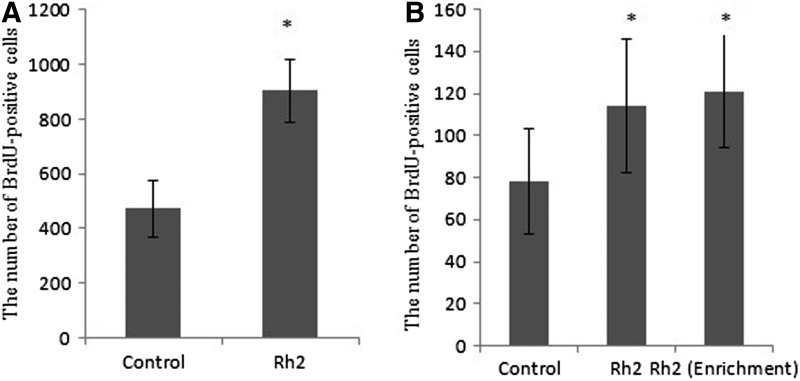
Newly generated cells in the adult mouse subgranular zone of DG can be quantified by immunostaining of BrdU (Fig. 3) incorporated into nuclei of dividing cells in the S phase.16,17 Subgranular zone of DG is the only region of the hippocampus in which adult neurogenesis occurs. In the present study, the effect of ginsenoside Rh2 supplement on DG cell genesis was evaluated by comparing the numbers of BrdU-positive cells in the subgranular zone of DG between control (n=6) and ginsenoside Rh2 group (n=6). As expected, ginsenoside Rh2 treatment gave rise to significant increase in the number of BrdU-labeled cells when compared with the control group (control group 472±64.7 BrdU-positive cells and ginsenoside Rh2 903±97.2 BrdU-positive cells). Theoretically, ginsenoside Rh2 might stimulate the quiescent stem cells in other subregions of DG; therefore, we evaluated the Cornu Ammonis fields, but only found a few scattered BrdU-positive cells in the hilar zone of the control group (44±8.6 compared with 49±10.7 in ginsenoside Rh2 group; P>.8).
FIG. 3.
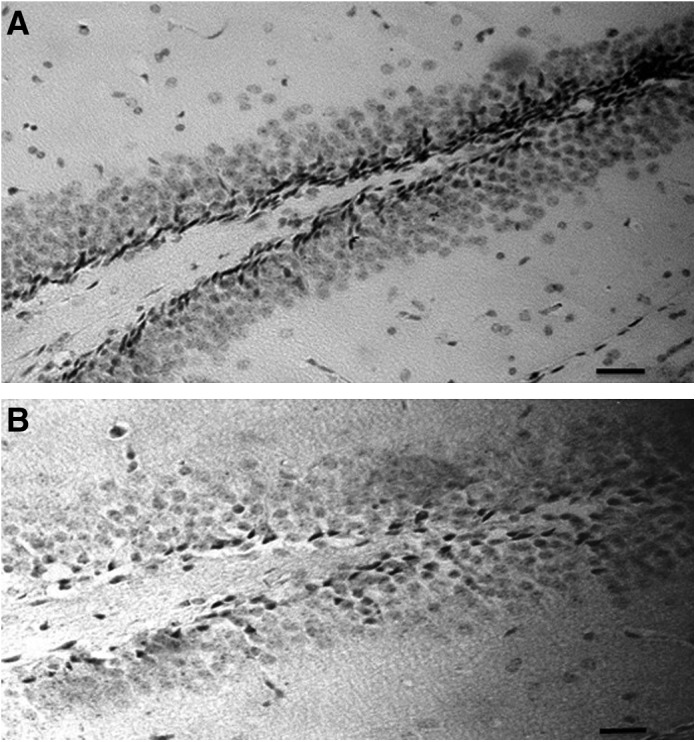
BrdU immunohistochemistry. (A) Neurogenesis (2 h after BrdU injection). At this stage, the nuclei of BrdU-labeled cells were dark and irregular in shape and localized to the subgranular zone. (B) Cell survival (30 days after BrdU injection) of mature granule cells localized to the subgranular zone and granule cell layer. Scale bar=20 μm.
Many newly generated cells normally die under standard laboratory conditions between 2 h and 2 weeks.18 Given that the new cells potentially function as neurons before the period of cell death,19 the survival of newly generated cells might play an important role in memory potentiation. In the present study, statistical analysis revealed that after 30 days administration the number of BrdU-labeled cells in ginsenoside Rh2 group (114±52.3 BrdU-positive cells) was significantly high compared with that of the control group mouse (78±39.1 BrdU-positive cells; Fig. 4B). These results indicated that ginsenoside Rh2 treatment promotes new cell survival, corresponding to the survival period after the proliferation of progenitors.20 In addition, ginsenoside Rh2 group under enriched condition (121±37.2 BrdU-positive cells) showed significant increase in the number of BrdU-positive cells compared with the control group, but not compared with ginsenoside Rh2 group in standard condition.
FIG. 4.
The effects of ginsenoside Rh2 on the subgranular zone of dentate gyrus neurogenesis and on the dentate gyrus cell survival. (A) Rh2 treated mice received BrdU injection for three consecutive days and were sacrificed 2 h after the last injection. (B) Mice received BrdU injection at day 1–3 and were sacrificed at day 30. The results shown are mean±SEM of BrdU-labeled cells in subregions of the hippocampus (n=6 per group). Rh2 significantly increased the cell proliferation and new-born cell survival. In addition, enriched conditions did not affect the cell survival comparing to Rh2 under standard conditions. Values were presented as means±SEM. Statistical comparisons were made using Student t-test. *P<.05 compared with control group.
To date, evidence for the effects of individual ginsenoside on memory and learning ability in mouse is increasing. Ginsenoside Rg1, a PPT saponin, has demonstrated ability to increase ischemia-induced proliferation and survival.12 Ginsenoside Rb1 could also facilitate the learning and memory ability in Scopolamine-induced impaired mouse.21 Ginsenoside Rh1 is a metabolites of Rg1, has demonstrated effects on improvement of memory in mice and increased excitability in rats.22 In addition, in the present study, there were not any significant side effects (food intake, control: 5.3±0.1 g/day and Rh2: 5.5±0.3 g/day; water intake, control: 5.1±0.4 mL/day and Rh2: 5.6±0.6 mg/day; body weight, control: 30.0±1.4 g and Rh2: 31.3±0.9 g) observed during the experimental period, which indicated that administration of Rh2 did not alter or induce stresses in the present study. The role of hippocampus in learning and memory and neurogenesis in the granule cell layer suggest an active role of new neurons in memory formation.23 In addition; new cells are able to be involved in the memory of functional networks in survival conditions during the death period. Therefore, the promotion of cell genesis and new cell survival may be a potent and efficient approach to improve learning and memory. In the present study, the protocol (Fig. 1) was utilized to evaluate the new cell survival in the DG of mouse since learning resulted in decreased cell genesis in the DG of adult rat.24 These BrdU staining results demonstrate a direct association between hippocampus-dependent learning and long-term ginsenoside Rh2-mediated treatment, indicating that neurogenesis and cell survival promotion may be associated with the improvement in spatial learning and memory.
The enhanced new cell survival is the other main finding of this work. The BrdU-positive cells were still able to be detected in the DG of mouse 45 days after the last BrdU injection in the Rh2 group. Control mice had few BrdU-positive cells. For newborn cells to be able to participate in learning process, they have to survive the death period and become integrated into the neurological system. Ginsenoside Rh2 treatment is likely a parallel contribution to the enhanced performance induced by exposure to an enriched environment in consideration of a survival-promoting effect on the proliferation of neuronal precursor cells in the DG.4 Consistent with the finding that ginsenoside Rh2 enhances cell survival of newly generated cells, previous reports showed Rh2 could stimulate pituitary adenylate cyclase-activationg polypeptide gene expression in type I rat brain astrocytes after β-amyloid peptide treatment.25 Further, ginsenoside Rh2 ameliorates Scopolamine-induced learning deficit in mice.26 Because there is little evidence in the literature for improvement in memory and learning by ginsenoside Rh2, the present study provided reasonable mechanism for ginsenoside Rh2-mediated regulation of memory and learning in mice.
In summary, ginsenoside Rh2-mediated memory and learning enhancement is a response to neurogenesis and new cell survival in DG of mouse. Our results suggest that long-term Rh2 administration may contribute to spatial learning by promoting the involvement of newly generated hippocampal cells in the DG of mouse or a parallel mechanism of the enriched environment. Understanding the potential mechanism of ginsenoside Rh2 on memory and learning improvement will contribute to the development of ginsenoside Rh2 as a functional dietary supplement. In addition, American ginseng leaf is an excellent source of ginsenoside Rh2 and the material is obtainable throughout the entire plant cycle, which provides a potential resource for pharmaceutical use.27
Author Disclosure Statement
The authors declare no competing financial interest.
References
- 1.Cameron HA. McKay R. Stem cells and neurogenesis in the adult brain. Curr Opin Neurobiols. 1998;8:677–680. doi: 10.1016/s0959-4388(98)80099-8. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson PS. Perfilieva E. Bjr-Eriksson T. Alborn AM. Nordborg C. Peterson DA. Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 3.Gould E. Reeves AJ. Fallah M. Tanapat P. Cross CG. Fuchs E. Hippocampal neurogenesis in adult old world primates. Proc Natl Acad Sci USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kempermann G. Kuhn HG. Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1999;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 5.Van Praag H. Kempermann G. Gag FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 6.Liu J. Solway K. Messing RO. Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parent JM. Yu TW. Leibowiz RT. Geschwind DH. Sloviter RS. Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemaire V. Koehl M. Le Moal M. Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder JS. Hong NS. Mcdonald RJ. Wojtowicz JM. A role for adult neurogenesis in the spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Clelland CD. Choi M. Romberg C. Clemenson GD., Jr Fragniere A. Tyers P. Jessberger S. Saksida LM. Barker RA. Gage FH. Bussey TJ. A function role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2005;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen TC. Yoshimura H. Matsuda S. Sakanaka M. Ginseng root prevents learning disability and neuronal loss in gerbils with 5-minute forebrain ischemia. Acta Neuropathol. 1996;91:15–22. doi: 10.1007/s004010050387. [DOI] [PubMed] [Google Scholar]
- 12.Shen LS. Zhang JT. Ginsenoside Rg1 increases ischemia-induced cell proliferation and survival in the dentate gyrus of adult gerbils. Neurosci Lett. 2003;344:1–4. doi: 10.1016/s0304-3940(03)00318-5. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH. Cho SY. Lee JH. Jeong SM. Yoon IS. Lee BH. Lee JH. Pyo MK. Lee SM. Chung JM. Kim S. Rhim H. Oh JW. Nah SY. Neuroprotective effects of ginsenoside Rg3 against homocysteine-induced excitotoxicity in rat hippocampus. Brain Res. 2007;1136:190–199. doi: 10.1016/j.brainres.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 14.Bae EA. Han MJ. Choo MK. Park SY. Kime DH. Metabolism of 20(S)-and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25:58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 15.Morris RGM. Garrud P. Pawlins JNP. O'Keefe J. Place navigation impaired in rats with hippocampal leisons. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 16.Miller MW. Nowakowski RS. Use of bromodeoxyuridine- immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- 17.Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kempermann G. Gast D. Kronenberg G. Yamaguchi M. Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 19.Gould E. Beylin A. Tanapat P. Reeves A. Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 20.Hastings NB. Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Wang YZ. Chen J. Chu SF. Wang YS. Wang XY. Chen NH. Zhang JT. Improvement of memory in mice and increase of hippocampal excitability in rats by ginsenoside Rg1's metabolites ginsenoside Rh1 and protopanaxatriol. J Pharmacol Sci. 2009;109:504–510. doi: 10.1254/jphs.08060fp. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q. Sun LH. Jia W. Liu XM. Dang HX. Mai WL. Wang N. Steinmetz A. Wang YQ. Xu CJ. Comparison of ginsenoside Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice. Phytother Res. 2010;24:1748–1754. doi: 10.1002/ptr.3130. [DOI] [PubMed] [Google Scholar]
- 23.Shors TJ. Towsend DA. Zhao M. Kozorovitskiy Y. Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrogini P. Orsini L. Mancini C. Ferri P. Ciaroni S. Cuppini R. Learning may reduce neurogenesis in adult rat dentate gyrus. Neuroscience. 2004;359:13–16. doi: 10.1016/j.neulet.2003.12.123. [DOI] [PubMed] [Google Scholar]
- 25.Shieh PC. Tsao CW. Li JS. Wu HT. Wen YJ. Kou DH. Cheng JT. Role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the action of ginsenoside Rh2 against beta-amyloid-induced inhibition of rat brain astrocytes. Neurosci Lett. 2008;434:1–5. doi: 10.1016/j.neulet.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Yang JH. Han SJ. Ryu JH. Jang IS. Kim DH. Ginsenoside Rh2 ameliorates scopolamine-induced learning deficit in mice. Biol Pharm Bull. 2009;32:1710–1715. doi: 10.1248/bpb.32.1710. [DOI] [PubMed] [Google Scholar]
- 27.David GP. David DK. Generation of ginsenosides Rg3 and Rh2 from North American ginseng. Phytochemisty. 2004;65:337–344. doi: 10.1016/j.phytochem.2003.11.020. [DOI] [PubMed] [Google Scholar]