Abstract
Reliable diagnosis of traumatic brain injury (TBI) is a major public health need. Glial fibrillary acidic protein (GFAP) is expressed in the central nervous system, and breakdown products (GFAP-BDP) are released following parenchymal brain injury. Here, we evaluate the diagnostic accuracy of elevated levels of plasma GFAP-BDP in TBI. Participants were identified as part of the prospective Transforming Research And Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Study. Acute plasma samples (<24 h post-injury) were collected from patients presenting with brain injury who had CT imaging. The ability of GFAP-BDP level to discriminate patients with demonstrable traumatic lesions on CT, and with failure to return to pre-injury baseline at 6 months, was evaluated by the area under the receiver operating characteristic curve (AUC). Of the 215 patients included for analysis, 83% had mild, 4% had moderate, and 13% had severe TBI; 54% had acute traumatic lesions on CT. The ability of GFAP-BDP level to discriminate patients with traumatic lesions on CT as evaluated by AUC was 0.88 (95% confidence interval [CI], 0.84–0.93). The optimal cutoff of 0.68 ng/mL for plasma GFAP-BDP level was associated with a 21.61 odds ratio for traumatic findings on head CT. Discriminatory ability of unfavorable 6 month outcome was lower, AUC 0.65 (95% CI, 0.55–0.74), with a 2.07 odds ratio. GFAP-BDP levels reliably distinguish the presence and severity of CT scan findings in TBI patients. Although these findings confirm and extend prior studies, a larger prospective trial is still needed to validate the use of GFAP-BDP as a routine diagnostic biomarker for patient care and clinical research. The term “mild” continues to be a misnomer for this patient population, and underscores the need for evolving classification strategies for TBI targeted therapy. (ClinicalTrials.gov number NCT01565551; NIH Grant 1RC2 NS069409)
Key words: clinical trial, health-related quality of life, outcome, post-concussion syndrome, TBI
Introduction
Traumatic brain injury (TBI) diagnostics currently rely on neurological examination and radiographic imaging. A blood-based biomarker that is disease/syndrome specific, such as troponin and creatine kinase (CK)-MB in acute cardiac injury, would enable the rapid diagnosis and appropriate triage for acute treatment, clinical trial stratification, and follow-up rehabilitation care plans of brain-injured patients. The ideal biomarker would be informative across the spectrum of TBI, from concussion to coma, allow for point-of-care testing, and provide an objective diagnostic for targeted treatment.
TBI diagnostic biomarkers have been examined in recent years, with potential candidates being neuron-specific enolase (NSE), glial protein S-100 beta (S-100β), myelin basic protein (MBP), glial fibrillary acidic protein (GFAP), and, recently, ubiquitin c-terminal hydrolase (UCH-L1).1,2 Investigation of GFAP-breakdown products (GFAP-BDP) in severe TBI has recently been reported by Mondello et al., with association to injury severity, intracranial lesions, and mortality.3 In moderate to mild TBI, GFAP-BDP has been investigated by Papa et al. with associations to injury severity as well as positive CT findings and the need for neurosurgical intervention.4 GFAP is an intermediate filament protein that is expressed by several cell types, including astrocytes, in the central nervous system, and is thought to maintain mechanical strength of cells. The exact mechanisms leading to elevation of GFAP and GFAP-BDPs are not completely understood, and may be caused by such phenomena as astrocyte reactivity or damage, such as that seen in brain damage. The detection of elevated GFAP-BDPs could, therefore, potentially be used as a measure of brain injury, with the added diagnostic benefit of the ability to be detected in the peripheral blood.
The Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Study is a National Institute of Neurological Disorders and Stroke (NINDS)-funded multicenter, prospective, collaboration among four United States centers to develop, test and refine TBI common data elements (TBI-CDEs) for research in four domains: demographics, neuroimaging, biomarkers, and outcome measures. A unique feature of the target TBI population under investigation is that it spans the entire range of TBI from mild to severe. Enrollment included a large cohort of mild TBI patients with negative neuroimaging results, as well as mild TBI patients discharged from the emergency department (ED).
The purpose of this study was to prospectively examine the diagnostic value of a plasma biomarker, GFAP-BDP, in a large prospective cohort of TBI patients. The relationship of early serum level of GFAP-BDP with injury severity and neurological outcome in TBI was assessed.
Methods
Study population
Subjects were identified and recruited upon arrival at one of three level I trauma centers as part of the multicenter prospective TRACK-TBI study (Yue et al, 2013).5 Study protocols were approved by the institutional review boards of participating centers (San Francisco General Hospital, University of Pittsburgh Medical Center [UPMC], University Medical Center Brackenridge [UMCB]). All participants or their legal authorized representatives gave written informed consent. At follow-up outcome assessments, participants whose consent had been previously given by legal authorized representative, if neurologically improved such as to be cognizant, gave consent for continuation in the study.
To be eligible for the TRACK-TBI study, patients had to present within 24 h of injury, and have a history of trauma to the head sufficient to be triaged to non-contrast head CT using the American College of Emergency Physicians/Centers for Disease Control (ACEP/CDC) evidence-based joint practice guideline.6 Details of loss of consciousness, amnesia, and source of trauma were recorded upon screening, and informed consent was obtained. Glasgow Coma Scale (GCS) score was assessed by a neurosurgeon at admission and was reconfirmed by study personnel at the time of biomarker collection.
Sample collection and measurement of GFAP-BDP
Blood samples were collected from subjects who consented to genetic and proteomic analysis within 24 h of injury. All samples were dated and time stamped to compare with time of injury. The TBI-CDE Biospecimens and Biomarkers Working Group Guidelines for plasma preparation were followed.7 Samples were centrifuged and plasma aliquots stored at −80°C for future batch processing. UPMC and UMCB batch shipped samples, overnight on dry ice, to University of California San Francisco (UCSF). All samples were stored in a de-identified manner, with a unique study number specific to site and subject. A central database was maintained by the coordinating center (UCSF) with each site entering site-specific data for final statistical reporting. Blinded sample analysis occurred in a single laboratory (Banyan Biomarkers, Alachua, FL) using a sandwich enzyme-linked immunosorbent assay (ELISA) to GFAP-BDP. The GFAP ELISA utilized a proprietary mouse monoclonal antibody for solid phase immobilization, and a proprietary polyclonal rabbit antibody for detection.4,8 The test sample was allowed to react sequentially with the capture and detection antibodies, resulting in GFAP molecules being sandwiched between the two antibodies. The antibodies detected both whole GFAP molecules and GFAP-BDPs, potentially resulting in a more complete measure of GFAP levels in circulation.8 Detection was mediated by addition of a tertiary anti-rabbit-horseradish peroxidase (HRP) conjugated antibody and a colorimetric (tetramethylbenzidine [TMB]) substrate. Quantitative determination of the biomarker concentration was achieved by comparing the unknown sample results to a standard curve obtained from the same assay. All samples were analyzed in duplicate concomitantly with calibrators prepared in compatible matrix. Specifically, a serial dilution of the calibrator protein was prepared, and aliquots were assayed in the same volume and under the same conditions as the samples. The calibrator signal intensities were used to generate a dose response curve and to calculate the sample concentrations using a four parameter logistic function (Mars Software for OPTIMA reader). The same number of samples, quality controls, and calibrators were used for each assay (dilution factor of 1). From high concentration to low, the previously reported intra-assay coefficient of variance for the ELISA was 4.3–7.8%, and the inter-assay coefficient of variance was 7.8–14.3%.4 The estimated limit of detection (LOD) for GFAP was ∼0.1 ng/mL.
Evaluation of CT scans according to TBI-CDE
All patients underwent CT imaging of the brain at the time of initial presentation to the ED. Each patient's head CT was characterized using the recommendations of the TBI-CDE Neuroimaging Working Group.9,10 The Neuroimaging TBI-CDEs are consensus-based recommendations for data collection regarding specific radiological features, data definitions needed to characterize injuries, and best practices needed to optimize and harmonize imaging data acquisition for TBI research. Each CT was de-identified, electronically uploaded to a central imaging database, and reviewed by a blinded central reader, and imaging features were extracted and entered into the TRACK-TBI database.
Outcome evaluation
Patient outcomes included mortality and neurological assessment at 6 months after injury. The primary outcome measure was the 6-month Glasgow Outcome Scale-Extended score (GOS-E).10 The GOS-E provides eight categories of outcome: Dead, Vegetative State, Lower Severe Disability, Upper Severe Disability, Lower Moderate Disability, Upper Moderate Disability, Lower Good Recovery, Upper Good Recovery. Ratings are based on patient consciousness, independence, ability to work, social and leisure activities, social relationships, and other sequelae of TBI. Upper Good Recovery (GOS-E score of 8) indicates return to pre-injury baseline with no residual effects of the TBI.
Statistical analysis
Descriptive statistics with means and proportions were used to describe categorical variables (site, presentation, demographics). Biomarker levels were treated as continuous data measured in nanograms per milliliter (ng/mL). Data were assessed for equality of variance and distribution. Intracranial lesions shown on initial CT were scored and analyzed with GFAP-BDP as the dependent variable, and the Student's t test was used to compare means across lesion groups. We assessed the ability of GFAP-BDP to separate patients with different injury patterns and outcomes; this is quantified as the area under the receiver operating characteristic curve (AUC). The AUC evaluates whether those with higher predicted risk are more likely to have a more severe injury (positive CT finding/poor GCS) or a poor outcome (mortality/unfavorable outcome) among all possible pairs of patients with different findings. In line with the current statistical consensus, AUC's of 0.8–0.9 are considered very good, those of 0.7–0.8 are considered adequate, and an AUC<0.7 is considered poor. Further, univariate and multivariate logistic regression were used to assess the ability of GFAP-BDP to predict outcome. Data were analyzed using Stata 11 (StataCorp, College Station, TX) and PASW (version 19.0; IBM Corporation, Somers, NY).
Results
Baseline demographics and CT imaging
There were 215 TRACK-TBI subjects with plasma samples processed according to the TBI-CDE recommendations, of which 145 had GOS-E scores at 6 months (67.44%). The study sample comprised the full spectrum of TBI encountered at three high-volume level 1 trauma centers. As seen in Table 1, the majority of the subjects (83%) were classified as having mild TBI (admission GCS between 13 and 15), 4% as having moderate TBI (GCS 9–12), and 13% as having severe TBI (GCS 3–8). The mean age was 42±18 years; 73% were male. The most common mechanism of injury was fall (36%), followed by motor vehicle accident (27%). CT scans were abnormal in 43% of subjects with mild TBI, in 78% of subjects with moderate TBI, and in 96% of subjects with severe TBI. Demographic analysis of patients lost to follow-up at 6 months revealed no significant difference in age or gender, but a significant difference in admission GCS score was present (p=0.019). Of patients lacking 6 month data, 94% sustained mild TBI, whereas 78% of patients with 6 month follow-up data sustained mild TBI. The relation of GFAP-BDP to patient age was investigated using linear regression, and no significant association was found.
Table 1.
Demographics and CT Imaging of Study Population
| Mild (n=179) | Moderate (n=9) | Severe (n=27) | |
|---|---|---|---|
| GCS Score (mean±SD) | 14.80±0.44 | 11.22±0.67 | 3.59±1.31 |
| Age (mean±SD) | 42.5±18.0 | 44.1±19.5 | 39.2±18.9 |
| Gender (% male) | 69.8% | 100.0% | 81.5% |
| Positive CT findings | 42.5% | 77.8% | 96.3% |
Distribution of Glasgow Coma Scale (GCS) score on admission to emergency department, age, gender, and positive intracranial findings on admission CT across mild (GCS 13-15), moderate (GCS 9-12), and severe (GCS 3-8) traumatic brain injury (TBI).
GFAP-BDP and abnormal head CT findings
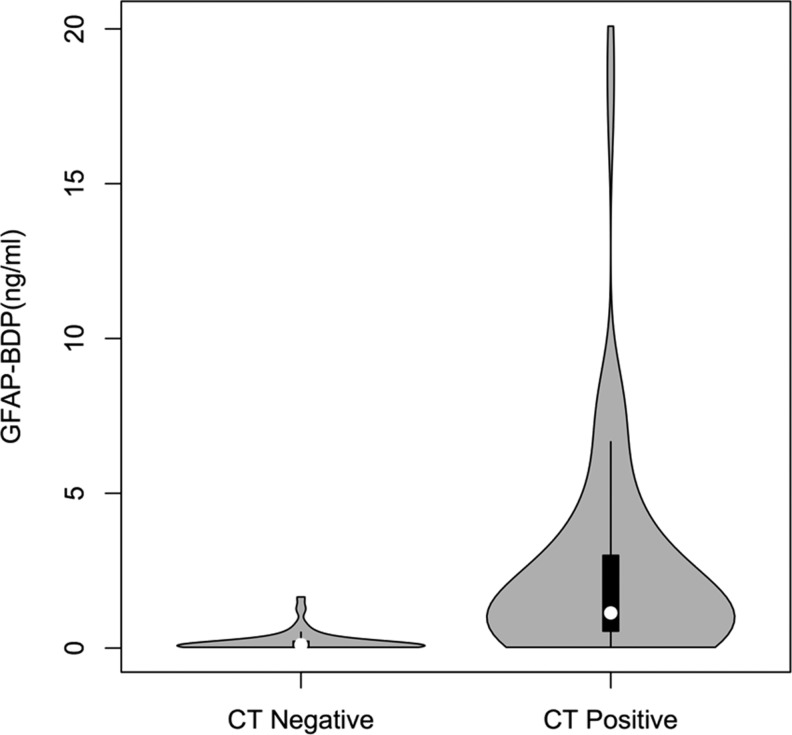
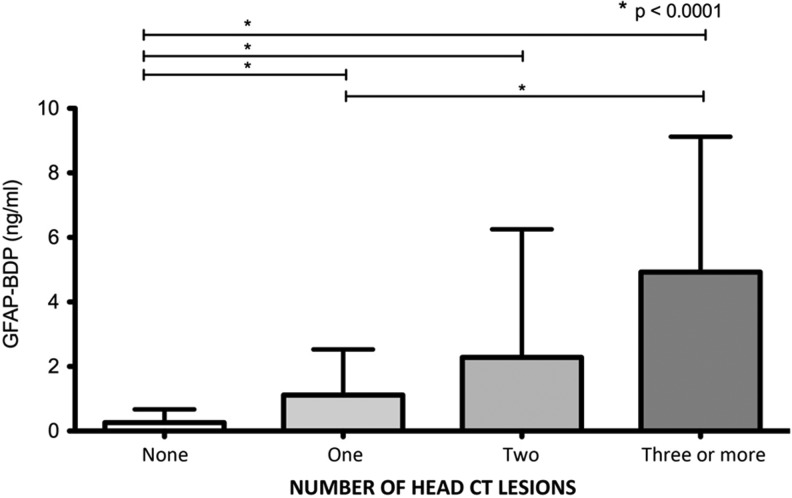
Plasma samples obtained within 24 h of injury (mean 10.9 h, SD 6.4 h, min 0.5 h, max 23.4 h) were analyzed individually in duplicate using a sandwich ELISA to measure GFAP-BDP levels. TBI-CDE-defined pathoanatomic features on the initial head CT scan included: subdural hematoma, subarachnoid hemorrhage (SAH), and contusion. When compared with subjects with a negative head CT scan, GFAP-BDP levels were significantly higher in those with evidence of traumatic pathoanatomic features (CT negative, n=106, GFAP-BDP=0.26±0.41 ng/mL; CT positive, n=109, GFAP-BDP=2.88±3.74 ng/mL; p<0.01; Fig. 1). When compared with GOS-E 6 month score, positive CT scan was significantly related (ANOVA, Sidak pairwise analysis, p<0.00001). With increasing severity of CT findings, there was an associated elevation of plasma GFAP-BDP levels (Fig. 2). Subjects with isolated subdural hematoma, SAH, or contusion had significant elevation of GFAP-BDP (mean range 1.06–4.92 ng/mL) compared with subjects with no intracranial pathology (mean 0.26 ng/mL). With multiple pathoanatomic features, the biomarker levels were further increased.
FIG. 1.
Violin and box plot of glial fibrillary acidic protein and breakdown products (GFAP-BDP) levels in traumatic brain injury (TBI) subjects with negative head CT for intracranial lesions versus subjects with evidence of pathoanatomic features. Box plots are shown in black with density distribution of GFAP-BDP values in gray. Patients negative for intracranial lesions on CT (CT-, n=106) had a mean plasma GFAP-BDP level of 0.26 ng/mL (SD 0.41 ng/mL), whereas patients positive for any intracranial lesion types (CT+, n=109) had a mean plasma GFAP-BDP level of 2.88 ng/mL (SD 3.74 ng/mL).
FIG. 2.
Mean plasma glial fibrillary acidic protein and breakdown products (GFAP-BDP) levels (ng/mL) for increasing numbers of lesions seen on admission head CT after acute TBI. Lesion types include epidural hematoma (EDH), acute subdural hemorrhage (ASDH), traumatic subarachnoid hemorrhage (tSAH), contusion, and intraventricular hemorrhage (IVH). Patients with no intracranial lesion (number of lesions=None) had statistically significant lower mean GFAP-BDP levels than those with one, two, and three or more lesion types (p<0.0001). Patients with one lesion type also had statistically significant lower mean GFAP-BDP levels than those with three or more lesion types (p<0.0001).
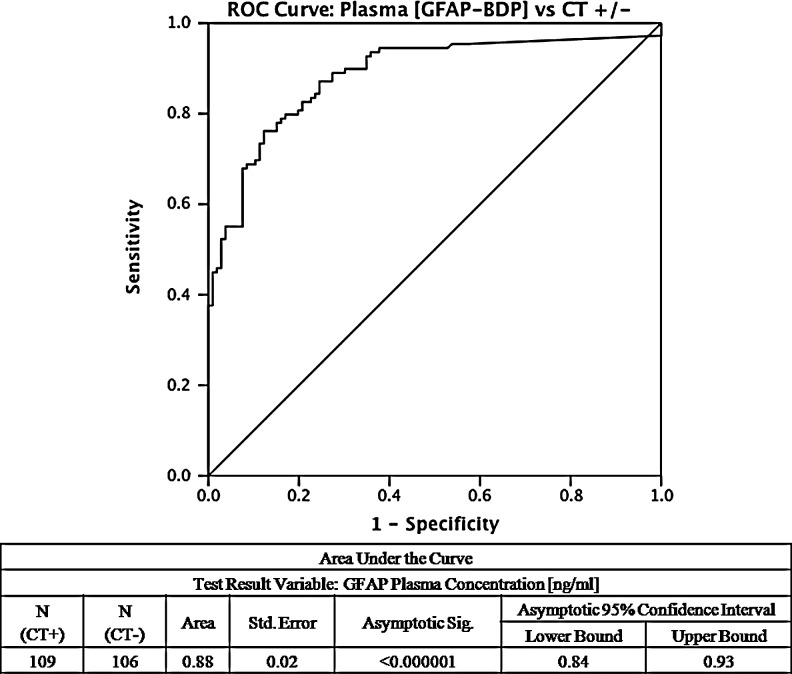
To assess the diagnostic performance of GFAP-BDP, the AUC for GFAP-BDP that was calculated to discriminate patients with traumatic lesions on head CT was 0.88 (95% confidence interval [CI], 0.84–0.93, p<0.000001) (Fig. 3). Age and GCS were tested as covariates in the receiver-operating-characteristic curve, and GCS was found to significantly contribute (p<0.01). When the curve was re-calculated, the result was unchanged from the non-controlled model.
FIG. 3.
Receiver operating-characteristic curve for diagnosing traumatic brain injury (TBI) subjects with pathologic CT features. The area under the curve (AUC) demonstrates that glial fibrillary acidic protein and breakdown products (GFAP-BDP) levels are able to discriminate between subjects with and without radiographic evidence of TBI, AUC 0.88 (95% CI, 0.84–0.93).
GFAP-BDP and injury severity
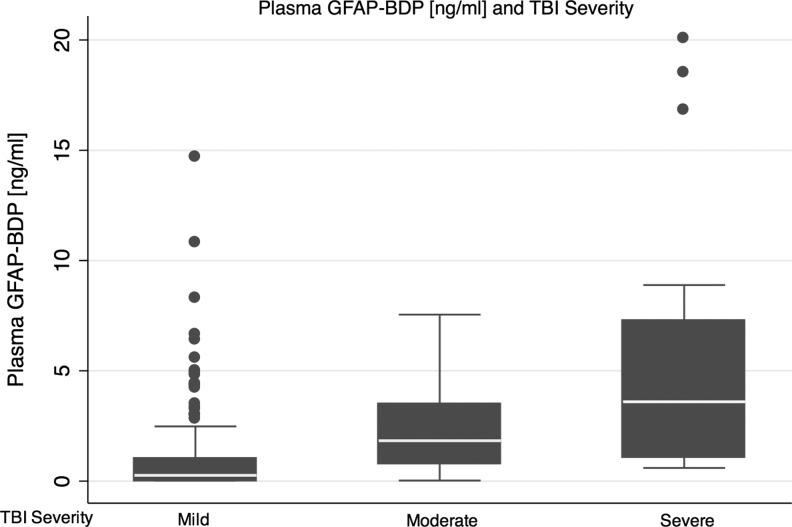
Serum measurement of GFAP-BDP in the first 24 h after TBI reliably distinguished injury severity, as assessed by the GCS. Mean serum GFAP-BDP levels increased significantly with decreasing GCS at presentation (ANOVA, Sidak pairwise analysis p<0.01); this persisted following adjustment for age, sex, and injury mechanism (p<0.01). The ability of the GFAP-BDP level to discriminate between patients with mild and moderate-to-severe injuries, as measured by the AUC, was 0.87 (95% CI, 0.81–0.93). The discriminatory ability of GFAP-BDP in assessing mild-moderate versus severe injury was 0.84 (95% CI, 0.77–0.91). A graphical box-plot display of the distribution of GFAP-BDP concentrations at each level of severity of TBI (mild, moderate, and severe) is shown in Figure 4.
FIG. 4.
Box plots displaying the concentration distribution of glial fibrillary acidic protein and breakdown products (GFAP-BDP) for each level of traumatic brain injury (TBI) severity, mild (Glasgow Coma Scale [GCS] score 13–15), moderate (GCS 9–12) and severe (GCS 3–8). Median (lower quartile, upper quartile) plasma GFAP-BDP concentration is 0.263 (0.025, 1.033) for mild TBI, 1.831 (0.772, 3.483) for moderate TBI, and 3.596 (1.09, 7.272) for severe TBI.
GFAP-BDP and outcome
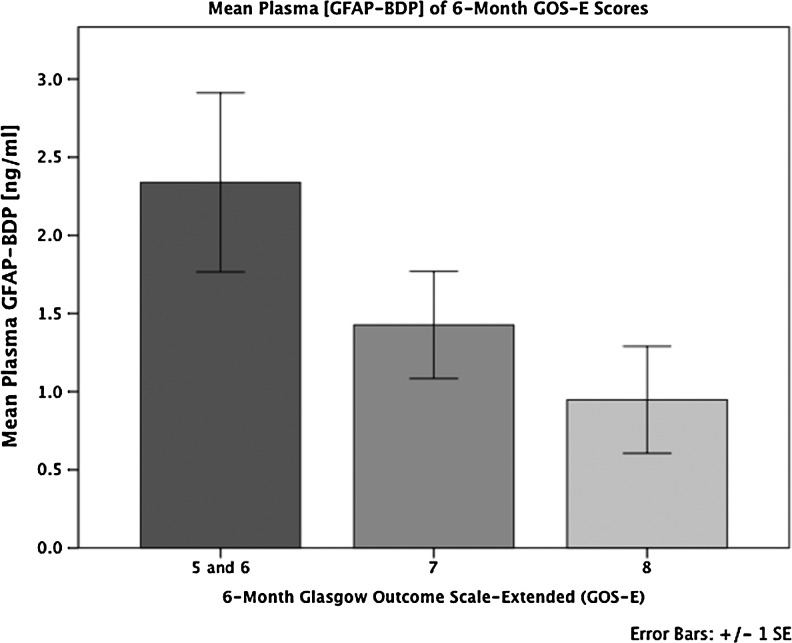
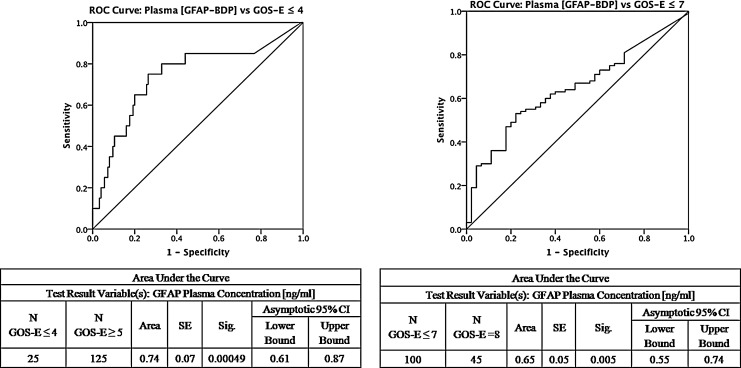
The overall median GOS-E at 6 months post-injury was 7 (interquartile range, 2), with 83% of the entire sample achieving some level of functional recovery (GOS-E ≥5, moderate disability or better). Significant predictors of unfavorable functional outcome (GOS-E 1–4) using increased age, poor GCS, and presence of traumatic lesion(s) on CT. Elevated GFAP-BDP levels were also significantly associated with lack of return to baseline function at 6 months (GOS-E ≤7) (odds ratio [OR] 2.07; 95% CI 1.30–3.55, p=0.006 at the 0.68 ng/mL GFAP-BDP cutoff). With increasingly poor outcome as measured by GOS-E score, there was an associated elevation of serum GFAP-BDP levels (Fig. 5). The ability of GFAP-BDP to discriminate between the likelihood of an unfavorable functional 6 month outcome (GOS-E 1–4), as measured by the AUC, was 0.74 (95% CI, 0.61–0.87) (Fig. 6a). Furthermore, we evaluated the predictive performance of GFAP-BDP for full recovery (GOS-E 8). The ability of GFAP-BDP to discriminate between the likelihood of a full recovery (return to normal life, with no problems related to the injury) was 0.65 (95% CI, 0.55–0.74) (Fig. 6b). Age and GCS were tested as covariates in both receiver-operating-characteristic curves, and neither was found to be significant.
FIG. 5.
Mean levels (+/− SD) of plasma glial fibrillary acidic protein and breakdown products (GFAP-BDP) concentration across 6 month Glasgow Outcome Scale-Extended (GOS-E) scores. Higher GFAP-BDP levels are associated with greater detraction from full global recovery. The mean GFAP-BDP difference trends toward significance (p=0.067, ANOVA), especially between traumatic brain injury (TBI) patients with GOS-E=8 versus those with GOS-E=5 and 6 (p=0.055, ANOVA) at 6 months post-injury.
FIG. 6.
Receiver operating-characteristic curve for predicting global recovery of traumatic brain injury (TBI) subjects at 6 months post-injury using the eight point Glasgow Outcome Scale – Extended (GOS-E). A GOS-E score of 8 signifies full recovery to baseline function, whereas a score ≤7 signifies deficits in recovery. GOS-E of ≤4 signifies an unfavorable functional recovery. The area under the curve (AUC) in the left panel demonstrates that glial fibrillary acidic protein and breakdown products (GFAP-BDP) are able to adequately discriminate between subjects of favorable versus unfavorable functional recovery, area under the curve (AUC) 0.74 (95% CI, 0.61–0.87). Right panel demonstrates that GFAP-BDP are able to somewhat discriminate between subjects who have made a full versus a partial recovery, AUC 0.65 (95% CI, 0.55–0.74).
Univariate and multivariate logistic regression models were also created to assess the predictive power of GFAP-BDP on 6 month outcomes. In univariate analysis, GFAP-BDP was significant for both unfavorable outcomes (OR 1.21; 95% CI 1.07–1.36) with a pseudo-R2 of 0.0999, and for return to baseline (OR 1.03; 95% CI 1.03–1.55) with a pseudo-R2 of 0.0469. Controlling for age, GCS, and positive CT in a multivariate regression model, GFAP-BDP was no longer a significant predictor of either unfavorable or return to baseline outcomes. Positive CT scan was the only significant predictor in the return to baseline model, with an OR of 3.42 (95% CI 1.28–9.15) and pseudo-R2 of 0.1176. It is of note that both positive CT scan and GFAP-BDP were found to be multicollinear with GCS in the model.
Cutoff for serum GFAP-BDP level as a diagnostic test for TBI
We evaluated the cutoff for diagnostic performance of serum GFAP-BDP level to determine ORs for CT findings and 6 month neurological outcome. The optimal GFAP-BDP cutoff value of 0.68ng/mL had a sensitivity of 73%, a specificity of 89%, and a positive predictive value of 87% for identifying patients with intracranial findings on CT (Table 2). The cutoff point of a serum GFAP-BDP level of 0.68 ng/mL produced a 21.61 OR for a positive head CT (p<0.0001) and a 2.07 OR for failure to return to pre-injury baseline (p=0.006).
Table 2.
Diagnostic Performance of GFAP-BDP in Differentiating CT Pathology and Neurologic Outcome after TBI
| GFAP-BDP cutoff (ng/mL) | Sensitivity | Specificity | Negative predictive value | Positive predictive value | Odds ratio (CT+) | p-value (CT+) | Odds ratio (GOS-E≤7) | P-Value (GOS-E≤7) |
|---|---|---|---|---|---|---|---|---|
| 0.40 | 0.80 (0.71–0.87) | 0.81 (0.72–0.89) | 0.80 (0.71–0.87) | 0.81 (0.72–0.88) | 17.00 (8.66–33.40) | p<0.0001 | 1.85 (1.12–3.04) | 0.014 |
| 0.44 | 0.79 (0.70–0.86) | 0.83 (0.74–0.89) | 0.79 (0.70–0.86) | 0.83 (0.74–0.89) | 18.28 (9.22–36.25) | p<0.0001 | 1.91 (1.16–3.18) | 0.009 |
| 0.48 | 0.79 (0.70–0.86) | 0.84 (0.75–0.90) | 0.79 (0.71–0.86) | 0.83 (0.75–0.90) | 19.58 (9.79–39.16) | p<0.0001 | 1.87 (1.12–3.10) | 0.013 |
| 0.52 | 0.78 (0.69–0.85) | 0.85 (0.76–0.91) | 0.79 (0.70–0.86) | 0.84 (0.75–0.90) | 19.92 (9.91–40.06) | p<0.0001 | 2.00 (1.19–3.35) | 0.006 |
| 0.56 | 0.76 (0.67–0.84) | 0.86 (0.77–0.92) | 0.78 (0.69–0.85) | 0.85 (0.76–0.91) | 19.36 (9.60–39.07) | p<0.0001 | 1.89 (1.13–3.17) | 0.012 |
| 0.60 | 0.74 (0.65–0.82) | 0.88 (0.80–0.93) | 0.77 (0.68–0.84) | 0.86 (0.77–0.92) | 20.70 (10.05–42.61) | p<0.0001 | 1.92 (1.13–3.25) | 0.012 |
| 0.64 | 0.74 (0.65–0.82) | 0.88 (0.80–0.93) | 0.77 (0.68–0.84) | 0.86 (0.77–0.92) | 20.70 (10.05–42.61) | p<0.0001 | 1.92 (1.13–3.25) | 0.012 |
| 0.68 | 0.73 (0.64–0.81) | 0.89 (0.81–0.94) | 0.76 (0.68–0.83) | 0.87 (0.78–0.92) | 21.61 (10.35–45.10) | p<0.0001 | 2.07 (1.20–3.55) | 0.006 |
| 0.72 | 0.72 (0.63–0.80) | 0.89 (0.81–0.94) | 0.76 (0.67–0.83) | 0.87 (0.78–0.93) | 20.63 (9.91–42.94) | p<0.0001 | 2.01 (1.17–3.45) | 0.008 |
| 0.76 | 0.70 (0.60–0.78) | 0.89 (0.81–0.94) | 0.74 (0.65–0.81) | 0.86 (0.77–0.92) | 18.04 (8.72–37.31) | p<0.0001 | 2.17 (1.25–3.79) | 0.004 |
| 1.00 | 0.60 (0.50–0.69) | 0.92 (0.85–0.96) | 0.69 (0.61–0.76) | 0.89 (0.79–0.95) | 18.10 (8.00–40.92) | p<0.0001 | 2.40 (1.29–4.46) | 0.002 |
Sensitivity, specificity, predictive values, and odds ratios (OR) of successive GFAP cutoff levels (ng/mL) in differentiating presence of intracranial lesions as seen on admission head CT, and corresponding odds ratios for predicting presence of less-than-full recovery as measured by the Glasgow Outcome Scale-Extended (GOS-E) at 6 months post-injury. The highlighted row displays the GFAP-BDP cutoff optimized for diagnosing CT+ odds ratio (OR=21.61) and area under the curve (AUC) (Fig. 3).
GFAP-BDP, glial fibrillary acidic protein and breakdown products.
Discussion
This prospective, multicenter study of TBI patients examined the diagnostic performance of a serum biomarker for the early diagnosis of TBI. In patients with exposure to head trauma, acute (within 24 h) serum measurement of GFAP-BDP reliably distinguished the presence and severity of CT scan findings at initial injury, and functional outcome at 6 months after injury. In our data, the optimal cutoff point for diagnosis of TBI was a serum GFAP-BDP level of 0.68 ng/mL.
TBI remains one of the greatest challenges in medicine and public health. Our study focused on biomarkers of mild TBI, because diagnosis of more severe TBI is apparent by clinical and imaging assessment. The epidemiological and financial burden of underdiagnosed mild TBI is high, and the clinical benefit of early intervention could be substantial. The effects of mild TBI have recently been highlighted in specific at-risk populations. McCrea et al. reported that college football players exhibited cognitive impairment and balance problems after concussion,12 and although these symptoms resolved, other studies have investigated the effects of repeated insults over time.13 More recently, in a study of United States military personnel with mild TBI, persistent abnormalities revealed on diffusion tensor imaging (DTI) were consistent with traumatic axonal injury in some subjects, although none had detectable intracranial injury on CT.14 In a similar study, soldiers with mild TBI were significantly more likely to report poor general health, missed work days, medical visits, and post-concussive symptoms than were soldiers with other injuries, although the effects were confounded by post-traumatic stress disorder (PTSD) and depression.15 In broader populations of adults with mild TBI, the likelihood of major depressive disorder and epilepsy increased, although effects were also confounded by prior history.16,17 A literature review found that the prevalence of chronic pain was greater in patients with mild TBI than in those with moderate or severe TBI.18
The diagnosis and treatment of TBI is hindered by the lack of a definitive biomarker, especially for mild TBI or concussion. Current medical imaging methods (CT, MRI, DTI, and functional MRI [fMRI]) rarely provide definitive biological indicators of TBI-induced damage.19 No serum biomarker is currently in routine clinical use. Because of the appreciation of the medical need, there has been heightened interest in identifying molecular markers for diagnosing mild TBI. To be attractive for use in the clinic, such markers should be easy to access and interpret as well as fast and inexpensive to process. Serum protein tests are attractive for these reasons, and an increasing body of evidence has been developed. In a series of studies, Vos et al. examined the utility of S100B, GFAP, and NSE as biomarkers in TBI and found reproducible effects of varying magnitudes. In patients who died, median serum levels of GFAP were increased 33-fold and S100B increased 2-fold. In unfavorable compared to favorable outcome, GFAP was increased 20-fold and S100B was increased 2-fold.20 Mean serum concentrations at hospital admission after SAH were increased (S100B threefold and GFAP twofold) compared with the upper limit of normal reference values.21 Vos and colleagues reported that, in severe TBI patients, median serum levels of S100B, GFAP, and NSE were raised 18-fold (S100b), 5-fold (GFAP), and 2-fold (NSE), respectively, compared with normal reference values. GFAP levels>1.5 ug/L strongly predicted death (OR 5.8) and poor outcome (OR 8.8).1 Others have reported similar results, with predictive power for GFAP, as measured by AUC, ranging from 0.98 to 0.84.22,23 It is of note that serum markers combined with clinical markers may be particularly useful. Using the IMPACT model with age, GCS, and pupillary response factors, Czeiter et al. were able to increase predictive power with the addition of GFAP in cerebrospinal fluid (CSF) and serum.24
The diagnostic performance of GFAP-BDP, as indicated in the current study by receiver operator curve analysis, is on a par with that of troponin in myocardial ischemia and brain natriuretic peptide in congestive heart failure.25–27 Our findings are also consistent with previous reports in terms of predictive power and measured levels of serum GFAP in TBI.4 We found that serum GFAP-BDP distinguished injury severity assessed by GCS, adjusted for age, sex, and injury mechanism. The ability of GFAP-BDP level to discriminate between patients with mild and moderate-to-severe injuries measured by the AUC was 0.87. GFAP-BDP levels were greater in patients with CT scans positive for traumatic lesions (0.26±0.41 ng/mL vs. 2.88±3.74 ng/mL; p<0.01), which is consistent with the previous findings of Papa et al. 2012.4 The ability of GFAP-BDP to discriminate between the likelihood of an unfavorable 6 month outcome, measured by the AUC, was adequate at 0.74, and the ability of GFAP-BDP to discriminate between the likelihood of a full recovery was 0.65. The optimal GFAP-BDP cutoff value was 0.68 ng/mL for identifying patients who failed to return to pre-injury baseline, and resulted in a 21.61 OR for a positive head CT and a 2.07 OR for failure to return to pre-injury baseline. Although the cutoff value is higher, with a lower sensitivity and higher specificity than that reported by Papa et al. (0.035 ng/mL) to identify positive CT findings, our analysis included all severity levels of TBI as well as 6 month outcomes.4
In addition to the reproducibility described, this study has other strengths, as well as potential limitations. To our knowledge, this is the largest prospective biomarker study in a mild TBI population. Other GFAP assays have been developed, but the advantages of our method include commercial availability and centrally read results.28,29 However, in terms of study design, we did not measure GFAP levels over time or in control subjects. Further, ∼33% of patients with GFAP data available were lost to follow-up by 6 months. Also, as with previous studies, we found GFAP to be a low abundance protein, detectable in ng/mL. Finally, it is also increasingly understood that GFAP levels may be associated with other physiological processes and comorbidities. In patients undergoing aortic valve replacement, S100B and GFAP increased, possibly because cardiac surgery with cardiopulmonary bypass may cause cerebral inflammation, glial cell injury, and blood–brain barrier (BBB) dysfunction without biochemical signs of neuronal damage.30 Children with sickle cell disease (SCD) had higher plasma GFAP than did healthy pediatric controls, and GFAP among children with SCD may be associated with subclinical brain injury.31 NSE and S100B can be released into serum during operations by extracranial sources, and although assessment of neurocognitive decline after surgery has been hampered by heterogeneous testing techniques, GFAP may be a sensitive marker whose extracranial sources are antigenically different from the brain-derived form.32
Through the TRACK-TBI study, we examined the diagnosis and triage of patients presenting to the ED with suspected TBI. The results demonstrate that measurement of serum GFAP-BDP may improve the ability of clinicians to identify TBI patients who may require further medical evaluation and management. Use of GFAP-BDP as a serum biomarker for TBI should lead to more accurate diagnosis and management of TBI.
Contributor Information
Collaborators: Transforming Research and Clinical Knowledge in Traumatic Brain Injury investigators including:
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Vos P.E. Lamers K.J. Hendriks J.C. van Haaren M. Beems T. Zimmerman C. van Geel W. de Reus H. Biert J. Verbeek M.M. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62:1303–1310. doi: 10.1212/01.wnl.0000120550.00643.dc. [DOI] [PubMed] [Google Scholar]
- 2.Papa L. Lewis L.M. Silvestri S. Falk J.L. Giordano P. Brophy G.M. Demery J.A. Liu M.C. Mo J. Akinyi L. Mondello S. Schmid K. Robertson C.S. Tortella F.C. Hayes R.L. Wang K.K. J. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. Trauma Acute Care Surg. 2012;72:1335–1344. doi: 10.1097/TA.0b013e3182491e3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondello S. Papa L. Buki A. Bullock M.R. Czeiter E. Tortella F.C. Wang K.K. Hayes R.L. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit. Care. 2011;15:R156. doi: 10.1186/cc10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papa L. Lewis L.M. Falk J.L. Zhang Z. Silvestri S. Giordano P. Brophy G.M. Demery J.A. Dixit N.K. Ferguson I., et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 2012;59:471–483. doi: 10.1016/j.annemergmed.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yue J.K. Vassar M.J. Lingsma H. Cooper S.R. Yuh E.L. Mukherjee P. Puccio A.M. Gordon W. Okonkwo D.O. Valadka A. Schnyer D.M. Maas A. Manley G.T. Casey S.S. Cheong M. Dams-O'Connor K. Hricik A.J. Knight E.E. Kulubya E.S. Menon D. Morabito D.J. Pacheco J.L. Sinha T.K. Transforming research and clinical knowledge in traumatic brain injury (TRACK-TBI) pilot: Multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma. 2013 doi: 10.1089/neu.2013.2970. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jagoda A.S. Bazarian J.J. Bruns J.J., Jr Cantrill S.V. Gean A.D. Howard P.K. Ghajar J. Riggio S. Wright D.W. Wears R.L. Bakshy A. Burgess P. Wald M.M. Whitson R.R. American College of Emergency Physicians, and Centers for Disease Control and Prevention. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann. Emerg. Med. 2008;52:714–748. doi: 10.1016/j.annemergmed.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Manley G.T. Diaz–Arrastia R. Brophy M. Engel D. Goodman C. Gwinn K. Veenstra T.D. Ling G. Ottens A.K. Tortella F. Hayes R.L. Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch. Phys. Med. Rehabil. 2010;91:1667–1672. doi: 10.1016/j.apmr.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Zoltewicz J.S. Scharf D. Yang B. Chawla A. Newsom K.J. Fang L. Characterization of antibodies that detect human GFAP after traumatic brain injury. Biomark Insights. 2012;7:71–79. doi: 10.4137/BMI.S9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duhaime A.C. Gean A.D. Haacke E.M. Hicks R. Wintermark M. Mukherjee P. Brody D. Latour L. Riedy G. Common Data Elements Neuroimaging Working Group Members, and Pediatric Working Group Members. Common data elements in radiologic imaging of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010;91:1661–1666. doi: 10.1016/j.apmr.2010.07.238. [DOI] [PubMed] [Google Scholar]
- 10.Whyte J. Vasterling J. Manley G.T. Common data elements for research on traumatic brain injury and psychological health: current status and future development. Arch. Phys. Med. Rehabil. 2010;91:1692–1696. doi: 10.1016/j.apmr.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Wilson J.T. Pettigrew L.E. Teasdale G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 12.McCrea M. Guskiewicz K.M. Marshall S.W. Barr W. Randolph C. Cantu R.C. Onate J.A. Yang J. Kelly J.P. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. J.A.M.A. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 13.Prins M. Alexander D. Giza C.C. Hovda D. Repeat mild traumatic brain injury: mechanisms of cerebral vulnerability. J. Neurotrauma. 2012;30:30–38. doi: 10.1089/neu.2012.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mac Donald C.L. Johnson A.M. Cooper D. Nelson E.C. Werner N.J. Shimony J.S. Snyder A.Z. Raichle M.E. Witherow J.R. Fang R. Flaherty S.F. Brody D.L. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoge C.W. McGurk D. Thomas J.L. Cox A.L. Engel C.C. Castro C.A. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 16.Bombardier C.H. Fann J.R. Temkin N.R. Esselman P.C. Barber J. Dikmen S.S. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. J.A.M.A. 2010;303:1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen J. Pedersen M.G. Pedersen C.B. Sidenius P. Olsen J. Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373:1105–1110. doi: 10.1016/S0140-6736(09)60214-2. [DOI] [PubMed] [Google Scholar]
- 18.Nampiaparampil D.E. Prevalence of chronic pain after traumatic brain injury: a systematic review. J.A.M.A. 2008;300:711–719. doi: 10.1001/jama.300.6.711. [DOI] [PubMed] [Google Scholar]
- 19.Niogi S.N. Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J. Head Trauma Rehabil. 2010;25:241–255. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- 20.Vos P.E. Jacobs B. Andriessen T.M. Lamers K.J. Borm G.F. Beems T. Edwards M. Rosmalen C.F. Vissers J.L. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology. 2010;75:1786–1793. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]
- 21.Vos P.E. van Gils M. Beems T. Zimmerman C. Verbeek M.M. Increased GFAP and S100beta but not NSE serum levels after subarachnoid haemorrhage are associated with clinical severity. Eur. J. Neurol. 2006;13:632–638. doi: 10.1111/j.1468-1331.2006.01332.x. [DOI] [PubMed] [Google Scholar]
- 22.Honda M. Tsuruta R. Kaneko T. Kasaoka S. Yagi T. Todani M. Fujita M. Izumi T. Maekawa T. Serum GFAP is a highly specific biomarker for traumatic brain injury in humans compared with S-100B and neuron-specific enolase. J. Trauma. 2010;69:104–109. doi: 10.1097/TA.0b013e3181bbd485. [DOI] [PubMed] [Google Scholar]
- 23.Pelinka L.E. Kroepfl A. Leixnering M. Buchinger W. Raabe A. Redl H. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J. Neurotrauma. 2004;21:1553–1561. doi: 10.1089/neu.2004.21.1553. [DOI] [PubMed] [Google Scholar]
- 24.Czeiter E. Mondello S. Kovacs N. Sandor J. Gabrielli A. Schmid K. Tortella F. Wang K.K. Hayes R.L. Barzo P. Ezer E. Doczi T. Buki A. Brain injury biomarkers may improve the predictive power of the IMPACT outcome calculator. J. Neurotrauma. 2012;29:1770–1778. doi: 10.1089/neu.2011.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maisel A.S. Krishnaswamy P. Nowak R.M. McCord J. Hollander J.E. Duc P. Omland T. Storrow A.B. Abraham W.T. Wu A.H. Clopton P. Steg P.G. Westheim A. Knudsen C.W. Perez A. Kazanegra R. Herrmann H.C. McCullough P.A. Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. New Engl. J. Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 26.Peacock W.F., 4th De Marco T. Fonarow G.C. Diercks D. Wynne J. Apple F.S. Wu A.H. ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N. Engl. J. Med. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 27.Reichlin T. Hochholzer W. Bassetti S. Steuer S. Stelzig C. Hartwiger S. Biedert S. Schaub N. Buerge C. Potocki M. Noveanu M. Breidthardt T. Twerenbold R. Winkler K. Bingisser R. Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N. Engl. J. Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 28.Vissers J.L. Mersch M.E. Rosmalen C.F. van Heumen M.J. van Geel W.J. Lamers K.J. Rosmalen F.M. Swinkels L.M. Thomsen J. Herrmann M. Rapid immunoassay for the determination of GFAP in serum. Clin. Chim. Acta. 2006;366:336–340. doi: 10.1016/j.cca.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Van Geel W.J. de Reus H.P. Nijzing H. Verbeek M.M. Vos P.E. Lamers K.J. Measurement of GFAP in blood: an analytical method. Clin. Chim. Acta. 2002;326:151–154. doi: 10.1016/s0009-8981(02)00330-3. [DOI] [PubMed] [Google Scholar]
- 30.Reinsfelt B. Ricksten S.E. Zetterberg H. Blennow K. Fredén–Lindqvist J. Westerlind A. Cerebrospinal fluid markers of brain injury, inflammation, and blood–brain barrier dysfunction in cardiac surgery. Ann. Thorac. Surg. 2012;94:549–555. doi: 10.1016/j.athoracsur.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 31.Savage W.J. Barron–Casella E. Fu Z. Dulloor P. Williams L. Crain B.J. White D.A. Jennings J.M. Van Eyk J.E. Debaun M.R. Everett A. Casella J.F. Plasma GFAP levels in children with sickle cell disease. Am. J. Hematol. 2011;86:427–429. doi: 10.1002/ajh.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seco M. Edelman J.J. Wilson M.K. Bannon P.G. Vallely M.P. Serum biomarkers of neurologic injury in cardiac operations. Ann. Thorac. Surg. 2012;94:1026–1033. doi: 10.1016/j.athoracsur.2012.04.142. [DOI] [PubMed] [Google Scholar]