Abstract
Significance
Postnatal vasculogenesis mediated via endothelial progenitor cells (EPCs) contributes to re-endothelialization and augments neovascularization after ischemia and tissue injury, providing a novel therapeutic application. However, controversy exists with respect to the origin, identification, and contributions of the EPCs to neovascularization, necessitating further study.
Recent Advances
Bone marrow (BM) or circulating cells expressing cd133/vascular endothelial growth factor receptor 2 include those with endothelial progenitor capacity. Increasing evidence suggests that there are additional BM-derived (myeloid; mesenchymal cells) and non-BM-derived (peripheral and cord-blood; tissue-resident) cell populations which also give rise to endothelial cells (ECs) and contribute to re-endothelialization and growth factor release after ischemia and tissue injury. Currently, EPCs are being used as diagnostic markers for the assessment of cardiovascular and tumor risk/progression. Techniques aimed at enhancing ex vivo expansion and the therapeutic potential of these cells are being optimized.
Critical Issues
Mobilization and EPC-mediated neovascularization are critically regulated. Stimulatory (growth factors, statins, and exercise) or inhibitory factors (obesity, diabetes, and other cardiovascular diseases) modulate EPC numbers and function. Recruitment and incorporation of EPCs require a coordinated sequence of signaling events, including adhesion, migration (by integrins), and chemoattraction. Finally, EPCs differentiate into ECs and/or secrete angiogenic growth factors. These cells are highly plastic, and depending on the microenvironment and presence of other cells, EPCs transdifferentiate and/or undergo cell fusion and become cells of a different lineage. Therefore, in vitro culture conditions should be optimized to mimic the in vivo milieu to fully characterize the biological function and contribution of EPCs to postnatal vasculogenesis.
Future Directions
Advances in characterization of the EPC biology and enhancement of EPC functions are required. In addition, innovative tissue-engineered carrier matrices that permit embedding of EPCs and provide optimal conditions for EPC survival and endothelial outgrowth will further contribute to EPC-mediated therapeutic applications in wound healing and ischemia repair.

Swathi Balaji, PhD
Scope and Significance
Wound healing is a complex but well-orchestrated process, comprising overlapping phases—homeostasis, inflammation, proliferation, and maturation—which involve interactions between different tissue structures, a large number of resident and infiltrating cell types, multiple growth factors, cytokines, and chemokines.1 Neovascularization is essential for the survival, repair, and remodeling of wounded and/or ischemic tissue. Endothelial progenitor cells (EPCs) are identified as bone marrow (BM)–derived endothelial precursor cells that contribute to neovascularization.2–4 Since Asahara et al. first identified circulating EPCs in 1997,5 increasing evidence suggests that BM-derived EPCs functionally contribute to neovascularization in several models of tissue injury and remodeling, including wound healing, myocardial ischemia, retinopathy, stroke, peripheral vascular disease, as well as tumor growth. During the last decade, significant research has been conducted to elucidate the physiologic role of EPCs in tissue repair and their deficiency in several disease states.
EPCs are mainly located within the stem cell niche in BM, along with some circulating populations in the peripheral blood. The process by which EPCs contribute to new vessel formation in adults is termed postnatal vasculogenesis, and it occurs via four interrelated steps. When injury or tissue damage occurs, EPCs are thought to mobilize from the BM into the circulation and home to tissue repair sites under the guidance of signals such as hypoxia, growth factors, chemoattractant signals, and chemokines. EPCs then invade and migrate at the same sites, and differentiate into mature endothelial cells (ECs) and/or regulate pre-existing ECs via paracrine or juxtacrine signals. During these four steps, EPCs interact with different physiological compartments, namely, BM, peripheral blood, blood vessels, and the site of tissue injury and remodeling. The success of each step depends on the ability of EPCs to interact, adapt, and respond to multiple molecular cues. However, specific molecular mechanisms have not been fully defined. The present review is an effort to summarize the research that has been conducted to elucidate the underlying mechanisms that govern EPC biology and function and their role in therapeutic angiogenesis, as well as strategies aimed at enhancing the contribution of EPCs to tissue repair.
Translational Relevance
Neovascularization is an essential mechanism in determining tissue repair outcomes and maintenance. Until recently, postnatal neovascularization was thought to depend mainly on angiogenesis (a process by which new vessels are formed by the activation, proliferation, and migration of ECs). More recently, postnatal vasculogenesis is becoming evident as a major contributor to adult neovascularization after injury or ischemia.6 During embryonic development, vasculogenesis is defined as the process where precursor cells termed angioblasts and/or hemangioblasts migrate and differentiate into ECs that coalesce to form a primitive vascular plexus and de novo vessels. The existence of postnatal vasculogenesis, which is mediated by a population of progenitors to vascular ECs during adulthood (identified in both peripheral blood and BM), is being extensively studied as a mediator for both pro- and antiangiogenic therapies in tissue repair and cancer research, respectively.7 Given the biological contribution of EPCs in different types of vascular pathologies, most studies have focused on defining the phenotype of EPCs, the molecular mechanisms regulating EPC function, and the quantitative determination of EPC contribution to physiological and/or pathological postnatal vasculogenesis.8 More recent studies have focused on the clinical and therapeutic applications of this cell population, such as using EPCs as a diagnostic marker for the assessment of cardiovascular and tumor risk/progression and autologous transplantation to improve neovascularization and tissue repair. Further, techniques aimed at enhancing ex vivo expansion and the therapeutic potential of these cells such as epigenetic and genetic modifications of these cells, as well as growth factor therapy, are also being extensively studied.9,10
Clinical Relevance
An important aspect of EPC-based therapeutic approaches is the magnitude of incorporation of transplanted or in-situ–mobilized EPCs into the newly forming vessels. A huge variation (∼1–50%) is reported so far, which may be due to differences in the target tissues studied, genetic differences in the animal strains used, and lack of standardized techniques to track and trace the lineage of these cells. Perhaps the most important factor is the heterogeneity of EPCs based on their isolation and culture protocols.11 Regardless of the lineage, it has been validated in vivo that autologous, immunologically neutral EPCs isolated from BM aspirates, peripheral blood, and cord blood constitute convenient sources for retransplantation for improved therapeutic outcomes.12 EPCs are used for nonthrombogenic re-endothelialization of vascular grafts, heart valve replacements, and intravascular/cardiac stent devices. EPCs directly injected or infused into sites of ischemic or vessel injury incorporate into the vessels or produce cytokines and growth factors that have positive therapeutic effects on improving regional flow, inhibiting cellular apoptosis, and improved function. Additionally, EPCs have demonstrated a broad plasticity, spontaneous cell fusion mechanisms, and ability to transdifferentiate into new lineages such as cardiomyocytes, liver cells, or neurons depending on the microenvironment, which may be used to enhance clinical repair outcomes. The enhancement of EPC functions combined with advances in tissue-engineered carrier matrices that permit embedding of EPCs and provide physiological, optimal conditions for EPC survival and endothelial outgrowth can contribute to therapeutic angiogenesis in wound healing and ischemia repair.13
Discussion of Findings and Relevant Literature
EPC biology and definition
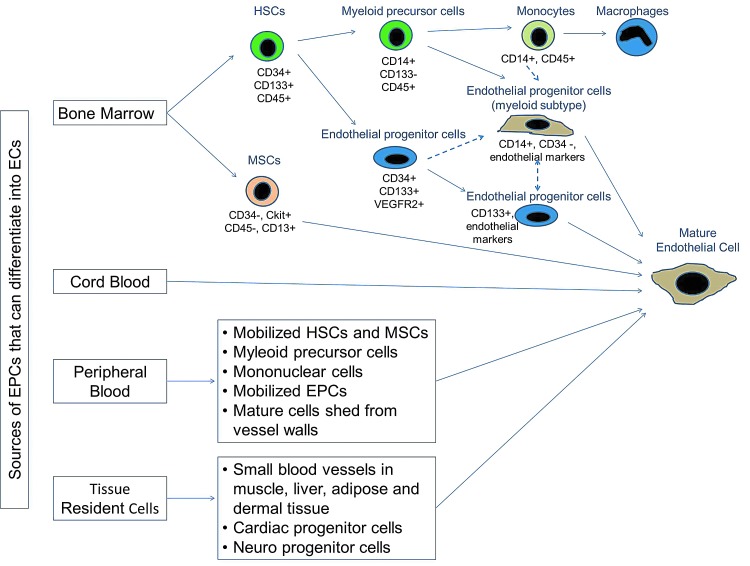
The definition and characteristics of EPCs are a work in progress. EPCs are believed to be differentiated from hemangioblasts along with other hematopoietic stem cells. Progenitor cells have been distinguished from stem cells due to the lack of self-renewing ability. However, EPCs are characterized as lineage-committed adult stem cells, as they have degrees of stemness, including self-renewal, clonogenicity, and differentiation capacity. Due to their unique characters of progressive differentiation, EPCs are usually described by cellular origin, their isolation methods, and their surface markers. Several cell surface markers have been used for EPC identification, such as vascular endothelial growth factor receptor 2 (VEGFR-2), Sca-1, CD34, and CD133.14 Unfortunately, there is no specific surface marker that definitely distinguishes EPCs. Peichev et al.15 characterized EPCs as VEGFR-2+/CD133+/CD34+ cells. Recently, Tian et al.16 employed Sca-1 as a BM cell marker and used VE-cadherin and E-selectin as endothelial markers to identify EPCs. However, markers such as CD31, von Willebrand factor, and VE-cadherin may identify cells in a more advanced stage of endothelial maturation. Currently, investigators characterize EPCs by including, at the minimum, antigenic markers defining the stemness and hematopoietic lineage (humans: CD34 and CD133; mice: CD34, c-kit, or Sca-1) in combination with markers demonstrating endothelial commitment (humans: KDR; mice: Flk-1) along with morphological, functional, and clonal expansion characteristics. It is established that there are lineage and functional heterogeneities within the EPC population, and it has a dynamic phenotype in space and time; however, the spectrum of phenotypes has not yet been fully defined (Fig. 1).
Figure 1.
Origin and differentiation of endothelial progenitor cells (EPCs). Several sources have been identified for progenitor cells that can differentiate into endothelial cells. Primarily, hematopoietic stem cells (HSCs) resident in the bone marrow (BM) niche give rise to a population of cells that differentiate into myeloid progenitor or endothelial progenitor subtypes. These subsets differentiate into their respective mature cell type. Alternative pathways (dashed lines) to derive EPCs have been proposed, where EPCs arise directly from HSCs or via a myeloid progenitor/monocyte intermediate. Cell surface markers differentiate cells along the proposed pathway of EPC ontogeny. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
In normal homeostatic conditions, there are a low number of circulating EPC populations in the peripheral blood. EPCs reside within a stem cell niche in the BM characterized by low oxygen tension and high levels of stromal cell-derived factor-1 (SDF-1), a potent chemoattractant for EPCs. There are also low numbers of circulating EPC populations in the peripheral blood. Peripheral tissue hypoxia under trauma or wound-healing conditions results in increased production of EPC-mobilizing factors (such as granulocyte colony–stimulating factor [G-CSF], VEGF, basic fibroblast growth factor [bFGF], placental growth factor, erythropoietin, or SDF-1) to a concentration greater than that in the BM.17,18 These factors activate endothelial nitric oxide synthase, leading to an increased production of nitric oxide, which regulates the enzymatic activity of matrix metalloproteinases (MMPs).19 In particular, activated MMP-9 leads to the release of a soluble kit ligand from EPCs in the BM niche, resulting in EPC release and mobilization into the peripheral circulation.20 Once in circulation, EPCs respond to chemokine signaling in the tissues undergoing active remodeling and home to the injury site. SDF-1 and CXCR4 are identified as critical mediators for the ischemia-specific recruitment of circulating progenitor cells. The other major chemokines and respective receptors that regulate EPC activation and homing are interleukin 8 (IL-8) and CXCR2; growth-regulated oncogene-a and CXCR1; CCL5 and CCR5; and C-C chemokine and chemokine (C–C motif ) receptors 2 and 5.21 Upon interaction with tissue-specific chemokines, EPCs become activated and initiate integrin-mediated adhesion to endothelial vascular cells and consequently transendothelial migration into the sites of vascular and tissue remodeling (Fig. 2). Once circulating EPCs have crossed the endothelial monolayer, they migrate through the blood vessel basement membrane and through the interstitial extracellular matrix (ECM) to exert their functions. EPC invasion depends on the activity of extracellular proteases mainly MMP-9 that breakdown and remodel the ECM components at the vessel basement membrane and also in the interstitial space. Once at the site of tissue repair, EPCs contribute to new vessel formation in multiple ways: direct incorporation into neovessels, differentiation into mature ECs, and production of paracrine/juxtacrine signals. The process of differentiation of EPCs into ECs comprises integrin-mediated adhesion of EPCs to ECM components, in particular, fibronectin, followed by growth factor-induced proliferation and survival.22 Angiogenic growth factors, including the VEGF family, the angiopoietin group, and EGF, have been implicated. Further maturation and acquisition of an endothelial phenotype are suggested to be mediated by histone deacetylase regulation of the transcription factor HoxA. In addition to differentiating into ECs, EPCs have been shown to produce multiple growth factors that have paracrine effects such as VEGF, SDF-1, insulin-like growth factor 1, monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1a, and platelet-derived growth factor. In combination, these factors can act on different cell types that lead to an overall response that promotes angiogenesis and tissue regeneration.23
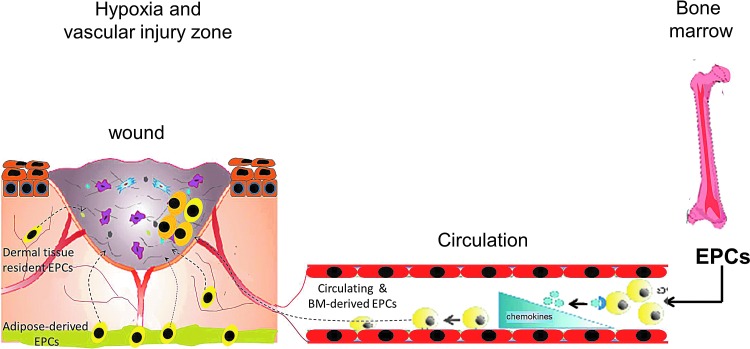
Figure 2.
EPCs mobilize in response to hypoxia induced by trauma or vascular injury. In normal homeostatic conditions, EPCs reside within a stem cell niche in the BM. Peripheral tissue hypoxia under trauma or wound healing or vascular injury conditions results in increased production of EPC-mobilizing chemokines and growth factors to a concentration greater than that in the BM, resulting in EPC release and mobilization into the peripheral circulation. Once in circulation, EPCs respond to chemokine signaling in the tissues undergoing active remodeling and home to the injury site. Concomitantly, circulating progenitor cells, tissue-resident, and adipose-derived stem cells respond to the chemokine signaling and home to the active tissue-remodeling site. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Role of integrins in EPC biology and function
EPCs express several integrin subunits (α1–α6, α9, αv; β1–β3, β5, β7). Interestingly, the integrin expression profile of EPCs is a dynamic process. Integrins get activated and/or upregulated at specific steps of the EPC course from BM to tissue repair sites, directly in response to the multiple molecular cues (tissue-specific ECM and cytokine factors), and reflect the adaptation of these cells to different conditions that regulate their mobilization, homing, transendothelial migration, invasion, and differentiation (Fig. 3).24
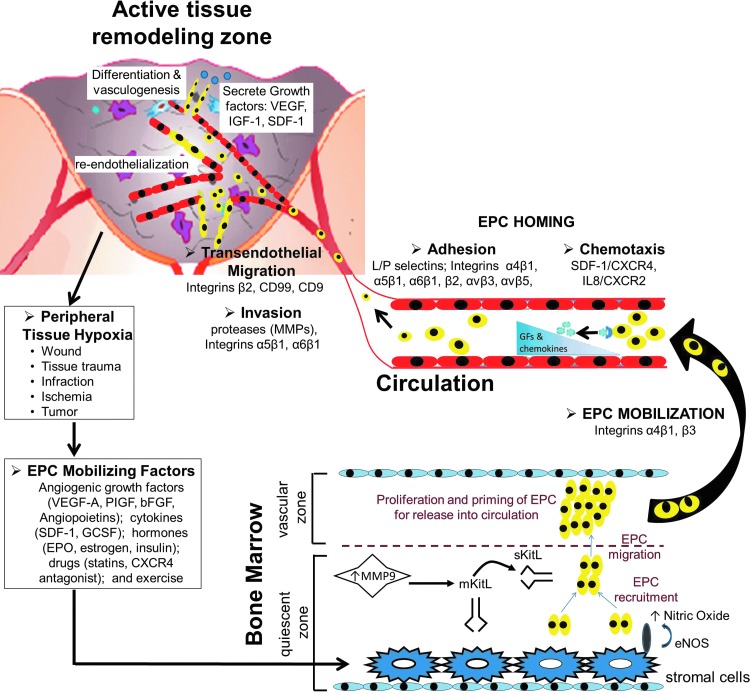
Figure 3.
BM-derived EPC stages during adult vasculogenesis—hypoxia/cytokine-driven EPC mobilization from BM to EPC homing and contribution to neovascularization. Circulating EPC count in peripheral blood is very low under normal homeostatic conditions. Hypoxia induced by tissues undergoing active remodeling after injury or ischemia increases the EPC-mobilizing factors, resulting in increase of peripheral blood EPCs. The life cycle of BM-derived EPCs is a complex cascade, which begins with the EPC-mobilizing signals, including angiogenic growth factors, cytokines, hormones, drugs, or exercise, releasing the EPCs from BM. This is mediated via increased matrix metalloproteinase-9 (MMP-9) activity in the quiescent zone of the BM niche, which cleaves the membrane-bound stem cell cytokine mKitL expressed by stromal cells to liberate soluble sKitL. sKitL recruits EPCs and then stimulates them to migrate to the vascular zone of the BM, which is more permissive for proliferation and priming of EPCs for subsequent mobilization into peripheral blood. Additionally, nitric oxide produced by stromal cells is crucial for EPC translocation. Homing and recruitment of EPCs into the active remodeling sites require a coordinated multistep process, including chemoattraction, adhesion, transendothelial migration, and tissue invasion. Locally enhanced levels of cytokines and growth factors provide a chemotaxic trigger for EPCs to home to the sites of remodeling. Recruited EPCs adhere to the endothelium and start transendothelial migration and invasion into the extravascular tissue, where they exert their effects by integration into neovasculature, re-endothelialization, in situ differentiation, and and/or cytokine production. A nonexhaustive list of various cytokines, growth factors, and integrins implicated in each stage of the EPC cycle is illustrated. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The main integrins regulating the retention of EPCs in the BM microenvironment are α4 and β3. α4-Integrins have an essential role in embryogenesis, hematopoiesis, and adhesion to vascular cell adhesion molecule I (VCAM-I) and promote adhesive interactions between EPC and BM stromal cells. β3-Integrins have an essential role in angiogenesis and hemostasis. Recent studies using both in vitro assays as well as in vivo studies (in integrin-null or conditional knockout mice models) demonstrate that inhibiting the availability of α4- and β3-integrins results in enhanced mobilization of EPCs from the BM niche into circulation.25
After being mobilized from the BM into circulation, EPCs recognize the signals present at the site of vessel injury and remodeling. They then home to the repair site by a direct interaction between molecular targets expressed in homing tissues and the integrins expressed by EPCs. One of the major integrin subunits regulating EPC homing to active angiogenic sites is the β2-integrin.26 β2-Integrins mediate the adhesion of peripheral blood-derived EPCs to activated ECs, and are also essential in recognizing multiple members of the intercellular adhesion molecule family, fibrinogen, and polysaccharides. Several studies have also shown the importance of β2-integrin activation in the neovascularization capacity of these cells in vivo. Additionally, studies using pharmacological activation of this integrin conformation demonstrate increased EPC adhesion, migration, and neovascularization-promoting capacity of intravenously injected EPCs. Interestingly, inhibition of β2-integrins leads only to a partial inhibition of EPC homing, suggesting that other integrins may also regulate this essential step of EPC biology. β1-Integrins are the other major integrin family identified in regulating EPC homing to angiogenic sites and their adherence to activated ECs, VCAM-I, and cellular fibronectin. In addition to recognizing molecular homing signals expressed by activated ECs, EPCs can recognize and adhere directly to the exposed ECM components. This is of particular interest in ischemia and wound healing, where vessel obstruction and reduced oxygen supply result in death and detachment of the ECs lining the vessel walls, exposing the underlying basement membrane components. Several of the members of the β1-integrin family facilitate direct homing of EPCs to denuded vessels. α5β1 is also highly expressed in EPCs, which facilitates the binding of these cells to the exposed fibronectin on vessels. α6β1 is another β1-integrin that promotes EPC homing to the sites of ischemia and vascular remodeling and has been shown to be essential for skin homeostasis and regulates endothelial tube formation.27 It is a laminin-binding integrin and is regulated by factors such as VEGF and bFGF, which are the major growth factors that regulate wound healing.28,29 EPC adhesion to denuded vessels is also mediated by integrins αvβ3 and αvβ5. Once EPCs adhere at specific homing sites, they need to migrate through the endothelial monolayer and invade through the underlying blood vessel basement membrane and the interstitial ECM to arrive at tissue repair sites, where they can exert their functions. It has been demonstrated that the transendothelial migration is mediated mainly by the β2-integrins and the molecular signaling of MCP-1 and VEGF. It is well recognized that both integrins and extracellular proteases are essential to modulate EPC invasion and migration along the ECM.
The two interdependent processes, EPC differentiation and direct incorporation of EPCs into neovessels/production of cytokines, comprise the functional activity of EPCs. The integrin expression profile in EPCs is variable throughout endothelial differentiation. Some integrins are expressed only in undifferentiated EPCs, while some integrins are expressed only during differentiation. Integrin subunits α4, α5, and αv, which are fibronectin-binding integrins, are expressed throughout EPC differentiation, suggesting that direct interaction of EPCs with fibronectin is essential for EPC differentiation.25 In fact, fibronectin has been shown to be essential for embryonic vasculogenesis, and several studies have demonstrated a synergy between fibronectin and VEGF in regulating EPC differentiation via binding to α5β1. The integrin–ECM interactions not only mediate different aspects of the endothelial differentiation process but also influence EPC paracrine factor production. Several studies have shown that overexpression of β5-integrins results in increased growth factor production by EPCs. Interestingly, EPCs plated on different matrices express different levels of multiple cytokines, further suggesting that paracrine factor production by EPCs is governed by integrin interactions with ECM.
These data clearly demonstrate that integrins play a crucial role in modulating several aspects of EPC biology and function. Taking into consideration the contributions of structural and morphogenic signals to EPC biology, several groups have proposed targeted manipulations of the EPC integrin–ECM interactions.30 This can potentially be accomplished by tissue engineering approaches of ECM modification or manipulations of the integrin presentation on EPCs, which can further promote EPC function and associated clinical tissue repair outcomes.
In vitro culture, selection, and enhancement of cultured EPCs
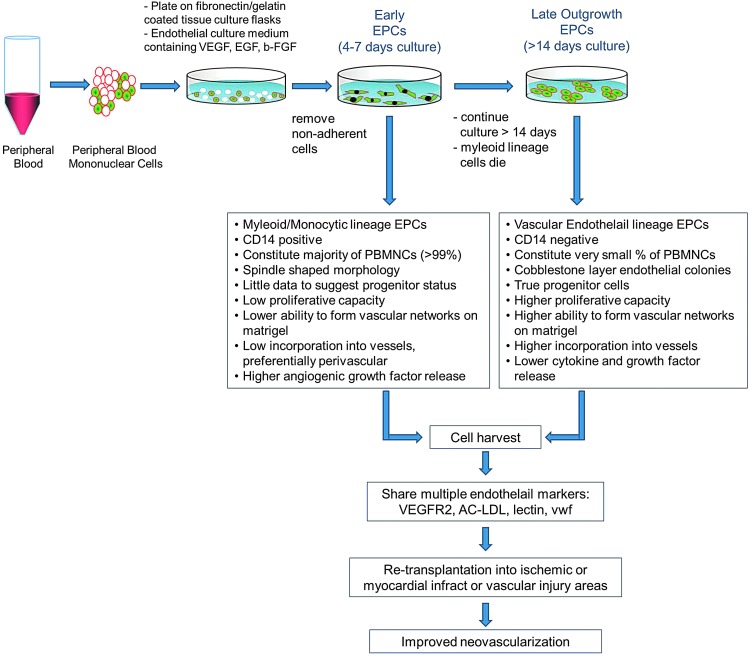
Optimizing the in vitro culture of EPCs is a growing field of research interest. Most assays use circulating EPCs obtained from peripheral blood in in vitro culture assays for identification of EPCs as biomarkers for cardiovascular diseases (CVDs), for analysis of EPC intracellular signaling pathways, or for enriching EPCs for therapeutic angiogenesis applications. These studies suggest that there are at least two different types of EPCs: early- and late-outgrowth EPCs. While both populations demonstrate the capacity to improve neovascularization in preclinical models, they differ with respect to their capacity to differentiate into ECs and to physically form new blood vessels. Early EPCs are cells with myeloid/hematopoietic characteristics and share features with immune cells, particularly monocytes/macrophages. These cells are obtained from short-term cultures of 4–7 days in vitro primarily generated by culturing peripheral blood mononuclear cells on fibronectin in a VEGF-containing medium. These cells are usually referred to as the circulating angiogenic cell population. Most studies suggest that these short-term cultures (early EPCs) predominantly enhance vessel formation by providing a potent mixture of growth factors that support angiogenesis. Late EPCs are often called out growth EPCs and have a more mature EC phenotype. These cells lack hematopoietic and myeloid markers and have lower cytokine release and different growth patterns, and are usually obtained from long-term cultures of at least 2–4 weeks in vitro.31 Yoder et al.32 showed that when culturing peripheral blood mononuclear cells on collagen for >14 days, mature ECs with a high proliferative capacity are obtained. These cells have the ability to form colonies and are named endothelial colony-forming cells (ECFCs). The late EPCs may generate ECs and thereby physically contribute to formation of new capillaries.33 Several studies have shown that the colony-forming ability is dependent on age and presence of CVDs. Because of these properties, the late EPC cultures are being used as biomarkers for prevalence of CVD, and significant research is being conducted to further refine the protocols to culture ECFCs, and develop humanized larger-scale culture assays to use these cells (Fig. 4).
Figure 4.
In vitro culture of EPCs: early-versus-late EPCs. Autologous EPCs for transplantation applications are grown from whole peripheral blood mononuclear cells. In vitro culture of these EPCs constitutes two distinct cell populations: early- and late-outgrowth EPCs. The majority of endothelial-like cells interpreted to be EPCs appear to originate from monocytic, CD14-positive cells (early EPCs). There are little data to support their endothelial stem or progenitor status. Alternately, a small population of the early EPCs when cultured for >14 days retains the true progenitor status. These cells are CD14-negative and demonstrate an increased capacity to proliferate and assemble into capillary tubes (late-outgrowth EPCs). Both populations demonstrate an ability to differentiate into endothelial cells and promote neovascularization in vivo, possibly through different mechanisms, that is, supply of angiogenic versus physical integration into newly forming vessels. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Another typical feature of long-term outgrowing cells is that they form vascular structures in vitro in the absence of coculture, whereas the short-term cultured cells require the interaction with ECs and particularly promote vascular network formation of mature ECs in vitro. Although early versus late EPCs are well-accepted phenotypes, the possibility that late EPCs originate from a rare population of cells within the early EPCs has to be acknowledged, and some cultured cells may have both activities. Additionally, the environment may influence the cell fate and therapeutic benefit, and the specific culture conditions may promote endothelial differentiation of myeloid cells. Moreover, the in vivo environment may influence cell fate and function. For instance, hypoxic stress or pharmacological modulators of epigenetic enzymes were shown to change the epigenetic signature of endothelial marker genes in cultured EPCs. This is of particular interest, because recent studies have shown that although fully mature ECs formed vessels in Matrigel, under in vivo ischemic conditions, they failed to improve neovascularization.
Additionally, cultured cells are a derivative of the circulating cells and may be a useful product to engineer blood vessels in vitro. However, it is unclear whether the cells obtained after in vitro cultures are indeed the same cells that are circulating in the body. The EPC phenotype is dynamic, lending them to be able to reprogrammed in vitro by the artificial environment. The existence of in vivo counterparts of cell types defined by colony assays has still to be elucidated. The functional assessment of ECFCs is more in its early stages than the study of short-term cultured EPCs, which have undergone a thorough critique and re-evaluation. Several groups have recently suggested that using CD34+ or CD133+ hematopoietic cells instead of peripheral blood mononuclear cells as the starting material will result in better clonal selectivity.
Interestingly, recent studies demonstrate the existence of intercellular communication among EPCs and their environment. These occur either by direct cell-to-cell contact or cell-contact–independent mechanisms. These can mediate the transfer of the epigenetic material, including proteins and nucleic acids to EPCs. This governs the phenotypic regulation of the cell-fate decisions, including their commitment and differentiation. For example, Badorff et al.34 reported that a small percentage of human peripheral blood EPCs differentiated toward a cardiomyocyte cell fate when cocultured with rat cardiomyocytes, suggesting transdifferentiation potential that is influenced by the local cellular environment. Other studies have indicated that transplanted BM-derived cells transdifferentiate into new cell types such as liver cells, cardiomyocytes, or neurons in vivo. This information suggests mechanisms that modulate EPC biology and their function by perhaps altering the transcriptional profile.
Clinical interest in therapeutic angiogenesis and advances in stem and progenitor cell biology has led to proposals to combine autologous cell therapy with ex vivo genetic and epigenetic manipulations of these cells. This added selection and expansion of populations with desirable attributes have the potential to enhance clinical efficacy in vivo.35 Lastly, the logical extrapolation of this concept to clinical use in the future is the utilization of high-throughput technology to clonally expand a patient's EPCs, optimize them, and potentially derive a lifetime supply of these cells when required by the patient.
Vascular insufficiency in chronic wounds
Several studies have demonstrated that the number of EPCs (both in circulation and in BM), as well as their function, is associated with CVDs and/or cardiovascular risk factors, including diabetes, hypertension, abnormal lipid metabolism, hypercholesterolemia, atherosclerosis, rheumatic diseases, smoking, and obesity.11 In addition, the number of circulating EPCs can be a risk biomarker for cardiovascular events, and, in combination with their functional ability, can be used as a marker to determine the severity of vascular sequelae in chronic conditions such as diabetes and atherosclerosis. Age and exercise also have a significant impact on the level of EPCs.
One of the functions of EPCs is to be incorporated into neovessels, contributing to neovascularization. Animal models have estimated that EPCs contribute anywhere between 2 and 25% of ECs in newly formed vessels. Transplantation of EPCs has the potential to be a promising approach in the therapy of diseases associated with deficit in angiogenesis, such as ischemic and chronic wounds and wounds associated with peripheral vascular diseases.36 The most prevalent forms of chronic wounds are leg ulcers caused by vascular insufficiency and foot ulcerations associated with diabetic complications. The role of EPCs in diabetic tissue repair has been an active area of investigation.37 Reduced levels and impaired function of EPCs, including impairments in mobilization, proliferation, integrin profile, adhesion to matrix molecules, incorporation into blood vessels, and differentiation, have been described in both type 1 and type 2 diabetic patients.38,39 Diabetic EPCs demonstrate impaired angiogenic paracrine secretory ability and impaired response to activating stimuli contributing to impaired EPC recruitment for re-endothelialization after vascular injury in diabetes.40 Several studies suggest that EPC numbers in diabetes may decrease because of increased apoptosis. The impairment in BM-derived EPCs in diabetes likely contributes to the pathogenesis of vascular complications as well as wound-healing mechanisms in both the BM and within the peripheral wound. Diabetic hyperglycemic conditions are associated with oxidative stress due to increased generation of reactive oxygen species, advanced glycation endproducts on the basement membrane, and reduced expression of growth factors such as VEGF, HIF-1, SDF-1, and nitric oxide signaling, which contributes to EPC dysfunction. Although systemic delivery of cytokines such as G-CSF and VEGF-A can induce the release of progenitor cells from the BM, the nonspecific effects on release of other white cells and platelets or the leaky-capillary effect make these factors unsuitable to treat diabetic patients with nonhealing chronic wounds. Additionally, single-factor therapy may not promote all phases of EPC biology for wound healing purposes, which may in part explain why clinical data as of yet have been discouraging.
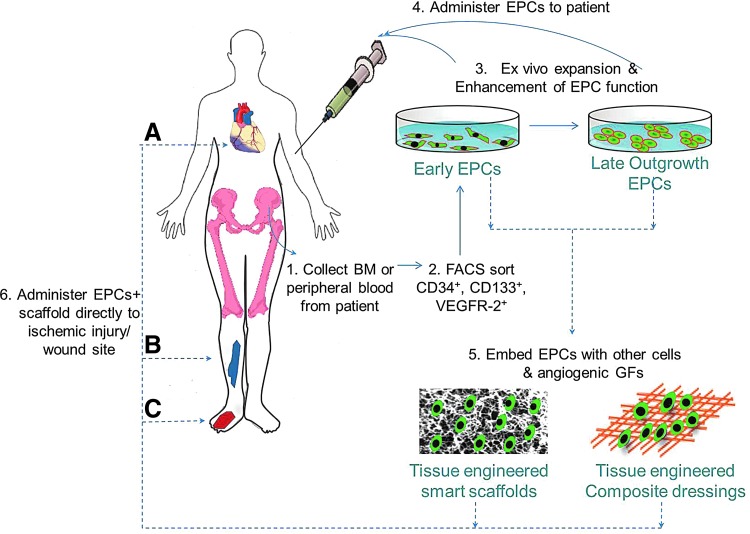
Clinical management of diabetic wound healing and complications relies mainly on wound management with dressings and pharmacological therapies that can improve endothelial repair and regeneration. However, very little to modest influence of these treatments on wound healing and end-organ function demonstrates a need for therapeutic interventions aimed to accelerate neovascularization and tissue formation. Using various in vitro and in vivo models, including transgenic and knockout animals, there is a clear demonstration that it is possible to modulate the wound healing progress with EPC treatment. EPC-based therapy with synergized augmentation of all the EPC functions in diabetic wounds may provide therapeutic benefit.41 EPCs can be used in different ways to improve therapeutic angiogenesis, by localized wound implantation, systemic transplantation, and/or use in tissue-engineered wound dressings. While direct injection of EPCs into circulation or into the injury site is a therapeutic option that is shown to be safe in animal models and clinical trials, the rate of incorporation, survival, and therapeutic efficacy were shown to be poor in various CVD settings. Concomitantly, local gene therapy to deliver vulnerary or pro-wound healing cytokines and growth factors has emerged as an alternative tool to ameliorate wound pathology and significantly enhance neovascularization and improve the wound healing outcome in chronic wounds as well as in myocardial ischemic remodeling. While both EPCs and gene therapy are powerful tools, neither treatments achieved the anticipated level of success in the clinical settings when administered by themselves. In this context, several groups hypothesized that combining the two approaches, where EPCs can be harvested from the patient, genetically modified ex vivo, and the corrected autologous cells can be reintroduced into the patient, might overcome the limitations of each individual treatment, to promote neovascularization and wound healing. Availability of these newer and novel techniques demonstrates a great future potential for therapeutic angiogenesis and regenerative medicine applications (Fig. 5).
Figure 5.
A proposed pathway for EPC contribution to ischemic and cutaneous wound healing and therapeutic angiogenesis. Therapeutic angiogenesis using EPCs can be harnessed by isolating these cells for patients, expanding them in vitro to an appropriate number, and enhancing their therapeutic potential using ex vivo genetic manipulations (gene, liposome, or growth factor therapy) and administering them back to the patient by systemic or local infusion at ischemic (A, B) or wound site (C). Alternately, EPCs can be embedded along with other cells or vulnerary growth factors in innovative tissue-engineered carrier matrices (scaffolds or dressings) that provide physiological, optimal conditions for EPC survival and endothelial outgrowth. Direct application of these EPC-infused scaffolds may further contribute to therapeutic angiogenesis for applications in wound healing and ischemia repair. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Clinical trials
Bench-to-bedside translational work has led to a number of phase I and II clinical trials involving EPC therapy for a variety of diseases.42 About 35% of registered clinical trials involving EPCs are performed in the United States (more information can be obtained at ClinicalTrials.gov). Several of these studies aimed to evaluate the effects of intrinsic disease states (including cardiomyopathy, hypertension, coronary artery disease, ischemia, stroke, liver cirrhosis, diabetes, oxidative stress, and obesity) and extrinsic factors such as food, exercise, and aging on EPC mobilization and function. The role of growth hormones, pharmacological drugs, including statins, ACE inhibitors, and cancer drugs on EPC mobilization and function is also being studied. There is a growing interest in using EPCs in wound repair, evidenced by an increasing number of trials recruiting patients for EPC transplantation in ischemia, stroke, and wound healing. In fact, a significant lab-to-clinic ascent of cell-based therapeutics has been aided by the establishment of consortiums consisting of thought leaders from academia, industry, the National Institutes of Health (NIH), and the U.S. Food and Drug Administration. However, there remain unanswered questions before realization of large-scale application of cell transplantation in patients. Multiple technical issues such as lack of randomization of the sample population, multivariates in the treatment, including dose, route, and timing of cell transplantation, and variable severity of clinical scenarios make it difficult to determine the role of EPCs as biomarkers for risk stratification, or the extent to which drugs and/or EPC therapy is efficacious. Nonetheless, EPC-based strategies will likely have great importance in the future therapeutic angiogenesis.
Therapeutic perspectives: novel strategies to enhance EPC numbers and function
Many of the recently described strategies to improve EPC function have focused on use of angiogenic growths factors and cytokines. It is now acknowledged that the hostile wound environment in settings, such as chronic wound healing and ischemic myocardial infarction characterized by hypoxia, increased inflammation, and increased free radicals, has an adverse effect on survival and function of mobilized/transplanted EPCs, thereby compromising their full therapeutic benefit.7 Modulation of wound inflammation by either systemic or local delivery of anti-inflammatory factors can confer improved stem cell survival and function, and improved clinical efficacy of EPCs.
In this context, several studies have demonstrated that administration of IL-10, a potent anti-inflammatory cytokine, significantly blunts inflammation and enhances tissue repair and remodeling after cutaneous wound healing and myocardial infarction.43–45 There is also growing evidence that IL-10 plays a role not only in immunoregulation and inhibition of proinflammatory cytokine synthesis but in directly regulating the growth and survival of noninflammatory cells as well. Several studies have demonstrated that BM mononuclear cells (BM-MNCs) as well as mesenchymal stem cells (MSCs) have the ability to immunoregulate and improve tissue repair through IL-10 secretion.46–48 BM-MNCs transplanted in the infarcted mouse hearts secreted significant amounts of IL-10, and the cardiac protection was associated with decreased T-lymphocyte accumulation, reactive hypertrophy, and myocardial collagen deposition.49 MSCs have demonstrated the ability to immunoregulate through multiple independent pathways, including inducing IL-10 secretion by macrophages.
Recent studies by Krishnamurthy et al. further demonstrate that IL-10 has a direct effect on EPC biology, function, and their angiogenic potential.50 They demonstrated that IL-10–deficient EPCs have diminished protection against inflammatory stimuli and hypoxia-induced cell death. Using IL-10 loss-of-function and gain-of-function studies in transgenic and BM transplant animal models, they demonstrated that IL-10 has a role on EPC mobilization from BM after myocardial injury, which appears to be mediated through the SDF-1/CXCR4 and STAT-3/VEGF signaling mechanisms. IL-10–deficient EPCs have reduced functional expression of CXCR4 and are associated with reduced SDF-1-induced EPC migration in vitro. To demonstrate the role that IL-10 can play in improving EPC function and associated tissue repair outcome, coadministration of IL-10 enhances the retention and survival of the intramyocardially transplanted EPCs associated with improved neovascularization and left ventricular function, as opposed to transplantation of EPCs alone after myocardial infarction. It is suggested that by protecting the EPCs in a harsh microenvironment, IL-10 increases the retention and numeric availability of these cells to participate in wound healing. Together, these data suggest a novel angiogenic role for IL-10, a well-known immunoregulator, in improving EPC-mediated therapeutic angiogenesis in wound healing.
Take-Home Messages.
Basic science advances
Postnatal vasculogenesis is mediated by EPCs, progenitors to vascular ECs that exist in adult life.
EPCs normally reside in the vascular niche of the BM, with a very low percentage (few thousand cells/mL blood) in circulation in healthy subjects.
Under the guidance of hypoxia, growth factors, and chemoattractant signaling, EPCs mobilize from the BM into the circulation and home to the tissue repair sites to contribute to neovascularization and healing, mediated by their surface integrins.
Clinical science advances
Clinical trials utilizing EPCs for ischemic and wound treatment have been favorable. Because these trials are primarily therapeutic innovations, they do not provide mechanistic information of how EPCs promote wound healing.
There is a need to develop in vitro culture methods and readouts to positively predict the effects of EPCs in vivo.
Relevance to clinical care
Major advantages of EPC-based cellular therapy are as follows:
These cells can be isolated from a patient and amenable to autologous transplantation or developing tissue-engineered matrices to cover the wounds, without the risk of infection associated with the introduction of foreign bodies or rejection of allografted cells.
Using a high-throughput technology, a single isolation can be a lifetime repository of cells for the patient.
These cells can also be genetically modified to overexpress vulnerary transgenes that can potentially augment the wound milieu and redirect chronic wound toward a regenerative wound-healing progression.
There are diverse possibilities for future research, including development of sophisticated delivery systems for EPC therapy and growth factors.
Conclusions and Future Developments of Interest
Research into angiogenesis was revolutionized by the discovery of circulating EPCs, and the potential of this newly identified cell population in wound healing, regenerative medicine and cancer therapy soon became apparent. After many years of preclinical research, the first clinical studies have been initiated to assess the potential of these cells to repair tissues after ischemic injury, wound healing, and cancer. The future research challenge is to identify and evaluate the methods that increase EPC homing and incorporation. This will enable the targeted delivery of EPCs to the site of interest. Continued characterization of EPCs both in vitro and in vivo combined with recent advances in nanotechnology and tissue engineering51 for local delivery of cells, growth factors, or DNA that encodes vulnerary growth factors will enable the clinical use of EPCs for therapeutic angiogenesis and wound healing.
Acknowledgments and Funding Sources
This work was supported by grants from The National Institutes of General Medical Sciences K08 GM098831-01 and the Wound Healing Society Foundation 3M Award to S.G.K.
Author Disclosure and Ghostwriting
There are no competing financial interests for any author. The content of this article is expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Swathi Balaji received her PhD in Biomedical Engineering from the University of Cincinnati and is now a postdoctoral research fellow at Cincinnati Children's working in the laboratory of Dr. Sundeep Keswani. She specializes in understanding the mechanisms underlying fetal regenerative wound healing. Alice King is a University of Cincinnati general surgery resident, who is completing a 2 year postdoctoral research fellowship in the Keswani Laboratory. Timothy M. Crombleholme is surgeon-in-chief at Children's Hospital Colorado and has had extensive NIH funding to study the role of EPCs in wound healing and neovascularization. Sundeep G. Keswani is a pediatric and fetal surgeon at Cincinnati Children's Hospital Medical Center and is the Principle Investigator of the wound healing research laboratory, which focuses on elucidating the mechanisms underlying the fetal scarless wound healing and the BM contribution to wound repair. He is also the director of Pediatric Wound Care Center at Cincinnati Children's Hospital.
Abbreviations and Acronyms
- bFGF
basic fibroblast growth factor
- BM
bone marrow
- BM-MNCs
BM mononuclear cells
- CVD
cardiovascular disease
- CXCR4
CXC chemokine receptor type 4
- ECs
endothelial cells
- ECFCs
endothelial colony-forming cells
- ECM
extracellular matrix
- EPCs
endothelial progenitor cells
- G-CSF
granulocyte colony-stimulating factor
- HSCs
hematopoietic stem cells
- IL
interleukin
- MCP-1
monocyte chemotactic protein 1
- MMPs
matrix metalloproteinases
- MSCs
mesenchymal stem cells
- NIH
National Institutes of Health
- SDF-1
stromal cell-derived factor 1
- VCAM-I
vascular cell adhesion molecule–I
- VEGF
vascular endothelial growth factor
- VEGFR-2
vascular endothelial growth factor receptor 2
References
- 1.Ko SH. Nauta A. Wong V. Glotzbach J. Gurtner GC. Longaker MT. The role of stem cells in cutaneous wound healing: what do we really know? Plast Reconstr Surg. 2011;127(Suppl 1):10S. doi: 10.1097/PRS.0b013e3181fbe2d8. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T. Masuda H. Takahashi T. Kalka C. Pastore C. Silver M. Kearne M. Magner M. Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 3.Kalka C. Masuda H. Takahashi T. Kalka-Moll WM. Silver M. Kearney M. Li T. Isner JM. Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Real C. Caiado F. Dias S. Endothelial progenitors in vascular repair and angiogenesis: how many are needed and what to do? Cardiovasc Hematol Disord Drug Targets. 2008;8:185. doi: 10.2174/187152908785849071. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Ribatti D. Vacca A. Nico B. Roncali L. Dammacco F. Postnatal vasculogenesis. Mech Dev. 2001;100:157. doi: 10.1016/s0925-4773(00)00522-0. [DOI] [PubMed] [Google Scholar]
- 7.Roncalli JG. Tongers J. Renault MA. Losordo DW. Endothelial progenitor cells in regenerative medicine and cancer: a decade of research. Trends Biotechnol. 2008;26:276. doi: 10.1016/j.tibtech.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Zammaretti P. Zisch AH. Adult “endothelial progenitor cells.” Renewing vasculature. Int J Biochem Cell Biol. 2005;37:493. doi: 10.1016/j.biocel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Peng LH. Tsang SY. Tabata Y. Gao JQ. Genetically-manipulated adult stem cells as therapeutic agents and gene delivery vehicle for wound repair and regeneration. J Control Release. 2012;157:321. doi: 10.1016/j.jconrel.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Smadja DM. Cornet A. Emmerich J. Aiach M. Gaussem P. Endothelial progenitor cells: characterization, in vitro expansion, and prospects for autologous cell therapy. Cell Biol Toxicol. 2007;23:223. doi: 10.1007/s10565-007-0177-6. [DOI] [PubMed] [Google Scholar]
- 11.Urbich C. Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 12.Fadini GP. Losordo D. Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balaji S. Vaikunth SS. Lang SA. Sheikh AQ. Lim FY. Crombleholme TM. Narmoneva DA. Tissue-engineered provisional matrix as a novel approach to enhance diabetic wound healing. Wound Repair Regen. 2012;20:15. doi: 10.1111/j.1524-475X.2011.00750.x. [DOI] [PubMed] [Google Scholar]
- 14.Schatteman GC. Dunnwald M. Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol. 2007;292 doi: 10.1152/ajpheart.00662.2006. H1. [DOI] [PubMed] [Google Scholar]
- 15.Peichev M. Naiyer AJ. Pereira D. Zhu Z. Lane WJ. Williams M. Oz MC. Hicklin DJ. Witte L. Moore MA. Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952. [PubMed] [Google Scholar]
- 16.Tian F. Liang PH. Li LY. Inhibition of endothelial progenitor cell differentiation by VEGI. Blood. 2009;113:5352. doi: 10.1182/blood-2008-08-173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aicher A. Heeschen C. Mildner-Rihm C. Urbich C. Ihling C. Technau-Ihling K. Zeiher AM. Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 18.De Falco E. Porcelli D. Torella AR. Straino S. Iachininoto MG. Orlandi A. Truffa S. Biglioli P. Napolitano M. Capogrossi MC. Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 19.Dimmeler S. Fleming I. Fisslthaler B. Hermann C. Busse R. Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 20.Heissig B. Hattori K. Dias S. Friedrich M. Ferris B. Hackett NR. Crystal RG. Besmer P. Lyden D. Moore MA. Werb Z. Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida Y. Kimura A. Kuninaka Y. Inui M. Matsushima K. Mukaida N. Kondo T. Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J Clin Invest. 2012;122:711. doi: 10.1172/JCI43027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caiado F. Real C. Carvalho T. Dias S. Notch pathway modulation on bone marrow-derived vascular precursor cells regulates their angiogenic and wound healing potential. PLoS One. 2008;3:e3752. doi: 10.1371/journal.pone.0003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caiado F. Dias S. Endothelial progenitor cells and integrins: adhesive needs. Fibrogenesis Tissue Repair. 2012;5:4. doi: 10.1186/1755-1536-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin G. Ii M. Silver M. Wecker A. Bord E. Ma H. Gavin M. Goukassian DA. Yoon YS. Papayannopoulou T. Asahara T. Kearney M. Thorne T. Curry C. Eaton L. Heyd L. Dinesh D. Kishore R. Zhu Y. Losordo DW. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;203:153. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chavakis E. Aicher A. Heeschen C. Sasaki K. Kaiser R. El Makhfi N. Urbich C. Peters T. Scharffetter-Kochanek K. Zeiher AM. Chavakis T. Dimmeler S. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouvard C. Gafsou B. Dizier B. Galy-Fauroux I. Lokajczyk A. Boisson-Vidal C. Fischer AM. Helley D. Alpha6-integrin subunit plays a major role in the proangiogenic properties of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1569. doi: 10.1161/ATVBAHA.110.209163. [DOI] [PubMed] [Google Scholar]
- 28.Ehrbar M. Metters A. Zammaretti P. Hubbell JA. Zisch AH. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J Control Release: official journal of the Controlled Release Society. 2005;101:93. doi: 10.1016/j.jconrel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Smadja DM. Bieche I. Helley D. Laurendeau I. Simonin G. Muller L. Aiach M. Gaussem P. Increased VEGFR2 expression during human late endothelial progenitor cells expansion enhances in vitro angiogenesis with up-regulation of integrin alpha(6) J Cell Mol Med. 2007;11:1149. doi: 10.1111/j.1582-4934.2007.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho H. Balaji S. Sheikh AQ. Hurley JR. Tian YF. Collier JH. Crombleholme TM. Narmoneva DA. Regulation of endothelial cell activation and angiogenesis by injectable peptide nanofibers. Acta Biomater. 2012;8:154. doi: 10.1016/j.actbio.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang SJ. Zhang H. Wei YJ. Su WJ. Liao ZK. Hou M. Zhou JY. Hu SS. Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res. 2006;16:577. doi: 10.1038/sj.cr.7310075. [DOI] [PubMed] [Google Scholar]
- 32.Yoder MC. Mead LE. Prater D. Krier TR. Mroueh KN. Li F. Krasich R. Temm CJ. Prchal JT. Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulati R. Jevremovic D. Peterson TE. Chatterjee S. Shah V. Vile RG. Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 34.Badorff C. Brandes RP. Popp R. Rupp S. Urbich C. Aicher A. Fleming I. Busse R. Zeiher AM. Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 35.Roncalli J. Tongers J. Renault MA. Losordo DW. Biological approaches to ischemic tissue repair: gene- and cell-based strategies. Expert Rev Cardiovasc Ther. 2008;6:653. doi: 10.1586/14779072.6.5.653. [DOI] [PubMed] [Google Scholar]
- 36.Suh W. Kim KL. Kim JM. Shin IS. Lee YS. Lee JY. Jang HS. Lee JS. Byun J. Choi JH. Jeon ES. Kim DK. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells. 2005;23:1571. doi: 10.1634/stemcells.2004-0340. [DOI] [PubMed] [Google Scholar]
- 37.Georgescu A. Alexandru N. Constantinescu A. Titorencu I. Popov D. The promise of EPC-based therapies on vascular dysfunction in diabetes. Eur J Pharmacol. 2011;669:1. doi: 10.1016/j.ejphar.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 38.Capla JM. Grogan RH. Callaghan MJ. Galiano RD. Tepper OM. Ceradini DJ. Gurtner GC. Diabetes impairs endothelial progenitor cell-mediated blood vessel formation in response to hypoxia. Plast Reconstr Surg. 2007;119:59. doi: 10.1097/01.prs.0000244830.16906.3f. [DOI] [PubMed] [Google Scholar]
- 39.Kim KA. Shin YJ. Kim JH. Lee H. Noh SY. Jang SH. Bae ON. Dysfunction of endothelial progenitor cells under diabetic conditions and its underlying mechanisms. Arch Pharm Res. 2012;35:223. doi: 10.1007/s12272-012-0203-y. [DOI] [PubMed] [Google Scholar]
- 40.Callaghan MJ. Ceradini DJ. Gurtner GC. Hyperglycemia-induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxid Redox Signal. 2005;7:1476. doi: 10.1089/ars.2005.7.1476. [DOI] [PubMed] [Google Scholar]
- 41.Madonna R. De Caterina R. Cellular and molecular mechanisms of vascular injury in diabetes—part II: cellular mechanisms and therapeutic targets. Vascul Pharmacol. 2011;54:75. doi: 10.1016/j.vph.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Rossig L. Hermann C. Haendeler J. Assmus B. Zeiher AM. Dimmeler S. Angiotensin II-induced upregulation of MAP kinase phosphatase-3 mRNA levels mediates endothelial cell apoptosis. Basic Res Cardiol. 2002;97:1. doi: 10.1007/s395-002-8381-2. [DOI] [PubMed] [Google Scholar]
- 43.Burchfield JS. Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1:4. doi: 10.1186/1755-1536-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon A. Kozin ED. Keswani SG. Vaikunth SS. Katz AB. Zoltick PW. Favata M. Radu AP. Soslowsky LJ. Herlyn M. Crombleholme TM. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen. 2008;16:70. doi: 10.1111/j.1524-475X.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 45.Krishnamurthy P. Rajasingh J. Lambers E. Qin G. Losordo DW. Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res. 2009;104:e9. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jui HY. Lin CH. Hsu WT. Liu YR. Hsu RB. Chiang BL. Tseng WY. Chen MF. Wu KK. Lee CM. Autologous mesenchymal stem cells prevent transplant arteriosclerosis by enhancing local expression of interleukin-10, interferon-gamma, and indoleamine 2,3-dioxygenase. Cell Transplant. 2012;21:971. doi: 10.3727/096368911X627525. [DOI] [PubMed] [Google Scholar]
- 47.Kawamoto A. Iwasaki H. Kusano K. Murayama T. Oyamada A. Silver M. Hulbert C. Gavin M. Hanley A. Ma H. Kearney M. Zak V. Asahara T. Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 48.Zhao W. Li JJ. Cao DY. Li X. Zhang LY. He Y. Yue SQ. Wang DS. Dou KF. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol. 2012;18:1048. doi: 10.3748/wjg.v18.i10.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burchfield JS. Iwasaki M. Koyanagi M. Urbich C. Rosenthal N. Zeiher AM. Dimmeler S. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ Res. 2008;103:203. doi: 10.1161/CIRCRESAHA.108.178475. [DOI] [PubMed] [Google Scholar]
- 50.Krishnamurthy P. Thal M. Verma S. Hoxha E. Lambers E. Ramirez V. Qin G. Losordo D. Kishore R. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ Res. 2011;109:1280. doi: 10.1161/CIRCRESAHA.111.248369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang EI. Bonillas RG. El-ftesi S. Ceradini DJ. Vial IN. Chan DA. Michaels JT. Gurtner GC. Tissue engineering using autologous microcirculatory beds as vascularized bioscaffolds. FASEB J. 2009;23:906. doi: 10.1096/fj.08-114868. [DOI] [PMC free article] [PubMed] [Google Scholar]