Abstract
Phase II detoxification enzymes are known to play essential roles in the detoxification and elimination of activated carcinogens during tumor initiation, while apoptosis is one of the most important chemopreventive targets for inhibiting tumor promotion in cancer. In this study, we investigated the cancer chemopreventive activity of two plant extracts, the ethanolic extract of Adenocaulon himalaicum (AHE) and the butanolic fraction of AHE (AHB). Both, the AHE and AHB induced NQO1 activity and had relatively high chemoprevention indices (CI=12.4). The AHE and AHB were associated with increased NQO1 and HO-1 mRNA levels via Nrf2-ARE pathway activation. In addition, the AHB increased CYP1A1 activity through AhR-XRE pathway activation. We also found that the AHE and AHB induced apoptosis, as evidenced by phosphatidylserine externalization, an increase in the sub-G0/G1 content, chromatin condensation, poly(ADP-ribose) polymerase cleavage, and p53 induction. These data suggest that AHE and AHB act as bifunctional inducers and that their chemopreventive effects result from the biphasic induction of phase II detoxification enzymes and apoptosis. Therefore, these results suggest that A. himalaicum plant extracts have potential for use as chemopreventive agents for the prevention and/or treatment of human cancers.
Key Words: Adenocaulon himalaicum, apoptosis, colon cancer, heme oxygenase, Nrf2, quinone reductase
Introduction
Carcinogenesis is an extremely complicated multistep process.1 Environmental exposure to carcinogens is an important risk factor during the early stages of carcinogenesis. Enhancing the body's carcinogen-detoxifying capability is a promising approach for cancer prevention.2 Some chemicals are capable of inhibiting the metabolic activation of procarcinogens to their ultimate electrophilic species or inhibiting their subsequent interactions with DNA. These agents are known as “cancer-blocking agents” because they stimulate the detoxification of carcinogens during the initiation of carcinogenesis.3 Other chemicals, known as “cancer-suppressing agents,” suppress the promotion and progression of carcinogenesis by inducing cell cycle arrest and apoptosis, thereby eliminating preneoplastic cells.4
Sulforaphane, found in cruciferous vegetables, is a well-known phytochemical that prevents chemical carcinogenesis by inducing phase II enzymes, including glutathione S-transferases (GSTs; EC 2.5.1.18) and quinone reductase (QR, also known as NAD(P)H:quinone oxidoreductase, NQO1; EC 1.6.5.2).5 Because phase I enzymes are involved in both bioactivation and detoxification of carcinogens, monofunctional inducers, which upregulate only phase II enzymes, are thought to be more closely related to chemoprevention than bifunctional inducers, which upregulate both phase I and II enzymes. However, bifunctional inducers have been shown to exert synergistic effects during cancer chemoprevention. Oltipraz, a plant-derived compound, is a promising bifunctional inducer that stimulates both phase II enzymes and phase I enzymes, such as cytochrome P450 (CYP).6 Oltipraz is now undergoing clinical trials for use as a cancer chemopreventive agent. Its underlying mechanism of action is as follows: the aryl hydrocarbon receptor (AhR), which can be induced by bifunctional inducers such as Oltipraz, heterodimerizes with the aryl hydrocarbon receptor nuclear translocator (Arnt) and activates the Nrf2 gene by binding to the xenobiotic response element (XRE) in the promoter region of Nrf2.7
Apoptosis is characterized by marked changes in cellular morphology, including chromatin condensation, membrane blebbing, the appearance of membrane-associated apoptotic bodies, internucleosomal DNA fragmentation, and poly (ADP-ribose) polymerase (PARP) cleavage.8 The p53 tumor suppressor protein plays a key role in apoptosis and cell cycle arrest. Alterations in the p53 gene have been associated with carcinogenesis in rodent models.9 A recent investigation into the relationship between apoptosis and Nrf2 in the N-nitrosobutyl (4-hydroxybutyl)-amine (BBN)–induced urinary bladder carcinogenesis model suggested that p53 and Nrf2, and their respective gene batteries, cooperatively contribute to cancer prevention.10
Adenocaulon himalaicum (Asteraceae), a perennial herb, is mainly found in Southeast Asia. Its aerial parts have been used to treat abscesses, hemorrhage, and inflammation in Korean folk medicine,11 and the sprouts of A. himalaicum are edible. However, its cancer preventive effects have not yet been reported. In this study, we have examined the cancer chemopreventive effects of an ethanolic extract of A. himalaicum (AHE) and its butanolic fraction (AHB) by investigating known anticancer mechanisms, including the induction of detoxification enzymes during the initiation stage and the induction of apoptosis during the promotion of carcinogenesis.
Materials and Methods
Plant materials
Whole A. himalaicum plants were collected at the Wild Vegetable Experiment Station, Gangwon ARES, Korea, and a voucher specimen (D-039) was stored at the Functional Food Center, KIST Gangneung Institute, Gangneung, Korea.
Extraction, isolation, and identification of the AHE
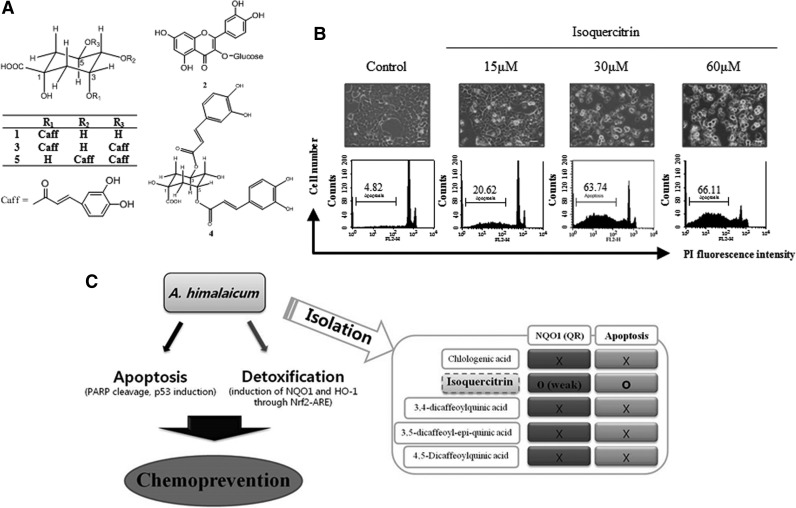
The air-dried whole plant of A. himalaicum (1.2 kg) was extracted with 94% EtOH 4 times (4×19.0 L) under reflux for 4 h and evaporated in vacuo to yield the total extract (149.36 g). This extract was then suspended in distilled water and sequentially partitioned with n-hexane, ethyl acetate, and n-butanol. The n-butanol fraction (19.06 g) was chromatographed on a Sephadex LH-20 column and eluted using MeOH. Ten fractions (fractions 1–10) were obtained. Fraction 4 (383.3 mg) was further chromatographed over silica gel using a stepwise gradient of CH2Cl2-MeOH-Water (5:1:0.1 to 2:1:0.1) and yielded compounds 2 (1.7 mg), 3 (6.7 mg) and 5 (4.1 mg). Further purification of fraction 7 (268.6 mg) by repeated Sephadex LH-20 column chromatography with EtOH yielded compounds 1 (8.9 mg) and 4 (13.6 mg). These compounds were identified using NMR spectroscopy. The chemical structures are shown in Fig. 7A. The analytical HPLC system was equipped with an Agilent Series 1200 liquid chromatography and YMC Hydrosphere C-18 column (4.6 mm×150 mm, 3 μm, Kyoto, Japan).
FIG. 7.
Isoquercitrin, a major active compound of the AHB that was isolated from the AHE, induces apoptosis in HCT116 cells. (A) Chemical structures of the compounds that were isolated from the AHB. (B) HCT116 cells were treated with isoquercitrin (15, 30, and 60 μM) for 48 h. Cellular morphology was observed using a phase contrast microscope (above) and the sub G0/G1 content (%) was evaluated via flow cytometric DNA content analysis using the PI labeling method (below; bar=20 μm). (C) Schematic diagram illustrating the elucidation of the bifunctional chemopreventive effects of an A. himalaicum extract and the compounds that were isolated from it.
Cell culture
HCT116 cells, Hepa1c1c7 cells and BPrc1 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). These cells were maintained at subconfluence in 95% air and 5% CO2 in a humidified atmosphere at 37°C. Minimum essential medium (MEM; Hyclone, Logan, UT, USA) was used for HCT116 cell cultivation and alpha–minimum essential medium (α-MEM; Hyclone) was used for Hepa1c1c7 and BPrc1 cell cultivation. The media were supplemented with 10% fetal bovine serum (FBS; Hyclone), penicillin (100 units/mL), and streptomycin (100 μg/mL).
Cell viability assay
The cytotoxicities of the AHE and its fractions were evaluated using a Cell Counting Kit (CCK-8; Dojindo Laboratories, Tokyo, Japan). In brief, the cells (5×103 cells/well) were plated on 96-well plates, incubated at 37°C for 24 h, and then given fresh medium. The cells were then treated with various concentrations of the AHE and its fractions and incubated at 37°C for an additional 24 h. Then, 10 μL of CCK-8 solution was added to 100 μL of media in the wells and incubated for another hour. The absorbance at 450 nm was measured and the absorbance at 600 nm was subtracted using a Synergy HT Multi-microplate Reader (Bio-Tek Instruments, Winooski, VT, USA). The data were reported as the percentage of cell growth relative to the respective controls (cells treated with solvent only) for each sample concentration.
QR activity assay
Induction of QR activity was determined using the Prochaska modified bioassay, with slight modifications.12,13 Hepa1c1c7 cells (5×103 cells/well) were plated on 96-well plates and incubated for 24 h prior to treatment. Sulforaphane (Calbiochem, San Diego, CA, USA) was used as a positive control. The absorbance at 610 nm was measured five times at 50 s intervals using a Synergy HT Multi-microplate Reader (Bio-Tek Instruments). Induction of QR activity was calculated by comparing the specific QR activity of the cells that had been treated with the extracts or fractions with the QR activity of vehicle-treated cells. Enzymatic activity was expressed as the concentration required to double QR activity (CD). The concentration resulting in a 50% inhibition of cell viability (chemoprevention index [CI]) was obtained by dividing the half-maximal cell growth inhibitory concentration (IC50) values by the CD values.
EROD activity assay
The 7-ethoxyresorufin-O-deethylase (EROD) activity was determined as a measure of CYP1A1 activity. The EROD activity was determined as previously described, with minor modifications.14 Hepa1c1c7 cells (10×104 cells/mL) were plated onto 24-well tissue culture plates and incubated for 24 h prior to treatment. The cells were then treated with various concentrations of the AHE or AHB and incubated for an additional 24 h. Fluorescence was measured using a Synergy HT Multi-microplate Reader at an excitation wavelength of 550 nm and an emission wavelength of 585 nm. Protein concentrations were determined according to the Bradford method using the Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA, USA). The fluorescence values were converted to picomoles using a calibration curve of resorufin fluorescence, and the specific activity was calculated as pmol of resorufin per mg of total protein per min.
Transient transfection and reporter gene assays
HCT116 cells (1×105 cells/mL) were cultured in 24-well tissue culture plates for 24 h and reached 40 to 50% confluence prior to transfection. The cells were transiently co-transfected with 0.25 μg of ARE-QR-CAT or XRE-QR-CAT reporter constructs containing either an antioxidant response element (ARE) or a XRE that had been derived from the rat QR gene15 and 0.02 μg of the pGL3-β-gal Vector using the Fugene 6 transfection reagent (Roche, Indianapolis, IN, USA) according to the manufacturer's recommended instructions. The pGL3-β-gal reporter plasmids were a gift from Dr. Son (Kangung-Wonju National University, Gangneung, Korea). After 24 h of treatment, the cells were lysed and assayed for CAT expression using a CAT-ELISA kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. CAT expression was normalized to β-galactosidase activity and the results are presented as fold induction over the control.
Total RNA extraction and real-time PCR analysis
Total RNA was extracted from the treated and untreated cell lines using RNeasy Mini Kit Columns (Qiagen, Valencia, CA, USA) according to the manufacturer's recommended protocol. The cDNA was prepared using random primers (Invitrogen, Carlsbad, CA, USA) and 200 U of SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's protocol. Quantitative real-time PCR was performed in triplicate in 384-well plates as previously described.13 Real-time PCR analysis was performed using an LC480 Detection System (Roche).
Flow cytometric cell cycle analysis
Cell cycle progression was evaluated using flow cytometric analysis, and the DNA content was measured using the propidium iodide (PI) labeling method, as previously described.16 To quantitate apoptotic cell death, we measured the externalization of phosphatidylserine, a specific phospholipid marker for apoptosis, which can be detected using flow cytometric analysis, as previously described.16 HCT116 cells (5×105 cells/dish) were seeded on 60 mm dishes and incubated at 37°C for 24 h and were then treated with each sample for 24 or 48 h. The data were obtained using Cell Quest Pro software.
Chromatin condensation
Chromatin condensation was observed using the Hoechst33342 staining method. HCT116 (3×105 cells/well) cells were plated on six-well plates, incubated at 37°C for 24 h, and treated with the AHE or AHB for 48 h. The cells were then treated with 20 μg/mL of Hoechst 33342 at 37°C for 15 min. After staining, both the detached and adherent cells were collected by trypsinization and washed with DPBS. The stained cells were visualized using a fluorescent microscope (Nikon TE2000U, Nikon, Kanagawa, Japan).
Western blot analysis
To prepare the nuclear and cytoplasmic fractions, the cells were treated with the AHE or AHB for 4 h, and the nuclear proteins were extracted using a Nuclear Extraction Kit (Sigma Chemical, St. Louis, MO, USA) according to the manufacturer's instructions. To prepare the total lysates, the cells were treated with either the AHE or AHB and then lysed and analyzed using Western blotting, as previously described.13
Immunofluorescence assay
HCT116 cells were grown on glass coverslips in 24-well plates. The cells were treated with either the AHE or AHB for 4 h, fixed with 4% paraformaldehyde at room temperature for 20 min, fixed with 100% methanol at −20°C for 15 min, and blocked with phosphate-buffered saline (PBS) containing 5% (v/v) goat serum and 0.3% Triton X-100 for 1 h. The cells were then incubated with the primary antibody at a 1:100 dilution in PBS containing 5% (v/v) goat serum and 0.3% Triton X-100 at 4°C overnight. The cells were washed with PBS and incubated with Alexa Fluor 488-conjugated anti-rabbit and Alexa Fluor 594-conjugated anti-goat secondary antibodies (Invitrogen) at 1:200 dilutions for another hour. The cells were then washed and mounted on glass slides with VECTASHIELD Mounting Medium and DAPI (Vector Laboratories, Inc., Cambridgeshire, United Kingdom). Images were obtained using a Leica TCS SP5 Confocal System (Leica, Wetzlar, Germany).
Statistics
Statistical analyses were performed using one-way ANOVA followed by either Dunnett's test or Bonferronis's test for multiple comparisons using GraphPad Prism 4 software (GraphPad Software, Inc., La Jolla, CA, USA). Differences were considered to be statistically significant at P<.05.
Results
The AHE and AHB increase phase II detoxification enzymes, QR and HO-1
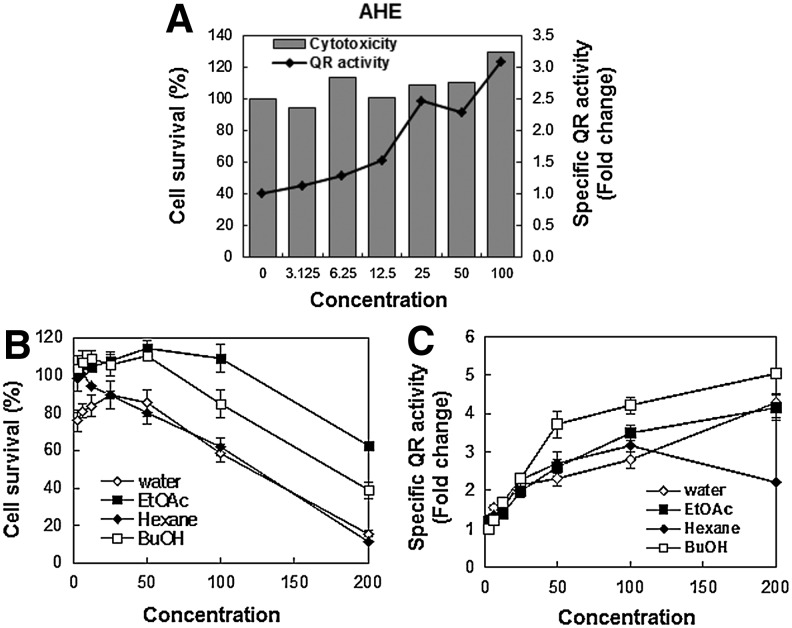
QR activity was measured in an effort to investigate the potential chemopreventive effects of the AHE using mouse hepatocarcinoma Hepa1c1c7 cells as a model cell line. Treatment with the AHE induced QR activity in a dose-dependent manner (Fig. 1A) and produced a doubling of QR activity (CD) at a concentration of 19.1 μg/mL with low cytotoxicity (IC50=197.7 μg/mL), resulting in a relatively high CI value (10.4; Table 1). Because treatment with the AHE stimulated high QR activity, we subjected the AHE to further fractionation with different organic solvents, which resulted in four fractions: n-hexane, ethyl acetate, n-butanol, and water. These four fractions also increased the QR activity (Fig. 1C) to varying degrees. The AHB displayed the highest CI value (12.4) of the four fractions, with an IC50 of 177.7 μg/mL and a CD value of 14.3 μg/mL (Table 1). Thus, the AHB was chosen for further investigation of the mechanisms governing the cancer chemopreventive effects.
FIG. 1.
NQO1 activity is induced by the AHE and its fractions in Hepa1c1c7 cells. (A) Measurement of AHE-induced QR activity in Hepa1c1c7 cells. The cells were treated with the indicated concentrations of the AHE (0–100 μg/mL) for 24 h. The cell survival ratio (%) (B) and specific QR activity (C) of the four AHE fractions in Hepa1c1c7 cells. Each value represents the mean±SD from three independent experiments. NQO1, NAD(P)H dehydrogenase (quinone 1); AHE, ethanolic extract of Adenocaulon himalaicum; QR, quinone reductase; SD, standard deviation.
Table 1.
Indicators of the Effects of Ethanolic Extract of Adenocaulon himalaicum and Its Fractions in Hepa1c1c7 and HCT116 Cells
| |
Hepa1c1c7 |
HCT116 |
||
|---|---|---|---|---|
| CD (μg/mL) | IC50 (μg/mL) | CI | IC50 (μg/mL) | |
| AHE | 19.1 | 197.7 | 10.4 | 151.7 |
| Hexane fraction | 17.5 | 102.2 | 5.8 | 73.2 |
| Ethyl acetate fraction | 24.7 | >200.0a | 8.1 | >200.0a |
| AHB | 14.3 | 177.0 | 12.4 | 134.5 |
| Water fraction | 27.7 | 106.6 | 3.8 | 136.7 |
| Sulforaphane (μM) | 1.17 | 14.96 | 12.8 | |
The highest limit of test concentration was 200 μg/mL. If the extract had no cytotoxicity, 200 μg/mL was used to calculate the CI value.
CD, concentration required to double QR activity; IC50 concentration required to inhibit cell growth by 50%; CI (chemoprevention index)=IC50/CD; AHE, ethanolic extract of Adenocaulon himalaicum; AHB, butanolic fraction of AHE; QR, quinone reductase.
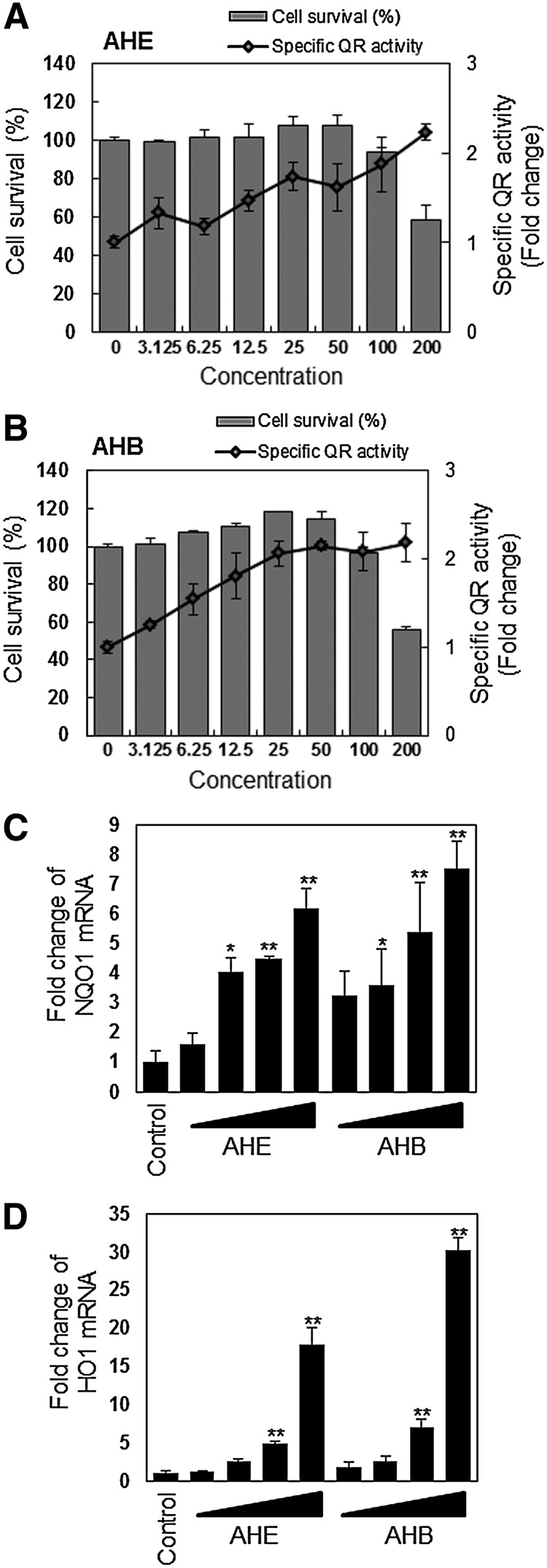
To determine whether the AHE and AHB could affect NQO1 (which is known as a QR gene) expression in HCT116 cells, we treated the cells for 24 h with various concentrations of the AHE or AHB. We observed a marked dose-dependent induction of NQO1 activity and mRNA expression (Fig. 2A–C). HO-1, another detoxification enzyme, was also dose-dependently increased following treatment with both fractions, although treatment with the AHB produced a stronger induction of both NQO1 and HO-1 mRNA expression compared to treatment with the AHE (Fig. 2D). These results suggest that AHE and AHB induce the transcription and translation of detoxification enzymes in human colorectal cancer cells and that this induction may occur as a result of bioactive components of the AHE and AHB.
FIG. 2.
NQO1 and HO-1 are induced by the AHE and its fractions in HCT116 cells. (A) Measurement of AHE-induced QR activity in HCT116 cells. The cells were treated with the indicated concentrations of the AHE (0–200 μg/mL) for 24 h. (B) Measurement of butanolic fraction of AHE (AHB)–induced QR activity in HCT116 cells. The cells were treated with the indicated concentrations of the AHB (0–200 μg/mL) for 24 h. Each value represents the mean±SD from three independent experiments. NQO1 (C) and HO-1 mRNA expression (D) in response to treatment with the AHE or AHB in HCT116 cells. The cells were treated with 25, 50, 100, and 200 μg/mL of the AHE or AHB for 24 h. The results represent the mean±SD; n=3. Asterisks denote significant differences compared to the control (*P<.05; **P<.01).
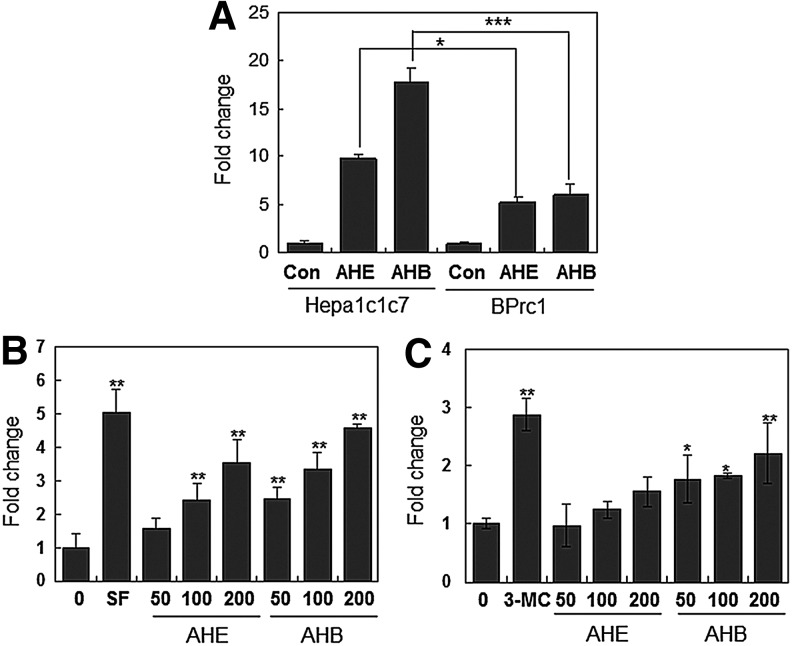
The AHE and AHB induce ARE and XRE activation
The majority of the genes encoding phase II detoxification and antioxidant enzymes contain two essential cis-acting elements, known as the ARE and XRE, in their promoter regions.15,17 To determine whether the induction of NQO1 by the A. himalaicum extracts was mediated via the ARE and/or XRE motifs, we examined Nqo1 mRNA levels in two different mouse hepatocarcinoma cell lines: Hepa1c1c7 (wild-type) and BPrc1 (Arnt-deficient). Treatment with the AHE and AHB significantly increased Nqo1 mRNA levels in Hepa1c1c7 cells. However, Nqo1 mRNA levels in BPrc1 cells were markedly decreased after treatment (Fig. 3A), suggesting that full induction of Nqo1 mRNA by the AHE and AHB requires a functional XRE system. To verify whether AHE- and/or AHB-inducible expression of NQO1 was mediated by both the ARE and XRE motifs, we performed reporter gene analysis using a construct containing the ARE or XRE core sequences from the upstream region of the rat Nqo1 gene. We found that treatment with the AHE and AHB induced activation of the reporter gene via the ARE and XRE in a dose-dependent manner (Fig. 3B, C). Although the AHE and AHB activated both the ARE and XRE motifs, they had a much stronger effect on the ARE than on the XRE, and showed a difference of approximately 25-fold in activation over the control.
FIG. 3.
Treatment with the AHE and the AHB induces ARE and XRE activation. (A) Real-time PCR analysis of AHE- or AHB-induced Nqo1 mRNA expression in Hepa1c1c7 and BPrc1 cells. The cells were treated with 50 μg/mL of the AHE or AHB for 48 h. The data are expressed as the mean±SD (n=3). Asterisks indicate significant differences compared to the vehicle-treated group (*P<.05; ***P<.001). (B, C) Activation of the ARE (B) and XRE (C) by the AHE or AHB was measured using an ARE- or XRE-containing reporter construct. The cells were treated with the indicated concentrations (50, 100, and 200 μg/mL) of the AHE for 24 h. A 5 μM concentration of sulforaphane (SF) or a 0.1 μM concentration of 3-methylcolanthrene (3-MC) was used as a positive control for ARE or XRE activation, respectively. The results represent the mean±SD; n=4. Asterisks denote significant differences as compared to the control (*P<.05; **P<.01). ARE, antioxidant response element; XRE, xenobiotic response element.
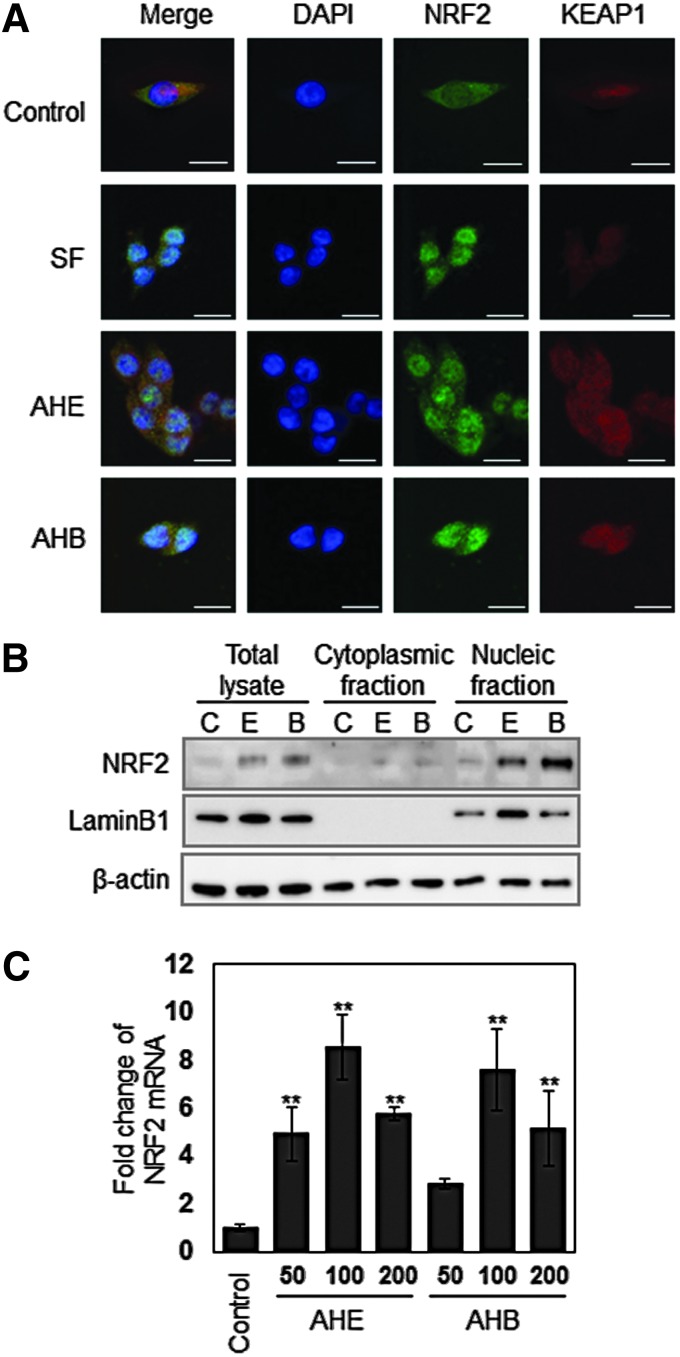
The AHE and AHB induce the nuclear translocation of Nrf2
Nrf2 is an important transcription factor that regulates ARE-driven genes such as NQO1, GST, and HO-1. The nuclear translocation of activated Nrf2 is an important upstream step in the mechanism underlying NQO1 expression.13 To investigate whether the AHE and AHB can induce Nrf2 translocation in HCT116 cells, we used an immunofluorescence assay to examine the intracellular distribution of Nrf2. In untreated HCT116 cells, Nrf2 fluorescence was distributed throughout the cells, including the cytoplasms and nuclei of the cells. However, following treatment with the AHE or AHB for 4 h, Nrf2 fluorescence was concentrated in the nuclei (Fig. 4A). In an effort to further confirm that Nrf2 nuclear translocation is induced by the AHE and AHB, we obtained the cytoplasmic and nucleic fractions of the cells and detected Nrf2 protein using Western blot analysis. The amount of Nrf2 protein in the nucleus was markedly increased, supporting the results of immunofluorescence (Fig. 4B). Treatment with the AHE and AHB also increased Nrf2 mRNA levels, as assessed using real-time PCR analysis (Fig. 4C). These data not only indicate that the AHE and AHB regulate Nrf2 through transcriptional activation and translocation to the nucleus but also that AHE- and AHB-mediated NQO1 induction involves the activation of the ARE promoter motif by Nrf2 in human colorectal carcinoma cells. In all of the experiments, the AHB showed much stronger effects than the AHE.
FIG. 4.
The AHE and AHB induce Nrf2 nuclear translocation. (A) Cellular distribution of Nrf2 in response to treatment with 100 μg/mL of the AHE or AHB in HCT116 cells. The cells were treated with 100 μg/mL of the AHE or AHB for 4 h, after which point an immunofluorescence assay was performed, followed by detection with confocal microscopy (bar=20 μm). (B) Nuclear Nrf2 accumulation in response to treatment with the AHE or AHB in HCT116 cells as assessed using Western blotting. The cells were treated with 100 μg/mL of the AHE or AHB for 4 h. The figure shows a representative image from three independent experiments. (C) Nrf2 mRNA expression in response to treatment with the AHE or AHB in HCT116 cells. The cells were treated with 50, 100, and 200 μg/mL of the AHE or AHB for 24 h. The results represent the mean±SD; n=3. An asterisk denotes a significant difference as compared to the control (**P<.01). Color images available online at www.liebertpub.com/jmf
The AHE and AHB induce the phase I detoxification enzyme CYP1A1
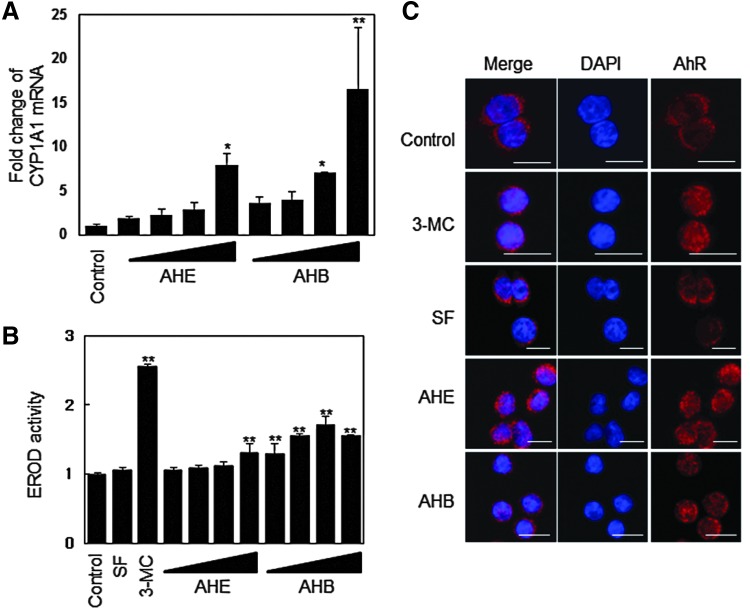
Phase I detoxification enzymes are regulated by the XRE,15,17 whereas phase II detoxification enzymes are regulated by both the ARE and XRE.18 In the present study, because the AHE and AHB activated the XRE in a dose-dependent manner, with the AHB fraction displaying greater effects than the AHE extract (Fig. 3C), we investigated the effects of the AHE and AHB on the expression of CYP1A1, an important phase I enzyme. We found that the AHE and AHB increased CYP1A1 mRNA levels in a dose-dependent manner (Fig. 5A), while only the AHB significantly induced CYP1A1 activity at 25–100 μg/mL, as measured by the EROD assay in Hepa1c1c7 cells (Fig. 5B).
FIG. 5.
The AHE and AHB increase CYP1A1 mRNA levels and induce EROD activity by inducing AhR accumulation in the nucleus. (A) CYP1A1 mRNA expression in response to treatment with the AHE or AHB in HCT116 cells. HCT116 cells were treated with 25, 50, 100, and 200 μg/mL of the AHE or AHB for 24 h. (B) EROD activity in response to treatment with the AHE or AHB in Hepa1c1c7 cells. 3-MC (0.1 μM) was used as a positive control. HCT116 cells were treated with 25, 50, 100, and 200 μg/mL of the AHE or AHB for 24 h. (C) Cellular distribution of the AhR in response to treatment with 100 μg/mL of the AHE or AHB in HCT116 cells. The cells were treated with 100 μg/mL of the AHE, 100 μg/mL of the AHB, 5 μM of SF, or 0.1 μM of 3-MC for 3 h, after which point an immunofluorescence assay was performed, followed by detection by confocal microscopy (bar=20 μm). The results represent the mean±SD; n=3. Asterisks denote significant differences compared to the control (*P<.05; **P<.01). EROD, 7-ethoxyresorufin-O-deethylase; AhR, aryl hydrocarbon receptor; 3-MC, 3-methylcolanthrene. Color images available online at www.liebertpub.com/jmf
We used an immunofluorescence assay to determine whether the AhR, a key transcription factor that directly binds to the XRE, accumulates in the nucleus in response to treatment with the AHE or AHB. AhR fluorescence was distributed in the cytoplasm of untreated cells and in cells treated with sulforaphane (SF), a well-known monofunctional inducer. However, after treatment with the AHE or AHB for 3 h, the AhR was detected in both the cytoplasm and the nucleus (Fig. 5C). Similar results were shown in cells that had been treated with 3-methylcolanthrene (3-MC), a powerful AhR inducer. These results suggest that the AHE and AHB also exert a chemopreventive effect through the AhR-mediated induction of phase I enzymes in addition to the induction of phase II detoxification enzymes.
The AHE and AHB induce antiproliferative activity and apoptosis
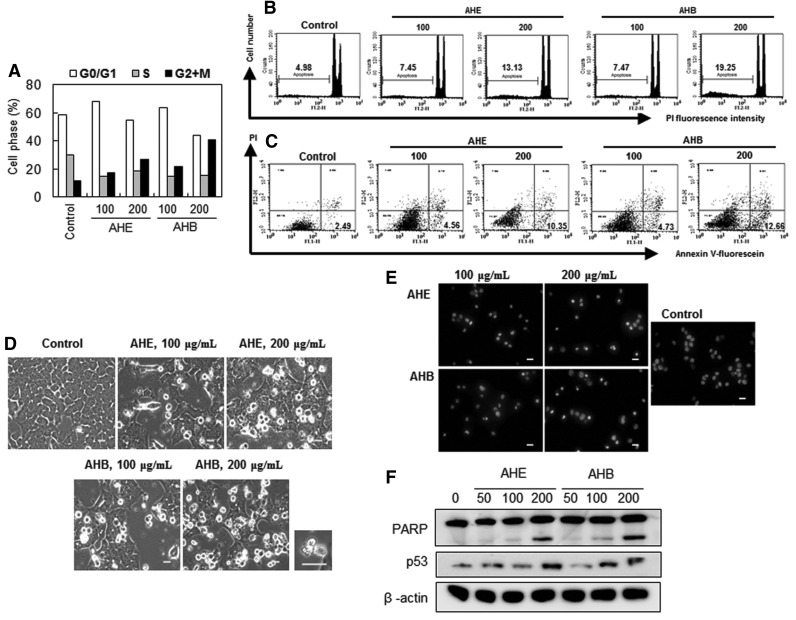
Treatment with 100 or 200 μg/mL of the AHE increased the proportion of the cell population in the G2/M phase, which was accompanied by a slight decrease in the cell population at S phase (Fig. 6A). The AHE caused G2/M arrest in 17.2% and 26.7% of cells, and the AHB caused even greater G2/M arrest (21.6%, and 40.5% of cells) at concentrations of 100 and 200 μg/mL, respectively. To determine whether AHE- and AHB-mediated inhibition of cell proliferation was due to apoptosis, we measured the levels of phosphatidylserine in the outer membrane. Apoptosis occurred in 4.98% of the solvent-treated control cells, while treatment with 200 μg/mL of the AHE and AHB caused apoptosis in 13.13% and 19.25% of the treated cells, respectively (Fig. 6B). These results suggested that treatment with the AHE and AHB potentiates apoptosis in HCT116 cells. A double labeling assay, in which annexin V was combined with PI, revealed that HCT116 cells that had been treated with 200 μg/mL of the AHE or AHB exhibited a dose-dependent increase in annexin V+/PI− (Fig. 6C), indicating that both the AHE and AHB induce apoptosis in HCT116 cells.
FIG. 6.
The AHE and AHB induce apoptosis in HCT116 cells. HCT116 cells were treated with the AHE or AHB (100 and 200 μg/mL) for 48 h. (A) The cell cycle distribution and (B) sub G0/G1 content (%) were evaluated using flow cytometric analyses measuring the DNA content according to the PI labeling method. (C) Phosphatidylserine externalization was measured using flow cytometric analysis after annexin V-fluorescein and PI staining. The cell population (%) for each quadrant is shown. The lower right quadrant (annexin V-fluorescein positive and PI negative) represents the early apoptotic cell population (%), indicated in bold. (D) Cellular morphology was observed using a phase contrast microscope. Phase contrast microscopy images were then taken. A magnified picture of apoptotic cells treated with 200 μg/mL of the AHB is shown on the right hand side of the original picture (bar=20 μm). (E) Chromatin condensation was observed using fluorescence microscopy after Hoechst33342 staining (bar=20 μm). (F) PARP cleavage and p53 levels were observed using Western blot analysis. The cells were treated with the indicated concentrations (50, 100, and 200 μg/mL) of the AHE or AHB for 48 h. All data are representative of three independent experiments. PI, propidium iodide; PARP, poly(ADP-ribose) polymerase.
Morphological changes due to apoptosis were also observed in the cells after treatment with 100 and 200 μg/mL of the AHE or AHB (Fig. 6D). These changes included the appearance of apoptotic bodies (Fig. 6D) and chromatin condensation (Fig. 6E) in cells that had been treated with 200 μg/mL of the AHB. In addition, both the AHE and AHB induced PARP cleavage, as indicated by the diminution of the parent PARP band and the concomitant dose-dependent accumulation of the PARP cleavage fragment (Fig. 6F). We also found that p53, an upstream regulator of apoptosis, became upregulated as the concentrations of the AHE or AHB were increased (from 50 to 200 μg/mL; Fig. 6F). These results suggested that p53 contributes to the antiproliferative effects and apoptosis induced by the AHE and AHB.
Isoquercitrin, a major compound isolated from the AHB, induces apoptosis but does not induce QR activity
We analyzed the chemical composition of the active fraction, the AHB, and isolated 5 major compounds: chlologenic acid (1), isoquercitrin (2), 3,4-dicaffeoylquinic acid (3), 3,5-dicaffeoyl-epi-quinic acid (4), and 4,5-dicaffeoylquinic acid (5) (Fig. 7A). We further investigated the induction of apoptosis and QR activity. Isoquercitrin (2) displayed potent antiproliferative activity, with an IC50 value of 29.61 μM for 48 h in HCT116 cells. It also appeared to increase apoptosis in HCT116 cells, as indicated by the increase in the sub G0/G1 content (%) and apoptotic morphological changes (Fig. 7B). However, isoquercitrin (2) only very weakly induced QR activity (data not shown). The other compounds (1, 3–5) did not induce apoptosis or QR activity (data not shown).
Discussion
In the present study, we investigated the cancer chemopreventive actions of the A. himalaicum extracts, AHE and AHB, on human cancer cells. Induction of phase II detoxification enzymes, such as QR and GST, is well known to block tumor initiation. Therefore, we chose to measure QR activity as a biomarker in an effort to screen the tumor-blocking effects of the target fractions of an A. himalaicum extract. The Hepa1c1c7 mouse hepatocarcinoma cell line is a popular model for use in the QR assay because of its low basal levels, and high inducibility, of QR. In HCT116 cells, QR activity was induced by the AHE or AHB, but the inducibility was not high (Fig. 2A, B).
In the present study, we have defined the AHE and AHB as bifunctional inducers because they induce not only phase II detoxification enzymes, including NQO1 (also known as QR) and HO-1, by activating the ARE and XRE promoter elements but also the phase I enzyme, CYP1A1, through XRE activation. We also observed synergistic induction of NQO1 and HO-1 mRNA as a result of significant ARE and XRE promoter motif activation by the AHB fraction but not the AHE extract (Fig. 2C, D). These results support the results of a previous report that stated that the transcriptional activation of NQO1 gene promoters can be regulated by two key cis-acting elements, the ARE and XRE.19
The ARE motif is known to be activated by Nrf2 and its binding complex, whereas the XRE is known to be activated by the AhR-Arnt system.6,19 In our experiments, Nrf2 rapidly accumulated in the nucleus when the cells were treated with the AHE or AHB (Fig. 4A). Our results also imply that the AHB activates the AhR-Arnt system because the AHB induced CYP1A1 by activating the XRE motif, leading to nuclear accumulation of the AhR (Figs. 3C and 5). Nrf2 plays an essential role in protecting against oxidative/electrophilic stress.20 In contrast, the AhR-Arnt system has two functions, which are classified as “adaptive” (the enhanced metabolism of xenobiotic metabolizing enzymes) and “toxic” (the adverse effects of dioxin-like compounds via the induction of CYP1A1 enzymes).21 Despite its toxic function, activation of the AhR-Arnt may facilitate detoxification by transcriptional regulation of Nrf2 depending on the balance of its adaptive and toxic functions within the cell.7 Nrf2 is known to contain three XRE-like elements that are regulated by the AhR-XRE pathway, and multiple copies of the ARE.7 Therefore, most of the bifunctional inducers, including the AHE and AHB, may not only induce Nrf2 nuclear accumulation but may also regulate Nrf2 transcription levels (Fig. 4C). One of the explanations for this phenomenon is that the AHE and AHB may increase Nrf2 mRNA by AhR-XRE binding in the Nrf2 promoter region. Therefore, Nrf2 could be one of the downstream targets of the AhR. It is possible that phase II detoxification enzymes may be synergistically activated by the AHE or AHB through the activation of both the ARE and XRE motifs, thus facilitating cancer prevention.
The AHE and AHB also stimulated Nrf2 translocation into the nucleus by releasing Nrf2 from the Keap1–Nrf2 complex (Fig. 4A, Supplementary Fig. S1; Supplementary Data are available at www.liebertpub.com/jmf). Nrf2 cellular protein levels were increased concurrently with Nrf2 translocation into the nucleus. This change in the cellular distribution of Nrf2 is a key event in the induction of phase II detoxification enzymes. However, it remains unclear whether the AHE and AHB inhibit KEAP-1 by directly interacting to protect Nrf2 from the ubiquitin-dependent degradation pathway, or whether they activate Nrf2 directly by phosphorylation through the PI3K or p38 pathways.22
Tumorigenesis is a multistep process that results from responses to various factors. Foods contain many components that can act as effective cancer preventive agents due to their synergistic actions. A. himalaicum is an abundant plant in Asia, and it has been traditionally consumed as food. We have focused on the chemopreventive effects of both A. himalaicum whole extract and its fractions. In this study, a range of concentrations of the AHE extract and AHB fraction induced detoxification enzyme activity, while they inhibited cancer cell growth at high concentrations by inducing apoptosis. These results illustrate that the AHE and AHB prevent cancer very efficiently by blocking and suppressing different steps in the carcinogenesis process.
The well-known p53 tumor suppressor gene plays a key role in a cell's decision to enter cell cycle arrest or undergo apoptosis.23 In our study, both the AHE and AHB induced cell cycle arrest in the G2/M phase and induced apoptosis at high concentrations. The role played by p53 in G2/M cell-cycle arrest is complex and involves multiple targets that affect either the cell cycle or the mitotic machinery.24 In a recent study, synergistic chemoprevention was shown to occur as a result of crosstalk between Nrf2 and p53.10 The synergistic induction observed in this study also suggests that the AHE and AHB might exert dual chemopreventive effects through the cooperation between Nrf2 and p53.
According to previous reports, activation of the AhR pathway could increase the induction of apoptosis via caspase-3 activation.25,26 In our case, the AHE and AHB induced the AhR pathway and its detoxification enzymes, including CYP1A1, and inducing p53-mediated apoptosis at 100–200 μg/mL. We speculated that AHE- and AHB-mediated induction of p53-mediated apoptosis might originate from AhR pathway activation.
Many flavonoids have been shown to activate chemoprevention and these results have been verified in manyarticles.27,28 A flavonoid, isoquercitrin, the O-glycoside of quercetin, displayed antiproliferative activity,29 but its QR-inducing activity was very weak.30 Our data were in agreement with the results of the previous study. Therefore, we concluded that isoquercitrin is a major active component of the AHB that contributes to the antiproliferative and apoptosis-inducing activity of the AHB. According to the results of our recent study, chlorogenic acid (1) and 3,5-dicaffeoylquinic acid (5) have antigenotoxic activity.31 Therefore, isoquercitrin (2), chlorlogenic acid (1), and 3,5-dicaffeoylquinic acid (5) can be used as marker compounds for the standardization of functional materials (such as the AHE and AHB) for cancer chemoprevention. However, we could not decipher the active components of the AHB that were responsible for inducing QR activity. Therefore, future detailed chemical analyses may be required to uncover the active compounds that are responsible for the induction of QR activity.
Taken together, our data indicate that the A. himalaicum extracts, the AHE and AHB, exert their chemopreventive effects through the induction of detoxification enzymes as a result of Nrf2-ARE and AhR-XRE pathway activation and through the induction of apoptosis. These bifunctional effects could potentially be applied to the development of cancer chemopreventive agents for the prevention of human colorectal cancer. The chemopreventive effects of the AHE and AHB should first be confirmed with in vivo efficacy and cytotoxicity assays prior to further application.
Supplementary Material
Acknowledgments
This work was supported by a KIST-Gangneung Institute Intramural Research Grant (2Z03560).
Author Disclosure Statement
The authors do not have any competing financial interests.
References
- 1.Lichtenstein P. Holm NV. Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 2.Kensler TW. Qian GS. Chen JG. Groopman JD. Translational strategies for cancer prevention in liver. Nat Rev Cancer. 2003;3:321–329. doi: 10.1038/nrc1076. [DOI] [PubMed] [Google Scholar]
- 3.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 4.de Kok TM. van Breda SG. Manson MM. Mechanisms of combined action of different chemopreventive dietary compounds: a review. Eur J Nutr. 2008;47(Suppl 2):51–59. doi: 10.1007/s00394-008-2006-y. [DOI] [PubMed] [Google Scholar]
- 5.Prestera T. Holtzclaw WD. Zhang Y. Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci USA. 1993;90:2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Ferrec E. Lagadic-Gossmann D. Rauch C, et al. Transcriptional induction of CYP1A1 by oltipraz in human Caco-2 cells is aryl hydrocarbon receptor- and calcium-dependent. J Biol Chem. 2002;277:24780–24787. doi: 10.1074/jbc.M111319200. [DOI] [PubMed] [Google Scholar]
- 7.Miao W. Hu L. Scrivens PJ. Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 8.Berger NA. Petzold SJ. Identification of minimal size requirements of DNA for activation of poly(ADP-ribose) polymerase. Biochemistry. 1985;24:4352–4355. doi: 10.1021/bi00337a015. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki K. Sukata T. Yamamoto S, et al. High susceptibility of p53(+/−) knockout mice in N-butyl-N-(4-hydroxybutyl)nitrosamine urinary bladder carcinogenesis and lack of frequent mutation in residual allele. Cancer Res. 1998;58:3806–3811. [PubMed] [Google Scholar]
- 10.Iida K. Itoh K. Maher JM, et al. Nrf2 and p53 cooperatively protect against BBN-induced urinary bladder carcinogenesis. Carcinogenesis. 2007;28:2398–2403. doi: 10.1093/carcin/bgm146. [DOI] [PubMed] [Google Scholar]
- 11.Kwon HC. Lee KR. An acetylene and a monoterpene glycoside from Adenocaulon himalaicum. Planta Med. 2001;67:482–484. doi: 10.1055/s-2001-15806. [DOI] [PubMed] [Google Scholar]
- 12.Fahey JW. Dinkova-Kostova AT. Stephenson KK. Talalay P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. Methods Enzymol. 2004;382:243–258. doi: 10.1016/S0076-6879(04)82014-7. [DOI] [PubMed] [Google Scholar]
- 13.Lee SB. Kim CY. Lee HJ. Yun JH. Nho CW. Induction of the phase II detoxification enzyme NQO1 in hepatocarcinoma cells by lignans from the fruit of Schisandra chinensis through nuclear accumulation of Nrf2. Planta Med. 2009;75:1314–1318. doi: 10.1055/s-0029-1185685. [DOI] [PubMed] [Google Scholar]
- 14.Sinal CJ. Bend JR. Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Mol Pharmacol. 1997;52:590–599. doi: 10.1124/mol.52.4.590. [DOI] [PubMed] [Google Scholar]
- 15.Favreau LV. Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 16.Kang K. Lee HJ. Kim CY, et al. The chemopreventive effects of Saussurea salicifolia through induction of apoptosis and phase II detoxification enzyme. Biol Pharm Bull. 2007;30:2352–2359. doi: 10.1248/bpb.30.2352. [DOI] [PubMed] [Google Scholar]
- 17.Rushmore TH. Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- 18.Okey AB. Riddick DS. Harper PA. The Ah receptor: mediator of the toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. Toxicol Lett. 1994;70:1–22. doi: 10.1016/0378-4274(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 19.Giudice A. Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays. 2006;28:169–181. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen T. Sherratt PJ. Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 21.Kohle C. Bock KW. Activation of coupled Ah receptor and Nrf2 gene batteries by dietary phytochemicals in relation to chemoprevention. Biochem Pharmacol. 2006;72:795–805. doi: 10.1016/j.bcp.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Eggler AL. Gay KA. Mesecar AD. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res. 2008;52(Suppl 1):S84–S94. doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- 23.Valenzuela MT. Guerrero R. Nunez MI, et al. PARP-1 modifies the effectiveness of p53-mediated DNA damage response. Oncogene. 2002;21:1108–1116. doi: 10.1038/sj.onc.1205169. [DOI] [PubMed] [Google Scholar]
- 24.Brown L. Boswell S. Raj L. Lee SW. Transcriptional targets of p53 that regulate cellular proliferation. Crit Rev Eukaryot Gene Expr. 2007;17:73–85. doi: 10.1615/critreveukargeneexpr.v17.i1.50. [DOI] [PubMed] [Google Scholar]
- 25.Kim JY. Chung JY. Park JE, et al. Benzo[a]pyrene induces apoptosis in RL95–2 human endometrial cancer cells by cytochrome P450 1A1 activation. Endocrinology. 2007;148:5112–5122. doi: 10.1210/en.2007-0096. [DOI] [PubMed] [Google Scholar]
- 26.Tomokiyo A. Maeda H. Fujii S, et al. Alternation of extracellular matrix remodeling and apoptosis by activation of the aryl hydrocarbon receptor pathway in human periodontal ligament cells. J Cell Biochem. 2012;113:3093–3103. doi: 10.1002/jcb.24186. [DOI] [PubMed] [Google Scholar]
- 27.Lee-Hilz YY. ter Borg S. van Berkel WJ. Rietjens IM. Aarts JM. Shifted concentration dependency of EpRE- and XRE-mediated gene expression points at monofunctional EpRE-mediated induction by flavonoids at physiologically relevant concentrations. Toxicol In Vitro. 2008;22:921–926. doi: 10.1016/j.tiv.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Yao H. Xu WZ. Shi XL. Zhang Z. Dietary flavonoids as cancer prevention agents. J Environ Sci Heal C. 2011;29:1–31. doi: 10.1080/10590501.2011.551317. [DOI] [PubMed] [Google Scholar]
- 29.Yoon H. Lee CY. Effect of selected phytochemicals on cell proliferation in A549 lung cancer cells. Food Sci Biotechnol. 2010;19:1063–1068. [Google Scholar]
- 30.Yang J. Liu RH. Induction of phase II enzyme, quinone reductase, in murine hepatoma cells in vitro by grape extracts and selected phytochemicals. Food Chem. 2009;114:898–904. [Google Scholar]
- 31.Kang K. Jho EH. Lee HJ, et al. Youngia denticulata protects against oxidative damage induced by tert-butylhydroperoxide in HepG2 cells. J Med Food. 2011;14:1198–1207. doi: 10.1089/jmf.2010.1557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.