Abstract
Significance
Coronary artery disease is a major cause of morbidity and mortality as the loss of functional myocardium drives progressive ventricular remodeling and subsequent heart failure. Medical management has significantly improved outcomes for acute myocardial infarction (MI); however, improved strategies are needed to regenerate functional myocardium and prevent the progression to heart failure. Cytotherapy using cardiac progenitor cells (PCs) to regenerate functional myocardium holds tremendous potential; however, a better understanding of PC biology is needed.
Recent Advances
Reports of cardiac regeneration in lower animals have been reported in the last decade. However, just recently, two separate models of mammalian cardiac regeneration have been published and offer potential to better define PC biology, including PC recruitment, differentiation, proliferation, and integration.
Critical Issues
Numerous clinical trials have been completed or are ongoing to evaluate possible cytotherapy options in the treatment of acute and chronic ischemic cardiac disease. To date, results have demonstrated improvements in cardiac function as a result of paracrine effects of cytotherapy, but regeneration of functional myocardium has yet to be observed.
Future Directions
Future translation of cardiac PC biology from these models is necessary to promote regenerative cardiac healing following MI and to prevent the progression to heart failure following the loss of functional myocardium. Knowledge gained from mammalian models of cardiac regeneration will allow for the development of therapeutic regimens in the treatment of heart failure.

Michael W. Morris, Jr., MD
Scope and Significance
Coronary artery disease is a major cause of morbidity and mortality as a result of acute myocardial infarction (MI) or subsequent heart failure with one in six American deaths secondary to coronary artery disease.1 Modern medical therapy has significantly improved outcomes for acute MI; however, strategies are needed to induce regeneration of lost myocardium and prevent the progression to heart failure, as the mortality from heart failure following MI has remained ∼50% since 1950.2 This fact highlights the clinical significance of cytotherapy in the prevention of ventricular remodeling and subsequent heart failure.
Translational Relevance
Cytotherapy offers tremendous therapeutic potential, but a better understanding of cardiac progenitor cell (PC) biology is necessary before the hope of functional cardiac regeneration is realized. Two separate reports were published just recently, describing mammalian cardiac regeneration in fetal sheep and neonatal mice.3,4 These two models offer the potential to better define progenitor cell biology, including recruitment, differentiation, proliferation, and integration during regeneration. Both reports demonstrate restoration of functional myocardium, and in particular, the fetal sheep model demonstrated that regeneration can occur after an MI in a clinically relevant model.
Clinical Relevance
Literature over the last 15 years has challenged the idea of the heart as a terminally differentiated organ, resulting in clinical trials to investigate cardiac PCs (CPCs) in the treatment of acute and chronic ischemic cardiac disease. To date, results have demonstrated a transient benefit of CPCs in the treatment of cardiac disease via paracrine mediated production of chemokines and other growth factors without restoration of functional myocardium or myocardial cell mass.2 Therefore, it is apparent that more research is necessary before CPC-mediated cytotherapy becomes a reality in the treatment of ischemic cardiac disease.
Background
The process of wound healing follows an orderly, coordinated, and overlapping series of events in response to tissue injury. This response can be divided into phases, beginning with the inflammatory phase, followed by the proliferative phase, and finally the remodeling phase. Following injury, this process is very similar in a variety of tissues and results in repair of the damaged tissue, but at the cost of scar formation or fibrosis. The degree of fibrosis and subsequent dysfunction ranges from a simple cosmetically undesirable scar to significant impairments in tissue function. These impairments are generally tissue- or organ-specific and include contractures in the skin, strictures in bowel or vascular anastomosis, pulmonary or liver fibrosis, or ventricular fibrosis and heart failure following an MI. One factor that influences the degree of fibrosis and subsequent remodeling is the load or ongoing work required of the injured tissue.
In the heart, loss of functional myocardial tissue following an MI may result in immediate failure of the heart to perform work. If the MI does not immediately result in mortality, the heart undergoes repair by replacing the necrotic myocardium with fibroblasts, which secrete collagen and result in scar formation. Fibroblasts and collagen are unable to perform the work of cardiac myocytes, and remodeling of this ventricular scar over time results in ventricular dilation and the development of heart failure.5
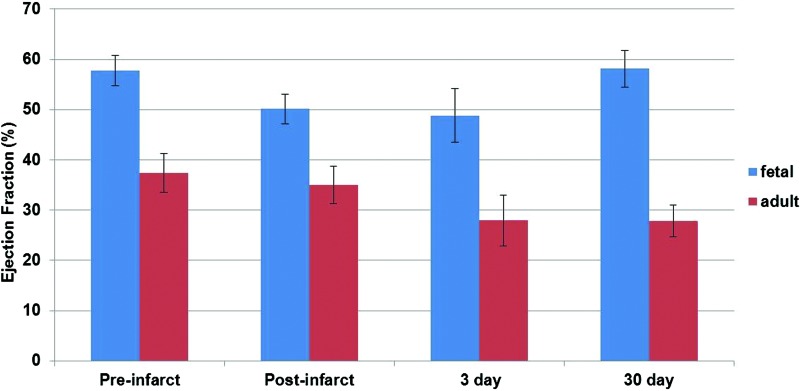
In contrast to reparative healing or scar formation in the adult, the fetal response to MI is regenerative. Thirty days following MI, fetal cardiac function as measured by the ejection fraction was shown to return to baseline, whereas the function of the adult heart demonstrated a progressive decline in function, as shown in Fig. 1.3 The regenerative potential of the fetus is also well established in skin and tendon wounds.6–9 These fetal tissues remain under tension and continue to perform work after injury, but heal in a regenerative fashion. Thus, the differential response between the fetus and the adult is of significant interest to researchers and clinicians in cardiovascular medicine.
Figure 1.
Ejection fraction (EF) as evaluated by echocardiography in fetal and adult sheep pre- and postinfarct, as well as 3 and 30 days following myocardial infarction (MI). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Fetal sheep demonstrated the potential for cardiac regeneration following apical infarct of the left ventricle (LV), while neonatal mice demonstrated a transient potential for cardiac regeneration following apical amputation.3,4 These studies implicated the recruitment of CPCs to the infarct, where they differentiated and restored functional myocardium; however, the source of these cells and whether they are derived from existing adult myocytes, resident, or circulating CPC populations remains to be determined. The development of a better understanding of CPC biology, including the factors that regulate the proliferation, differentiation, and mobilization of these cells, is essential for the development of potential treatment modalities to restore functional myocardium in the adult following MI.
Discussion of Findings and Relevant Literature
Cardiac regeneration in animals
Cardiac regeneration was first reported in newts10 and more recently in zebrafish, which regenerate the cardiac apex following surgical resection.11 Regeneration occurs as residual cardiac myocytes undergo dedifferentiation with dissolution of sarcomeric structures to generate new cardiac myocytes, which can proliferate and functionally integrate into the injured myocardium.12 It is important to note that cardiac regeneration in the zebrafish occurs through the proliferation of existing cardiac myocytes and not from the influence of progenitor or stem cells.
The idea of mammalian cardiac regeneration dates back to rodent studies in the 1960s.13 A single report on the transient regenerative potential of murine, neonatal hearts suggests that dedifferentiation and proliferation are responsible for cardiac regeneration.4 However, a recent report argues that CPCs play an important role in mammalian cardiac regeneration.3 This idea is supported by evidence, which demonstrates the ability of activated fetal CPCs in the mouse to generate all myocyte progeny up to day one of life.14
Investigation of markers involved in cardiac development identified the combination of three transcription factors, the gata 4 transcription factor gene (Gata4), the myocyte-specific enhancer factor 2C gene (Mef2c), and the t-box 5 gene (Tbx5), capable of inducing cardiac myocyte-like behavior from murine postnatal fibroblasts.15 Multiple investigators utilized these induced murine fibroblasts in an attempt to effect cardiac regeneration, but conflicting results have been published.16–18 Qian et al. used Gata4, Mef2c, and Tbx5 to induce cardiac myocyte-like behavior from postnatal cardiac and dermal fibroblasts.16 Song et al. utilized these same transcription factors plus a fourth, the heart and neural crest derivative expressed protein 1 gene (Hand1), to induce cardiac myocyte function from mouse tail tip and cardiac fibroblasts.17 Both reports demonstrated improved cardiac function, electrical coupling, and inhibition of ventricular remodeling following treatment, but superior results were noted with the addition of the fourth transcription factor. A third investigator utilized the same three genes as Qian et al. in murine tail tip and cardiac fibroblast, but did not demonstrate induced electrochemical or functional change of cardiac fibroblasts, furthermore, transplantation of induced cells resulted in diminished cell survival.16,18 Collectively, these reports provide mixed results regarding the use of induced cardiac fibroblasts to restore myocardial cell mass, but do provide targets for future research that may improve functional integration of CPCs or induced pluripotent cells to treat ischemic heart disease.
Cardiac regeneration in humans
The report of cardiac myocyte division following MI challenged the longstanding idea of the human heart as a terminally differentiated organ with quiescent cells.19 Multipotent CPCs were identified within the heart and have been shown to be activated by an ischemic insult.20,21 Two published labeling studies demonstrate convincing evidence for cellular renewal in the human heart. In the first study, carbon-14 dating allowed evaluation of human cardiac myocyte renewal in 12 human subjects at autopsy. Results demonstrated cardiac myocyte turnover at 1% per year at age 25 years that decreased to 0.45% at age 75 years, with <50% of cardiac myocytes renewed within a person's lifespan.22 In the second labeling study, hearts of cancer patients treated with iododeoxyuridine, a radiation sensitizer which intercalates into DNA, were examined postmortem. Immunohistochemical analysis demonstrated cardiac myocyte renewal of 22% per year with complete cardiac myocyte renewal multiple times in a person's lifespan.23 The results of these two studies vary widely; however, these studies demonstrate cardiac myocyte renewal and support the notion of cardiac regeneration in humans. In combination, these results demonstrate the presence of CPCs within the adult myocardium, and the ability for cardiac renewal. However, cardiac regeneration has not been witnessed, and this is most likely secondary to insufficient CPC number or impaired function in an adult.
Genetic fate mapping and the heart fields
Genetic fate mapping is a sophisticated tool that allows identification of embryonic tissues and their progeny. Cardiac cells derived from the mesoderm are divided into the first and second heart fields, which contribute to the development of specific structures and demonstrate distinctive cell surface markers. Table 1 lists the marker, corresponding heart field, and resulting progeny. Cells of both heart fields demonstrate Nkx2.5 expression.24,25 Figure 2 demonstrates the recruitment of Nkx2.5+ CPCs to the fetal infarct 30 days following MI. A recent fate-mapping study in transgenic mice also addresses the potential of cardiac regeneration by proliferation of pre-existing cardiomyocytes versus CPCs.26 This study demonstrated regeneration of adult mammalian cardiomyocytes after MI by CPCs; however, CPCs did not produce cardiomyocytes during normal aging. These results demonstrate differential cardiac regeneration, with mammals regenerating the myocardium from CPCs, but with zebrafish regenerating the myocardium via dedifferentiation and proliferation of existing myocytes.13 Elaborate fat-mapping studies have provided much knowledge in regard to the cell lineage of potential CPCs; however, further definition of CPCs capable of post-MI cardiac regeneration in humans is necessary. Future identification of these CPCs by a cell-surface marker may grant identification of their resident location and then allow direct experimentation to evaluate function.
Table 1.
Cardiac progenitor cell surface markers, the corresponding heart field, and the resultant progeny
| CPC Markers | First or Second Heart Field | PC Progeny |
|---|---|---|
| Ckit | First | Cardiac myocytes of the bilateral atria left ventricle |
| Nkx 2.5 | Both | Cardiac myocytes of the bilateral atria, and ventricles |
| IsI-1 | Second | Cardiac myocytes of both atria, the right ventricle, and conductions system |
CPC, cardiac progenitor cell; PC, progenitor cell.
Figure 2.
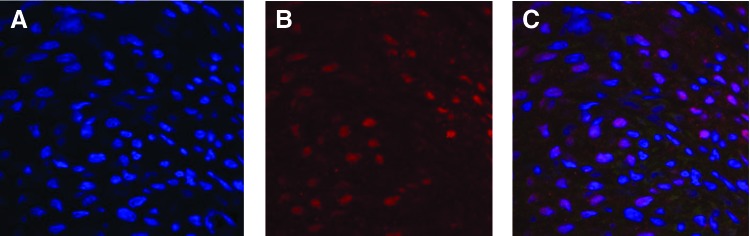
Fetal infarct at 30 days after MI stained for (A) nuclei using DAPI, (B) nkx2.5+ cardiac progenitor cells using immunohistochemistry, and (C) merged DAPI and nkx2.5 immunohistochemistry. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Cytotherapy and clinical trials
The potential therapeutic benefit of bone marrow-derived progenitor cells (BM PCs) and other noncardiac PCs coupled with the epidemic healthcare burden of ischemic cardiac disease resulted in a rush to multiple clinical trials for research and development of cytotherapeutic options.24 Investigators pursued the potential of BM PCs based on the assumption that these cells of mesodermal origin could differentiate into cells of cardiac lineage.24 Granulocyte-colony stimulating factor (G-CSF), a growth factor known to stimulate mobilization and differentiation of hematopoietic stem cells, was initially investigated. FIRSTLINE AMI, a randomized clinical trial, evaluated the therapeutic effect and safety of G-CSF versus placebo on the mobilization of CD34+ cells from bone marrow and the subsequent effect on cardiac function following first ST elevation MI.27 G-CSF administration, after percutaneous intervention, demonstrated inhibition of LV remodeling, and improved cardiac function without increased risk of atherosclerosis at 4 months. However, these results differ from those reported in REVIVAL 2, a double-blinded, randomized trial designed to evaluate stem cell mobilization after acute MI with treatment of G-CSF versus placebo.28 REVIVAL 2 did not demonstrate a significant change in cardiac function or LV remodeling, and similar results were presented by the G-CSF STEMI trial, with no improvement in cardiac function between the G-CSF-treated and placebo groups.29 A published meta-analysis with combined data on 445 patients from 10 studies, including the FIRSTLINE AMI, REVIVAL 2, and G-CSFSTEMI trials, demonstrated no significant benefit in the treatment of acute MI with G-CSF, and concluded that mobilized bone marrow stem cells were not pluripotent, but rather were multipotent PCs able to differentiate into endothelial or hematopoietic origin.30
Following the initial report on G-CSF, clinical trials were initiated to investigate the direct regenerative potential of transplanted BM PCs.12 The BOOST trial was a randomized, controlled study that included 60 patients treated according to standards of practice, and then additionally with placebo or intracoronary infusion of autologous bone marrow cells administered after percutaneous coronary intervention.31 Results demonstrated significantly improved cardiac function with attenuated LV remodeling at 6 months in the treatment group without increased risk of arrhythmia or restenosis.
REPAIR AMI is the largest randomized, double-blinded study that compared intracoronary infusion of BM PCs versus placebo following acute MI.32 This multicenter, clinical trial included 204 patients and demonstrated the benefits of BM PCs, with improvement in LV function at 4 months, and decreased intervention rates, mortality, and MI recurrence at 12 months post-BM PC infusion. Re-evaluation of this study population 2 years after initial publication demonstrated continued benefits, with significantly improved LV contractility in the BM PC-treated group.33
The first American clinical trial to investigate the benefits of intracoronary BM PCs following MI was the TIME study.34 This randomized, double-blind study demonstrated similar results as the BOOST and REPAIR AMI studies, but BM PCs were given via standardized, continuous infusion within 7 days of MI. This trial evaluated 45 patients with an ST elevation MI in the anterior distribution. Results demonstrated a significant improvement in cardiac function, but no improvement in LV remodeling.
Since intracoronary infusions of BM PCs within 7 days following MI demonstrated improved cardiac function, the National Heart, Blood, and Lung Institute funded a randomized, double-blinded study to investigate the effect of delayed infusions. Rationale for late infusion resulted from the ideas that unstable patients would not tolerate infusion, and few centers offering initial percutaneous coronary intervention possess the expertise necessary to offer cytotherapy, and therefore, coronary infusions would be delayed until transfer of a stable patient to a capable facility.34 In the LATE TIME study, autologous bone marrow stem cells were administered through the coronary circulation of patients 2–3 weeks after percutaneous intervention for first MI. Results failed to demonstrate a benefit of delayed administration of BM PCs over placebo, thus highlighting the effect of BM PCs as most beneficial in early healing.
The STAR-heart study evaluated the clinical effect of BM PCs administered to patients with chronic heart failure secondary to ischemic coronary disease.35 Included patients presented with heart failure 8.5±3.2 years after percutaneous treatment for acute MI. A total of 391 patients were included and received standard medical therapy alone (n=200) versus standard therapy with coronary infusion of BM PCs within the infarct distribution (n=191). Patients were followed up to 5 years, and the results demonstrated significantly decreased mortality, and improved cardiac function in the BM PC-treated cohort.
Initial reports on BM PCs presented evidence of improved cardiac function, inhibition of LV remodeling, and subsequent heart failure. However, the reported improvements in cardiac function by BM PCs are now attributed to paracrine effects on residual cardiac myocytes rather than regeneration and integration of new cells.2 Whether by induced mobilization or direct transplantation, BM PCs have yet to demonstrate genesis of cardiac myocytes likely related to intrinsic, multipotent potential that limits differentiation. Therefore, medical research is focused on other progenitor populations able to regenerate functional myocardium without risk of an increased risk of ectopy or neoplastic transformation.
Autologous skeletal myoblast implants represented a potential cytotherapeutic option with advantages of low neoplastic and immunologic rejection risks, and therefore, the Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial was initiated to evaluate safety and potential efficacy of transplanted myoblasts in the treatment of LV remodeling.36 This double-blinded, randomized clinical trial was a multicenter study that evaluated the effect of transplanted autologous myoblasts versus placebo at the time of coronary artery bypass graft (CABG) in patients with pre-existing left heart failure. Results demonstrated inhibition of LV remodeling with only a high myoblast dose, but failed to demonstrate a benefit of implanted myoblasts on cardiac function, and furthermore, demonstrated increased risk of early postoperative arrhythmias. The study concluded that a better cell type and delivery method were necessary for the treatment of acute and chronic coronary disease processes.36
Ckit+ CPCs within the human heart demonstrate the potential to form all types of cells within the heart. The SCIPIO study represents the first clinical trial to evaluate the direct effect of ckit+ CPCs on heart failure following MI.37 This randomized, phase 1 clinical trial compares postoperative infusion of ckit+ CPCs to placebo following CABG. This study is ongoing, however, the initial results demonstrate safety of infusion and suggest efficacy in prevention of remodeling and subsequent heart failure.37 Phase 2 trials will likely follow as the initial results with human CPCs are promising and ischemic cardiomyopathy represents a significant healthcare burden. Future studies may help further define the function of ckit+ CPCs in regeneration and healing following acute and chronic ischemic insult.
Cardiosphere-derived cells (CCs) represent ckit+, multipotent clones derived from endogenous cardiac tissue capable of cardiac regeneration following MI.24,38 A second prospective study, CADUCEUS, recently published initial phase 1 data on CCs cultured from endomyocardial biopsies.39 Autologous CCs were administered via coronary infusion up to 3 months post-MI, and initial results demonstrated safety of coronary infusions. Additionally, results of this phase I study demonstrated therapeutic cardiac regeneration with a significantly increased myocardium, cardiac function, and inhibition of remodeling with a decreased scar.39
Human embryonic stem cells (ESCs) demonstrate the potential to create all cell lines in all organ systems. However, issues of availability, cultural perception, and unknown mechanistic behavior currently limit the therapeutic potential of ESCs. Investigation of cardiac regeneration by embryonic progenitors demonstrated improved cardiac function with ESC transplantation following MI.40 However, functional integration into the infarcted myocardium has yet to be demonstrated, and therefore, ESC transplantation may carry significant risk of arrhythmia.40 Additional studies on recruitment, differentiation, proliferation, and most importantly integration are necessary to unlock the potential of cardiomyogenesis by these embryonic progenitors. Table 2 summarizes the clinical trial results for previously investigated PC therapies.
Table 2.
Progenitor cell types and outcomes in clinical trials
| PC Types | Clinical Trial Outcomes |
|---|---|
| BM PC | Beneficial outcomes are attributed to paracrine effects on residual cardiac myocytes rather than regeneration of new cells |
| Ckit+ CPC | Phase I clinical trial demonstrated safety and preliminary results suggest efficacy for inhibition of infarct remodeling and development of heart failure |
| Skeletal muscle myoblasts | Phase II clinical trial failed to conclusively demonstrate improvement in cardiac function. |
| Cardiosphere-derived cells | These ckit+ cells demonstrated safety in phase I clinical trial and initial results demonstrate regeneration of myocardium, restoration of cardiac function, and inhibition of remodeling with decreased scar. |
| Embryonic stem cells | No clinical trials at this time. |
BM PC, bone marrow-derived progenitor cell.
Adjuncts to cytotherapy
Stromal-derived factor 1-alpha (SDF-1α, CXCL12) is a highly conserved chemokine thought to play an important role in the stimulation and homing of CPCs following MI.41 SDF-1α binds to CXCR4, a G protein coupled receptor, and triggers intracellular signaling, which results in mobilization of cells to the site of injury. Increased expression of SDF-1α following MI42 establishes a gradient for CPCs recruitment43 that results in the recruitment of CPCs to the infarct and border zone at the time of acute MI.44,45
Numerous studies demonstrate a multifactorial benefit of SDF-1α expression following MI via recruitment, attenuated inflammatory response, decreased apoptosis, and improved cardiac function following MI, but cardiac regeneration has yet to be reported following SDF-1α treatment.43,46,47 The level of SDF-1α expression correlates with the number of PCs recruited.48 However, the exact mechanisms for recruitment and homing are not yet understood and are the focus of strategies to improve cytotherapy. Previous reports demonstrated the therapeutic benefit of G-CSF following MI with improved cardiac myocyte survival, decreased LV remodeling, decreased inflammation and scar, blunted apoptosis, and improved cardiac function as cells mobilize to the site of injury.49,50 Interactions between G-CSF and SDF-1α have been established, however, G-CSF alone or in combination has yet to conclusively demonstrate functional or gross cardiac regeneration in multiple clinical studies.
Thymosin beta-4 (TB4) is a secreted peptide known to attenuate, inflammation, fibrosis, and apoptosis in multiple organ systems.51 Specifically, TB4 demonstrates a cardioprotective effect with decreased fibrosis, apoptosis, and infarct size following MI.52 These beneficial effects directly result from upregulated antiapoptotic, anti-inflammatory, and antioxidative genes as demonstrated in neonatal cardiomyocytes.53 These effects may not be limited to wound healing as PC differentiation and mobilization are triggered by TB4 binding of actin.51,52 The effect of these cytotherapy adjuncts on PCs and post-MI outcomes is summarized in Table 3.
Table 3.
Adjuncts to cytotherapy and their effects on progenitor cells and cardiac healing
| Adjuncts to Cytotherapy | Effect on PCs | Reported Effect Post–Myocardial Infarction |
|---|---|---|
| Stromal-derived factor 1α | Stimulates mobilization and homing. | Attenuated inflammatory response, decreased apoptosis, and improved cardiac function. |
| Granulocyte-colony stimulating factor | Stimulates mobilization and differentiation of hematopoietic PCs. | Improved cardiac myocyte survival and cardiac function |
| Decreased apoptosis, inflammation, and left ventricle remodeling. | ||
| Thymosin β 4 | Stimulates differentiation and mobilization. | Decreased fibrosis, apoptosis, and infarct size |
| Increased expression of antiapoptotic, anti-inflammatory, and antioxidative genes. |
Conclusion
Over the last two decades, science has demonstrated the regenerative capacity of the heart, and initial reports of cardiac regeneration in lower animals fostered analysis of cardiac regeneration in the mammalian heart. Efforts have resulted in numerous clinical trials to assess potential PCs and delivery methods for the treatment of acute and chronic ischemic cardiac disease. Populations of CPCs have been defined with techniques such as genetic fate mapping; however, the identity of CPCs capable of left ventricular regeneration remains undetermined. As a result, mammalian cardiac regeneration remains unrealized in the adult, and therefore, the fetal sheep and neonatal mouse models for cardiac regeneration offer potential to further define CPC niches and biology necessary for regeneration.
Cardiac regeneration remains elusive in the adult heart; however, cardiac renewal is well reported although this ability diminishes with advancing age. This diminished capacity for regeneration and renewal with senescence is likely related to the PC number, which also decreases with age. The limited benefit of adjuncts or the paracrine-mediated effect of cytotherapy is likely related to the senescent population of CPCs in the adult, which lacks the critical number necessary to effectively regenerate the myocardial mass.
CPC function can be evaluated early in development when CPC numbers remain high, and results may be translated to influence a positive environment for recruitment, proliferation, differentiation, and integration in the adult. Further studies are necessary to define gene expression that results in a regenerative phenotype such as previously reported for Gata4, Mef2c, and Tbx5.15–18 Additionally, evaluation of adjunct-mediated changes to CPC function can be investigated with the hope of translating knowledge gained to adult regeneration.
Elucidation of CPC biology and resident CPC location may allow for better therapeutic regimens with adjuncts alone thereby circumventing problems of recruitment, proliferation, differentiation, and integration previously reported with cytotherapy. Alternatively, with CPC biology and location defined, these cells can be harvested, expanded ex vivo, and be administered back to a patient with the aim of induced cardiac regeneration. Therefore, the established models represent significant potential for advancement of knowledge concerning CPCs.
Currently, two mammalian models of cardiac regeneration exist in the neonatal mouse and fetal sheep. Although the mouse model demonstrates dedifferentiation as a mechanism for regeneration, it does not rule out the influence of CPCs. Therefore, both models provide potential to define CPC biology specifically related to recruitment, proliferation, differentiation, and integration. However, only the fetal sheep model allows investigation of cardiac regeneration following ischemic injury as occurs following acute MI. Future translation of CPC function from these models is necessary to modulate reparative to regenerative healing following MI in the adult. Once processes are better defined within these models, knowledge gained will allow for the development of better therapeutic regimens in the treatment of ischemic heart disease.
Take-Home Messages.
Cardiac regeneration in fetal and early neonatal hearts results in restoration of organ architecture and function.
Cardiac regeneration in lower animals occurs by dedifferentiation and proliferation of existing cardiac myocytes.
Evidence demonstrates dedifferentiation in mammalian cardiac regeneration; however, reports demonstrate CPC function is necessary for mammalian cardiac regeneration.
CPCs have been defined and participate in cardiac myocyte renewal during aging, but are unable to trigger cardiac regeneration and are likely related to decreased number of CPCs with aging.
Fate-mapping studies have identified multipotent CPCs and associated progeny.
BM PCs, peripheral PCs, skeletal myoblasts, ckit+ CPCs, CCs, and ESCs demonstrate benefits in the treatment of ischemic cardiac disease, but PC integration and regeneration of functional myocardium have not been demonstrated in clinical trials.
Adjuncts to cytotherapy demonstrate a benefit to residual myocardium following ischemic insult; however, adjuncts have failed to induce cardiac regeneration.
The mammalian models for cardiac regeneration offer potential to study CPC recruitment, differentiation, proliferation, and integration with the hope of translating knowledge gained to improve clinical outcomes with cytotherapy following MI.
Abbreviations and Acronyms
- BM PC
bone marrow-derived progenitor cell
- CABG
coronary artery bypass graft
- CC
cardiosphere-derived cell
- CPC
cardiac progenitor cell
- ESC
embryonic stem cell
- Gata4
gata 4 transcription factor gene
- G-CSF
granulocyte-colony stimulating factor
- Hand1
heart and neural crest derivative expressed protein 1 gene
- LV
left ventricle
- Mef2c
myocyte-specific enhancer factor 2C gene
- MI
myocardial infarction
- PC
progenitor cell
- SDF-1α
stromal-derived factor 1-alpha
- TB4
Thymosin beta-4
- Tbx5
t-box 5 gene
Acknowledgments and Funding Sources
This work was supported by the National Institutes of Health grant 1T32HL105324 through the Center for Excellence in Cardiovascular and Renal Research at the University of Mississippi Medical Center.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article were expressly written by the authors listed. No ghostwriters were used to write this article.
About The Authors
Dr. Michael Morris recently completed a research fellowship under his mentors, Dr. Kenneth Liechty and Dr.Chris Anderson, as an NIH-funded research fellow at the University of Mississippi Medical Center (UMMC). He completed the first 3 years of a general surgery residency at UMMC, and returned for the last 2 years of training in July of 2013 after completion of his research fellowship. His recent research efforts focused on fetal regenerative cardiac healing. He is a resident fellow of the American College of Surgeons, member of the Wound Healing Society, and resident member of the Association for Academic Surgery and the Southeastern Surgical Congress. In addition, he has authored or coauthored multiple articles for peer-reviewed publications. He wishes to pursue a fellowship in pediatric surgery and a career in academic medicine following completion of training. Dr. Kenneth Liechty serves as the Chair of Surgery and Director of Fetal Medicine at Nemours Children's Hospital in Orlando, FL. He received his undergraduate degree in 1989 and his MD in 1994 from the University of Utah. He completed a general surgery residency at the University of Pennsylvania School of Medicine. He completed a 3-year research fellowship in fetal surgery and a clinical fellowship in pediatric surgery at the Children's Hospital of Philadelphia. A fellow of the American College of Surgeons and the American Academy of Pediatrics, Liechty is a member of several professional organizations, including the American Pediatric Surgical Association, the Wound Healing Society, and the Association for Academic Surgery. He serves as an ad-hoc reviewer for a number of professional publications, including the Journal of Clinical Investigation, the Journal of Pediatric Surgery, and Pediatric Research. In addition, he has authored or coauthored more than four dozen articles for peer-reviewed publications. Dr. Liechty is funded by the NIH for his wound-healing research and has extensive experience with fetal wound-healing models.
References
- 1.Roger VL. Go AS. Lloyd-Jones DM. Adams RJ. Berry JD. Brown TM. Carnethon MR. Dai S. de Simone G. Ford ES. Fox CS. Fullerton HJ. Gillespie C. Greenlund KJ. Hailpern SM. Heit JA. Ho PM. Howard VJ. Kissela BM. Kittner SJ. Lackland DT. Lichtman JH. Lisabeth LD. Makuc DM. Marcus GM. Marelli A. Matchar DB. McDermott MM. Meigs JB. Moy CS. Mozaffarian D. Mussolino ME. Nichol G. Paynter NP. Rosamond WD. Sorlie PD. Stafford RS. Turan TN. Turner MB. Wong ND. Wylie-Rosett J. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy D. Kenchaiah S. Larson MG. Benjamin EJ. Kupka MJ. Ho KK. Murabito JM. Vasan RS. Longterm trends in the incidence of and survival with heart failure. NEJM. 2002;347:1397. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 3.Herdrich BJ. Danzer E. Davey M. Allukian M. Englefield V. Gorman JH. Gorman RC. Liechty KW. Regenerative healing following fetal myocardial infarction. Eur J Cardiothorac Surg. 2010;38:691. doi: 10.1016/j.ejcts.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porrello ER. Mahmoud AI. Simpson E. Hill JA. Richardson JA. Olson EN. Sadek HA. Transient regenerative potential of neonatal mouse heart. Science. 2011;331:1078. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutton MG. Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 6.Rowlatt U. Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol. 1979;381:353. doi: 10.1007/BF00432477. [DOI] [PubMed] [Google Scholar]
- 7.Longaker MT. Whitby DJ. Adzick NS. Crombleholme TM. Langer JC. Duncan BW. Bradley SM. Stern R. Ferguson MW. Harrison MR. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25:63. doi: 10.1016/s0022-3468(05)80165-4. [DOI] [PubMed] [Google Scholar]
- 8.Beredjiklian PK. Favata M. Cartmell JS. Flanagan CL. Crombleholme TM. Soslowsky LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31:1143. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- 9.Herdrich BJ. Danzer E. Davey MG. Bermudez DM. Radu A. Zhang L. Zhang Z. Soslowsky LJ. Liechty KW. Fetal tendon wound size modulates wound gene expression and subsequent wound phenotype. Wound Repair Regen. 2010;18:543. doi: 10.1111/j.1524-475X.2010.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberpriller JO. Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool. 1974;187:249. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 11.Poss KD. Wilson LG. Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 12.Steinhauser ML. Lee RT. Regeneration of the heart. EMBO Mol Med. 2011;3:701. doi: 10.1002/emmm.201100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laflamme MA. Murry CE. Heart regeneration. Nature. 2011;473:326. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira-Martins J. Ogórek B. Cappetta D. Matsuda A. Signore S. D'Amario D. Kostyla J. Steadman E. Ide-Iwata N. Sanada F. Iaffaldano G. Ottolenghi S. Hosoda T. Leri A. Kajstura J. Anversa P. Rota M. Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ Res. 2012;110:701. doi: 10.1161/CIRCRESAHA.111.259507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Ieda M. Fu JD. Delgado-Olguin P. Vedantham V. Hayashi Y. Bruneau BG. Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian L. Huang Y. Spencer CI. Foley A. Vedantham V. Liu L. Conway SJ. Fu JD. Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song K. Nam YJ. Luo X. Qi X. Tan W. Huang GN. Acharya A. Smith CL. Tallquist MD. Neilson EG. Hill JA. Bassel-Duby R. Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JX. Krane M. Deutsch MA. Wang L. Rav-Acha M. Gregoire S. Engels MC. Rajarajan K. Karra R. Abel ED. Wu JC. Milan D. Wu SM. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:50. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beltrami AP. Urbanek K. Kajstura J. Yan SM. Finato N. Bussani R. Nadal-Ginard B. Silvestri F. Leri A, et al. Evicence that the human cardiac myocytes divide after myocardial infarction. N Eng J Med. 2001;344:1750. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 20.Beltrami AP. Barlucchi L. Torella D. Baker M. Limana F. Chimenti S. Kasahara H. Rota M. Musso E. Urbanek K, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 21.Urbanek K. Torella D. Sheikh De Angelis A. Nurzynska D. Silvestri F. Beltrami CA. Bussani R. Beltrami AP. Quaini F. Bolli R. Leri A. Kajstura J. Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. PNAS. 2005;102:8692. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergmann O. Bhardwaj RD. Bernard S. Zdunek S. Barnabé-Heider F. Walsh S. Zupicich J. Alkass K. Buchholz BA. Druid H. Jovinge S. Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kajstura J. Urbanek K. Perl S. Hosoda T. Zheng H. Ogórek B. Ferreira-Martins J. Goichberg P. Rondon-Clavo C. Sanada F. D'Amario D. Rota M. Del Monte F. Orlic D. Tisdale J. Leri A. Anversa P. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Ptaszek LM. Mansour M. Ruskin JN. Chien KR. Towards regenerative therapy for cardiac disease. Lancet. 2012;379:933. doi: 10.1016/S0140-6736(12)60075-0. [DOI] [PubMed] [Google Scholar]
- 25.Buckingham M. Meilhac S. Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh PC. Segers VF. Davis ME. MacGillivray C. Gannon J. Molkentin JD. Robbins J. Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ince H. Petzsch M. Kleine HD. Schmidt H. Rehders T. Körber T. Schümichen C. Freund M. Nienaber CA. Preservation from left ventricular remodeling by front-integrated revascularization and stem cell liberation in evolving acute myocardial infarction by use of granulocyte-colony-stimulating factor (FIRSTLINE-AMI) Circulation. 2005;112:3097. doi: 10.1161/CIRCULATIONAHA.105.541433. [DOI] [PubMed] [Google Scholar]
- 28.Zohlnhöfer D. Ott I. Mehilli J. Schömig K. Michalk F. Ibrahim T. Meisetschläger G. von Wedel J. Bollwein H. Seyfarth M. Dirschinger J. Schmitt C. Schwaiger M. Kastrati A. Schömig A REVIVAL-2 Investigators. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295:1003. doi: 10.1681/01.asn.0000926832.82033.c5. [DOI] [PubMed] [Google Scholar]
- 29.Engelmann MG. Theiss HD. Hennig-Theiss C. Huber A. Wintersperger BJ. Werle-Ruedinger AE. Schoenberg SO. Steinbeck G. Franz WM. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. J Am Coll Cardiol. 2006;48:1712. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 30.Zohlnhöfer D. Dibra A. Koppara T. de Waha A. Ripa RS. Kastrup J. Valgimigli M. Schömig A. Kastrati A. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. J Am Coll Cardiol. 2008;51:1429. doi: 10.1016/j.jacc.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 31.Wollert KC. Meyer GP. Lotz J. Ringes-Lichtenberg S. Lippolt P. Breidenbach C. Fichtner S. Korte T. Hornig B. Messinger D. Arseniev L. Hertenstein B. Ganser A. Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 32.Schachinger V. Erbs S. Elsasser A. Haberbosch W. Hambrecht R. Holschermann H. Yu J. Corti R. Mathey DG. Hamm CW. Suselbeck T. Assmus B. Tonn T. Dimmeler S. Zeiher AM REPAIR-AMI Investigators. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 33.Assmus B. Rolf A. Erbs S. Elsässer A. Haberbosch W. Hambrecht R. Tillmanns H. Yu J. Corti R. Mathey DG. Hamm CW. Süselbeck T. Tonn T. Dimmeler S. Dill T. Zeiher AM. Schächinger V REPAIR-AMI Investigators. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3:89. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 34.Traverse JH. Henry TD. Ellis SG. Pepine CJ. Willerson JT. Zhao DX. Forder JR. Byrne BJ. Hatzopoulos AK. Penn MS. Perin EC. Baran KW. Chambers J. Lambert C. Raveendran G. Simon DI. Vaughan DE. Simpson LM. Gee AP. Taylor DA. Cogle CR. Thomas JD. Silva GV. Jorgenson BC. Olson RE. Bowman S. Francescon J. Geither C. Handberg E. Smith DX. Baraniuk S. Piller LB. Loghin C. Aguilar D. Richman S. Zierold C. Bettencourt J. Sayre SL. Vojvodic RW. Skarlatos SI. Gordon DJ. Ebert RF. Kwak M. Moyé LA. Simari RD Cardiovascular Cell Therapy Research Network. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strauer BE. Yousef M. Schannwell CM. The acute and long-term effects of intracoronary Stem cell Transplantation in 191 patients with chronic heARt failure: the STAR-heart study. Eur J Heart Fail. 2010;12:721. doi: 10.1093/eurjhf/hfq095. [DOI] [PubMed] [Google Scholar]
- 36.Menasché P. Alfieri O. Janssens S. McKenna W. Reichenspurner H. Trinquart L. Vilquin JT. Marolleau JP. Seymour B. Larghero J. Lake S. Chatellier G. Solomon S. Desnos M. Hagège AA. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 37.Bolli R. Chugh AR. D'Amario D. Loughran JH. Stoddard MF. Ikram S. Beache GM. Wagner SG. Leri A. Hosoda T. Sanada F. Elmore JB. Goichberg P. Cappetta D. Solankhi NK. Fahsah I. Rokosh DG. Slaughter MS. Kajstura J. Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Chimenti I. Smith RR. Li TS. Gerstenblith G. Messina E. Giacomello A. Marbán E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makkar RR. Smith RR. Cheng K. Malliaras K. Thomson LE. Berman D. Czer LS. Marbán L. Mendizabal A. Johnston PV. Russell SD. Schuleri KH. Lardo AC. Gerstenblith G. Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiba Y. Fernandes S. Zhu WZ. Filice D. Muskheli V. Kim J. Palpant NJ. Gantz J. Moyes KW. Reinecke H. Van Biber B. Dardas T. Mignone JL. Izawa A. Hanna R. Viswanathan M. Gold JD. Kotlikoff MI. Sarvazyan N. Kay MW. Murry CE. Laflamme MA. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyle AJ. Yeghiazarians Y. Shih H. Hwang J. Ye Jianqin Sievers R. Zheng D. Palasubramanian J. Palasubramanian D. Karschimkus C. Whitbourn Jenkins A. Wilson AM. Myocardial production and release of MCP-1 and SDF-1 following myocardial infarction: differences between mice and man. J Transl Med. 2011;9:150. doi: 10.1186/1479-5876-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J. Chemaly E. Liang L. Kho C. Lee A. Park J. Altman P. Schecter AD. Hajjar RJ. Tarzami ST. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. Am J Pathol. 2010;176:1705. doi: 10.2353/ajpath.2010.090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbott JD. Huang Y. Liu D. Hickey R. Krause DS. Giordano FJ. Stromal cell-derived factor-1 alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 44.Unzek S. Zhang M. Mal N. Mills WR. Laurita KR. Penn MS. SDF-1 recruits cardiac stem cell-like cells that depolarize in vivo. Cell Transplant. 2007;16:879. doi: 10.3727/096368907783338271. [DOI] [PubMed] [Google Scholar]
- 45.Hatzistergos KE. Quevedo H. Oskouei BN. Hu Q. Feigenbaum GS. Margitich IS. Mazhari R. Boyle AJ. Zambrano JP. Rodriguez JE. Dulce R. Pattany PM. Valdes D. Revilla C. Heldman AW. McNiece I. Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Askari AT. Unzek S. Popovic ZB. Goldman CK. Forudi F. Kiedrowski M. Rovner A. Ellis SG. Thomas JD. DiCorleto PE. Topol EJ. Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 47.Saxena A. Fish JE. White MD. Yu S. Smyth JW. Shaw RM. DiMaio JM. Srivastava D. Stromal cell-derived factor-1 alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghadge SK. Mühlstedt S. Ozcelik C. Bader M. SDF-1α as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129:97. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Harada M. Qin Y. Takano H. Minamino T. Zou Y. Toko H. Ohtsuka M. Matsuura K. Sano M. Nishi J. Iwanaga K. Akazawa H. Kunieda T. Zhu W. Hasegawa H. Kunisada K. Nagai T. Nakaya H. Yamauchi-Takihara K. Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11:305. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 50.Baldo MP. Rodrigues SL. Mill JG. Acute effects of granulocyte colony-stimulating factor on early ventricular arrhythmias after coronary occlusion in rats. J Pharmacol Pharmacother. 2012;3:39. doi: 10.4103/0976-500X.92508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldstein AL. Hannappel E. Sosne G. Kleinman HK. Thymosin β4: a multi-functional regenerative peptide. Basic properties and clinical applications. Expert Opin Biol Ther. 2012;12:37. doi: 10.1517/14712598.2012.634793. [DOI] [PubMed] [Google Scholar]
- 52.Gajzer DC. Balbin J. Chaudhry HW. Thymosin β4 and cardiac regeneration: are we missing a beat? Stem Cell Rev. 2013;9:303. doi: 10.1007/s12015-012-9378-3. [DOI] [PubMed] [Google Scholar]
- Wei C. Kumar S. Kim IK. Gupta S. Thymosin Beta 4 protects cardiomyocytes from oxidative stress by targeting anti-oxidative enzymes and anti-apoptotic genes. PLoS One. 2012;7:e42586. doi: 10.1371/journal.pone.0042586. [DOI] [PMC free article] [PubMed] [Google Scholar]