Abstract
Electrospinning is a popular technique to fabricate tissue engineering scaffolds due to the exceptional tunability of fiber morphology that can be used to control scaffold mechanical properties, degradation rate, and cell behavior. Although the effects of modulating processing or solution parameters on fiber morphology have been extensively studied, there remains limited understanding of the impact of environmental parameters such as humidity. To address this gap, three polymers (poly(ethylene glycol) [PEG], polycaprolactone [PCL], and poly(carbonate urethane) [PCU]) were electrospun at a range of relative humidities (RH=5%–75%) and the resulting fiber architecture characterized with scanning electron microscopy. Low relative humidity (<50%) resulted in fiber breakage for all three polymers due to decreased electrostatic discharge from the jet. At high relative humidity (>50%), three distinct effects were observed based on individual polymer properties. An increase in fiber breakage and loss of fiber morphology occurred in the PEG system as a result of increased water absorption at high relative humidity. In contrast, surface pores on PCL fibers were observed and hypothesized to have formed via vapor-induced phase separation. Finally, decreased PCU fiber collection occurred at high humidity likely due to increased electrostatic discharge. These findings highlight that the effects of relative humidity on electrospun fiber morphology are dependent on polymer hydrophobicity, solvent miscibility with water, and solvent volatility. An additional study was conducted to highlight that small changes in molecular weight can strongly influence solution viscosity and resulting fiber morphology. We propose that solution viscosity rather than concentration is a more useful parameter to report in electrospinning methodology to enable reproduction of findings. In summary, this study further elucidates key mechanisms in electrospun fiber formation that can be utilized to fabricate tissue engineering scaffolds with tunable and reproducible properties.
Introduction
Tissue engineering aims to provide a temporary matrix and appropriate cues to facilitate regeneration of damaged tissue. To achieve functional repair, the biomaterial scaffold needs to have appropriate mechanical properties to restore function to the damaged tissue while supporting cell growth throughout remodeling. In addition to material chemistry, the 3D microarchitecture of the scaffold plays a large role in graft properties and cell behavior. Given that the fabrication technique dictates this microarchitecture, enormous efforts have been made to develop strategies that are both highly tunable and exhibit fine control of the resulting architecture. Electrospinning has gained popularity in recent years as a technique to generate fibrous scaffolds with high porosities, large surface area-to-volume ratios, and nano- to micron-sized fiber diameters.1–4 The relative ease of modulating fiber architecture provides a means to control scaffold properties. For example, fiber alignment and fiber diameter have been shown to influence mechanical properties,5–8 degradation rate,9,10 and cell growth.10–15 Thus, electrospun scaffold properties can be tailored to meet the specific design criteria of a wide range of tissues. Due in part to this tunability, electrospun scaffolds have been investigated for regeneration of vasculature,16–18 nerve,19–21 skin,22–24 and bone.25,26
Electrospinning involves pumping a polymer solution at a constant rate through a needle tip that is placed a set distance away from a grounded or oppositely charged collector. When a voltage is applied at the needle tip, the electrostatic forces within the droplet overcome the surface tension of the droplet at the needle tip, causing a liquid jet to erupt from the needle. The electrically charged jet undergoes a bending instability, which causes it to rapidly whip in multiple expanding loops as it travels to the collector. This whipping process causes stretching and thinning into micro- and nanometer diameter fibers and facilitates solvent evaporation.4 The fiber mesh can then be removed from the collector and utilized for various tissue engineering applications. The fiber morphology and diameter are dependent on a number of processing parameters that are typically divided into the intrinsic properties of the solution (e.g., polymer, concentration, conductivity, polarity, and surface tension of the solvent) and the operational conditions (e.g., strength of the applied electric field, distance to the collector, and flow rate). An understanding of electrospun fiber formation and modulation of fiber morphology is necessary for precisely control of scaffold properties. To this end, systematic studies have examined the roles of various processing and solution parameters on fiber morphology (Table 1).
Table 1.
Effects of Processing and Solutions Parameters on Electrospun Fiber Morphology
| Effect on fiber morphology | |
|---|---|
| Processing parameters | |
| Applied voltage | Increasing applied voltage can result in |
| Increased beading27–31 | |
| Decreased fiber diameter29,32–35 | |
| Distance to collector | Increasing distance to collector can result in |
| Decreased fiber diameter35,36 | |
| Decreased fiber wetness33,34 | |
| Flow rate | Increasing flow rate can result in |
| Increased fiber wetness37 | |
| Increased bead formation30,36–40 | |
| Solution parameters | |
| Polymer molecular weight | Increasing molecular weight can result in decreased beading29,41–43 |
| Polymer concentration | Increasing concentration can result in |
| Decreased beading27–30,32–38,42–54 | |
| Increased fiber diameter28,31,38,41,42,45–48,51,53,55,56 | |
| Solution conductivity | Increasing conductivity through the addition of salt can result in |
| Defect-free fibers27,39,50,57 | |
| Smaller diameter fibers45,47 | |
| Solvent dielectric constant | Increasing dielectric constant can result in decreased bead formation40,49,58 |
Fong et al. proposed that fiber formation is dependent on the balance of forces caused by surface tension, density of net charges on the jet, and solution viscosity.50 Surface tension drives conversion of the liquid jet into one or many spherical droplets to reduce surface area (Rayleigh instability), whereas the electrostatic repulsion between charges on the jet opposes this force and favors increased surface area and the formation of a thin jet. Viscoelastic forces in a polymer solution resists deformation changes in shape and also supports the formation of fibers. By shifting the balance of these forces, a range of fiber morphologies can be achieved. For example, bead formation can be eliminated by enhancing the effects of viscoelastic and charge repulsion forces over surface tension effects.59 Although there are well-documented effects of solution concentration on fiber morphology,27,33,44,50,60,61 it is the resultant change in solution viscosity as described above that is the root cause of these changes to the electrospun fiber morphology. Given that small variations in molecular weight and distribution are typical in different batches of polymers and can strongly influence viscosity, it is often impossible to reproduce fiber architecture by using the solution concentration reported in electrospinning literature. In addition, environmental parameters such as humidity are often not reported and have a strong impact on fiber morphology, which makes reproduction of electrospun architectures from the literature even more difficult. The effects of humidity on fiber morphology are poorly understood and/or there are contradictory effects that have been observed that appear to be dependent on properties such as the type of polymer, polymer–solvent combination, molecular weight, polymer hydrophilicity, and size of the electrospun structure.62–66 Both the incomplete reporting of electrospinning conditions and the limited understanding of environmental effects on fiber formation make it difficult to predict and reproduce electrospun scaffold microarchitecture.
In this study, we aim to further elucidate of the process of fiber formation under various electrospinning conditions in order to improve control of fiber morphology and scaffold reproducibility. Three commonly used polymeric biomaterials (poly(carbonate urethane) [PCU], polycaprolactone [PCL], poly(ethylene glycol) [PEG]) were electrospun through a range of relative humidity (RH) levels (5%–75%) and the effect on fiber morphology was monitored with scanning electron microscopy (SEM). These polymers represent a broad range of hydrophobicity and therefore will provide predictive information of the effect of humidity on fiber formation for a wide range of polymeric biomaterials. To highlight the effect of viscosity, two batches of the commercially available PCU with different molecular weights were electrospun while maintaining either concentration or viscosity constant. The overall goal of this work is to elucidate mechanisms of electrospun fiber formation and utilize this understanding to provide a systematic method to fabricate scaffolds with tunable and reproducible properties for tissue engineering applications.
Materials and Methods
Materials
PEG (molecular weight [Mw]=35,000 Da; Sigma Aldrich), PCL (Mw=70,000 Da; Scientific Polymer Products), and PCU (Carbothane® PC3575A, Mw=217,000 Da; Lubrizol Advanced Materials) were purchased and used as received. The PCU had a polycarbonate diol soft segment and a 4,4′-methylenebis(cyclohexyl isocyanate) (H12MDI) hard segment. A second batch of PCU with Mw=241,000 Da was purchased to study the effects of solution viscosity on fiber morphology. All other chemicals were purchased from Sigma Aldrich and used as received.
Water contact angle
Films were fabricated by spin coating (Spincoat G3P-8; Specialty Coating Systems) the electrospinning solutions onto glass slides at 750 rpm for 50 s and allowed to dry in a fume hood for 24 h. Static water contact angle was determined using an optical tensiometer (CAM 200; KSV Instruments). A 5-μL droplet of deionized water was placed on the film and contact angle was measured after allowing the surface to equilibrate after 60 s (n=5).
Rheology
The zero shear viscosity was measured for the polymer solutions using a rheometer with a cone-plate configuration (Physica MCR 301; Anton Paar, n=4). Temperature of the cone and plate were kept constant at 20.5°C. The data are displayed as mean±standard deviation for each composition. A Student's t-test was performed to determine any statistically significant differences between compositions. All tests were carried out at a 99% confidence interval (p<0.01).
Electrospinning
PEG, PCL, and PCU were each mixed with solvent (Table 2) at ambient conditions to make the electrospinning solutions. The polymer solution was poured into a 10 mL glass syringe with a blunted 20-gauge needle tip and the solution was pumped at a constant rate of 1.0 mL/h using a syringe pump (KDS100; KDScientific). Fibers were collected onto a grounded 6-inch-square copper plate that was covered with a PET film to facilitate removal of the fiber mesh. For PCL and PEG, the collector was positioned 12 cm away from the needle tip and a voltage of 10 kV (ES30P-5W/DDPM, Gamma Scientific) was applied at the needle tip. PCU was electrospun at a distance of 40 cm with 15 kV applied voltage. No additional methods were implemented to concentrate or direct fibers on the collector. Electrospinning was performed for 10 min for each run. Solution viscosity studies were all performed at ambient conditions (50% RH, 20°C). PCU fibers spun for the viscosity study were electrospun onto a 5-mm-diameter negatively charged (−5 kV) stainless steel rotating mandrel placed 50 cm from the needle tip.
Table 2.
Polymer Solutions and Properties Used for Electrospinning
|
Solution properties | |||
|---|---|---|---|
| Polymer | Solvent | Solution viscosity (Pa·s) | Concentration (wt%) |
| PCU | N,N-dimethylacetamide (DMAc) | 10.3±0.4 | 25 |
| PCL | chloroform:N,N-dimethylformamide (DMF) 80:20 | 9.5±4.8 | 18 |
| PEG | chloroform | 45.3±4.2 | 25 |
|
Solvent properties | |||
|---|---|---|---|
| Solvent | Boiling point (°C) | Water miscibility | Dielectric constant |
| Water | 100 | — | 80.0 |
| Chloroform | 61 | Immiscible | 4.8 |
| DMF | 153 | Miscible | 36.7 |
| DMAc | 166 | Miscible | 37.8 |
PEG, poly(ethylene glycol); PCU, poly(carbonate urethane); PCL, polycaprolactone.
Electrospinning was performed in a sealed acrylic box to maintain constant humidity levels. To increase relative humidity, a humidifier (ReptiFogger; Zoo Med) with a humidity controller (HygroTherm, Zoo Med) was used. To decrease relative humidity, compressed air was pumped through Drierite™ and into the sealed box until the humidity reached the desired level. Each polymer was electrospun at 5%, 20%, 35%, 50%, 60%, and 75% RH (±5% RH). Relative humidity was recorded at the beginning and end of each run using a hygrometer (HI 9569, Hanna Instruments, accuracy±4%). Temperature of the room was controlled at 20.5°C and was measured and recorded in the electrospinning chamber at the beginning and end of each run.
Electrospun fiber characterization
Specimens approximately 7×7 cm were cut from the center of each fiber mesh to avoid edge effects (three specimens per mesh). Fiber morphology was observed using SEM (JEOL NeoScope JCM-5000) at 5 kV accelerating voltage. Before imaging, specimens were coated with 4 nm of gold using a sputter coater (Sputter Coater 108; Cressingtion Scientific Instruments). Fiber morphology was analyzed on specimens from four separate runs at each relative humidity level. Images at 2000×, 1000×, and 500× magnifications of each mesh specimen were analyzed to determine representative morphologies. The percentage broken fibers was determined by counting the number of broken fibers out of the total that crossed the midline of each 1000× image. Fiber diameter was measured from the first 5 fibers to cross the midline of each 2000× micrograph. Fiber density was measured using photo-editing software (GIMP) on 2000× magnification micrographs. First, a transparent layer was created on which visible fibers were fully traced in a solid black color using a paintbrush tool. The total pixels drawn in the transparent layer (equal to the total area of deposited fibers) was determined from the histogram. Fiber density was equal to the total fiber area (pixels) divided by the total image area (pixels).
Results and Discussion
Effects of solution viscosity
First, the effect of solution viscosity on electrospun fiber morphology was investigated by mixing solutions of PCU (217 kDa) in DMAc at concentrations ranging from 15 to 20 wt%. As expected, increasing solution concentration resulted in increased viscosity. Representative images of fibers spun at low, intermediate, and high viscosity (Fig. 1) revealed beaded fibers at low viscosity (7.2±1.7 Pa·s), uniform fibers at an intermediate viscosity (10.1±0.5 Pa·s), and larger fibers at high viscosity (22.5±1.4 Pa·s) (fiber diameter increased from 1.2±0.6 μm to 3.5±0.9 μm). As expected, the lower concentration solution had insufficient viscosity to resist fiber deformation without defect at the given electric field. One scenario of bead formation in electrospun fibers occurs when the surface tension in the charged jet is sufficient to change the jet into droplets to reduce surface area.50 This is opposed by viscoelastic forces in the jet that resist changes to the fiber shape. Therefore, it was hypothesized that the low concentrations studied here generated jets with insufficient viscoelastic forces to fully suppress droplet breakup due to the Rayleigh instability. In contrast, the increased viscosity of the higher concentration solutions created higher viscoelastic forces that resisted the axial stretching during whipping, resulting in larger fiber diameter. These observed effects of concentration/viscosity agree with literature reports.42,46,48,50 From these results, ∼10 Pa·s viscosity was selected as the viscosity where dry, uniform fibers were collected for this system.
FIG. 1.
(A) Solution viscosity of PCU (217 kDa) in DMAc as a function of concentration, n=4, *p<0.01. (B–D) Scanning electron micrographs of PCU (217 kDa) electrospun at varying solution viscosities: (B) 7 Pa·s, (C) 13 Pa·s, and (D) 23 Pa·s. PCU, poly(carbonate urethane).
The effect of solution viscosity on scaffold reproducibility was then examined by comparing two different lots of commercially available PCU. We hypothesize that a small change in molecular weight due to batch-to-batch variability causes a corollary change in viscosity that influences electrospun fiber morphology. Gel permeation chromatography analysis of 2 different batches of PCU revealed that the molecular weights differed by ∼24 kDa (Mw=241 kDa, PDI=2.3; Mw=217 kDa, PDI=2.3). This relatively small change in molecular weight was correlated with a 20% reduction in viscosity of 17 wt% polymer solutions (10 and 8 Pa·s, respectively). The higher molecular weight PCU (241 kDa) electrospun at 17 wt% concentration and 10 Pa·s viscosity resulted in dry, uniform fibers (Fig. 2A). The lower molecular weight PCU (217 kDa) solution electrospun at the same concentration (17 wt%) had a decreased viscosity (8 Pa·s) and the resulting fiber morphology under identical spinning conditions was characterized as beads-on-strings (Fig. 2B). It was assumed that the reduced viscosity resulted in an imbalance in viscous solution force and electrostatic force necessary for uniform fiber formation, as described above. To obtain uniform fiber morphologies, the viscosities of the low- and high-molecular-weight solutions were matched, rather than the concentrations. When the lower molecular weight solution concentration was increased to 18 wt%, the viscosity increased to 10 Pa·s, which was comparable to the higher molecular weight solution at 17 wt%. This solution resulted in dry, uniform fibers that were similar to the higher molecular weight PCU (Fig. 2C). These results highlight that solution viscosity, rather than concentration, is a better determinant of fiber morphology and is a more useful parameter to report in electrospinning methodology to enable reproduction of findings.
FIG. 2.

Scanning electron micrographs of PCU spun at different molecular weights, concentrations, and viscosities: (A) 241±2 kDa, 17 wt%, 10 Pa·s, (B) 217±2 kDa, 17 wt%, 8 Pa·s, and (C) 217±2 kDa, 18 wt%, 10 Pa·s. MW, molecular weight.
Effects of relative humidity
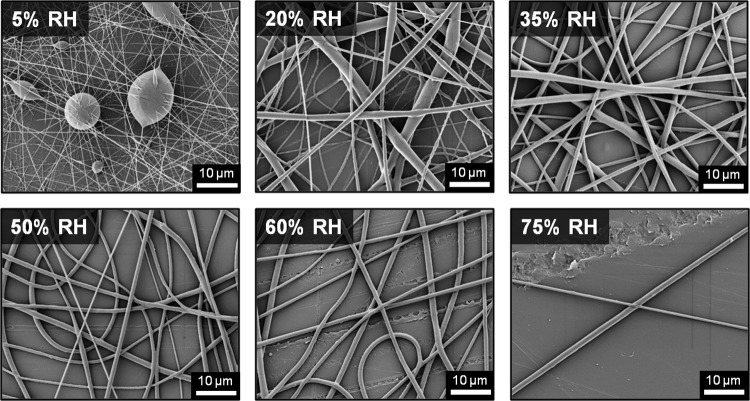
The effects of relative humidity on electrospun fiber morphology were hypothesized to be influenced by polymer hydrophobicity, solvent properties, and applied charge. The static contact angle of each polymer was used as a relative measure of hydrophobicity. The PCU was the most hydrophobic (110.6°±5.2°), PCL had an intermediate hydrophobicity (84.1°±1.6°), and PEG was hydrophilic (14.6°±1.6°). Solvents for each polymer were selected such that each would fully dissolve and facilitate the electrospinning process. A list of solution characteristics were identified to elucidate differences in the solutions that could potentially affect fiber formation and morphology (Table 2). Solvent boiling point was used as a relative measure of volatility and therefore fiber drying rate. The miscibility of water with the solvent was used as a relative predictor of the likelihood of water absorption at high humidity levels. Finally, solvent dielectric constant relative to water was used to provide a measure of solution conductivity. Electrospinning parameters were first determined at 50% RH such that dry, uniform fibers were produced. PCL and PEG could be electrospun using the same processing parameters, but it was necessary to increase the distance from tip to collector and the applied voltage to fabricate dry PCU fibers due to the low volatility of DMAc. A corollary increase in the applied voltage was necessary to accelerate the jet to the collector over this larger distance. These parameters were then held constant while humidity was varied from 5% to 75%.
At low humidity (<50% RH), PEG meshes were characterized by broken fibers with the number of breakages increasing as the relative humidity decreased. Uniform, continuous fibers formed at 50% RH and increasing relative humidity to 60% resulted in broken fibers of distinctly larger diameter and ball-shaped fiber ends. Fibrous morphology was not evident at 75% RH; instead, a polymer sheet indicative of jet beading was observed (Fig. 3). Fiber breakage at low relative humidity was attributed to the increased electrostatic charge on the spinning jet due to reduced water vapor in the air. During electrospinning, a portion of charge from the electrospinning jet is discharged to the water vapor molecules in the atmosphere.67 This is facilitated by the high dielectric constant of water (Table 2), indicating that it has a high energy storage capacity by means of polarization. Therefore, a decrease in water vapor (low humidity environment) results in a decreased amount of electrostatic discharge since fewer water molecules are available for charge transfer. As a result, the charge density on the jet is higher at low humidity causing fiber breakage. This is most likely a different mechanism than the bead or bead-on-string morphologies seen with low viscosity solutions that is driven by surface tension, described above. We hypothesize that the fiber breakage observed at low humidity is due to increased stretching of the jet during whipping after sufficient solvent evaporation to retain fiber morphologies. At high relative humidity, the fiber breakage and loss of morphology was attributed to water absorption and polymer dissolution given the known solubility of PEG in water. PCL fibers also exhibited a morphology characterized by broken fibers below 50% RH but formed thicker fibers with surface pores above 50% RH (Fig. 4). The number and density of surface pores increased as relative humidity was increased. Broken fibers at low humidity (<50% RH) was attributed to the same decreased electrostatic charge dissipation as described for PEG. The effect of increased electrostatic force was demonstrated by increasing the applied voltage on a run that otherwise formed continuous fibers. PCL formed continuous fibers when electrospun at 50% RH and 10 kV applied voltage, whereas broken fibers, similar in morphology to the low humidity runs, formed when the voltage was increased to 16 kV (Fig. 5). The presence of surface pores at high humidity (>50% RH) was hypothesized to be caused by vapor-induced phase separation (VIPS).60,63,68–71 Typically, a hydrophobic polymer, high volatility solvent, and a water-miscible solvent are necessary to facilitate VIPS. First, water vapor is absorbed into the jet due to the water-miscible solvent. Phase separation occurs as the hydrophobic polymer precipitates out of the solution when water is introduced, creating polymer-rich and polymer-poor regions within the jet. Finally, rapid evaporation of the highly volatile solvent locks in the phase-separated geometry, resulting in fibers with surface pores. Pores created by VIPS are typically irregularly shaped, as has been documented in the literature.60,62,63,70 In this study, DMF in the solvent mixture allows for water absorption, hydrophobic PCL precipitates out upon absorption of water, and the highly volatile chloroform evaporates rapidly to lock in the pore geometry.
FIG. 3.
Scanning electron micrographs of poly(ethylene glycol) (PEG) electrospun at relative humidity (RH) ranging from 5% to 75%.
FIG. 4.
Scanning electron micrographs of polycaprolactone (PCL) electrospun at relative humidity ranging from 5% to 75%.
FIG. 5.

Scanning electron micrographs of PCL electrospun at 50% RH and with either (A) 10 kV or (B) 16 kV applied voltage.
PCU fibers electrospun at low humidity (5% RH) resulted in beads connected by thin fibers, but increasing the RH (20%–75% RH) resulted in smooth, uniform fibers (Fig. 6). Additionally, fiber density decreased as relative humidity was increased from 50% to 75%. Similar to PEG and PCL, it was assumed that reduced charge dissipation at low humidities (<50% RH) resulted in increased electrostatic forces that stretch the jet during its flight to the collector. However, both PEG and PCL solutions had a shorter distance the collector and a higher drying rate than PCU as indicated by solvent boiling point (Table 2). This increased drying rate permitted sufficient fiber solidification before jet breakage to retain fiber morphologies, whereas the slower drying PCU had fiber retraction into beads after jet breakage resulting in the observed large beads and nanofibers. Unlike PCL, the PCU did not exhibit a porous surface morphology at high RH (60%–75% RH) despite being a hydrophobic polymer in a water-miscible solvent. It was hypothesized that the lack of surface pores on PCU was a result of the low volatility of DMAc that does not permit rapid evaporation to lock in the phase-separated geometry. Instead, a decrease in fiber density as relative humidity increases was observed and attributed to the increased amount of water vapor that facilitates electrostatic discharge. In this scenario, the electric field is unable to selectively direct the jet toward the collector after discharge resulting in reduced fiber collection. This effect was also observed when the distance to the collector was increased, which increases jet discharge independent of humidity (Fig. 7). Fibers collected over 50 cm while maintaining humidity at 50% RH resulted in a decreased fiber density (0.36±0.07 and 0.05±0.01 fibers/image area for 40 and 50 cm, respectively) due to greater electrostatic discharge, similar to meshes electrospun at high relative humidity. Literature reports of electrospinning a solution with these properties (hydrophobic polymer, water miscible solvent, and low volatility) have shown it is also possible to fabricate fibers with porous cores.68,69
FIG. 6.
Scanning electron micrographs of poly(carbonate urethane) (PCU) electrospun at relative humidity ranging from 5% to 75%.
FIG. 7.

Scanning electron micrographs of PCU electrospun at 50% RH for 10 min and at a distance of (A) 40 cm or (B) 50 cm.
In order to provide a general overview of the role of humidity on fiber formation, the fiber morphologies for PEG, PCL, and PCU at 5%–75% RH are summarized in Table 3. First, the amount of fiber breakage for PEG and PCL at low RH was quantified, and a general trend of decreased humidity resulting in increased fiber breakage was observed. This finding is expected given that decreased levels of electrostatic discharge are expected to result in increased fiber breakage, as described above. The differences in fiber morphology at high relative humidity were attributed to differences in polymer hydrophobicity, water miscibility, and volatility of the solvent. In addition to the morphologies demonstrated in this study, high humidity conditions can also generate surface pores via thermally induced phase separation (TIPS) during electrospinning. Based on the current findings and review of the literature, TIPS was generally associated with a hydrophobic polymer dissolved in a water miscible and highly volatile solvent. In this process, rapid evaporation of the solvent creates local decreases in temperature at the surface of the jet that results in water vapor condensation. These water droplets are trapped on the skin layer and create circular indentations. After the fiber solidifies and the water droplets evaporate, surface pores are created in the form of breath figures. Pores formed through TIPS are typically circular since the pores are molded by water droplets.62,65 A summary of the theories describing formation of pores on hydrophobic polymer fibers is illustrated in Figure 8.
Table 3.
Description of the Changes in Fiber Morphology in the Range of 5% to 75% Relative Humidity
| 5% RH | 20% RH | 35% RH | 50% RH | 60% RH | 75% RH | |
|---|---|---|---|---|---|---|
| PEG | 21%±22% broken fibers | 14%±18% broken fibers | 21%±18% broken fibers | 9%±10% broken fibers | Short, broken fibers with beading | Flat beads with surface morphology |
| PCL | 34%±18% broken fibers | 49%±17% broken fibers | 32%±16% broken fibers | 3%±4% broken fibers | Porous fibers | Porous fibers |
| PCU | Beads-on-strings | Both large and small diameter fibers | Increased fiber deposition | Smooth, uniform fibers | Decreased fiber deposition | Minimal fiber deposition |
RH, relative humidity.
FIG. 8.

Different fiber morphologies observed for hydrophobic polymer electrospun at high percent RH with different solvents.
Conclusions
These studies demonstrate the effects of both solution viscosity and relative humidity on the morphology of electrospun fibers. Importantly, we have illustrated the importance of reporting and matching solution viscosity, rather than concentration, and humidity for improved reproducibility of fiber architectures between studies. Key mechanisms governing fiber formation at different humidity levels were identified for three commonly used polymeric biomaterials of different physical properties. Previously unclear and contradictory mechanisms based on polymer and solvent properties have been elucidated to provide a system for predicting and tuning fiber morphology based on humidity and polymer hydrophobicity. Fiber breakage occurred for each of the polymers at low humidity, but the effects at high humidity were dependent on the polymer hydrophobicity as well as the solvent volatility and miscibility with water. These mechanisms are widely applicable to the design of electrospinning methodology for other synthetic polymers as well as natural polymers such as collagen, cellulose, and chitosan. Enhanced understanding of these fiber formation mechanisms provides better control of fiber morphology in order to fabricate tissue engineering scaffolds with improved properties.
Disclosure Statement
No competing financial interests exist.
References
- 1.Reneker D. Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7:216. [Google Scholar]
- 2.Hohman M. Shin M. Rutledge G. Brenner M. Electrospinning and electrically forced jets. I. Stability theory. Phys Fluids. 2001;13:2201. [Google Scholar]
- 3.Shin Y.M. Hohman M.M. Brenner M.P. Rutledge G.C. Electrospinning: a whipping fluid jet generates submicron polymer fibers. Appl Phys Lett. 2001;78:1149. [Google Scholar]
- 4.Doshi J. Reneker D.H. Electrospinning process and applications of electrospun fibers. J Electrost. 1995;35:151. [Google Scholar]
- 5.Lee K. Kim H. Ryu Y. Kim K. Choi S. Mechanical behavior of electrospun fiber mats of poly(vinyl chloride)/polyurethane polyblends. J Polym Sci Part B Polym Phys. 2003;41:1256. [Google Scholar]
- 6.Tan E.P.S. Ng S.Y. Lim C.T. Tensile testing of a single ultrafine polymeric fiber. Biomaterials. 2005;26:1453. doi: 10.1016/j.biomaterials.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Wong S.-C. Baji A. Leng S. Effect of fiber diameter on tensile properties of electrospun poly(ɛ-caprolactone) Polymer. 2008;49:4713. [Google Scholar]
- 8.Stylianopoulos T. Bashur C. Goldstein A. Guelcher S.A. Barocas V. Computational predictions of the tensile properties of electrospun fibre meshes: Effect of fibre diameter and fibre orientation. J Mech Behav Biomed Mater. 2008;1:326. doi: 10.1016/j.jmbbm.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K. Yu M. Zong X. Chiu J. Fang D. Seo Y.-S. Hsiao B.S. Chu B. Hadjiargyrou M. Control of degradation rate and hydrophilicity in electrospun non-woven poly(d,l-lactide) nanofiber scaffolds for biomedical applications. Biomaterials. 2003;24:4977. doi: 10.1016/s0142-9612(03)00407-1. [DOI] [PubMed] [Google Scholar]
- 10.Bolgen N. Menceloglu Y. Acatay K. Vargel I. Piskin E. In vitro and in vivo degradation of non-woven materials made of poly(ɛ-caprolactone) nanofibers prepared by electrospinning under different conditions. J Biomater Sci Polymer Edn. 2005;16:1537. doi: 10.1163/156856205774576655. [DOI] [PubMed] [Google Scholar]
- 11.Yang F. Murugan R. Wang S. Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 12.Chew S. Mi R. Hoke A. Leong K. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials. 2008;29:653. doi: 10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keun Kwon I. Kidoaki S. Matsuda T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: structural characteristics, mechanical properties and cell adhesion potential. Biomaterials. 2005;26:3929. doi: 10.1016/j.biomaterials.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Baiguera S. del Gaudio C. Fioravanzo L. Bianco A. Grigioni M. Folin M. In vitro astrocyte and cerebral endothelial cell response to electrospun poly(e-caprolactone) mats of different architecture. J Mater Sci Mater Med. 2010;21:1353. doi: 10.1007/s10856-009-3944-5. [DOI] [PubMed] [Google Scholar]
- 15.Gupta D. Venugopal J. Prabhakaran M. Dev V. Low S. Choon A. Ramakrishna S. Aligned and random nanofibrous substrate for the in vitro culture of Schwann cells for neural tissue engineering. Acta Biomater. 2009;5:2560. doi: 10.1016/j.actbio.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Xu C. Inai R. Kotaki M. Ramakrishna S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004;10:1160. doi: 10.1089/ten.2004.10.1160. [DOI] [PubMed] [Google Scholar]
- 17.Soletti L. Hong Y. Guan J. Stankus J. El-Kurdi M. Wagner W. Vorp D. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomater. 2010;6:110. doi: 10.1016/j.actbio.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong Y. Ye S. Nienponice A. Soletti L. Vorp D. Wagner W. A small diameter, fibrous vascular conduit generated from a poly(ester urethane)urea and a phospholipid polymer blend. Biomaterials. 2009;30:2457. doi: 10.1016/j.biomaterials.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bini T. Gao S. Tan T. Wang S. Lim A. Hai L. Ramakrishna S. Electrospun poly(L-lactide-co-glycolide) biodegradable polymer nanofibre tubes for peripheral nerve regeneration. Nanotechnology. 2004;15:1459. [Google Scholar]
- 20.Ghasemi-Mobarakeh L. Prabhakaran M. Morshed M. Nasr-Esfahani M. Ramakrishna S. Electrospun poly(e-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue enginering. Biomaterials. 2008;29:4532. doi: 10.1016/j.biomaterials.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Prabhakaran M. Venugopal J. Chyan T. Hai L. Chan C. Lim A. Ramakrishna S. Electrospun biocomposite nanofibrous scaffolds for neural tissue engineering. Tissue Eng Part A. 2008;14:1787. doi: 10.1089/ten.tea.2007.0393. [DOI] [PubMed] [Google Scholar]
- 22.Kumbar S.G. Nukavarapu S.P. James R. Nair L.S. Laurencin C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials. 2008;29:4100. doi: 10.1016/j.biomaterials.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X. Cui W. Li X. Jin Y. Electrospun fibrous mats with high porosity as potential scaffolds for skin tissue engineering. Biomacromolecules. 2008;9:1795. doi: 10.1021/bm800476u. [DOI] [PubMed] [Google Scholar]
- 24.Khil M. Cha D. Kim H. Kim I. Bhattarai N. Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mater Res Part B. 2003;67B:675. doi: 10.1002/jbm.b.10058. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimoto H. Shin Y. Terai H. Vacanti J. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 26.Li C. Vepari C. Jin H.-J. Kim H.J. Kaplan D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Jarusuwannapoom T. Hongrojjanawiwat W. Jitjaicham S. Wannatong L. Nithitanakul M. Pattamaprom C. Koombhongse P. Rangkupan R. Supaphol P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur Polym J. 2005;41:409. [Google Scholar]
- 28.Ki C.S. Baek D.H. Gang K.D. Lee K.H. Um I.C. Park Y.H. Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer. 2005;46:5094. [Google Scholar]
- 29.Geng X. Kwon O. Jang J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials. 2005;26:5427. doi: 10.1016/j.biomaterials.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 30.Zong X. Kim K. Fang D. Ran S. Hsiao B. Chu B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer. 2002;43:4403. [Google Scholar]
- 31.Deitzel J. Kleinmeyer J. Harris D. Tan N. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer. 2001;42:261. [Google Scholar]
- 32.Katti D.S. Robinson K.W. Ko F.K. Laurencin C.T. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res Part B. 2004;70B:286. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- 33.Lee J.S. Choi K.H. Ghim H.D. Kim S.S. Chun D.H. Kim H.Y. Lyoo W.S. Role of molecular weight of atactic poly(vinyl alcohol) (PVA) in the structure and properties of PVA nanofabric prepared by electrospinning. J Appl Polym Sci. 2004;93:1638. [Google Scholar]
- 34.Buchko C.J. Chen L.C. Shen Y. Martin D.C. Processing and microstructural characterization of porous biocompatible protein polymer thin films. Polymer. 1999;40:7397. [Google Scholar]
- 35.Lee K. Kim H. La Y. Lee D. Sung N. Influence of a mixing solvent with tetrahydrofuran and N,N-dimethylformamide on electrospun poly(vinyl chloride) nonwoven mats. J Polym Sci Part B Polym Phys. 2002;40:2259. [Google Scholar]
- 36.Yuan X. Zhang Y. Dong C. Sheng J. Morphology of ultrafine polysulfone fibers prepared by electrospinning. Polym Int. 2004;53:1704. [Google Scholar]
- 37.Jeun J. Lim Y. Nho Y. Study on morphology of electrospun poly(caprolactone) nanofiber. J Ind Eng Chem. 2005;11:573. [Google Scholar]
- 38.Zhang C. Yuan X. Wu L. Han Y. Sheng J. Study on morphology of electrospun poly(vinyl alcohol) mats. Eur Polym J. 2005;41:423. [Google Scholar]
- 39.Zuo W. Zhu M. Yang W. Yu H. Chen Y. Zhang Y. Experimental study on relationship between jet instability and formation of beaded fibers during electrospinning. Polym Eng Sci. 2005;45:704. [Google Scholar]
- 40.Wannatong L. Sirivat A. Supaphol P. Effects of solvents on electrospun polymeric fibers: preliminary study on polystyrene. Polym Int. 2004;53:1851. [Google Scholar]
- 41.Koski A. Yim K. Shivkumar S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater Lett. 2004;58:493. [Google Scholar]
- 42.Tao J. Shivkumar S. Molecular weight dependent structural regimes during the electrospinning of PVA. Mater Lett. 2007;61:2325. [Google Scholar]
- 43.Duan B. Dong C. Yuan X. Yao K. Electrospinning of chitosan solutions in acetic acid with poly(ethylene oxide) J Biomater Sci Polym Ed. 2004;15:797. doi: 10.1163/156856204774196171. [DOI] [PubMed] [Google Scholar]
- 44.Kim H. Kim K. Jin H. Chin I. Morphological characterization of electrospun nano-fibrous membranes of biodegradable poly(L-lactide) and poly(lactide-co-glycolide) Macromol Symp. 2005;224:145. [Google Scholar]
- 45.Zhao Z. Li J. Yuan X. Li X. Zhang Y. Sheng J. Preparation and properties of electrospun poly(vinylidene fluoride) membranes. J Appl Polym Sci. 2005;97:466. [Google Scholar]
- 46.Gupta P. Elkins C. Long T.E. Wilkes G.L. Electrospinning of linear homopolymers of poly(methyl methacrylate): exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer. 2005;46:4799. [Google Scholar]
- 47.Mit-uppatham C. Nithitanakul M. Supaphol P. Ultrafine electrospun polyamide-6 fibers: effect of solution conditions on morphology and average fiber diameter. Macromol Chem Phys. 2004;205:2327. [Google Scholar]
- 48.Jiang H. Fang D. Hsiao B.S. Chu B. Chen W. Optimization and characterization of dextran membranes prepared by electrospinning. Biomacromolecules. 2004;5:326. doi: 10.1021/bm034345w. [DOI] [PubMed] [Google Scholar]
- 49.Son W. Youk J. Lee T. Park W. The effects of solution properties and polyelectrolyte on electrospinning of ultrafine poly(ethylene oxide) fibers. Polymer. 2004;45:2959. [Google Scholar]
- 50.Fong H. Chun I. Reneker D. Beaded nanofibers formed during electrospinning. Polymer. 1999;40:4585. [Google Scholar]
- 51.Kenawy E.R. Layman J.M. Watkins J.R. Bowlin G.L. J.A. M. Simpson D.G. Wnek G.E. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials. 2003;24:907. doi: 10.1016/s0142-9612(02)00422-2. [DOI] [PubMed] [Google Scholar]
- 52.McKee M. Wilkes G. Colby R. Long T. Correlations of solution rheology with electrospun fiber formation of linear and branched polyesters. Macromolecules. 2004;37:1760. [Google Scholar]
- 53.Ryu Y. Kim H. Lee K. Park H. Lee D. Transport properties of electrospun nylon 6 nonwoven mats. Eur Polym J. 2003;39:1883. [Google Scholar]
- 54.Cha D. Kim H. Lee K. Jung Y. Cho J. Chun B. Electrospun nonwovens of shape-memory polyurethane block copolymers. J Appl Polym Sci. 2005;96:460. [Google Scholar]
- 55.Tong H. Wang M. Electrospinning of fibrous polymer scaffolds using positive voltage or negative voltage: a comparative study. Biomed Mater. 2010;5:1. doi: 10.1088/1748-6041/5/5/054110. [DOI] [PubMed] [Google Scholar]
- 56.Ding B. Kim H.-Y. Lee S.-C. Shao C.-L. Lee D.-R. Park S.-J. Kwag G.-B. Choi K.-J. Preparation and characterization of a nanoscale poly(vinyl alcohol) fiber aggregate produced by an electrospinning method. J Polym Sci Part B Polym Phys. 2002;40:1261. [Google Scholar]
- 57.Huang L. Nagapudi K. Apkarian R.P. Chaikof E.L. Engineered collagen-PEO nanofibers and fabrics. J Biomater Sci Polym Ed. 2001;12:979. doi: 10.1163/156856201753252516. [DOI] [PubMed] [Google Scholar]
- 58.Guarino V. Cirillo V. Taddei P. Alvarez-Perez M.A. Ambrosio L. Tuning size scale and crystallinity of PCL electrospun fibres via solvent permittivity to address hMSC response. Macromol Biosci. 2011;11:1694. doi: 10.1002/mabi.201100204. [DOI] [PubMed] [Google Scholar]
- 59.Li D. Xia Y. Electrospinning of nanofibers: reinventing the wheel? Adv Mater. 2004;16:1151. [Google Scholar]
- 60.Megelski S. Stephens J.S. Chase D.B. Rabolt J.F. Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules. 2002;35:8456. [Google Scholar]
- 61.Demir M. Yilgor I. Yilgor E. Erman B. Electrospinning of polyurethane fibers. Polymer. 2002;43:3303. [Google Scholar]
- 62.Medeiros E.S. Mattoso L.H.C. Offeman R.D. Wood D.F. Orts W.J. Effect of relative humidity on the morphology of electrospun polymer fibers. Can J Chem. 2008;86:590. [Google Scholar]
- 63.Huang L. Bui N.-N. Manickam S.S. McCutcheon J.R. Controlling electrospun nanofiber morphology and mechanical properties using humidity. J Polym Sci Part B Polym Phys. 2011;49:1734. [Google Scholar]
- 64.Hardick O. Stevens B. Bracewell D. Nanofibre fabrication in a temperature and humidity controlled environment for improved fibre consistency. J Mater Sci. 2011;46:3890. [Google Scholar]
- 65.Kim G.T. Lee J.S. Shin J.H. Ahn Y.C. Hwang Y.J. Shin H.S. Lee J.K. Sung C.M. Investigation of pore formation for polystyrene electrospun fiber: effect of relative humidity. Korean J Chem Eng. 2005;22:783. [Google Scholar]
- 66.Peresin M.S. Habibi Y. Vesterinen A.-H. Rojas O.J. Pawlak J.J. Seppälä J.V. Effect of moisture on electrospun nanofiber composites of poly(vinyl alcohol) and cellulose nanocrystals. Biomacromolecules. 2010;11:2471. doi: 10.1021/bm1006689. [DOI] [PubMed] [Google Scholar]
- 67.Kalayci V. Patra P. Kim Y. Ugbolue S. Warner S. Charge consequences in electrospun polyacrylonitrile (PAN) nanofibers. Polymer. 2005;46:7191. [Google Scholar]
- 68.Lin J. Ding B. Yu J. Hsieh Y. Direct fabrication of highly nanoporous polystyrene fibers via electrospinning. ACS Appl Mater Interfaces. 2010;2:521. doi: 10.1021/am900736h. [DOI] [PubMed] [Google Scholar]
- 69.Zheng J. Zhang H. Zhao Z. Han C.C. Construction of hierarchical structures by electrospinning or electrospraying. Polymer. 2012;53:546. [Google Scholar]
- 70.Casper C.L. Stephens J.S. Tassi N.G. Chase D.B. Rabolt J.F. Controlling surface morphology of electrospun polystyrene fibers: effect of humidity and molecular weight in the electrospinning process. Macromolecules. 2003;37:573. [Google Scholar]
- 71.Pai C.-L. Boyce M.C. Rutledge G.C. Morphology of porous and wrinkled fibers of polystyrene electrospun from dimethylformamide. Macromolecules. 2009;42:2102. [Google Scholar]