Abstract
Introduction
Neurocognitive dysfunction is a major symptom feature of schizophrenia and bipolar disorder. A prognostic relationship between cognition and community outcomes is well-documented in schizophrenia and increasingly recognized in bipolar disorder. However, specific associations amongst neurocognition, diagnosis, state symptomatology, and community functioning are unclear, and few studies have compared these relationships amongst patients with affective and non-affective psychoses in the same study. We examined neurocognitive, clinical, and community functioning in a cross-diagnostic sample of patients with psychotic disorders over a 6-month follow-up interval.
Method
Neurocognitive, clinical and community functioning were assessed in participants with schizophrenia (n=13), schizoaffective disorder (n=17), or bipolar disorder with psychosis (n=18), and healthy controls (n=18) at baseline and 6 months later.
Results
Neurocognitive functioning was impaired in all diagnostic groups and, despite reductions in primary symptoms, did not recover on most measures over the follow-up period. Neurocognitive impairment was not associated with diagnosis or clinical improvement. Several neurocognitive scores at baseline (but not diagnosis or clinical baseline or follow-up scores) predicted community functioning at follow-up.
Discussion
In one of the few studies to longitudinally examine neurocognition in association with clinical and outcomes variables in a cross diagnostic sample of psychotic disorders patients, neurocognitive deficits were pronounced across diagnoses and did not recover on most measures despite significant reductions in clinical symptoms. Baseline neurocognitive functioning was the only significant predictor of patients’ community functioning six months later. Efforts to recognize and address cognitive deficits, an approach that has shown promise in schizophrenia, should be extended to all patients with psychosis.
Keywords: Bipolar, Schizophrenia, Schizoaffective, neurocognitive, comparative, longitudinal
1. Introduction
Cognitive dysfunction is a core feature of schizophrenia (SZ), schizoaffective disorder (SZA), and bipolar disorder (BD) (Heinrichs and Zakzanis, 1998, Mesholam-Gately et al., 2009, Murphy and Sahakian, 2001). Some cross-sectional comparisons indicate that cognitive deficits are qualitatively similar but quantitatively different across diagnostic categories (typically: SZ<BD, SZA) (Altshuler et al., 2004, Krabbendam et al., 2005, Reichenberg et al., 2008). However, others report no differences between groups (Balanza-Martinez et al., 2005, Lewandowski et al., 2011a, Simonsen et al., 2011).
Cognitive impairment in adults with SZ appears to be significant but relatively stable (e.g. Hoff et al., 2005), although some cross-sectional evidence suggests that neurocognition may worsen with illness progression (Pukrop et al., 2006). Neurocognitive deficits in BD seem to present early in illness and remain largely stable (Mora et al., 2012, Torrent et al., 2012). A meta-analysis of neurocognition in euthymic BD showed deficits in most domains with medium to large effect sizes (Mann-Wrobel et al., 2011). However, some findings suggest that impairments are associated with illness duration and disease course (Denicoff et al., 1999, Robinson and Ferrier, 2006). Few studies have compared neuropsychological functioning across diagnostic groups longitudinally. A study of older adults with SZ or BD found similar trajectories of neurocognitive decline but greater variability in patients with BD (Depp et al., 2007). A three-year follow-up comparing patients with SZ and BD found that groups did not differ on most neuropsychological measures (Balanza-Martinez et al., 2005).
Cognition is associated with poorer functional outcomes in SZ, and an increasing literature supports the same relationship in BD (e.g. Bowie et al., 2010, Green, 2006, Mora et al., 2012). A recent meta-analysis reported that the strength of associations between cognition and community functioning was similar between patients with SZ or BD (Depp et al., 2012).
We examined neurocognitive, clinical, and community functioning in patients with SZ, SZA, or bipolar disorder with psychosis (BDP) over time. We have previously reported that at baseline all three patient groups exhibited significant and similar neurocognitive deficits (Lewandowski et al., 2011a). We hypothesized that a) all patient groups would exhibit deficits in neurocognition at follow-up, which would not differ by diagnosis, b) any observed cognitive change would not be associated with diagnosis or clinical state, and c) cognitive deficits at baseline, but not diagnosis or clinical symptoms, would predict community functioning at follow-up.
2. Method
2.1 Participants
Participants with SZ (n=13), SZA (n=17) or BDP (n=18), and healthy controls (n=18) ages 18–55 were recruited through the Schizophrenia and Bipolar Disorder Program at McLean Hospital. All procedures were approved by the McLean IRB. The present sample includes only subjects who returned for follow-up (56% of the initial sample). Non-completion was due primarily to an inability to re-contact subjects (39%). Four subjects (5%) declined to participate in the follow-up. The present subsample did not differ from the baseline sample on any demographic characteristics or clinical measures; however, fewer patients with SZ were inpatients at baseline (68% vs. 38%).
2.2 Materials
Clinical assessment included the Young Mania Rating Scale (YMRS), the Montgomery-Asberg Depression Rating Scale (MADRS), and the Positive and Negative Syndrome Scale (PANSS). Cognitive measures included the Brief Visuospatial Memory Test-Revised (BVMT), Hopkins Verbal Learning Test (HVLT), Trails A and B, Stroop Color and Word Test (Stroop), and Category Fluency. Community functioning was assessed using the Multnomah Community Ability Scale (MCAS), which measures daily living, social involvement and interest, and occupational/other meaningful activity. We administered an abbreviated version, eliminating items that assessed clinical symptoms (M3, M4, M17), substance abuse (M16), and intellectual functioning (M2) so as to measure community functioning in a way that was less directly associated with clinical and cognitive symptoms. The final version included 11 items scored 1–5 (5 indicating highest functioning) for a total of 55 points.
2.3 Procedures
Ascertainment, diagnostic and baseline procedures are described in detail elsewhere (Lewandowski et al., 2011a). Briefly, participants completed assessments at baseline and approximately 6 months later. Diagnosis was established using the SCID interview. All participants were prescribed psychiatric medications at the time of assessment. Chlorpromazine (CPZ) equivalents were calculated based on the recommendations of Baldessarini (2012).
2.4 Statistical Approach
Subjects were compared on demographic, clinical, and cognitive variables using ANOVA (continuous variables) or Chi-Square (categorical variables). Effect sizes were calculated (Cohen’s d) for cognitive variables. Neuropsychological variables were normed using published data and converted to standard scores. A neuropsychological composite score was calculated for each subject and change scores were calculated for neuropsychological and clinical variables. Linear regressions were conducted predicting neuropsychological change using diagnosis or clinical change as predictors, accounting for number of lifetime hospitalizations, inpatient vs. outpatient status at baseline, and CPZ equivalents. To examine community outcomes at follow-up, linear regressions were conducted predicting MCAS using baseline cognition and baseline or follow-up clinical variables as predictors after accounting for diagnosis and the above confounders.
3. Results
Patients did not differ by group on any demographic variable at baseline (Table 1). A greater proportion of patients with BDP were inpatients (BDP>SZ, SZA; p<.05). Groups differed on MCAS scores at baseline (BDP, SZA>SZ; p<.05) (Table 2). At follow-up, groups differed on PANSS P (SZ>SZA>BDP; p<.05) and MCAS (BDP, SZA>SZ; p<.05). Patients with BDP showed greater improvement on the YMRS (p<.05).
Table 1.
Demographic variables by diagnosis
| SZ (n=13) | SZA (n=17) | BDP (n=18) | HC (n=18) | Test Statistic | |
|---|---|---|---|---|---|
| Age | 42.2 (8.9) | 40.0 (8.4) | 34.9 (12.8) | 41.5 (8.5) | 2.08* |
| Educationa | 4.0 (1.2) | 4.6 (1.7) | 4.7 (1.0) | 6.3 (1.4) | 1.01*** |
| % Caucasian | 69% | 82% | 89% | 72% | 1.94 |
| % Female | 23% | 53% | 56% | 39% | 3.74 |
| # Lifetime Hosp. | 4.8 (2.0) | 4.8 (1.8) | 3.8 (1.9) | n/a | 1.52 |
| % Inpatient | 38% | 41% | 75% | n/a | 6.49* |
Education is coded based on the SCID Education and Work History scale: 1=grade 6 or less; 2= grade 7–12 (without graduating); 3= high school grad or equivalent; 4= part college; 5= graduated 2 year college; 6= graduated 4 year college; 7= part graduate/professional school; 8= completed graduate/professional school.
p <.05
p<.001
Education: HC>SZ, SZA, BDP, p<.01.
Age: HC> BDP, p<.05.
Inpatient: BDP> SZ, SZA, p < .05.
Table 2.
Clinical variables by diagnosis
| SZ (n=13) | SZA (n=17) | BDP (n=18) | F-statistic | |
|---|---|---|---|---|
| Baseline | ||||
| MCAS | 39.3 (8.5) | 45.1 (7.7) | 47.7 (7.0) | 4.21* |
| YMRS | 11.5 (10.3) | 13.1 (11.9) | 20.7 (15.4) | 2.28 |
| MADRS | 10.1 (7.6) | 11.0 (8.8) | 14.6 (11.7) | 0.97 |
| PANSS P | 18.7 (8.6) | 16.9 (8.3) | 16.2 (7.1) | 0.38 |
| PANSS N | 14.0 (3.9) | 11.5 (4.4) | 10.7 (7.1) | 1.43 |
| PANSS G | 28.0 (8.4) | 26.8 (9.2) | 30.7 (11.4) | 0.71 |
| CPZE | 534 (402) | 476 (384) | 294 (294) | 1.62 |
| Follow-up | ||||
| MCAS | 42.6 (6.6) | 46.4 (5.1) | 48.6 (6.1) | 3.80* |
| YMRS | 9.3 (11.5) | 8.1 (6.3) | 6.9 (6.3) | 0.35 |
| MADRS | 9.4 (8.4) | 10.7 (8.3) | 10.5 (12.0) | 0.07 |
| PANSS P | 17.2 (8.0) | 14.8 (5.7) | 10.8 (3.5) | 4.66* |
| PANSS N | 14.0 (5.1) | 13.6 (7.0) | 10.7 (3.0) | 1.98 |
| PANSS G | 28.8 (11.8) | 24.9 (6.9) | 23.5 (7.0) | 1.54 |
| Change Scores | ||||
| MCAS | 3.3 (4.6) | 1.0 (9.4) | 1.7 (5.4) | 0.40 |
| YMRS | −2.2 (11.3) | −5.1 (12.6) | −14.0 (15.7) | 3.23* |
| MADRS | −0.8 (7.4) | −0.3 (8.1) | −4.1 (12.0) | 0.80 |
| PANSS P | −1.5 (6.0) | −2.1 (9.3) | −5.9 (6.7) | 1.60 |
| PANSS N | 0.0 (6.0) | 2.1 (6.2) | 0.0 (7.6) | 0.52 |
| PANSS G | 0.8 (6.8) | −1.9 (10.7) | −7.2 (11.3) | 2.65 |
MCAS: Multnomah Community Ability Scale; YMRS: Young Mania Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; PANSS: Positive and Negative Syndrome Scale; CPZE: CPZ Equivalents
p <.05
Cognitive data are presented in Figure 1. At baseline and follow-up groups differed only on Trails B (BDP, SZA>SZ; p<.05). Groups differed in neurocognitive improvement over the follow-up only on the BVMT-R (BDP>SZA; p<.05) and Stroop Interference (BDP>SZA; p<.05). Control participants differed from patient groups on all neuropsychological measures at baseline and follow-up (p=.013 - p<.001); controls and patients did not differ on change scores. At Time 1 Trails B showed medium effects (SZ vs. SZA d = .67; SZ vs. BDP d = .73). At Time 2 Trails B showed large effects (SZ vs. SZA d = .75; SZ vs. BDP d = .90) as did the Composite (SZ vs. BDP d =.78); we found several medium effects including HVLT (SZ vs. SZA, d =.60; SZ vs. BDP d = .72), Stroop Color (SZ vs. BDP d =.63), and Stroop Interference (SZA vs. BDP d = .64). Change BVMT and Stroop Interference scores showed medium effects (SZA vs. BDP d = .70 and SZA vs. BDP d = .72 respectively).
Figure 1.
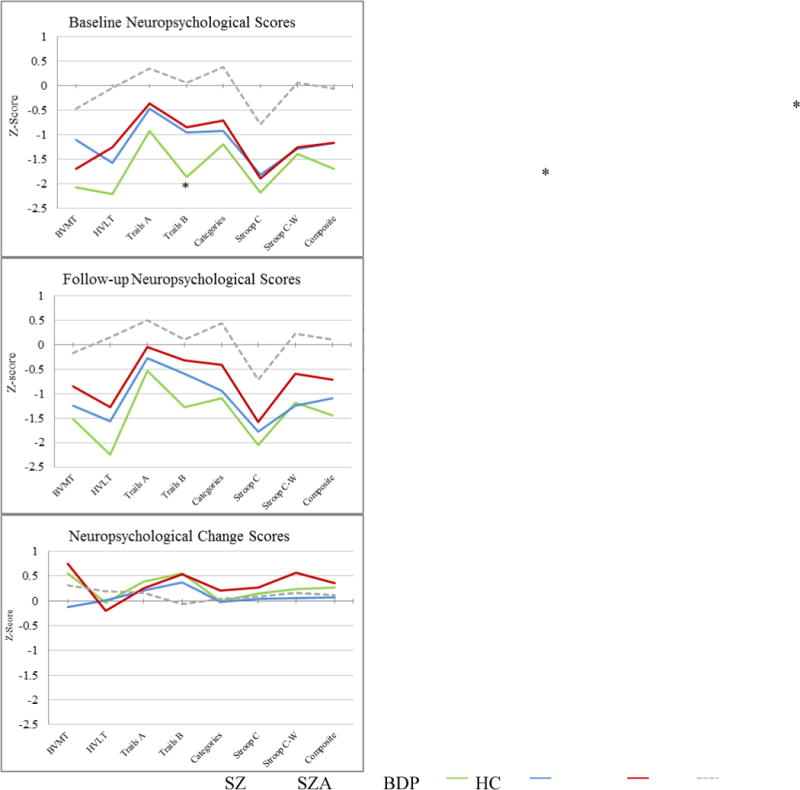
Age-adjusted z-scores for neuropsychological variables by diagnosisBVMT: Brief Visuospatial Memory Test-Revised, HVLT: Hopkins Verbal Learning Test-Revised, Categories: Category Fluency (Animals), Stroop C: Stroop Color, Stroop C-W: Stroop Color-Word
Linear regressions revealed that diagnosis did not predict cognitive change for the neurocognitive composite or any neurocognitive variable after accounting for severity measures and CPZ equivalents. Similarly, clinical change did not predict neurocognitive change for any neurocognitive measures. All neurocognitive scores at baseline predicted the neurocognitive composite at follow-up (p<.05 - p<.001).
MCAS and neurocognitive data were correlated at both time points (p<.01 for all associations; baseline r=0.27–0.40; follow-up r=0.34–0.63). Baseline neurocognitive scores were significantly correlated with follow-up MCAS scores (r = 0.38–0.60, p<.01). Linear regressions predicting MCAS scores at follow-up revealed that several baseline neurocognitive variables predicted MCAS at follow-up even after accounting for severity and diagnosis (Figure 2). Baseline BVMT (β=1.43; t=2.25, p<.05), Trails A (β=2.26; t=2.13, p<.05), and Stroop Interference (β=2.31; t=2.24, p<.05) predicted follow-up MCAS; the composite (β=2.27; t=1.99, p=.05) and Stroop Color (β=2.31; t=1.93, p=.06) approached significance. Clinical measures including diagnosis, baseline and follow-up clinical variables did not predict MCAS at follow-up.
Figure 2.
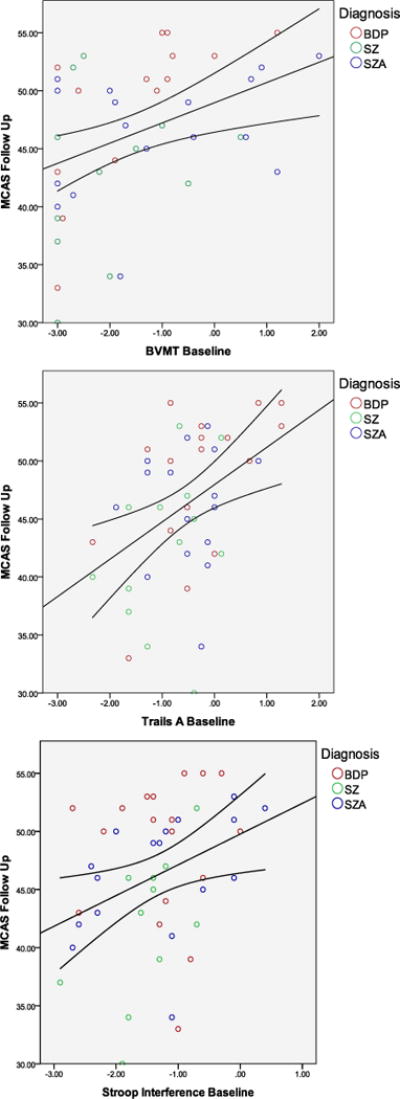
Cognitive scores at baseline by community functioning at follow-up
4. Discussion
Patients with SZ, SZA, and BDP exhibited cognitive deficits at baseline, which remained largely unchanged six months later despite clinical improvement in other domains. Indeed, of the change scores that revealed medium to large effects, one was in a worsening direction (BVMT: SZA T2<T1). Patients with SZ performed significantly worse than patients with BDP or SZA on Trails B at baseline and follow-up. No other group differences were observed at either time point. Effects sizes at both time points and change scores were typically in the small range for most non-significant findings although medium effects on HVLT, Stroop Color and Stroop Interference at follow-up were suggestive of better performance in the BD group on these measures. Patients with BDP evidenced greater improvement than patients with SZA on visuospatial memory and Stroop Interference. No other group differences in cognitive change were observed.
Community functioning was impaired in all patient groups at both assessment points; however, patients with SZ scored significantly worse than the other two patient groups at both assessments. Cognitive functioning at baseline predicted community functioning at follow-up even after accounting for diagnosis and symptom severity. Community functioning at follow-up was not predicted by diagnosis or clinical presentation. These findings support the concept of a core neurocognitive dimension that cuts across diagnostic boundaries, is relatively independent of clinical symptoms, and contributes in a similar way to community outcomes in patients with SZ, SZA or BDP.
Our study is limited by a small sample size, short follow-up duration, and a relatively high sample attrition. However, the follow-up subsample did not differ from the total sample on most measures. Cognitive functioning may improve along a flatter trajectory than clinical symptoms. Nonetheless, the observation that symptoms improved but cognition did not suggesting a decoupling of these dimensions.
The present findings suggest that patients with SZ, SZA, and BDP show similar neurocognitive deficits that do not fully recover in most domains over time, regardless of diagnosis or symptom change. Additionally, cognitive functioning was the only significant predictor of community outcomes in all patient groups. Treatments targeting neurocognitive dysfunction hold the promise of improving outcomes for all patients with psychotic disorders suffering from neurocognitive deficits (Lewandowski et al., 2011b). Additionally, identification of shared symptom dimensions such as neurocognition may improve understanding of the nature of brain abnormalities underlying psychotic disorders.
Acknowledgments
Role of Funding Source
This work was supported by Grant # MH 91210 (KEL); Grant #MH 78113 (MSK); Grant #R01MH094594 (DO); The Shervert Frazier Research Institute (KEL; BMC). This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers). The funding organizations had no further role in study design; in collection, analysis and interpretation of data; in the writing of this report; or in the decision to submit this work for publication.
Footnotes
Conflict of Interest
Kathryn Lewandowski is a paid consultant with Clintara; Matcheri Keshavan has received a grant from GlaxoSmithKline and Sunovion; Dost Ongur is currently on a research contract with Rules Based Medicine, Inc. The authors declare that they have no other disclosures of conflicts of interest in connection with the present work.
Contributors
KEL contributed to all aspects of this study. DO drafted portions of the manuscript and contributed to study design and subject recruitment; BMC and MSK drafted portions of the manuscript and consulted on statistical analyses. SHS contributed to the data collection, analyses, and drafting of the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol Psychiatry. 2004;56:560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Balanza-Martinez V, Tabares-Seisdedos R, Selva-Vera G, Martinez-Aran A, Torrent C, Salazar-Fraile J, Leal-Cercos C, Vieta E, Gomez-Beneyto M. Persistent cognitive dysfunctions in bipolar I disorder and schizophrenic patients: a 3-year follow-up study. Psychother Psychosom. 2005;74:113–119. doi: 10.1159/000083170. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ. Chemotherapy in Psychiatry. Springer Verlag; New York: 2012. [Google Scholar]
- Bowie CR, Depp C, McGrath JA, Wolyniec P, Mausbach BT, Thornquist MH, Luke J, Patterson TL, Harvey PD, Pulver AE. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. Am J Psychiatry. 2010;167:1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denicoff KD, Ali SO, Mirsky AF, Smith-Jackson EE, Leverich GS, Duncan CC, Connell EG, Post RM. Relationship between prior course of illness and neuropsychological functioning in patients with bipolar disorder. J Affect Disord. 1999;56:67–73. doi: 10.1016/s0165-0327(99)00028-2. [DOI] [PubMed] [Google Scholar]
- Depp CA, Mausbach BT, Harmell AL, Savla GN, Bowie CR, Harvey PD, Patterson TL. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord. 2012;14:217–226. doi: 10.1111/j.1399-5618.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depp CA, Moore DJ, Sitzer D, Palmer BW, Eyler LT, Roesch S, Lebowitz BD, Jeste DV. Neurocognitive impairment in middle-aged and older adults with bipolar disorder: comparison to schizophrenia and normal comparison subjects. J Affect Disord. 2007;101:201–209. doi: 10.1016/j.jad.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12. [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res. 2005;78:27–34. doi: 10.1016/j.schres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Krabbendam L, Arts B, van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80:137–149. doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Cohen BM, Keshavan MS, Ongur D. Relationship of neurocognitive deficits to diagnosis and symptoms across affective and non-affective psychoses. Schizophr Res. 2011a;133:212–217. doi: 10.1016/j.schres.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011b;41(2):225–241. doi: 10.1017/S0033291710001042. [DOI] [PubMed] [Google Scholar]
- Mann-Wrobel MC, Carreno JT, Dickinson D. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder: an update and investigation of moderator variables. Bipolar Disord. 2011;13:334–342. doi: 10.1111/j.1399-5618.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Mora E, Portella MJ, Forcada I, Vieta E, Mur M. Persistence of cognitive impairment and its negative impact on psychosocial functioning in lithium-treated, euthymic bipolar patients: a 6-year follow-up study. Psychol Med. 2012:1–10. doi: 10.1017/S0033291712001948. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ. Neuropsychology of bipolar disorder. Br J Psychiatry. 2001;41:s120–127. [PubMed] [Google Scholar]
- Pukrop R, Schultze-Lutter F, Ruhrmann S, Brockhaus-Dumke A, Tendolkar I, Bechdolf A, Matuschek E, Klosterkotter J. Neurocognitive functioning in subjects at risk for a first episode of psychosis compared with first- and multiple-episode schizophrenia. J Clin Exp Neuropsychol. 2006;28:1388–1407. doi: 10.1080/13803390500434425. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, Bromet E. Neuropsychological Function and Dysfunction in Schizophrenia and Psychotic Affective Disorders Schizophr. Bull. 2008;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006;8:103–116. doi: 10.1111/j.1399-5618.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Simonsen C, Sundet K, Vaskinn A, Birkenaes AB, Engh JA, Faerden A, Jonsdottir H, Ringen PA, Opjordsmoen S, Melle I, Friis S, Andreassen OA. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr Bull. 2011;37:73–83. doi: 10.1093/schbul/sbp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrent C, Martinez-Aran A, Bonnin Cdel M, Reinares M, Daban C, Sole B, Rosa AR, Tabares-Seisdedos R, Popovic D, Salamero M, Vieta E. Long-term outcome of cognitive impairment in bipolar disorder. J Clin Psychiatry. 2012;73:e899–905. doi: 10.4088/JCP.11m07471. [DOI] [PubMed] [Google Scholar]


