Abstract

Western gorillas (Gorilla gorilla) have been identified as the natural reservoir of the parasites that were the immediate precursor of Plasmodium falciparum infecting humans. Recently, a P. falciparum-like sequence was reported in a sample from a captive greater spot-nosed monkey (Cercopithecus nictitans), and was taken to indicate that this species may also be a natural reservoir for P. falciparum-related parasites. To test this hypothesis we screened blood samples from 292 wild C. nictitans monkeys that had been hunted for bushmeat in Cameroon. We detected Hepatocystis spp. in 49% of the samples, as well as one sequence from a clade of Plasmodium spp. previously found in birds, lizards and bats. However, none of the 292 wild C. nictitans harbored P. falciparum-like parasites.
Keywords: Malaria, Plasmodium falciparum, Hepatocystis, Non-human primates, Cercopithecus nictitans, Greater spot-nosed monkey
Malaria is one of the most devastating infectious diseases, with 300-500 million new cases and more than one million deaths occurring annually. Five species of Plasmodium, Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae and Plasmodium knowlesi, are known to cause malaria in humans. Among these, P. falciparum is particularly notable as it is the most malignant, being responsible for the overwhelming majority of human malaria mortality (Greenwood et al., 2005), and it is sufficiently divergent from most other species of Plasmodium that it has been placed in a separate subgenus, Laverania. Thus, the evolutionary origin of P. falciparum is of considerable interest. Until recently, the only other parasite known to fall within Laverania has been P. reichenowi, which infects chimpanzees (Pan troglodytes). It had been widely assumed that this reflects long-term host-parasite co-evolution, i.e., that the common ancestor of P. falciparum and P. reichenowi infected the common ancestor of humans and chimpanzees (Escalante et al., 1994). This view changed dramatically when several groups reported the detection, in samples from chimpanzees, bonobos and gorillas, of additional Plasmodium DNA sequences that fell within the Laverania clade (Ollomo et al., 2009; Rich et al., 2009; Duval et al., 2010; Krief et al., 2010; Prugnolle et al., 2010). However, it remains unclear how many different species of Laverania parasitise apes, which ape species are infected in the wild, and which of these represent the true source of human P. falciparum. These issues were seemingly resolved by an extensive molecular epidemiological study of wild chimpanzees and gorillas (Liu et al., 2010). Analysis of more than 2,000 ape faecal samples from throughout central Africa identified six distinct Laverania spp., three exclusively found in chimpanzees and three others found only in western gorillas (Gorilla gorilla). More importantly, in detailed phylogenetic analyses, all known human P. falciparum strains emerged from within the radiation of just one of the species infecting gorillas. Thus, these data identified western gorillas as the natural reservoir of the parasites that were the immediate precursor of human P. falciparum (Liu et al., 2010; Rayner et al., 2011).
However, this conclusion that human P. falciparum is of gorilla origin has recently been called into question (Prugnolle et al., 2011a). Screening blood samples from 338 captive and semi-free ranging monkeys representing 10 different primate species in Gabon, Prugnolle and colleagues (2011a) found P. falciparum-like sequences in one sample from a pet greater spot-nosed monkey (Cercopithecus nictitans). Although there was only a single positive sample, among 29 from this species, it was argued that P. falciparum could be present in wild monkeys, and that these monkeys, rather than gorillas, could have served as the original source of the parasites found in humans (Prugnolle et al., 2011a,b). If true, this would have profound implications for our understanding of past, and potential future, zoonotic transmissions of malaria parasites. The increasing human population density in African forests inhabited by apes and monkeys, together with changes in mosquito vector abundance due to deforestation and climate change, may alter the transmission dynamics of Plasmodium spp. from primates and other mammals (Keesing et al., 2010). In addition, malaria eradication strategies may create new ecological niches by reducing the burden of human malaria parasites. Thus, it is important to monitor the Plasmodium infections of non-human primates. To determine whether greater spot-nosed monkeys are indeed widely infected with P. falciparum-like parasites, we screened blood samples from nearly 300 wild C. nictitans, the first comprehensive survey of this species as a possible reservoir for human malaria.
We selected blood samples from existing specimen banks that were originally collected in Cameroon to assess the risk of zoonotic transmission of simian retroviruses (Aghokeng et al., 2010). The samples were collected at bushmeat markets and around logging concessions from freshly killed animals that had been hunted in the wild. The material had either been confiscated by the governmental anti-poaching program or came from hunters who had agreed to collaborate and who were educated about conservation of primates, as well as the risks associated with direct contact with primate blood, body fluids or tissues. Whole blood was collected and either stored in tubes at -20°C or spotted onto filter paper (Whatman plc, Kent, UK) or 903 filter paper cards (Schleicher & Schuell, Keene, NH, USA) and stored at ambient temperature. All samples were first subjected to mtDNA analysis to test for DNA integrity and to confirm the species of origin. This was done by amplifying a 386 bp fragment spanning the mitochondrial 12S rRNA gene using primers 12S-L1091 and 12S-H1478 (van der Kuyl et al., 2000). Blood samples from 292 C. nictitans were identified among material from 12 different locations across southern Cameroon (Fig. 1). These samples had been obtained between 1999 and 2004, and were collected at various times throughout the year, including 28% obtained in the months with lower rainfall (December to February). One sample site (CS7) comprised markets in the city of Yaounde, so the precise geographic origin of those samples is unknown, although they were most likely brought to the city from south and southeast Cameroon. The other 11sample sites were all located in forested areas.
Fig. 1.
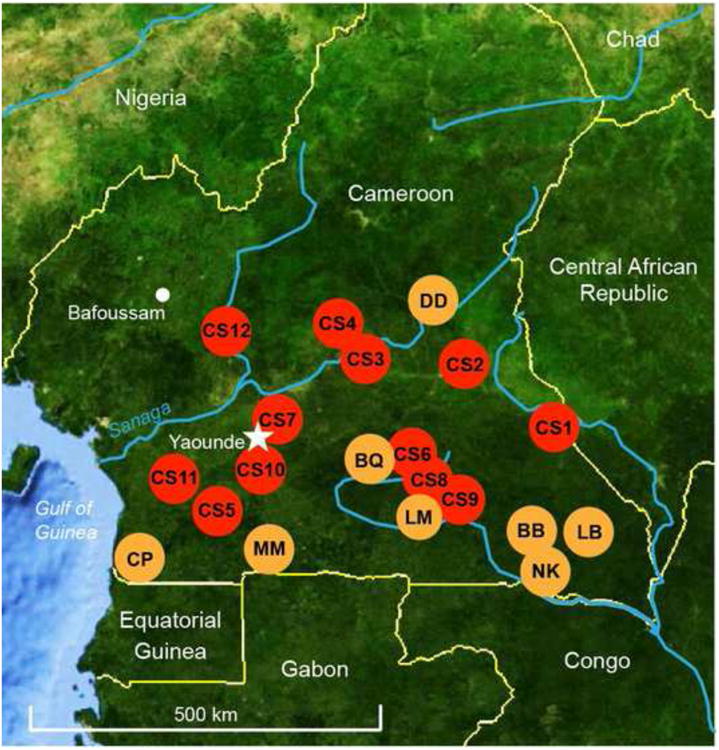
Collection sites of blood samples from greater spot-nosed monkeys in Cameroon. Red circles indicate 12 locations (CS1-CS12) where samples were collected from hunted animals. Orange circles denote sites where Plasmodium falciparum-like parasites were previously detected in wild gorilla populations (Liu et al., 2010).
Samples were screened for the presence of Plasmodium and related parasites by standard PCR amplification with primers that amplify a 939 bp mtDNA fragment encoding part of the cytochrome b gene (cytb); these primers have previously been shown to amplify cytb from a wide range of haemosporidian species (Liu et al., 2010). Briefly, nested PCR was performed, using DW2 and DW4 primers in the first round and Pfcytb1 and Pfcytb2 in the second round. For the first round, 5 μl DNA was used in a 50 ul reaction volume, containing 2 μl dNTPs (10 mM of each dNTP), 20 pmol of each primer (DW2 and DW4), 5 μl of PCR buffer and 0.75 μl Expand Long Template enzyme mix (Roche, Meylan, France). Cycling conditions included an initial denaturation step of 5 min at 94°C, followed by 35 cycles of denaturation (92°C, 20 s), annealing (50°C, 30 s) and elongation (72°C, 90s), and then a final elongation step of 10 min at 72°C. For the second round, 2 μl of the first-round product was used in a 50 μl reaction volume, under the same PCR conditions. Amplicons were gel-purified and sequenced directly. All new sequences were submitted to GenBank, under the accession numbers JQ070813-JQ070956.
Haemosporidian cytb sequences were amplified from 144 (49.2%) of the 292 C. nictitans samples (Table 1). Evidence of haemosporidian infection was found at almost all sampling locations; only sites CS1 and CS11, where in each case the sample size was very small (n = 2), yielded no positive samples. The high overall prevalence was not due to one or two specific sites; the fraction of positive samples ranged between 47% and 59% at four of the six sites with larger sample sizes (n > 10). The cytb sequences were aligned with representative sequences from haemosporidian species and phylogenetic trees were constructed using maximum likelihood methods, with a GTR+I+G nucleotide substitution model, and 1,000 bootstrap replicates, executed in PhyML (Guindon and Gascuel, 2003). All sequences, except one (S2138_CS8, discussed further below), fell clearly within the radiation of Hepatocystis parasites known to infect Old World monkey species (Fig. 2). Importantly, none of the new sequences were similar to the previously reported P. falciparum-like cytb sequence from a captive C. nictitans, and indeed none were found to cluster with the Laverania lineage (Fig 2).
Table 1.
Numbers and percentages of cytb positive samples from greater spot-nosed monkeys (Cercopithecus nictitans) at 12 different field sites in Cameroon.
| Site | Geographic area | n tested | n (%) positive for cytb PCR |
|---|---|---|---|
| CS1 | Gribe | 2 | 0 (0.0%) |
| CS2 | Bertoua | 4 | 2 (50.0%) |
| CS3 | Nanga Eboko | 2 | 2 (100%) |
| CS4 | North Sanaga | 1 | 1 (100%) |
| CS5 | Ebolowa | 40 | 10 (25.0%) |
| CS6 | Eboumetoum | 36 | 20 (55.6%) |
| CS7 | Yaoundé | 49 | 29 (59.2%) |
| CS8 | Mindourou | 99 | 55 (55.6%) |
| CS9 | Messok | 30 | 11 (36.7%) |
| CS10 | Nditam | 19 | 9 (47.4%) |
| CS11 | Mbalmayo | 2 | 0 (0.0%) |
| CS12 | Bipindi | 8 | 5 (62.5%) |
|
| |||
| Total | 292 | 144 (49.2%) | |
Fig. 2.
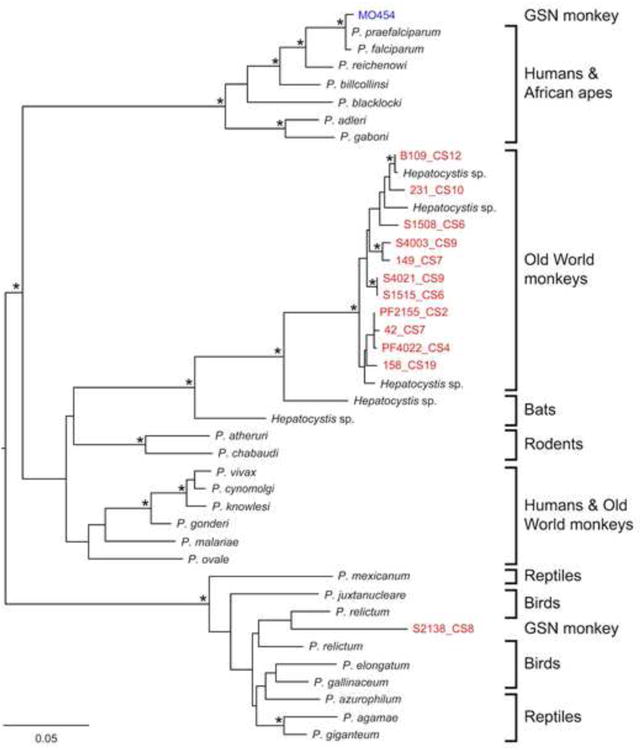
Evolutionary relationships of haemosporidian sequences from greater spot-nosed (GSN) monkeys (Cercopithecus nictitans). A maximum likelihood tree of mitochondrial cytb sequences (726 bp) is shown, with Plasmodium and Hepatocystis reference sequences in black, newly derived sequences highlighted in red (only a subset of Hepatocystis sequences is shown), and one Plasmodium falciparum-like sequence (MO454) previously reported from a captive GSN monkey (Prugnolle et al., 2011) in blue. Asterisks on branches indicate bootstrap values ≥ 80%; the scale bar represents 0.05 nucleotide substitutions per site. GenBank accession numbers for all sequences are listed in Supplementary Table S1.
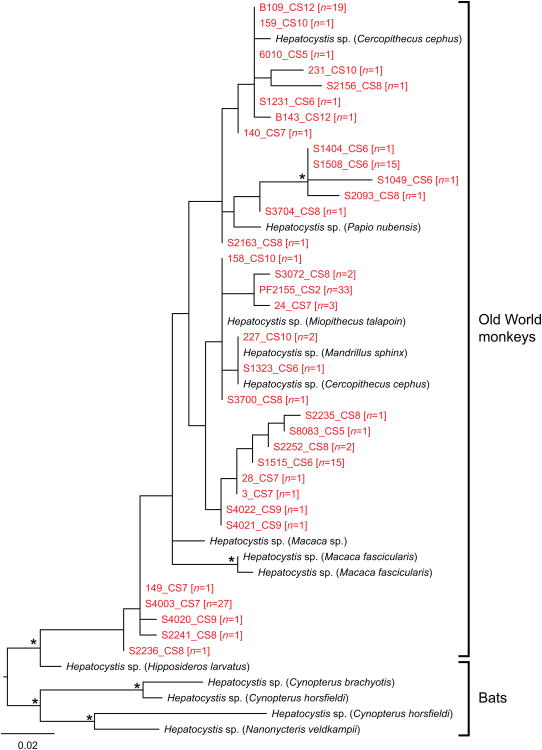
To investigate in greater detail the relationship between the Hepatocystis parasites infecting C. nictitans and those from other Old World monkeys, the new sequences were aligned with all distinct Hepatocystis cytb sequences available in GenBank. All of the Hepatocystis sequences from Old World monkeys, including those from C. nictitans, formed a monophyletic group within the radiation of previously reported Hepatocystis spp. from bats (Fig. 3). Among the clade of Hepatocystis sequences from monkeys, there was very little evidence of host-species specificity, with the C. nictitans-derived sequences completely interspersed among those from other African species (including guenons (Cercopithecus spp.), mandrills (Mandrillus sphinx) and baboons (Papio spp.)) and even Asian monkeys (macaques (Macaca spp.)). There was also no evidence for geographic clustering of specific haplotypes; multiple haplotypes were found at each sample site and haplotypes from, for example, Mindourou (CS8), spanned the entire tree. While the level of genetic divergence across this clade of monkey parasites was similar to that seen between P. falciparum and P. reichenowi (see Fig. 2), it is as yet unclear whether these Hepatocystis sequences comprise multiple taxa or a single widely dispersed species. However, the phylogeny shown in Fig. 3 is based on a very short sequence alignment; thus the addition of longer sequences, as well as sequences from the nuclear and apicoplast genomes, together with sequences from parasites from more host species, will be needed to resolve this issue.
Fig. 3.
Evolutionary relationships of Hepatocystis parasites. A maximum likelihood tree of mitochondrial cytb sequences (201 bp) is shown. Hepatocystis reference sequences are shown in black, with their respective host species indicated in parentheses; newly derived sequences from greater spot-nosed monkey samples are highlighted in red (the number of sequences with the same haplotype is shown in parentheses). Asterisks on branches indicate bootstrap support values ≥ 70%; the scale bar represents 0.02 nucleotide substitutions per site. GenBank accession numbers for all sequences are listed in Supplementary Table S1.
The cytb sequence from one C. nictitans sample (S2138_CS8) fell within a clade of “sauropsid” Plasmodium spp., previously found primarily in birds and lizards (Fig. 2). Due to the ubiquitous and high levels of Hepatocystis infections in these samples, it is theoretically possible that additional Plasmodium infections had been missed in the initial screening because the broad Haemosporidian cytb primers were amplifying primarily Hepatocystis targets and masking lower levels of infections with other species. To investigate this possibility, as well as to examine the distribution of this unclassified Plasmodium among greater spot-nosed monkeys, we re-screened all samples using new Plasmodium-specific primers, which do not co-amplify Hepatocystis spp. The same primers and conditions, as described above, were used for the first round, but the primers Cytb1SP1 (5′-AGAAAAATAATACAATAAATAAWCGA-3′) and Cytb2SP2 (5′-CWATYGGGTCAACAATGACTTTATTT-3′) were used for the second round. However, with the exception of S2138, none of the samples yielded an amplicon. Parasites falling within this “sauropsid” Plasmodium clade have been found infecting bats from both Africa (Madagascar) and Asia (Cambodia) (Duval et al., 2007), but have not been reported in primates. Thus, while parasites from this clade are certainly capable of infecting mammals, since we found only a single monkey sample to be infected with this lineage, it is not yet clear whether they infect primates generally.
In stark contrast to the widespread endemic infection with Hepatocystis parasites, we found no evidence of P. falciparum-like sequences in any of the 292 samples tested, either by screening with primers targeting all haemosporidian parasites, or with primers specific for Plasmodium spp. It is highly unlikely that this lack of detection of P. falciparum-like infections in wild C. nictitans was due to suboptimal amplification conditions. Using an identical primer set, we previously amplified Plasmodium cytb sequences from 286 (21%) of 1,390 chimpanzee and gorilla samples collected at numerous field sites across southern Cameroon and neighboring parts of the Republic of Congo and the Central African Republic (Liu et al., 2010). In those ape samples, these same primers detected six Plasmodium spp. within the Laverania subgenus, as well as P. ovale-like, P. malariae-like and P. vivax-like parasites (Liu et al., 2010). Moreover, these previous studies were done using faecal samples, which most likely have a substantially lower parasite burden than the blood samples analysed here.
Greater spot-nosed monkeys inhabit a wide range across western central Africa (Groves 2001). The pet monkey previously reported to be infected with a P. falciparum-like parasite was from Gabon. Our sample sites were all in Cameroon, but many were in the south of the country, where it borders Gabon. The mosquito vectors that transmit apicomplexan parasites between monkeys are not known and therefore their potential to transmit parasites between monkeys in Gabon and Cameroon cannot be estimated. However, for Laverania infections of wild apes, where the vectors are also unknown, it is clear that such distances and (unsurprisingly) political boundaries do not interrupt the flow of malarial parasites (Liu et al., 2010). Therefore, it is highly unlikely that wild C. nictitans in Gabon have a higher prevalence of infection with P. falciparum-like parasites than their conspecifics in Cameroon. Also, while prevalence of Plasmodium infections may vary across seasons, our samples were collected throughout the year. Thus, we conclude that P. falciparum-like infections must be extremely rare, or more likely absent, in wild greater spot-nosed monkeys. For example, if the individuals we sampled are representative, and the true prevalence (as detectable by our methods) was only 1%, there would still be a 95% probability of observing at least one infected individual among 292, whereas we saw none. Nonetheless, it cannot be excluded that greater spot-nosed monkeys are susceptible to Laverania parasites in captivity when exposed to mosquitoes not normally encountered in the wild, which appears to be the case for captive chimpanzees and bonobos (Duval et al., 2010; Krief et al, 2010).
In contrast to a recent proposal (Prugnolle et al., 2011a,b), we therefore conclude that greater spot-nosed monkeys are not natural carriers of P. falciparum-like parasites, and that there is no evidence to support the speculation that they could have served as the reservoir from which humans became infected. In contrast, P. falciparum-like parasites have been documented in wild gorillas at numerous sites in Cameroon (Fig. 1) and neighbouring countries, and these apes thus appear to have been the original host of P. falciparum infecting humans (Liu et al., 2010; Rayner et al., 2011; Sharp et al., 2011).
Supplementary Material
Highlights.
Wild Cercopithecus nictitans from Cameroon were screened for Plasmodium falciparum-related parasites.
Hepatocystis spp. were detected in 49% of the samples
None of the 292 wild C. nictitans harbored P. falciparum-like parasites.
Greater spot-nosed monkeys do not represent a natural P. falciparum reservoir
Acknowledgments
This work was supported in part by grants from the Agence Nationale de Recherche, France (ANR, Programme Blanc SVSE3, PRIMAL), the National Institutes of Health, USA (RO1 AI50529 AI91595), the Wellcome Trust, UK (090851) and Agence Nationale de Recherches sur le SIDA, France (ANRS 12125/12182). We thank the Cameroonian Ministries of Health, Environment and Research and the national ethics committee (Authorization N°259/CNE/SE/2011) for permission to perform this study and thank the field staff of Projet PRESICA in Cameroon for logistical support.
Footnotes
Note: Nucleotide sequence data reported in this paper are available in the GenBank database under Accession Numbers JQ070813-JQ070956.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghokeng AF, Ayouba A, Mpoudi-Ngole E, Loul S, Liegeois F, Delaporte E, Peeters M. Extensive survey on the prevalence and genetic diversity of SIVs in primate bushmeat provides insights into risks for potential new cross-species transmissions. Infect Genet Evol. 2010;10:386–396. doi: 10.1016/j.meegid.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval L, Fourment M, Nerrienet E, Rousset D, Sadeuh SA, Goodman SM, Andriaholinirina NV, Randrianarivelojosia M, Paul RE, Robert V, Ayala FJ, Ariey F. African apes as reservoirs of Plasmodium falciparum and the origin and diversification of the Laverania subgenus. Proc Natl Acad Sci USA. 2010;107:10561–10566. doi: 10.1073/pnas.1005435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval L, Robert V, Csorba G, Hassanin A, Randrianarivelojosia M, Walston J, Nhim T, Goodman SM, Ariey F. Multiple host-switching of Haemosporidia parasites in bats. Malar J. 2007;6:157. doi: 10.1186/1475-2875-6-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante AA, Ayala FJ. Phylogeny of the malarial genus Plasmodium, derived from rRNA gene sequences. Proc Natl Acad Sci USA. 1994;91:11373–11377. doi: 10.1073/pnas.91.24.11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BM, Bojang K, Whitty CJ, Targett GA. Malaria. Lancet. 2005;365:1487–1498. doi: 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- Groves CP. Primate Taxonomy. Smithsonian Institution Press; Washington: 2001. [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, Myers SS, Bogich T, Ostfeld RS. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krief S, Escalante AA, Pacheco MA, Mugisha L, Andre C, Halbwax M, Fischer A, Krief JM, Kasenene JM, Crandfield M, Cornejo OE, Chavatte JM, Lin C, Letourneur F, Grüner AC, McCutchan TF, Rénia L, Snounou G. On the diversity of malaria parasites in African apes and the origin of Plasmodium falciparum from bonobos. PLoS Path. 2010;6:e1000765. doi: 10.1371/journal.ppat.1000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Li Y, Learn GH, Rudicell RS, Robertson JD, Keele BF, Ndjango JB, Sanz CM, Morgan DB, Locatelli S, Gonder MK, Kranzusch PJ, Walsh PD, Delaporte E, Mpoudi-Ngole E, Georgiev AV, Muller MN, Shaw GM, Peeters M, Sharp PM, Rayner JC, Hahn BH. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature. 2010;467:420–425. doi: 10.1038/nature09442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollomo B, Durand P, Prugnolle F, Douzery E, Arnathau C, Nkoghe D, Leroy E, Renaud F. A new malaria agent in African hominids. PLoS Path. 2009;5:e1000446. doi: 10.1371/journal.ppat.1000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prugnolle F, Durand P, Neel C, Ollomo B, Ayala FJ, Arnathau C, Etienne L, Mpoudi-Ngole E, Nkoghe D, Leroy E, Delaporte E, Peeters M, Renaud F. African great apes are natural hosts of multiple related malaria species, including Plasmodium falciparum. Proc Natl Acad Sci USA. 2010;107:1458–1463. doi: 10.1073/pnas.0914440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prugnolle F, Ollomo B, Durand P, Yalcindag E, Arnathau C, Elguero E, Berry A, Pourrut X, Gonzalez JP, Nkoghe D, Akiana J, Verrier D, Leroy E, Ayala FJ, Renaud F. African monkeys are infected by Plasmodium falciparum nonhuman primate-specific strains. Proc Natl Acad Sci USA. 2011a;108:11948–11953. doi: 10.1073/pnas.1109368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prugnolle F, Durand P, Ollomo B, Ayala FJ, Renaud F. Host species sampling bias and Plasmodium falciparum origin paradigm shifts. Proc Natl Acad Sci USA. 2011b;108:E873. doi: 10.1073/pnas.1112821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner JC, Liu W, Peeters M, Sharp PM, Hahn BH. A plethora of Plasmodium species in wild apes: a source of human infection? Trends Parasitol. 2011;27:222–229. doi: 10.1016/j.pt.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich SM, Leendertz FH, Xu G, LeBreton M, Djoko CF, Aminake MN, Takang EE, Diffo JL, Pike BL, Rosenthal BM, Formenty P, Boesch C, Ayala FJ, Wolfe ND. The origin of malignant malaria. Proc Natl Acad Sci USA. 2009;106:14902–14907. doi: 10.1073/pnas.0907740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Liu W, Learn GH, Rayner JC, Peeters M, Hahn BH. Source of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 2011;108:E744–E745. doi: 10.1073/pnas.1112134108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Kuyl AC, Van Gennep DR, Dekker JT, Goudsmit J. Routine DNA analysis based on 12S rRNA gene sequencing as a tool in the management of captive primates. J Med Primatol. 2000;29:307–315. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.