Abstract
The voltage-dependent K+ channel responsible for the slowly activating delayed K+ current IKs is composed of pore-forming KCNQ1 and regulatory KCNE1 subunits, which are mutated in familial forms of cardiac long QT syndrome. Because KCNQ1 and KCNE1 genes also are expressed in epithelial tissues, such as the kidneys and the intestine, we have investigated the adaptation of KCNE1-deficient mice to different K+ and Na+ intakes. On a normal K+ diet, homozygous kcne1−/− mice exhibit signs of chronic volume depletion associated with fecal Na+ and K+ wasting and have lower plasma K+ concentration and higher levels of aldosterone than wild-type mice. Although plasma aldosterone can be suppressed by low K+ diets or stimulated by low Na+ diets, a high K+ diet provokes a tremendous increase of plasma aldosterone levels in kcne1−/− mice as compared with wild-type mice (7.1-fold vs. 1.8-fold) despite lower plasma K+ in kcne1−/− mice. This exacerbated aldosterone production in kcne1−/− mice is accompanied by an abnormally high plasma renin concentration, which could partly explain the hyperaldosteronism. In addition, we found that KCNE1 and KCNQ1 mRNAs are expressed in the zona glomerulosa of adrenal glands where IKs may directly participate in the control of aldosterone production by plasma K+. These results, which show that KCNE1 and IKs are involved in K+ homeostasis, might have important implications for patients with IKs-related long QT syndrome, because hypokalemia is a well known risk factor for the occurrence of torsades de pointes ventricular arrhythmia.
The slowly activating delayed K+ current, known as IKs, is formed by the assembly of two distinct subunits KCNQ1 and KCNE1 (formerly called KvLQT1 and IsK/MinK, respectively; refs. 1 and 2). KCNQ1 is a pore-forming K+ channel protein with six transmembrane domains, whereas KCNE1 is a single-transmembrane domain protein that acts as a regulatory subunit (3, 4). In humans, mutations in the genes encoding these two subunits are associated with long QT (LQT) syndrome, a familial disorder that predisposes to a polymorphic type of ventricular arrhythmia known as torsades de pointes that may lead to syncope and sudden death (5).
LQT syndrome includes two clinically specific syndromes that share similar cardiac abnormalities. The most frequent, called Romano–Ward syndrome, is autosomal dominant although some recessive cases also have been described (6, 7). Romano–Ward syndrome consists only in cardiac defects. Conversely, recessive and rarer Jervell and Lange–Nielsen (JLN) syndrome comprises bilateral deafness in addition to the cardiac phenotype (8). Null mutant mice with a targeted disruption of the kcne1 gene have been engineered (9). At the homozygous state, these mice represent a relevant animal model of the JLN syndrome. As in JLN patients, kcne1-deficient mice bore bilateral deafness from birth because of the absence of K+ secretion into the endolymph (9). Analysis of the cardiac phenotype of these mice has highlighted the important role of the IKs current in the adaptability of the duration of ventricular repolarization to heart rate changes (10).
In addition to the heart and the inner ear, KCNE1 is expressed in many epithelial tissues including the intestine and the kidneys where it was originally cloned (11), but there are few data, if any, regarding its function in these tissues. It is particularly important to evaluate whether the IKs current plays a role in the control of systemic K+ balance, because disturbance of plasma K+ concentration is a well known factor influencing the occurrence of ventricular arrhythmia (12, 13). Mutations in several genes encoding renal and/or intestinal K+ and Na+ transport proteins, such as colonic H+,K+-ATPase (14), Kir 1.1 K+ channel (ROMK1) (15), amiloride-sensitive epithelial Na+ channel (ENaC) (16), Na+,K+,2Cl− (17) and Na+,Cl− (18) cotransporters, or isoform 3 of the Na+,H+ exchanger (19), have been shown to produce chronic perturbations of K+ homeostasis. In this study, we show that kcne1 is another gene critically involved in K+ homeostasis and in plasma K+-mediated regulation of aldosterone and renin production. The results suggest that mutations in the KCNE1 gene may promote the apparition of torsades de pointes ventricular arrhythmia both directly by acting on ventricular repolarization and indirectly by influencing plasma K+.
Materials and Methods
kcne1-Deficient Mice and Experimental Design.
The mice were generated by the usual gene-targeting methodologies as previously described (9). The animals used in this study were 3–5 months old and were inbred on the 129/Sv genetic background. Mice were chronically maintained on a normal diet (0.9% K+, 0.3% Na+). For the experiments, they were fed high (3%) or low (0.05%) K+ chow (UAR, Epinay, France) or barley (low Na+ diet) for 2 weeks before measuring the molecular, biochemical, and physiological parameters. The animals were allowed free access to food and water in full compliance with the French Government animal welfare policy.
Plasma Analyses.
Blood was collected into heparin-treated capillary tubes from ketamine/xylazine-anesthetized mice (7.0 and 0.4 mg/100 g body weight, respectively) by puncture of the retrobulbar venous plexus. Samples were centrifuged and plasma was frozen and kept at −20°C. Aldosterone levels were determined on unextracted plasma by using a solid-phase 125I RIA kit (Immunotech, Marseille, France) with a very low crossreactivity with corticosterone (0.042% according to the manufacturer). Plasma creatinine and ion concentrations were determined by using an automatic liquid-phase analyzer (Hitachi, model 917, Roche, Basel). Osmolality and hematocrit were measured, respectively, with a Fiske One Ten Osmometer (Norwood, Massachusetts) and a Cobas Micro Hematology Analyzer (Roche Diagnostics). Plasma renin concentration (PRC) was determined by RIA of angiotensin I generated by incubation of the plasma at pH 8.5 in the presence of an excess of rat angiotensinogen (20).
Blood Pressure and Electrocardiogram Measurements.
Ketamine/xylazine-anesthetized mice (7.0 and 0.4 mg/100 g body weight, respectively) were equipped with a catheter [extruded phycoerythrin (PE)-10 heat-sealed to PE-50] inserted into the femoral artery. Mean blood pressure was measured with a pressure transducer (Cobe, Lakewood, CO) connected to a PowerLab/S system and analyzed with CHART ver. 3.4/S software (A. D. Instruments, Phymep, France). Values were collected three times at 10-min intervals during a 3-min period and averaged. Electrocardiography was performed in ketamine/xylazine-anesthetized mice as previously described (10).
Balance Studies.
Water and food intakes as well as urine and fecal outputs were determined by using individual metabolic cages (Marty Technology, Paris, France) during two consecutive 24-h periods that were averaged for each animal. Urinary ion concentrations were measured on the same automatic analyzer used for plasma samples. The feces were weighed, suspended overnight in 0.75 N nitric acid at 4°C, and centrifuged; Na+ and K+ content were determined in the supernatant with a flame photometer (model 480; Corning Medical and Scientific, Medfield, MA).
Ussing Chamber Experiments.
Mice were killed by decapitation. The distal colon was removed, stripped from the muscle layer, and mounted into an Ussing chamber (area, 0.07 cm2). The chamber (1 ml) was thermostated at 37°C and continuously perfused on both sides at a rate of 10–20 ml/min with a solution containing (in mM): 145 NaCl, 0.4 KH2PO4, 1.6 K2HPO4, 5 d-glucose, 1 MgCl2, and 1.3 calcium gluconate; the pH was adjusted to 7.4. Trans-epithelial resistance (Rte) was determined from the voltage deflection ΔVte caused by the injection of short current pulses (1 sec; 1.5 μA); the resistance of the empty chamber was subtracted. Equivalent short-circuit current (Isc) was calculated from trans-epithelial voltage (Vte) and Rte according to Ohm's law. The polarity of Isc and Vte was referred to the lumen (21). A minus sign indicated the lumen negative voltage as compared with the basolateral surface.
In Situ Hybridization and Reverse Transcription (RT)-PCR.
Anesthetized mice were perfused with 4% paraformaldehyde/PBS solution and adrenal glands were dissected and postfixed in the same solution for 2 h. Paraffin-embedded sections (5 μm) were dewaxed by incubation in xylene and used for in situ hybridization as previously described (22). The 5′ end of the KCNE1 cDNA sequence [nucleotides 1–512, GenBank accession no. X60457 (23)] inserted into pBluescript II SK(−) vector (Stratagene) was used as a template to generate [33P]UTP-labeled sense and antisense riboprobes. Slides were dipped into Amersham Pharmacia hypercoat LM-1 autoradiography emulsion, exposed at 4°C for 3 weeks, and then developed in Kodak D-19.
Reverse transcription was performed on adrenal gland total RNA (1 μg). KCNQ1 (650 bp) and KCNE1 (451 bp) cDNA fragments were PCR-amplified (32 cycles, 30 s at 94°C, 30 s at 60°C, 30 s at 72°C) using specific oligonucleotides (5′-CTGAGAAAGATGCGGTGAAC-3′ sense and 5′-TGGGGGTCAGCAGTGTCTCC-3′ antisense for KCNQ1) and (5′-CGACTGTTCTGCCCTTTCTG-3′ sense and 5′-CTCAGTGGTGCCCCTACAAT-3′ antisense for KCNE1). The specificity of the amplified fragments was checked by hybridization with internal oligonucleotides after Southern blotting.
Statistics.
All values are expressed as means ± SEM and have been compared by ANOVA. Student's t test was used for comparing the slopes of fecal and urinary K+ excretion in relation to plasma K+ levels.
Results
Plasma K+, Aldosterone, and Renin Levels.
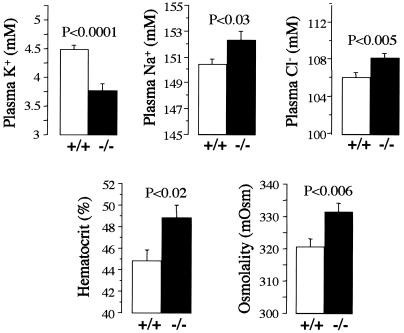
At steady state, on a normal K+ diet (0.9%), kcne1−/− mice have a lower plasma K+ concentration than wild-type mice (3.77 ± 0.12 vs. 4.48 ± 0.08 mM, n = 91, P < 0.0001; Fig. 1). This hypokalemia is probably underestimated because many indices indicate that kcne1−/− mice are slightly dehydrated. Hematocrit (48.8 ± 1.2 vs. 44.8 ± 1.1%, n = 16, P < 0.02), plasma osmolality (331.4 ± 2.8 vs. 320.6 ± 2.4 mOsm, n = 37, P < 0.006), plasma Na+ (152.26 ± 0.71 vs. 150.43 ± 0.38 mM, n = 59, P < 0.03), and Cl− concentrations (108.23 ± 0.49 vs. 106.13 ± 0.50 mM, n = 54, P < 0.005) are all increased in kcne1−/− mice compared with wild-type mice (Fig. 1). A tendency toward hypokalemia in kcne1−/− mice also is observed under a high (3%) K+ diet (4.51 ± 0.16 vs. 4.88 ± 0.13 mM, n = 51, P < 0.07) but not under a low (0.05%) K+ diet (2.88 ± 0.24 vs. 3.00 ± 0.11 mM, n = 42, P = 0.72).
Figure 1.
Blood analyses in kcne1−/− and wild-type mice at steady state on a normal diet. Plasma K+, Na+, Cl−, osmolality, and hematocrit were measured in blood collected from animals chronically fed a normal K+ (0.9%) and Na+ (0.3%) diet. In addition to the hypokalemia, kcne1−/− mice present signs of dehydration as shown by the elevation in plasma osmolality and hematocrit and the increased plasma Na+ and Cl− concentrations.
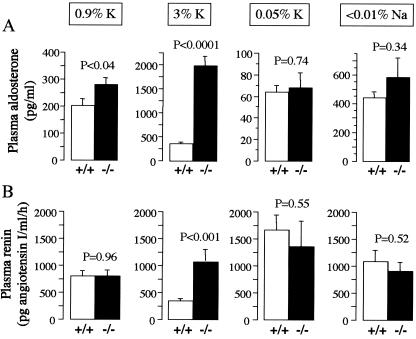
On a normal K+ diet, kcne1−/− mice have a higher plasma aldosterone concentration than wild-type mice (279.5 ± 27.3 vs. 201.8 ± 26.2, n = 72, P < 0.04; Fig. 2A). High K+ diet elevates plasma aldosterone 7.1-fold in kcne1−/− mice (279.5 ± 27.3 to 1,984.6 ± 208.8 pg/ml, P < 0.0001) vs. 1.8-fold in wild-type mice (201.8 ± 26.2 to 354.3 ± 39.7 pg/ml, P < 0.002). As a consequence, plasma aldosterone concentration is 5-fold higher in kcne1−/− mice than in wild-type mice under a high K+ diet (1,984.6 ± 208.8 vs. 354.3 ± 39.7 pg/ml, n = 51, P < 0.0001) (Fig. 2A). As expected, the low K+ diet diminishes plasma aldosterone levels but to a similar extent in kcne1−/− and wild-type mice (68.3 ± 13.4 vs. 64.1 ± 5.9 pg/ml, n = 44, P = 0.75). The low Na+ diet increases plasma aldosterone levels but no difference is observed between kcne1−/− and wild-type mice (583.2 ± 136.4 vs. 444.4 ± 39.9 pg/ml, n = 22, P = 0.34; Fig. 2A).
Figure 2.
Plasma aldosterone and renin levels in kcne1−/− and wild-type mice challenged with various K+ or Na+ diets. The dosages were performed on blood collected from animals maintained on a normal diet or fed high or low K+ diets or a low Na+ diet for 2 weeks. (A) The hyperaldosteronism in kcne1−/− mice is blunted by low K+ or Na+ diets but strongly exacerbated by high K+ intake. (B) PRC is not different between kcne1−/− and wild-type mice except on the high K+ diet, which does not decrease PRC in kcne1−/− mice. PRC is expressed as the amount of angiotensin I formed per ml of plasma per h of incubation.
Varying K+ dietary intakes also influences plasma renin levels. In wild-type mice, the high K+ diet reduces PRC from 803.4 ± 100.7 to 340.3 ± 47.6 pg angiotensin (angio) I per ml/h, n = 82, P < 0.003, whereas the low K+ diet increases PRC up to 1,672.2 ± 271.9 pg angio I per ml/h, n = 73, P < 0.004 (Fig. 2B). In contrast, in kcne1−/− mice, PRC is not affected either by the high K+ diet (809.4 ± 112.4 vs. 1,076.4 ± 231.2 pg angio I per ml/h, n = 76, P < 0.25) or by the low K+ diet (up to 1,362.7 ± 471.4 pg angio I per ml/h, n = 63, P < 0.12; Fig. 2B). The low Na+ intake does not significantly affect PRC in both kcne1−/− and wild-type mice (908.9 ± 154.7 and 1,079.9 ± 212.6 pg/ml, n = 22, P = 0.52; Fig. 2B).
Blood Pressure.
kcne1−/− and wild-type mice have similar mean blood pressure on the normal K+ diet (120.4 ± 3.1 vs. 118.6 ± 2.3 mmHg (1 mmHg = 133 Pa), n = 14, P = 0.38). As expected, blood pressure diminishes under a high K+ diet but similarly in kcne1−/− and wild-type mice (97.4 ± 2.3 vs. 95.2 ± 2.5 mmHg, n = 30, P = 0.52) despite the marked hyperaldosteronism and high PRC observed in kcne1−/− mice.
Electrocardiography.
The heart rate and QT interval vary according to plasma K+. Indeed, the QT/RR adaptation is exacerbated in hypokalemic kcne1−/− mice compared with normokalemic wild-type mice (slope: 0.97 ± 0.15 vs. 0.60 ± 0.07, n = 11, P < 0.05) yielding QT intervals of 105 and 90 ms, respectively, at a heart rate of 400 beats per minute.
Fecal and Urinary Excretion of K+ and Na+.
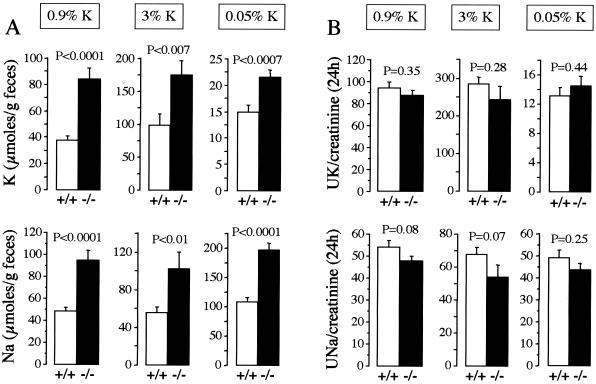
KCNE1 is expressed in both renal and intestinal epithelial cells where primary ion transport defects could explain part of the altered plasma K+ levels in kcne1−/− mice. Although the absolute K+ and Na+ intakes are not statistically different between kcne1−/− mice (499 ± 48 and 362 ± 35 μmol/day on the normal K+ diet, 1,996 ± 206 and 385 ± 45 μmol/day on the high K+ diet, 9.1 ± 0.5 and 385 ± 24 μmol/day on the low K+ diet) and wild-type mice (577 ± 54 and 392 ± 39 μmol/day on the normal K+ diet, 2,060 ± 173 and 396 ± 39 μmol/day on the high K+ diet, 9.6 ± 0.5 and 407 ± 23 μmol/day on the low K+ diet), a chronic loss of K+ and Na+ is indeed detected in the feces of kcne1−/− mice independently of the diet (Fig. 3A). kcne1−/− mice fed a normal, high, or low K+ diet excrete per gram of feces, respectively, 2.2-, 1.8-, or 1.4-fold as much K+ and 1.9-, 1.8-, or 1.8-fold as much Na+ as wild-type mice (Fig. 3A). Thus, kcne1−/− mice fed a normal K+ diet excrete 5.7% of the 24-h K+ intake in the feces vs. only 2.1% in wild-type mice. The percentages are, respectively, 3.3 and 1.9% on the high K+ diet and 68 and 48% on the low K+ diet. Likewise, kcne1−/− mice fed a normal, high, or low K+ diet excrete, respectively, 8.7, 9.6, or 14.8% of the 24-h Na+ intake in the feces compared with 3.7, 5.4, or 8.2% in wild-type mice.
Figure 3.
Fecal and urinary K+ and Na+ excretion in kcne1−/− and wild-type mice challenged with various K+ diets. Twenty-four-hour urinary and fecal excretion were determined on animals fed high or low K+ diets for 2 weeks. (A) Compared with wild-type mice, kcne1−/− mice lose K+ and Na+ in the feces for all K+ intakes. (B) Urinary K+ and Na+ excretion normalized to creatinine excretion are not different between kcne1−/− and wild-type mice whatever the K+ intake.
In contrast to fecal K+ and Na+ excretion, 24-h urinary K+ and Na+ excretion normalized to creatinine excretion are similar in kcne1−/− and wild-type mice fed normal, high, or low K+ diets (Fig. 3B). Note that on a normal K+ diet, 24-h urinary creatinine excretion (4.63 ± 0.36 vs. 4.28 ± 0.32 μmol, n = 37, P = 0.47) and plasma creatinine concentration (33.1 ± 1.2 vs. 33.0 ± 1.1 μM, n = 37, P = 0.99) are identical in kcne1−/− and wild-type mice. The recovery rates of ingested K+ and Na+ in the urine of kcne1−/− mice are 65.4 and 60.2% on the normal K+ diet, 50.4 and 61.8% on the high K+ diet, and 764.0 and 62.9% on the low K+ diet. For wild-type mice, the corresponding values are, respectively, 68.0 and 63.0% on the normal K+ diet, 51.8 and 64.9% on the high K+ diet, and 777.0 and 66.6% on the low K+ diet.
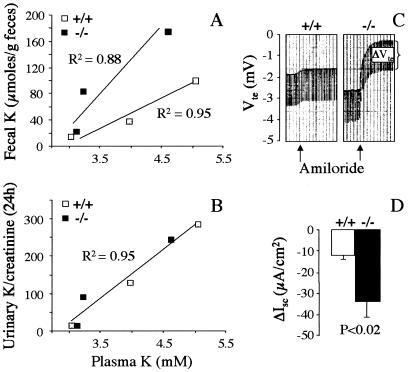
The adaptation of the intestine and the kidneys to varying K+ intake can be estimated by plotting mean values of plasma K+ concentrations and 24-h fecal or urinary K+ excretion rates measured in the mice fed different K+ diets. Fecal K+ excretion increases linearly with plasma K+ concentration much more rapidly in kcne1−/− mice than in wild-type mice (P < 0.01) suggesting a dysfunction of the intestine in secreting or absorbing K+ (Fig. 4A). In contrast, K+ excretion in the urine increases similarly in kcne1−/− and wild-type mice (P = 0.81) for increasing plasma K+ concentration (Fig. 4B).
Figure 4.
Adaptation of fecal and urinary K+ excretion to varying K+ plasma levels and ion transport assessment in the colon of kcne1−/− and wild-type mice. (A and B) Colon and renal functions were estimated by plotting mean values of plasma K+ levels and fecal or urinary 24-h K+ excretion reached by the mice on the various K+ diets. The overall adaptation of the kidneys is not affected in kcne1−/− mice in contrast to the intestine whose dysfunction provokes a chronic K+ wasting in the feces. (C and D) Short circuit currents in colonic mucosa of kcne1−/− and wild-type mice. A typical recording is shown in C. Arrows indicate the application of 10 μM amiloride. The averaged amiloride-sensitive calculated short circuit currents (ΔIsc) are shown in D.
Ion Transport in Distal Colon.
The increased fecal excretion of K+ and Na+ observed in kcne1−/− mice suggests an impaired electrolyte intestinal reabsorption. Electrogenic Na+ reabsorption in the distal colon was estimated by measuring the amiloride-sensitive short-circuit current (Isc) in Ussing chamber experiments. On a normal K+ diet, trans-epithelial voltage Vte (Fig. 4C) and amiloride-sensitive Isc are higher in kcne1−/− than in wild-type mice (−33.7 ± 7.5 vs. −11.7 ± 1.99 μA/cm2, n = 23, P < 0.02; Fig. 4D) indicating that the intestinal Na+ loss is not caused by an impaired Na+ reabsorption in distal colon. Electrogenic Cl− secretion is not affected by the lack of KCNE1 because forskolin (5 μM) in the presence of amiloride (10 μM) and indomethacin (1 μM) enhances Isc to the same extent (P = 0.59) in kcne1−/− (from −25.9 ± 7.0 to −130 ± 14.2 μA/cm2, n = 7) and wild-type mice (from −40.6 ± 6.2 to −136.2 ± 10.2 μA/cm2, n = 16).
Tissue Expression of KCNE1.
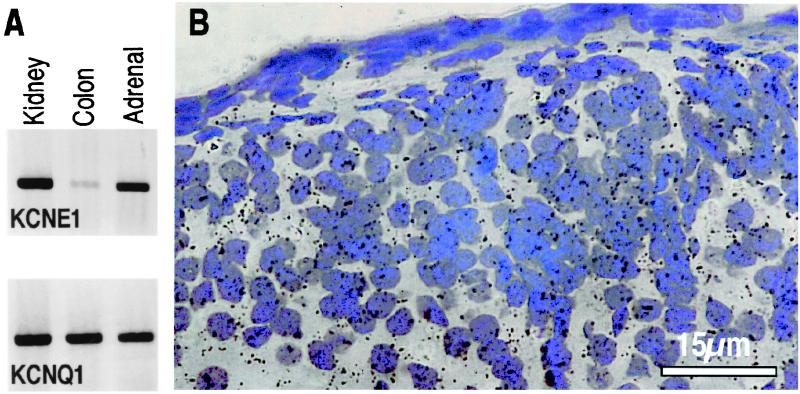
It is known from previous work that KCNE1 is expressed in renal proximal tubules (24) and in the colon (25). This is confirmed by RT-PCR experiments showing a clear expression of KCNE1 and KCNQ1 in the kidneys and colon (Fig. 5A). The KCNE1 signal in the colon is low compared with the KCNQ1 signal, which is in good agreement with the fact that KCNQ1 is associated mainly with KCNE3 in this organ to form the basolateral cAMP-activated K+ channel (26) whose activity does not seem to be affected in kcne1−/− mice. Both RT-PCR and in situ hybridization reveal the presence of KCNE1 mRNA in adrenal glands, particularly in the zona glomerulosa that synthesizes aldosterone (Fig. 5B). KCNE1 mRNA is also expressed in the zona reticularis and to a lesser extent in the zona fasciculata, but not in the medulla (data not shown).
Figure 5.
Tissue localization of KCNE1 and KCNQ1 expression. (A) RT-PCR analysis of KCNE1 and KCNQ1 mRNA in the kidneys, colon, and adrenal glands of wild-type mice. (B) In situ hybridization analysis of KCNE1 mRNA in the zona glomerulosa of toluidine blue counterstained adrenal sections of wild-type mice. No signal is detected in sections from kcne1−/− mice (data not shown).
Discussion
Null mutant kcne1 mice that lack the IKs current represent a model for the human Jervell and Lange–Nielsen syndrome that is characterized by cardiac and auditory defects (8–10). In addition to the heart and inner ear, KCNE1 also is expressed in several epithelial tissues (27, 28) where its functional role is still not clearly understood (29). In this study, we report that kcne1−/− mice at steady state on a normal K+ diet display chronic hypokalemia, hyperaldosteronism, dehydration, and Na+ and K+ fecal wasting. Such an association between hypokalemia and hyperaldosteronism could be explained by a primary epithelial defect and/or by a primary hyperaldosteronism subsequent to adrenal dysfunction.
When originating from epithelial defects, hypokalemia usually results from an increased K+ secretion triggered by up-regulation of Na+ reabsorption in the renal collecting tubule cells (30). Such a situation is observed in Liddle's syndrome where the primary defects are found in the amiloride-sensitive epithelial Na+ channel (ENaC) β and γ subunits (16, 31). In this syndrome, the constitutive activation of ENaC leads to an hypoaldosteronism acting as a compensatory mechanism to limit Na+ reabsorption and K+ wasting. Hypokalemia associated with an hyperaldosteronism can be observed in cases of primary Na+, K+, and/or Cl− transport defects occurring in the upstream segments of the nephron. Examples include Bartter's syndrome in which loss-of-function mutations are present in the Na+,K+,2Cl− cotransport NKCC2, inward rectifying K+ channel ROMK1, or Cl− channel CLCNKB of the thick ascending limb of Henle's loop, and Gitelman's syndrome that results from inactivating mutations of the Na+,Cl− cotransport NCC in the distal convoluted tubule (32). The KCNE1 protein has been detected in the apical membrane of proximal convoluted tubular cells (24) and, although kcne1−/− mice do not present overt signs of global renal dysfunction, a proximal K+ transport defect having significant consequences on systemic K+ balance and aldosteronemia cannot be excluded (33). Indeed, it has been shown recently that K+ flux through a KCNE1-associated channel contributes to K+ secretion and maintenance of the electrical driving force for Na+-coupled transport in the proximal tubule (34). It is known that proximal tubular defects can be almost completely masked at the whole kidney level by various compensatory phenomena as observed, for example, in isoform 3 Na+,H+ exchanger-deficient mice (19).
The chronic fecal Na+ and K+ wasting in kcne1−/− mice compared with wild-type mice indicates the presence of an intestinal epithelial ion transport abnormality. It is possible that some Na+ reabsorptive pathways like the isoform 3 of the Na+,H+ exchanger (19) might be affected by the lack of KCNE1 to explain the Na+ loss. The fecal Na+ losing process would occur in the small intestine and/or in the pancreas and could cause the chronic volume depletion and hyperaldosteronism despite the increased amiloride-sensitive Na+ reabsorption in the colon, which would act as a compensatory phenomenon limiting the fecal Na+ loss. The K+ wasting in kcne1−/− mice would be a direct consequence of the increased amiloride-sensitive Na+ reabsorption that mechanistically stimulates K+ secretion. This chronic loss of K+ in the feces may participate to some extent in the hypokalemia in kcne1−/− mice in a manner analogous to what is observed in colonic H+,K+-ATPase-deficient mice under K+ depletion (14).
The hypokalemia in kcne1−/− mice could also result from a primary hyperaldosteronism that can be either idiopathic or related to adrenal hyperplasia (35). This latter possibility can be excluded in kcne1−/− mice because no sign of cortical hyperplasia or tumor adenomas is observed at anatomical examination of adrenal glands (data not shown). One of the main characteristics of primary hyperaldosteronism consists of weak responsiveness to maneuvers designed to alter plasma aldosterone levels (35). Plasma K+ and angiotensin II are potent regulators of aldosterone secretion from the zona glomerulosa (36). In this study, low K+ or Na+ diets show, respectively, that the hyperaldosteronism is fully remediable and that adrenal aldosterone secretion can be stimulated normally in kcne1−/− mice. Hence, adrenal aldosterone synthesis and secretion pathways do not seem to be affected per se by the lack of KCNE1 in accordance with the normal anatomical structure of the glands.
Conversely, the increase in plasma aldosterone levels triggered by the high K+ diet is markedly exacerbated in kcne1−/− compared with wild-type mice, indicating that KCNE1 might be involved in the control of aldosterone production by extracellular K+. Plasma K+ concentration, which remains lower in kcne1−/− than in wild-type mice under the high K+ diet, cannot explain this abnormal aldosterone response. Rather, the presence of both KCNE1 and KCNQ1 in the zona glomerulosa cells suggests that the IKs current could be locally implicated in the regulation of aldosterone synthesis and secretion. In general, the process is mainly controlled by Ca2+ influx through plasma membrane voltage-dependent Ca2+ channels, probably of the T-type (37), which can be activated independently by elevation of extracellular K+ concentration or by angiotensin II. Binding of angiotensin II to AT1 receptor results in the inhibition of a resting K+ conductance, which causes a membrane depolarization sufficient to open the low-threshold T-type Ca2+ channels. The K+ channel underlying the angiotensin II-sensitive resting conductance does not present the biophysical properties of IKs and has been recently shown to be TASK1 (38), a two P-domain background channel (39). Simultaneous activation of Ca2+/calmodulin-dependent protein kinase II by angiotensin II also shifts the voltage range of activation of the T-type Ca2+ channels, thus increasing their open probability even at relatively polarized membrane potentials (37). On the other hand, even very small elevations of extracellular K+ concentration can cause a membrane depolarization leading to opening of the T-type Ca2+ channels, which in turn increases further the depolarization (40). This large depolarization is thought to activate voltage-dependent K+ channels that blunt the depolarizing response to inward currents and limit the Ca2+ influx and aldosterone secretion (40). These depolarization-activated K+ conductances in glomerulosa cells are not yet well characterized and their molecular nature is totally unknown (41). Our results strongly suggest that the IKs current is one of the conductances restraining aldosterone secretion. The low Na+ intake may elicit the same aldosterone response in kcne1−/− and wild-type mice, because angiotensin II causes a smaller depolarization than elevated K+ (37), which may not be sufficient to reach the threshold for opening the IKs current. Of note, the presence of KCNE1 mRNA in the zona reticularis and zona fasciculata cells of the adrenal cortex suggests that the IKs current could be similarly involved in the control of androgen and glucocorticoid secretion.
Dietary K+ is a known controller of renal renin synthesis (42). High K+ diet usually decreases PRC, whereas a low K+ diet has the opposite effect, as shown by this study in wild-type mice. PRC does not respond to dietary K+ load or deprivation in kcne1−/− mice. The resulting abnormally high PRC in kcne1−/− mice fed the high K+ diet could participate in the increased aldosterone response by increasing the circulating concentration of angiotensin II. The mechanism by which the lack of KCNE1 affects renin production is not known and is probably indirect because no evidence of KCNE1 expression is found in renal juxtaglomerular apparatus (data not shown). Despite the abnormal status of the renin-angiotensin-aldosterone system, kcne1−/− mice have normal blood pressure compared with wild-type mice even on the high K+ diet for which the hormonal imbalance is maximal.
Although one must remain cautious in extrapolating data from mice to humans, our finding that the IKs current plays a significant role in K+ homeostasis as well as in aldosterone and renin production may have implications in human pathology, particularly in congenital LQT syndromes. Mutations in the KCNE1 gene are very rare in humans, but those in the KCNQ1 gene represent more than 50% of all forms of LQT syndrome (5). Therefore, IKs is certainly the most frequently affected channel in this type of cardiac disorder. Mutations affecting the IKs current could favor ventricular arrhythmia both by prolonging ventricular repolarization and by inducing hypokalemia, which is a well known physiological trigger of torsades de pointes ventricular arrhythmia in humans (12, 13). In addition, the recent Randomized Aldactone Evaluation Study trial has clearly demonstrated a deleterious influence of elevated plasma aldosterone levels by showing that treatment with a mineralocorticoid receptor antagonist reduces the risk of death from progressive heart failure and the risk of cardiac sudden death (43). In view of the consequences of the kcne1 gene disruption on aldosterone production under a high K+ diet, it may be suggested that K+ load test could be applied to patients at risk to unmask a possible IKs defect that could lead to sudden death if not adequately treated. In addition, hypokalemia and volume depletion should be systematically investigated in humans with LQT syndrome who eat a normal K+ diet.
Acknowledgments
We thank Drs. David G. Warnock and Xavier Jeunemaitre for discussions and critical reading of the manuscript, Dr. François Alhenc-Gelas for the work conducted in his laboratory, and M. Larroque and M. Garcia for their expert assistance. We are grateful to the Department of Biochemistry in St-Roch Hospital at Nice for the biochemical measurements. The work was supported by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale (PROGRES no. 4P009D), the Association Française contre les Myopathies (France), Monsanto France, and Forschungsschwerpunktprogramm Baden-Württemberg.
Abbreviations
- IKs
slowly activating delayed K+ current
- LQT
long QT
- PRC
plasma renin concentration
- Isc
short-circuit current
References
- 1.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. Nature (London) 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 2.Sanguinetti M C, Curran M E, Zou A, Shen J, Spector P S, Atkinson D L, Keating M T. Nature (London) 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 3.Meneton P, Lesage F, Barhanin J. Semin Nephrol. 1999;19:438–457. [PubMed] [Google Scholar]
- 4.Sanguinetti M C. Trends Pharmacol Sci. 2000;21:199–201. doi: 10.1016/s0165-6147(00)01475-9. [DOI] [PubMed] [Google Scholar]
- 5.Priori S G, Barhanin J, Hauer R N, Haverkamp W, Jongsma H J, Kleber A G, McKenna W J, Roden D M, Rudy Y, Schwartz K, Schwartz P J, Towbin J A, Wilde A M. Circulation. 1999;99:518–528. doi: 10.1161/01.cir.99.4.518. [DOI] [PubMed] [Google Scholar]
- 6.Priori S G, Schwartz P J, Napolitano C, Bianchi L, Dennis A, DeFusco M, Brown A M, Casari G. Circulation. 1998;97:2420–2425. doi: 10.1161/01.cir.97.24.2420. [DOI] [PubMed] [Google Scholar]
- 7.Chouabe C, Neyroud N, Richard P, Denjoy I, Hainque B, Romey G, Drici M D, Guicheney P, Barhanin J. Cardiovasc Res. 2000;45:971–980. doi: 10.1016/s0008-6363(99)00411-3. [DOI] [PubMed] [Google Scholar]
- 8.Jervell A, Lange-Nielsen F. Am Heart J. 1957;54:59–68. doi: 10.1016/0002-8703(57)90079-0. [DOI] [PubMed] [Google Scholar]
- 9.Vetter D E, Mann J R, Wangemann P, Liu J Z, McLaughlin K J, Lesage F, Marcus D C, Lazdunski M, Heinemann S F, Barhanin J. Neuron. 1996;17:1251–1264. doi: 10.1016/s0896-6273(00)80255-x. [DOI] [PubMed] [Google Scholar]
- 10.Drici M D, Arrighi I, Chouabe C, Mann J R, Lazdunski M, Romey G, Barhanin J. Circ Res. 1998;83:95–102. doi: 10.1161/01.res.83.1.95. [DOI] [PubMed] [Google Scholar]
- 11.Takumi T, Ohkubo H, Nakanishi S. Science. 1988;242:1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- 12.Eckardt L, Haverkamp W, Borggrefe M, Breithardt G. Cardiovasc Res. 1998;39:178–193. doi: 10.1016/s0008-6363(98)00043-1. [DOI] [PubMed] [Google Scholar]
- 13.Roden D M. Clin Cardiol. 1997;20:285–290. doi: 10.1002/clc.4960200318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meneton P, Schultheis P J, Greeb J, Nieman M L, Liu L H, Clarke L L, Duffy J J, Doetschman T, Lorenz J N, Shull G E. J Clin Invest. 1998;101:536–542. doi: 10.1172/JCI1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon D B, Karet F E, Rodriguez-Soriano J, Hamdan J H, DiPietro A, Trachtman H, Sanjad S A, Lifton R P. Nat Genet. 1996;14:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 16.Shimkets R A, Warnock D G, Bositis C M, Nelson-Williams C, Hansson J H, Schambelan M, Gill J R, Jr, Ulick S, Milora R V, Findling J W. Cell. 1994;79:407–414. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 17.Simon D B, Karet F E, Hamdan J M, DiPietro A, Sanjad S A, Lifton R P. Nat Genet. 1996;13:183–188. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 18.Simon D B, Nelson-Williams C, Bia M J, Ellison D, Karet F E, Molina A M, Vaara I, Iwata F, Cushner H M, Koolen M, Gainza F J, Gitleman H J, Lifton R P. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 19.Schultheis P J, Clarke L L, Meneton P, Miller M L, Soleimani M, Gawenis L R, Riddle T M, Duffy J J, Doetschman T, Wang T, et al. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 20.Menard J, Catt K J. Endocrinology. 1972;90:422–430. doi: 10.1210/endo-90-2-422. [DOI] [PubMed] [Google Scholar]
- 21.Lohrmann E, Burhoff I, Nitschke R B, Lang H J, Mania D, Englert H C, Hropot M, Warth R, Rohm W, Bleich M, Greger R. Pflugers Arch. 1995;429:517–530. doi: 10.1007/BF00704157. [DOI] [PubMed] [Google Scholar]
- 22.Sibony M, Commo F, Callard P, Gasc J M. Lab Invest. 1995;73:586–591. [PubMed] [Google Scholar]
- 23.Honore E, Attali B, Romey G, Heurteaux C, Ricard P, Lesage F, Lazdunski M, Barhanin J. EMBO J. 1991;10:2805–2811. doi: 10.1002/j.1460-2075.1991.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimoto T, Tanabe Y, Shigemoto R, Iwai M, Takumi T, Ohkuubo H, Nakanishi S. J Membr Biol. 1990;113:39–47. doi: 10.1007/BF01869604. [DOI] [PubMed] [Google Scholar]
- 25.Busch A E, Suessbrich H. Trends Pharmacol Sci. 1997;18:26–29. doi: 10.1016/s0165-6147(96)01016-4. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder B C, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch T J. Nature (London) 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 27.Barhanin J, Attali B, Lazdunski L. Trends Cardiovasc Med. 1998;8:207–214. doi: 10.1016/s1050-1738(98)00013-9. [DOI] [PubMed] [Google Scholar]
- 28.Demolombe S, Franco D, de Boer P, Kuperschmidt S, Roden D, Pereon Y, Jarry A, Moorman A F, Escande D. Am J Physiol. 2001;280:C359–C372. doi: 10.1152/ajpcell.2001.280.2.C359. [DOI] [PubMed] [Google Scholar]
- 29.Bleich M, Warth R. Pflugers Arch. 2000;440:202–206. doi: 10.1007/s004240000257. [DOI] [PubMed] [Google Scholar]
- 30.Weiner I D, Wingo C S. J Am Soc Nephrol. 1997;8:1179–1188. doi: 10.1681/ASN.V871179. [DOI] [PubMed] [Google Scholar]
- 31.Pradervand S, Wang Q, Burnier M, Beermann F, Horisberger J D, Hummler E, Rossier B C. J Am Soc Nephrol. 1999;10:2527–2533. doi: 10.1681/ASN.V10122527. [DOI] [PubMed] [Google Scholar]
- 32.Simon D B, Lifton R P. Curr Opin Nephrol Hypertens. 1998;7:43–47. doi: 10.1097/00041552-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Giebisch G. Am J Physiol. 1998;43:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 34.Vallon, V., Grahammer, F., Richter, K., Bleich, M., Lang, F., Barhanin, J., Voelkl, H. & Warth, R. (2001) J. Am. Soc. Nephrol., in press. [DOI] [PubMed]
- 35.Biglieri E G, Kater C E, Mantero F. In: Hypertension: Pathophysiology, Diagnosis, and Management. 2nd Ed. Laragh J H, Brenner B M, editors. New York: Raven; 1995. pp. 2145–2162. [Google Scholar]
- 36.Delcayre C, Silvestre J S. Cardiovasc Res. 1999;43:7–12. doi: 10.1016/s0008-6363(99)00088-7. [DOI] [PubMed] [Google Scholar]
- 37.Chen X L, Bayliss D A, Fern R J, Barrett P Q. Am J Physiol. 1999;276:F674–F683. doi: 10.1152/ajprenal.1999.276.5.F674. [DOI] [PubMed] [Google Scholar]
- 38.Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. Mol Endocrinol. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- 39.Lesage F, Lazdunski M. In: Current Topics in Membranes. Kurachi Y, Jan L Y, Lazdunski M, editors. Vol. 46. San Diego: Academic; 1999. pp. 199–222. [Google Scholar]
- 40.Lotshaw D P. Endocrinology. 1997;138:4167–4175. doi: 10.1210/endo.138.10.5463. [DOI] [PubMed] [Google Scholar]
- 41.Lotshaw D P. J Membr Biol. 1997;156:261–277. doi: 10.1007/s002329900206. [DOI] [PubMed] [Google Scholar]
- 42.Linas S L. J Clin Invest. 1981;68:347–355. doi: 10.1172/JCI110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitt B, Zannad F, Remme W J, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]