Abstract
Docosahexaenoic acid is a long-chain polyunsaturated fatty acid that is found in large quantity in the brain and which has repeatedly been observed to be related in positive ways to both cognitive function and cardiovascular health. The mechanisms through which docosahexaenoic acid affects cognition are not well understood, but in this article, we propose a hypothesis that integrates the positive effects of docosahexaenoic acid in the cognitive and cardiovascular realms through the autonomic nervous system. The autonomic nervous system is known to regulate vital functions such as heart rate and respiration, and has also been linked to basic cognitive components related to arousal and attention. We review the literature from this perspective, and delineate the predictions generated by the hypothesis. In addition, we provide new data showing a link between docosahexaenoic acid and fetal heart rate that is consistent with the hypothesis.
1. The effects of DHA
Docosahexaenoic acid (DHA, 22:6n-3) is a long-chain poly unsaturated fatty acid (LC-PUFA) member of the n-3 fatty acid family found in all cell membranes. Because the accumulation of fatty acids in cell membranes is influenced by the kind and amount of n-3 fatty acids in the diet, there exists the potential for dietary fatty acids to influence many physiological functions.
1.1. Cardiovascular function
The best documented effects of DHA are in the realm of cardiovascular function. Studies in adults have shown that fish consumption and marine LC-PUFA supplementation lowers the risk of arrhythmias and reduces both blood pressure and heart rate (HR) [1,2]. Increased heart rate variability (HRV) with greater vagal predominance has also been reported with consumption of these fatty acids. Reduced HR and increased HRV results in higher stroke volume (a secondary effect of lower vascular resistance) and improved filling [3]. A meta-analysis of 30 randomized controlled trials provided evidence that fish oil consumption effectively lowers HR in humans, either directly or indirectly, by influencing cardiac electrophysiology and thereby improves autonomic tone, vascular resistance and ventricular efficiency that ultimately has a favorable effect on cardiovascular health [4].
1.2. Behavioral and cognitive development
It has also been established that DHA is important in early development. Over the past several decades, evidence has been accumulating on positive effects on sensory, cognitive, and behavioral function. The initial outcome measured was visual acuity and that showed positive effects of DHA status and supplementation, though not in all studies [5]. Less frequently, clinical trials of DHA supplementation during gestation and infancy have studied and found benefits on behavioral development, including improved performance on global developmental tests [6], higher order cognitive tasks and intelligence [7,8].
The biological effects of variable DHA in neuronal membranes include alterations in membrane biophysics and in the regulation of cell signaling and cell proliferation [9]. However, the causal path through which DHA affects behavioral and cognitive development is not at all clearly conceptualized. DHA is found in many brain structures [10], so its effects may be specifically linked to the timing and location of neuronal deposition during development. Effects on attention have been found with variable DHA exposure or status in early development [11,12].
Attention is among the most basic cognitive functions. The most fundamental conceptualization of the construct of attention is as a behavioral state that is closely associated with concepts of alertness and arousal [13,14] that enhances or facilitates learning [15]. The brain regions centrally responsible for initiating, sustaining, and modulating attention are among the same regions that mediate vital functions such as respiration, sleep-wake cycles, and cardiac activity and control [16].
In this article we will explore and develop the hypothesis that the effects of DHA on cardiac and cognitive function may be achieved through effects on the autonomic nervous system (ANS). To accomplish this, we will briefly review the nature of the ANS, the linkages between cardiac and cognitive function as mediated by the ANS and the relevant literature on the relation of DHA to ANS function. New data on the association between DHA supplementation in pregnancy and fetal HR that is in accord with the hypothesis will be presented. We will end with a delineation of the directions for future study that the hypothesis suggests.
2. The autonomic nervous system
The ANS maintains internal homeostasis of the organism by regulating functions of heart muscle, smooth muscle and hormone secretion [17]. The peripheral ANS is separated into two anatomically and functionally different divisions, the sympathetic nervous system and the parasympathetic (vagal) nervous system. The peripheral ANS is controlled by the central nervous system (CNS) through complex neuronal interconnections that form a functional entity known as the central autonomic network (CAN) [18]. The output of the CAN is mediated through preganglionic sympathetic and parasympathetic neurons that innervate the heart via the stellate ganglia and vagus nerve. The interaction of these inputs to the sino-atrial node of the heart is the source of variability and control of HR; thus HRV is an index of central-peripheral neural feedback and CNS-ANS integration. Through the CAN, the brain controls visceromotor, neuroendocrine, pain and behavioral responses essential for behavior, adaptability and survival [18].
Respiratory sinus arrhythmia (RSA) is the high-frequency HRV linked to the respiratory cycle and is a reflection of the phasic vagal control of the heart, (i.e., “vagal tone”). Respiration alters HR via the myelinated vagus, originating in the nucleus ambiguous and terminating at the sino-atrial node. HR increases during inspiration and decreases during expiration. On an electro cardiogram, this is seen as a shortening and lengthening of the R-R interval (heart period) that results in an oscillatory pattern in the HR trace at the same frequency as the respiratory cycle.
Recently, the broader health significance of autonomic balance and vagal function has been recognized. Autonomic imbalance occurs when one branch of the ANS dominates over the other, more often sympathetic hyperactivity or parasympathetic hypoactivity [19]. When the inhibitory influences of the parasympathetic nervous system are deficient, an autonomic imbalance occurs and this has been associated with increased morbidity and all-cause mortality [20]. The model of neurovisceral integration emphasizes the importance of higher order brain systems, such as the medial prefrontal cortex, that are involved in cognitive and affective processes, and proposes that inhibitory control over sympathoexcitatory circuits in the brainstem is vital to preservation of the organism and key to good health [21].
2.1. The ANS and modulation of HR in development
Early in gestation, fetal HR is primarily under control of the sympathetic nervous system [22]. As the parasympathetic nervous system matures, fetal HR decreases from an average 175 bpm in the first trimester to 140 bpm at term [23]. With increasing gestational age and increased influence from the parasympathetic nervous system, HR becomes more variable. Around 30 weeks gestational age (GA), distinct HR patterns associated with fetal activity states begin to emerge [24].
Developmental increases in cardiac vagal activity serve as an index of the developmental changes in the ability of the ANS to mediate physiological and behavioral activity [25]. This is the precursor for emotional, cognitive and behavioral regulation [26]. There is evidence that inter-individual differences in HR and HRV persist from prenatal to postnatal life [27,28]. Measures of heart period (R-R interval) and RSA are frequently used as an index of the physiological foundation of infant behaviors and to predict later developmental outcomes [29,30]. There are significant developmental increases in heart period and RSA from 4 months to 4 years of age [31] which continue until at least 7 years of age[32], paralleling the development of behavioral self-regulation[33]. Assessing vagal function, as indexed by RSA, has become a useful and important index of autonomic control and reactivity in studies of disease, development, and psychological research.
2.2. The ANS and cognition
The ANS has been linked with many cognitive functions, particularly with functions that facilitate learning. At the turn of the 20th century, the Yerkes-Dodson law [34] articulated the nature of the relationship between arousal and performance, thus implicitly integrating ANS output with cognitive ability. ANS functions were subsequently linked to many aspects of psycho logical function [35]. Perhaps the clearest link between ANS function and the fundamental steps of information processing involves the physiological changes to the occurrence of an event [36]. The orienting reflex (OR); which is a cluster of behavioral and physiological responses that occur in response to a novel or unexpected stimuli, has been historically linked to exploration and learning [37,38]. When a stimulus occurs, there is a clear behavioral response that indicates the initiation of attention (i.e., the direction of the sensory receptors toward the source or location of the stimulus). This behavioral response is also accompanied by autonomic indicators, such as changes in HR, respiration, skin conductance, pupil size and motor activity. Subsequent formulations attempted to refine and dissociate different response components from the autonomically driven OR; for example, Graham and Clifton [39] concluded that more intense or threatening stimuli tend to activate the sympathetic nervous system (e.g., cardiac acceleration) while stimuli eliciting interest or curiosity would activate parasympathetic responses (e.g., cardiac deceleration). The Laceys [40,41] proposed a similar, but more wide-ranging model termed “directional fractionation,” in which parasympathetic activation represented stimulus intake, and sympathetic activation represented stimulus avoidance [42]. In considering the link between ANS function and cognition, it is critical to note that parasympathetic driven responses (cardiac deceleration, slowed respiration, pupil dilation, reduced skin conductance) to stimuli or events have the presumed effect of reducing random or unwanted “noise” in the CNS, a fundamental characteristic of attention and a facilitator of learning [13]. Sympathetic activation more closely characterizes increased arousal which, as noted above, is a more phasic moderator of cognitive performance.
A discussion of the mechanisms through which ANS and cognitive functions are mediated are beyond the scope and page limitations of the current paper. It has been suggested that ANS changes in attention are a result of the behavioral events that occur in response to a stimulus or event [43]. However, the more widely accepted notion at this time [33,44] posits that the ANS changes seen at the initiation of cognitive functions such as attention and arousal are attributable to a common, neurally regulated mechanism. Evidence supports the existence of such a mechanism at the level of mammalian brain stem organization; this is mediated by a dual vagal complex and that links autonomic processes in particular with attention, action, and other psycho logical constructs such as emotion. Indeed, four ascending brainstem systems have been integrally implicated in attention and arousal, and in the modulation of various forms of cognitive behavior [45–48]. This suggests that measures of cardiovascular response to stimuli (in particular, measures of variability such as vagal tone derived from RSA) will be associated with various developmental and adult cognitive and affective outcomes. At this point, there is considerable evidence in its favor of this model [49]. The links between ANS function and cognition are bidirectional; that is, initiation of physiological changes will produce changes in attention and arousal, and initiation of attention and arousal will produce changes in ANS indicators.
Infant autonomic control is linked to the degree of maturation and integrity of the ANS. Small for gestational age and preterm infants with decreased vagal function are more vulnerable, less able to adjust quickly to stressful stimuli and are shown to have suboptimal neurodevelopmental outcomes [29,50,51]. Conversely, higher vagal activity and self-regulation in infants has been positively correlated with higher Bayley scores [52], better social skills [29], shorter visual fixation duration [53], and increased attention [54].
2.3. DHA and ANS function
DHA is the second most abundant LC-PUFA in the CNS and with arachidonic acid (AA) constitutes approximately 50% of the total fatty acids in neuronal membrane phospholipids [9]. The developing fetus receives maternal DHA by transfer across the placenta and DHA accumulates in the CNS during the third trimester. After birth, the infant receives DHA from mother’s milk or DHA-supplemented infant formula.
Between 30 and 34 weeks gestational age there is a period of rapid brain growth, myelination and synaptogenesis. Two important physiologic oscillators emerge during the third trimester: (1) the sleep-wake cycle where distinct quiet and active states are observed and (2) increased parasympathetic control over heart rhythms. While studies have investigated the importance of DHA intake for preterm and term infant visual and cognitive development, few studies have considered the influence of DHA on ANS maturation and regulation.
Observational studies of human milk-fed vs. formula-fed infants prior to 2002 when DHA was added to US infant formulas showed that human milk-fed infants had lower HR and higher HRV than their bottle formula-fed cohorts [55–57]. Recent experimental studies of LC-PUFA supplementation in human infants and non-human infant primates suggest that supplementation during infancy could have positive cardiovascular effects. Healthy Danish infants were supplemented with fish oil (or no oil) in cow’s milk or infant formula from 9 to 12 months of age. Fish oil supplementation effectively increased red blood cell DHA levels and lowered HR with a trend towards higher HRV. These findings suggest that adding fish oil to the diet had a beneficial effect on HR in healthy infants similar to the effect seen in adults [58].
Three-year-old Rhesus monkeys fed formulas containing DHA and AA as infants had significantly higher HRV when under stress than monkeys fed formulas without DHA and AA [59]. It is notable that the dietary intervention was only from birth until weaning after which both groups were fed ordinary monkey chow without DHA and AA. Early dietary supplementation of DHA and AA appears to have induced a cardiac programming effect that persisted well beyond the period of DHA and AA exposure.
Intrauterine exposure of DHA has also been shown to modulate sleep. Cheruku et al. [60] studied the sleep patterns of 1–2 day old infants and found that the infants born to women with higher plasma phospholipid DHA had more mature sleep-state patterning.
Healthy, human milk-fed newborns had superior arousability when newborn neurobehavioral outcomes were tested using the Brazelton Neonatal Behavioral Assessment Scale (NBAS). A few investigators have posited that enhanced arousability [61,62] and higher scores for orientation/engagement, emotional regulation, motor quality and total behavior scores using the NBAS [63] may be due to components in breast milk, including DHA. However, maternal breast milk DHA level is a reflection of maternal DHA stores available to the fetus during intrauterine transfer therefore; the positive behavioral effects seen in these studies are likely due to increased availability to the fetus in the 3rd trimester as opposed to DHA intake from breast milk after birth.
There have been several calls to examine the constructs of arousal and attention in studies of LC-PUFA status and supplementation in infancy and childhood [6,59]. Measures of attention appear to be particularly sensitive to DHA status, as evidenced by a number of studies showing positive results in infancy [11,12,64–66]. One study showed supplementation as specifically improving sustained attention performance in late childhood [67] although another showed improvements in most cognitive measures except attention [68].
There have been reports of abnormal LC-PUFA (DHA and/or AA) profiles in children with attention-deficit disorder [69–72]. These have motivated studies of remediation with supplementation of DHA, AA and/or gamma linolenic acid. Such supplementation universally increased LC-PUFAs in a positive direction; however, remediation was associated with improvement in symptoms in some [73,74] but not other trials [75,76].
3. New data: fetal HR and DHA supplementation
Fetal biomagnetometry has emerged as a reliable and sensitive tool for monitoring fetal ANS development [77–79]. Biomagnetic cardiac signals (magnetocardiogram or MCG) are measured by a detector that is inductively coupled to a superconducting quantum interference device (SQUID) that acts as a low-noise, high-gain, current-to-voltage converter. Fetal MCG offers the precision required for measures of HRV based on accurate detection of R-R intervals from 24 weeks GA to term. We have used MCG to obtain measures of fetal HR, HRV and fetal movements as part of a fetal neurobehavioral assessment [80].
The study was approved by the University of Kansas Medical Center Human Subjects Committee (protocol #9297). A total of 40 subjects were studied between 24 and 38 weeks gestation. The MCG was recorded using an investigational 83 channel dedicated fetal biomagnetometer (CTF systems, subsidiary of VSM MedTech Ltd.) housed in a magnetically shielded room to reduce the interference from external environmental magnetic fields. The data were acquired with a 300 Hz sampling rate over an 18min recording session and digitally filtered offline. Multivariate data were presented to an Infomax ICA algorithm implemented in EEGLAB toolbox [81] in order to separate contributions from spatially distinct electrophysiologic sources. The fiducial points (R peaks) were automatically estimated by a template matching algorithm applied on the root-mean-square signal across channels. False negative and false positive detections were manually corrected. Fetal activity state was determined from the HR pattern [82]. Indices of HRV were determined from Poincaire plots [83] where SD1 is a measure of short-term HRV, SD2 is a measure of overall HRV and the SD1:SD2 ratio is a measure of sympatho- vagal balance.
We recorded maternal prenatal vitamin and supplement use. All women reported taking prenatal vitamins. Out of these women, 10 reported consuming DHA or DHA/EPA supplements based on the recommendation of their health care provider or by their own choice. The average daily dose of DHA was 200 mg. All 10 women took the supplements during the 2nd and 3rd trimester. Fetal sex, activity state and GA were matched to an unsupplemented fetus and mean HR and HRV indices were compared. When possible, subjects were followed longitudinally but it was not possible to obtain data at every time point in all subjects. The total number of subjects (supplemented plus unsupplemented control) for each GA tested are: 24 wks n = 6, 28 wks n = 16, 32wks n = 18, 36 wks n = 16, 38 wks n = 12.
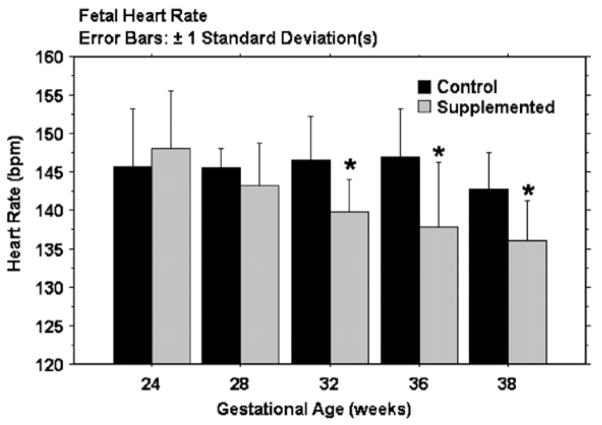
Fetal HR from 24 to 38 weeks GA is shown in Fig. 1. There was no group difference in fetal HR at 24 and 28 weeks. However, from 32 to 38 weeks, fetal HR was significantly lower in the supplemented group when compared to unsupplemented. (32 wks, p = .01, 36wks, p = .02, 38 wks, p = .03). Overall HRV (SD2) and the SD1:SD2 ratio did not differ between groups (data not shown). There was no difference in short-term HRV (SD1) at 24 and 28 weeks GA but at 32 weeks, short-term variability was higher in the supplemented group and this persisted to term. The difference was significant at 36 weeks (p = .02) (Fig. 2).
Fig. 1. Mean fetal HR(±1 SD) in control (unsupplemented) and supplemented groups across gestational age.
Mean HR for the supplemented group begins to diverge from the control group at 32 weeks and is consistently and significantly lower at 32, 36 and 38 weeks.
Fig. 2. Short term heart rate variability.
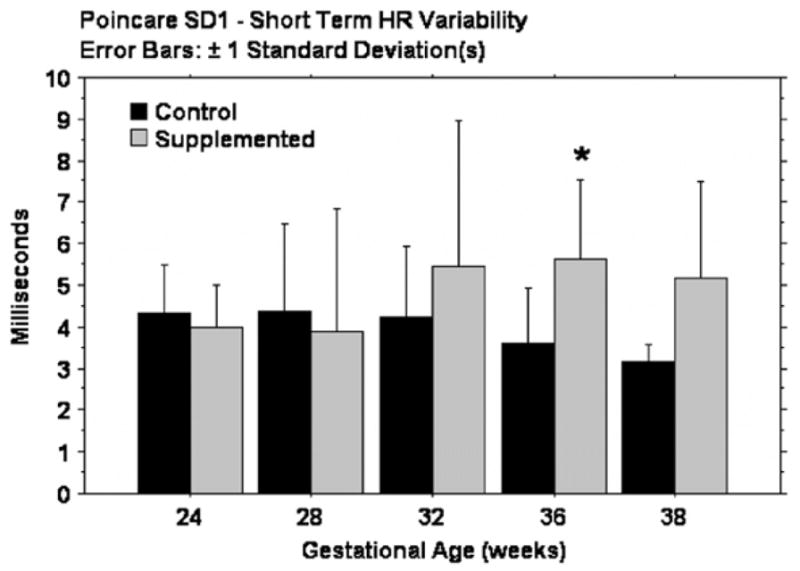
There is no group difference in Poincare SD1, a measure of short term heart rate variability, between 24 and 28 weeks GA. At 32 weeks, short term HRV is higher in the supplemented group, significant at 36 weeks.
These are preliminary data and, as such, have several limitations. This was a convenience sample of women who consumed DHA or DHA/EPA supplements during the 2nd and 3rd trimester of pregnancy, drawn from a larger pool of women with uncomplicated, singleton pregnancies. DHA intake was not controlled or monitored, there was no placebo group and we have no measure of maternal DHA status in either group. However, our preliminary, observational findings suggest that maternal DHA or DHA/EPA supplementation during pregnancy may result in lower fetal HR and higher cardiac vagal control and a larger, controlled study is planned.
4. Summary and conclusions
There were three aims of this paper. The first was to propose a hypothesis that sought to integrate the effects of DHA seen in both the cardiovascular and cognitive realms. This was done by invoking mechanisms common to the ANS which serves both vital physiological functions as well as fundamental steps in the initiation of information processing. Second, we sought to develop this hypothesis by reviewing the literature on the effects of DHA and links between ANS, DHA and cognitive function. Finally, we provided new data showing possible relations between prenatal DHA or DHA/EPA supplementation and fetal cardiac measures that are consistent with the hypothesis. We realize that we have made a series of preliminary arguments here, but it is our hope that this paper will spur researchers to consider the involvement of the ANS as a basic system that is affected by DHA we well as a mechanism through which DHA affects both physiological and behavioral outcomes, Furthermore, we hope that researchers will also seek to include measures of autonomic function in studies of DHA supplementation and status in the future.
References
- 1.Theobald HE, Goodall AH, Sattar N, Talbot DC, Chowienczyk PJ, Sanders TA. Low-dose docosahexaenoic acid lowers diastolic blood pressure in middle-aged men and women. J Nutr. 2007;137:973–978. doi: 10.1093/jn/137.4.973. [DOI] [PubMed] [Google Scholar]
- 2.Horrocks LA, Yeo YK. Health benefits of docosahexaenoic acid (DHA) Pharmacol Res. 1999;40:211–225. doi: 10.1006/phrs.1999.0495. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D. Fish, n-3 fatty acids and cardiovascular haemodynamics. J Cardiovasc Med (Hagerstown) 2007;8(Suppl 1):S23–S26. doi: 10.2459/01.JCM.0000289279.95427.e2. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 5.SanGiovanni JP, Parra-Cabrera S, Colditz GA, Berkey CS, Dwyer JT. Meta analysis of dietary essential fatty acids and long-chain polyunsaturated fatty acids as they relate to visual resolution acuity in healthy preterm infants. Pediatrics. 2000;105:1292–1298. doi: 10.1542/peds.105.6.1292. [DOI] [PubMed] [Google Scholar]
- 6.Carlson SE, Neuringer M. Polyunsaturated fatty acid status and neurodevelopment: a summary and critical analysis of the literature. Lipids. 1999;34:171–178. doi: 10.1007/s11745-999-0351-2. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JT, Bellinger DC, Connor WE, Shaywitz BA. A quantitative analysis of prenatal intake of n-3 polyunsaturated fatty acids and cognitive development. Am J Prev Med. 2005;29:366–374. doi: 10.1016/j.amepre.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics. 2003;111:e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 9.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh AT, Anthony JC, Diersen-Schade DA, Rumsey SC, Lawrence P, Li C, et al. The influence of moderate and high dietary long chain polyunsaturated fatty acids (LCPUFA) on baboon neonate tissue fatty acids. Pediatr Res. 2007;61:537–545. doi: 10.1203/pdr.0b013e318045bec9. [DOI] [PubMed] [Google Scholar]
- 11.Carlson SE, Werkman SH. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until two months. Lipids. 1996;31:85–90. doi: 10.1007/BF02522416. [DOI] [PubMed] [Google Scholar]
- 12.Werkman SH, Carlson SE. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until nine months. Lipids. 1996;31:91–97. doi: 10.1007/BF02522417. [DOI] [PubMed] [Google Scholar]
- 13.Colombo J. The development of visual attention in infancy. Annu Rev Psychol. 2001;52:337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- 14.Colombo J, Richman WA. Infant timekeeping: attention and temporal estimation in 4-month-olds. Psychol Sci. 2002;13:475–479. doi: 10.1111/1467-9280.00484. [DOI] [PubMed] [Google Scholar]
- 15.Steinmetz JE. Brain substrates of classical eyeblink conditioning: a highly localized but also distributed system. Behav Brain Res. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- 16.Richards JE. The development of sustained visual attention in infants from 14 to 26 weeks of age. Psychophysiology. 1985;22:409–416. doi: 10.1111/j.1469-8986.1985.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 17.Ravits JM. AAEM minimonograph #48: autonomic nervous system testing. Muscle Nerve. 1997;20:919–937. doi: 10.1002/(sici)1097-4598(199708)20:8<919::aid-mus1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Benarroch EE. The central autonomic network: functional organization, dysfunction and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 19.Malliani A, Lombardi F, Pagani M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J. 1994;71:1–2. doi: 10.1136/hrt.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- 22.Renou P, Newman W, Wood C. Autonomic control of fetal heart rate. Am J Obstet Gynecol. 1969;105:949–953. doi: 10.1016/0002-9378(69)90103-3. [DOI] [PubMed] [Google Scholar]
- 23.Pillai M, James D. The development of fetal heart rate patterns during normal pregnancy. Obstet Gynecol. 1990;76:812–816. doi: 10.1097/00006250-199011000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Feldman R. From biological rhythms to social rhythms: physiological precursors of mother-infant synchrony. Dev Psychol. 2006;42:175–188. doi: 10.1037/0012-1649.42.1.175. [DOI] [PubMed] [Google Scholar]
- 25.Porges SW, Doussard-Roosevelt JA, Portales AL, Suess PE. Cardiac vagal tone: stability and relation to difficultness in infants and 3-year-olds. Dev Psychobiol. 1994;27:289–300. doi: 10.1002/dev.420270504. [DOI] [PubMed] [Google Scholar]
- 26.Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal brake predicts child behavior problems: a psychobiological model of social behavior. Dev Psychobiol. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 27.DiPietro JA, Costigan KA, Pressman EK, Doussard-Roosevelt JA. Antenatal origins of individual differences in heart rate. Dev Psychobiol. 2000;37:221–228. doi: 10.1002/1098-2302(2000)37:4<221::aid-dev2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 28.Thomas PW, Haslum MN, MacGillivray I, Golding MJ. Does fetal heart rate predict subsequent heart rate in childhood? Early Hum Dev. 1989;19:147–152. doi: 10.1016/0378-3782(89)90125-4. [DOI] [PubMed] [Google Scholar]
- 29.Doussard-Roosevelt JA, McClenny BD, Porges SW. Neonatal cardiac vagal tone and school-age developmental outcome in very low birth weight infants. Dev Psychobiol. 2001;38:56–66. [PubMed] [Google Scholar]
- 30.Dipietro JA, Irizarry RA, Hawkins M, Costigan KA, Pressman EK. Cross-correlation of fetal cardiac and somatic activity as an indicator of antenatal neural development. Am J Obstet Gynecol. 2001;185:1421–1428. doi: 10.1067/mob.2001.119108. [DOI] [PubMed] [Google Scholar]
- 31.Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Dev Psychobiol. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Marshall PJ, Stevenson-Hinde J. Behavioral inhibition, heart period and respiratory sinus arrhythmia in young children. Dev Psychobiol. 1998;33:283–292. [PubMed] [Google Scholar]
- 33.Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 34.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit formation. J Comp Neurol Psychol. 1908;18:459–482. [Google Scholar]
- 35.Ruckmick CA. The systematic position of emotion. Psychol Rev. 1936;43:417–426. [Google Scholar]
- 36.Sokolov EN. Perception and the Conditioned Reflex. The MacMillan Company; New York: 1963. p. 312. [Google Scholar]
- 37.Sechenov IM. Reflexes of the Brain. MIT Press; Cambridge, MA: 1863/1965. [Google Scholar]
- 38.Pavlov IP. Conditional Reflexes. Dover Publications; New York: 1927. [Google Scholar]
- 39.Graham FK, Clifton RK. Heart-rate change as a component of the orienting response. Psychol Bull. 1966;65:305–320. doi: 10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- 40.Lacey JI, Lacey BC. Verification and extension of the principle of autonomic response-stereotypy. Am J Psychol. 1958;71:50–73. [PubMed] [Google Scholar]
- 41.Libby WL, Jr, Lacey BC, Lacey JI. Pupillary and cardiac activity during visual attention. Psychophysiology. 1973;10:270–294. doi: 10.1111/j.1469-8986.1973.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 42.Hess EH. How Your Eyes Reveal Hidden Thoughts and Emotions. Van Nostrand Reinhold; Oxford, England: 1975. The Tell-Tale Eye. [Google Scholar]
- 43.Obrist PA, Webb RA, Sutterer JR. Heart rate and somatic changes during aversive conditioning and a simple reaction time task. Psychophysiology. 1969;5:696–723. doi: 10.1111/j.1469-8986.1969.tb02872.x. [DOI] [PubMed] [Google Scholar]
- 44.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doty RW. Brainstem influences on forebrain processes, including memory. In: Spear NE, Spear LP, Woodruff ML, editors. Neurobehavioral Plasticity: Learning, Development and the Response to Brain Insults. Lawrence Erlbaum Associates, Inc; Hillsdale, JH: 1995. pp. 349–370. [Google Scholar]
- 46.Parasuraman R, Warm JS, See JE. Brain systems of vigilance. In: Parasuraman R, editor. The Attentive Brain. MIT Press; Cambridge, MA: 1998. pp. 221–256. [Google Scholar]
- 47.Robbins TW. Arousal and attention: psychopharmacological and neuropsychological studies in experimental animals. In: Parasuraman R, editor. The Attentive Brain. MIT Press; Cambridge, MA: 1998. pp. 189–220. [Google Scholar]
- 48.Robbins TW, Everitt BJ. Arousal systems and attention. In: Gazzaniga M, editor. The Cognitive Neurosciences. MIT Press; Cambridge, MA: 1995. pp. 703–720. [Google Scholar]
- 49.Porges SW, Doussard-Roosevelt J. Early physiological patterns and later behavior. In: Reese HW, Franzen MD, editors. Biological and Neuropsychological Mechanisms: Life-Span Developmental Psychology. England: Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1997. pp. 163–179. [Google Scholar]
- 50.Arditi H, Feldman R, Eidelman AI. Effects of human contact and vagal regulation on pain reactivity and visual attention in newborns. Dev Psychobiol. 2006;48:561–573. doi: 10.1002/dev.20150. [DOI] [PubMed] [Google Scholar]
- 51.Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, Scanlon KB. Vagal regulation of heart rate in the prediction of developmental outcome for very low birth weight preterm infants. Child Dev. 1997;68:173–186. [PubMed] [Google Scholar]
- 52.DeGangi GA, DiPietro JA, Greenspan SI, Porges SW. Psychophysiological characteristics of the regulatory disordered infant. Infant Behav Dev. 1991;14:37–50. [Google Scholar]
- 53.Richards JE. Respiratory sinus arrhythmia predicts heart rate and visual responses during visual attention in 14 and 20 week old infants. Psychophysiology. 1985;22:101–109. doi: 10.1111/j.1469-8986.1985.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 54.Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: assessments at twelve weeks of age. Child Dev. 1998;69:624–635. [PubMed] [Google Scholar]
- 55.DiPietro JA, Larson SK, Porges SW. Behavioral and heart rate pattern differences between breast-fed and bottle-fed neonates. Dev Psychol. 1987;23:467–474. [Google Scholar]
- 56.Zeskind PS, Marshall TR, Goff DM. Rhythmic organization of heart rate in breast-fed and bottle-fed newborn infants. Early Dev Parenting. 1992;1:79–87. [Google Scholar]
- 57.Butte NF, Smith EO, Garza C. Heart rates of breast-fed and formula-fed infants. J Pediatr Gastroenterol Nutr. 1991;13:391–396. doi: 10.1097/00005176-199111000-00009. [DOI] [PubMed] [Google Scholar]
- 58.Lauritzen L, Christensen JH, Damsgaard CT, Michaelsen KF. ISSFAL. Cairns; North Queensland, Australia: 2006. Heart rhythm is affected by fish oil supplementation in healthy Danish infants; p. 129. [Google Scholar]
- 59.Hibbeln JR, Ferguson TA, Blasbalg TL. Omega-3 fatty acid deficiencies in neurodevelopment, aggression and autonomic dysregulation: opportunities for intervention. Int Rev Psyhchiatry. 2006;18:107–118. doi: 10.1080/09540260600582967. [DOI] [PubMed] [Google Scholar]
- 60.Cheruku SR, Montgomery-Downs HE, Farkas SL, Thoman EB, Lammi-Keefe CJ. Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. Am J Clin Nutr. 2002;76:608–613. doi: 10.1093/ajcn/76.3.608. [DOI] [PubMed] [Google Scholar]
- 61.Hart SL, Boylan LM, Carroll SR, Musick YA, Kuratko C, Border BG, et al. Brief report: newborn behavior differs with docosahexaenoic acid levels in breast milk. J Pediatr Psychol. 2006;31:221–226. doi: 10.1093/jpepsy/jsj069. [DOI] [PubMed] [Google Scholar]
- 62.Horne RS, Parslow PM, Ferens D, Watts AM, Adamson TM. Comparison of evoked arousability in breast and formula fed infants. Arch Dis Child. 2004;89:22–25. [PMC free article] [PubMed] [Google Scholar]
- 63.Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Wright LL, Langer JC, et al. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118:e115–e123. doi: 10.1542/peds.2005-2382. [DOI] [PubMed] [Google Scholar]
- 64.Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, et al. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004;75:1254–1267. doi: 10.1111/j.1467-8624.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- 65.Willatts P, Forsyth JS. The role of long-chain polyunsaturated fatty acids in infant cognitive development. Prostaglandins Leukot Essent Fatty Acids. 2000;63:95–100. doi: 10.1054/plef.2000.0198. [DOI] [PubMed] [Google Scholar]
- 66.Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Influence of long-chain polyunsaturated fatty acids on infant cognitive function. Lipids. 1998;33:973–980. doi: 10.1007/s11745-998-0294-7. [DOI] [PubMed] [Google Scholar]
- 67.Vaisman N, Kaysar N, Zaruk-Adasha Y, Pelled D, Brichon G, Zwingelstein G, et al. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: effect of dietary n-3 fatty acids containing phospholipids. Am J Clin Nutr. 2008;87:1170–1180. doi: 10.1093/ajcn/87.5.1170. [DOI] [PubMed] [Google Scholar]
- 68.Osendarp SJ, Baghurst KI, Bryan J, Calvaresi E, Hughes D, Hussaini M, et al. Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in Australia and Indonesia. Am J Clin Nutr. 2007;86:1082–1093. doi: 10.1093/ajcn/86.4.1082. [DOI] [PubMed] [Google Scholar]
- 69.Burgess JR, Stevens L, Zhang W, Peck L. Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am J Clin Nutr. 2000;71:327S–330S. doi: 10.1093/ajcn/71.1.327S. [DOI] [PubMed] [Google Scholar]
- 70.Colter AL, Cutler C, Meckling KA. Fatty acid status and behavioural symptoms of attention deficit hyperactivity disorder in adolescents: a case- control study. Nutr J. 2008;7:8. doi: 10.1186/1475-2891-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Germano M, Meleleo D, Montorfano G, Adorni L, Negroni M, Berra B, et al. Plasma, red blood cells phospholipids and clinical evaluation after long chain omega-3 supplementation in children with attention deficit hyperactivity disorder (ADHD) Nutr Neurosci. 2007;10:1–9. doi: 10.1080/10284150601153801. [DOI] [PubMed] [Google Scholar]
- 72.Young GS, Maharaj NJ, Conquer JA. Blood phospholipid fatty acid analysis of adults with and without attention deficit/hyperactivity disorder. Lipids. 2004;39:117–123. doi: 10.1007/s11745-004-1209-3. [DOI] [PubMed] [Google Scholar]
- 73.Joshi K, Lad S, Kale M, Patwardhan B, Mahadik SP, Patni B, et al. Supplementation with flax oil and vitamin C improves the outcome of Attention Deficit Hyperactivity Disorder (ADHD) Prostaglandins Leukot Essent Fatty Acids. 2006;74:17–21. doi: 10.1016/j.plefa.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Sorgi PJ, Hallowell EM, Hutchins HL, Sears B. Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutr J. 2007;6:16. doi: 10.1186/1475-2891-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr. 2001;139:189–196. doi: 10.1067/mpd.2001.116050. [DOI] [PubMed] [Google Scholar]
- 76.Stevens L, Zhang W, Peck L, Kuczek T, Grevstad N, Mahon A, et al. EFA supplementation in children with inattention, hyperactivity and other disruptive behaviors. Lipids. 2003;38:1007–1021. doi: 10.1007/s11745-006-1155-0. [DOI] [PubMed] [Google Scholar]
- 77.Van Leeuwen P, Lange S, Bettermann H, Gronemeyer D, Hatzmann W. Fetal heart rate variability and complexity in the course of pregnancy. Early Hum Dev. 1999;54:259–269. doi: 10.1016/s0378-3782(98)00102-9. [DOI] [PubMed] [Google Scholar]
- 78.Wakai RT, Wang M, Martin CB. Spatiotemporal properties of the fetal magnetocardiogram. Am J Obstet Gynecol. 1994;170:770–776. doi: 10.1016/s0002-9378(94)70280-2. [DOI] [PubMed] [Google Scholar]
- 79.Verklan MT, Padhye NS, Brazdeikis A. Analysis of fetal heart rate variability obtained by magnetocardiography. J Perinat Neonatal Nurs. 2006;20:343–348. doi: 10.1097/00005237-200610000-00014. [DOI] [PubMed] [Google Scholar]
- 80.DiPietro JA. Neurobehavioral assessment before birth. Ment Retard Dev Disabil Res Rev. 2005;11:4–13. doi: 10.1002/mrdd.20047. [DOI] [PubMed] [Google Scholar]
- 81.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 82.Pillai M, James D. Behavioural states in normal mature human fetuses. Arch Dis Child. 1990;65:39–43. doi: 10.1136/adc.65.1_spec_no.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamen PW, Krum H, Tonkin AM. Poincare plot of heart rate variability allows quantitative display of parasympathetic nervous activity in humans. Clin Sci (Lond) 1996;91:201–208. doi: 10.1042/cs0910201. [DOI] [PubMed] [Google Scholar]