Abstract
Insight into the mechanisms of anti-tumor immunity has generated great enthusiasm for the treatment of patients with advanced melanoma. Particularly, negative regulators of the immune system, called immunologic checkpoints, have been found to play important roles in restraining otherwise effective anti-tumor immunologic responses. Therapies that target these negative regulator checkpoints, such as those directed against cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death-1 receptor (PD-1), have demonstrated promising clinical results. Treatment is generally well tolerated, but a novel spectrum of side effects, termed immune-related adverse events, has been experienced. Unfortunately not all patients respond to these therapies and evaluation of biomarkers predictive of response is ongoing. Based upon their unique mechanisms of action, radiographic assessment of response differs from methods traditionally applied to cytotoxic chemotherapy. We expect ongoing and future efforts combining agents that target immunologic checkpoints with other immunotherapeutic approaches, targeted therapy, chemotherapy, and radiotherapy may additionally be beneficial.
Keywords: melanoma, immunotherapy, ipilimumab, tremelimumab, monoclonal antibody, programmed death-1 receptor, cytotoxic T-lymphocyte antigen 4
Introduction
The promise of harnessing the immune system to treat cancer has captivated medical researchers for many years. Dr. William Coley may have been the first to describe the power of the immune system to treat cancer after he discovered bacterial products (Coley’s toxins) could result in dramatic disease regressions in patients with cancer.1,2 Since that time, a variety of immunotherapeutic strategies to elicit anti-tumor immunity have been attempted. Many of these treatment modalities have been applied to patients with melanoma, a disease model long believed to be responsive to immune system manipulation.
Immunotherapy for melanoma can largely be divided into several strategic approaches: therapeutic cancer vaccines, adoptive cell therapy, cytokine therapy, and immunomodulatory antibody therapy. Therapeutic cancer vaccination is a strategy of enhancing active immunity, primarily through activation of T cells, to recognize and destroy a patient’s tumors. A variety of platforms have been used in therapeutic vaccination programs including small epitopes/peptides,3 DNA,4,5 dendritic cells,6 and whole tumor cell preparations7 with mixed clinical results. Adoptive cell therapy is a method of administering highly selected tumor-reactive T cells to patients following lymphodepleting chemotherapy. While this approach has shown clinical benefit for some patients,8 technical aspects of the procedure have limited more widespread use. Cytokine therapy, primarily interferon alpha-2b and interleukin-2 (IL-2), have been widely used in patients with high-risk localized and metastatic melanoma, respectively. For patients with high-risk melanoma after complete surgical resection, a pooled analysis of adjuvant trials has shown interferon-alpha to significantly prolong relapse-free survival.9 IL-2 has been shown to induce durable remissions in a small subset of patients with metastatic disease, albeit with significant treatment related toxicity,10 and there is evidence combining IL-2 with the gp100 vaccine increases the response rate and progression-free survival of patients with melanoma compared to IL-2 alone.11 Though therapeutic cancer vaccines, adoptive cell therapy and cytokine therapy have each demonstrated efficacy in particular contexts for patients with melanoma, significant enthusiasm currently exists for a fourth immunotherapeutic strategy-- use of immunomodulatory antibody therapy.
Immunomodulatory antibody therapy refers to the use of monoclonal antibodies that directly enhance the function of components of the anti-tumor immune response such as T cells or block immunologic checkpoints that would otherwise restrain effective anti-tumor immunity. At present, antibodies that block immunosuppressive checkpoints are more clinically developed than those that stimulate aspects of the immune system. In this review, we will trace the major preclinical and clinical studies that led to the development of clinically active immune checkpoint targeting antibodies, discuss the lessons learned through the development of this novel therapeutic strategy, and suggest avenues for future research.
Cytotoxic T-Lymphocyte Antigen 4 Blockade: Ipilimumab and Tremelimumab
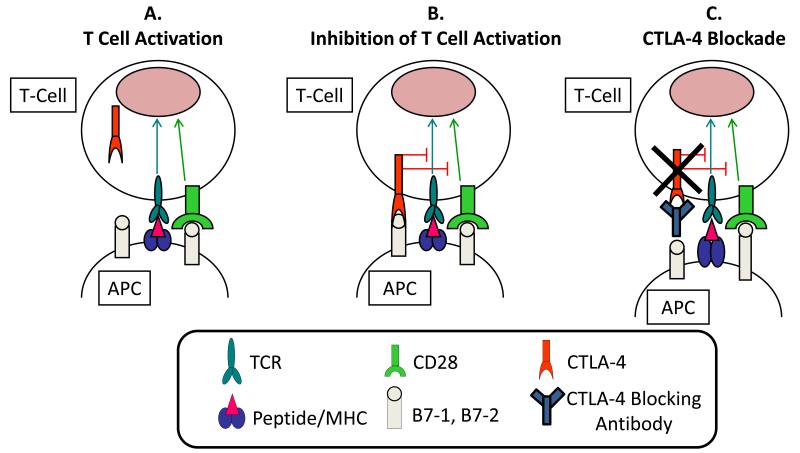
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is a member of the CD28:B7 immunoglobulin superfamily and is normally expressed at low levels of the surface of naïve effector T-cells and regulatory T cells (Tregs).12 After stimulation of a naïve T cell through the T cell receptor (TCR, Figure 1A), CTLA-4 localizes to the plasma membrane and competes with CD28 for B7, ultimately turning off T cell receptor signaling (Figure 1B).13,14 Antibodies that target CTLA-4 prevent the attenuating function of CTLA-4 and thereby enhance T cell function (Figure 1C).
Figure 1.
Panel “A” shows T cell activation involves binding of the T cell receptor (TCR) to a peptide antigen bound to the major histocompatibility complex (MHC) on the surface of an antigen presenting cell (APC). This process also involves the interaction of CD28 on T cells with the B7 molecules on APC. Following T cell activation, panel “B” shows CTLA-4 is up-regulated and expressed on the cell surface of effector T cells and functions as an inhibitory molecule, outcompeting CD28 in the binding to B7, and causing inhibition of T cell activation and function. Antibodies that block CTLA-4 such as ipilimumab and tremelimumab bind to and inhibit the function of CTLA-4 and, thus, enhance T cell function as shown in panel “C”.
Over 20 years of research have established the foundations for the therapeutic potential of therapies targeting CTLA-4. CTLA-4 was first described in 1987 as a member of the immunoglobulin superfamily,15 and subsequent studies continued to describe its role in the inhibition of immune responses.16 Studies in mice suggested that functional CTLA-4 is essential for viability as CTLA-4 (−/−) mice die from diffuse lymphoproliferation and autoimmunity one month after birth.17 Based upon this improved understanding of the importance of CTLA-4 as an immunologic checkpoint, Dr. James Allison hypothesized that targeting CTLA-4 with monoclonal antibodies may be a strategy to enhance anti-tumor immunity. In 1996, the first preclinical report was published that demonstrated CTLA-4 blockade with monoclonal antibody therapy could result in tumor regressions in mice.18
Pilot clinical studies of CTLA-4 blockade in patients followed several years later with ipilimumab (Yervoy™, previously MDX-010; Medarex, Princeton, NJ, USA, Bristol Myers-Squibb, Princeton, NJ, USA). A phase I study was reported in 2002 showing ipilimumab treatment with a single dose of 3mg/kg resulted in two partial responses out of 17 treated patients with unresectable melanoma.19 Subsequent studies focused on establishing appropriate dosing (0.3mg/kg vs. 3mg/kg vs. 10mg/kg) and schedule and suggested that the dose of ipilimumab was relevant. In a randomized, dose-finding phase II study, the highest administered dose, 10mg/kg, resulted in higher response rates compared to the 3mg/kg dose, albeit with increased side effects.20
Ultimately, US Food and Drug Administration (FDA) approval at the 3mg/kg dose was based upon an overall survival benefit seen in a randomized phase III trial comparing ipilimumab (3mg/kg) with or without the gp100 peptide vaccine vs. gp100 alone for patients with previously treated, unresectable stage III or stage IV melanoma.21 The results of this study were truly remarkable as this was the first time an agent has demonstrated an overall survival benefit in a phase III trial for patients with advanced melanoma. Median overall survival in the combination ipilimumab and gp100 peptide vaccine arm (10.0 months) was similar to the ipilimumab alone arm (10.1 months) but significantly higher than the gp100 peptide vaccine alone arm (6.4 months). Survival for the ipilimumab alone group compared to the gp100 peptide vaccine alone group at one (45.6% vs. 25.3%) and two years (23.5% vs. 13.7%) was significantly improved, attesting to the promise of ipilimumab in creating durable anti-tumor immunity for a subgroup of patients. A more recent randomized phase III study has demonstrated the benefit of ipilimumab in the front-line setting.22 Patients who received dacarbazine in combination with ipilimumab (10mg/kg) had improved overall survival compared to patients who received dacarbazine alone. A survival advantage for ipilimumab-treated patients was reported at 1 year (47.3% vs. 36.3%), 2 years (28.5% vs. 17.9%), and 3 years (20.8% vs. 12.2%) again highlights the possibility for the long-term durable survival benefit of ipilimumab.
Though ipilimumab is the only FDA approved antibody targeting the immunologic checkpoint CTLA-4, tremelimumab (previously CP-675,206 or ticilimumab; Pfizer, New York, NY) is another monoclonal antibody targeting CTLA-4 that has undergone clinical evaluation. Since the half-life of tremelimumab is longer than ipilimumab, tremelimumab is dosed less frequently; 15 mg/kg every 3 months was selected as the recommended phase II dose based upon results from a phase I/II study.23 Similar to ipilimumab, in phase I and II studies of tremelimumab, durable responses were seen in a subset of patients, with one phase II study demonstrating a 21% clinical benefit (stable diseases or response) rate.24 Tremelimumab was compared to dacarbazine and temozolomide in a randomized, open-label phase III trial.25 After the data safety monitoring board (DSMB) determined that the second interim analysis had crossed the prospectively defined futility boundaries for improvement in overall survival, the study was halted. These results may have been complicated, however, by the relatively short period of time patients had been followed in this study and by unintentional crossover in the control arm that may have had access to ipilimumab in concomitant clinical trials. Recently updated interim results, demonstrate a non-significant trend in overall survival favoring the tremelimumab group (p=0.14),26 and further development of tremelimumab is under consideration, now through Medimmune.
Programmed Death-1 Receptor Blockade
Though CTLA-4 blockade remains the most thoroughly investigated and clinically developed method for targeting immunologic checkpoints, programmed death-1 (PD-1) receptor is another immune checkpoint and highly promising therapeutic target. PD-1 is expressed on T cells, B cells, and myeloid cells after activation,27 and its ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC), are expressed on tumor cells, antigen presenting cells (APCs), and other cells in the tumor microenvironment.28 The interaction of PD-1 with its ligand is felt to function as an immunologic checkpoint which tumors may use to defend themselves against anti-tumor immune responses through multiple immunosuppressive pathways including the induction of T cell apoptosis (Figure 2).29 The interaction of PD-1 on T-cells and PD-L1 on tumor cells may additionally render tumor cells more resistant to T cell mediated apoptosis.30 Further preclinical evidence of PD-1’s role as an immunologic checkpoint comes from the finding that PD-1 deficient mice develop systemic and organ-specific autoimmune diseases,31,32 though not to the degree of the fatal lymphoproliferation seen in CTLA-4 (−/−) mice.17
Figure 2.
T cells are believed to play an important role in anti-tumor immunity as demonstrated in panel A with the “X” indicating tumor destruction. Many tumors are believed to escape this T cell mediated attack by high levels of expression of PD-L1 (B7-H1), Panel B. When PD-L1 interacts with the PD-1 receptor on T cells, T cell function is impaired through a variety of mechanisms including induction of apoptosis, suppression of proliferation, and inhibition of T cell cytokine production. This interaction is therefore believed to impair anti-tumor immunity. Therapeutic antibodies that block PD-1 prevent the inhibitory interaction between PD-L1 and PD-1 and may be helpful in restoring T cell mediated anti-tumor immunity (Panel C).
Several PD-1 inhibitory antibodies are already in clinical development including; BMS-936558 (previously MDX-1106, Bristol-Myers Squibb, Princeton, NJ, USA), CT-011 (Cure Tech, Yavne, Israel), and MK-3475 (Merck, Whitehouse Station, NJ). BMS-936558 has been the most extensively studied PD-1 blocking antibody. In the first in-human phase I study, BMS-936558 was administered to 39 patients with advanced solid tumors in escalating dose cohorts (0.3mg/kg, 1mg/kg, 3mg/kg, and 10mg/kg).33 Treatment overall was well tolerated with one case of colitis, one case of hypothyroidism, and two cases of polyarticular arthropathies. Remarkably, one patient with metastatic colon cancer had a complete response and two patients (melanoma and renal cell cancer) achieved a partial response. Another phase I protocol limited to patients with metastatic castrate-resistant prostate cancer, renal cell carcinoma, melanoma, and non-small cell lung cancer, treated 16 patients with BMS-936558 dosed every two weeks. Six of the 16 patients have responded, and the study is ongoing.
CT-011 is another PD-1 blocking antibody which has demonstrated efficacy in hematologic malignancies and is currently being tested for patients with solid tumors, specifically colorectal cancer (Clinicaltrials.gov identifier, NCT00890305) and melanoma (NCT01435369). In an open-label, dose-escalation, phase I study of 17 patients with advanced hematologic malignancies, CT-011 was well tolerated and the maximum-tolerated dose (MTD) was not reached. CT-011 resulted in a complete response for a patient with follicular lymphoma and clinical benefit for 33% of study participants.34
MK-3475 is currently being evaluated in a first-in-human, dose-escalation study for patients with carcinomas (NCT1295827). Results thus far indicate MK-3475 is well tolerated, and the MTD has not yet been reached. At the completion of the dose-escalation portion of the study, an extension arm, involving patients with melanoma and non-small cell lung cancer (NSCLC) will commence. Evaluation is too premature to determine clinical efficacy of MK-3475.
While monoclonal antibodies have been the most clinically developed molecules inhibiting PD-1, AMP-224 (Amplimmune, Gaithersburg, MD and GlaxoSmithKline, London, UK) is a fusion-protein of PD-L2 and an antibody Fc portion. Though the precise mechanism of AMP-224 is not as clear as antibodies which directly target PD-1, AMP-224 similarly inhibits PD-1 function, thereby disinhibiting T cell activity. A phase I trial is underway testing AMP-224 for patients with solid tumors and cutaneous T-cell lymphoma (NCT01352884). Genentech (San Francisco, CA) is additionally developing strategies to inhibit interactions involving PD-1.
Programmed Death-1 Ligand Blockade
In addition to approaches which directly target PD-1, targeting the ligand of PD-1, PD-L1, is an attractive alternative strategy for abrogating this important immunologic checkpoint. Research involving a murine model of leukemia has shown that an antibody directed against PD-L1 resulted in decreased tumor burden and prolonged survival.35 BMS-936559 (previously MDX-1105, Bristol-Myers Squibb, Princeton, NJ, USA), a PD-L1 blocking antibody, has been developed and is being tested in a Phase I open-label, dose escalation study for patients with advanced solid tumors (NCT00729664).
Lessons Learned in the Clinical Development of Agents that Target Immune Checkpoints
Unique Spectrum of Adverse Events
Antibodies that target CTLA-4 and PD-1 have been generally well tolerated but can be associated with a host of novel side effects, presumed to be linked to the activation of the immune system by blocking immunologic checkpoints. Collectively the spectrum of side effects has been described as immune-related adverse events (irAEs). Using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0, in the first phase III trial of ipilimumab, patients were affected by irAEs involving the skin (rash/vitiligo/pruritis; 43.5% any grade, 1.5% grade 3-4), the bowel (diarrhea/colitis; 29.0% any grade, 7.6% grade 3-4), the liver (hepatitis/ rise in liver enzymes; 3.8% any grade, 0% grade 3-4), and the endocrine system (hypophysitis, thyroiditis, adrenal insufficiency; 7.6% any grade, 3.8% grade 3-4).21 Studies of tremelimumab demonstrated similar irAEs to ipilimumab.24 Interestingly, irAEs were slightly different when ipilimumab was combined with dacarbazine for treatment naïve patients.22 There was an increased proportion of hepatitis (31.6%; grade 3-4) and a lower degree of colitis (4.9%; grade 3-4), suggesting that the toxicity profile for ipilimumab can be shifted based on the combining partner since dacarbazine has a low but documented ability to raise transaminase levels as monotherapy.
More rarely, uveitis, conjunctivitis, neuropathy, myopathy, and nephritis have been known to occur. IrAEs are typically responsive to interruption or discontinuation of CTLA-4 blockade in combination with immunosuppressive drugs such as corticosteroids or occasionally TNF-blocking antibodies. Although the immunosuppressive properties of these medicines could theoretically be a concern with treatment intended to enhance anti-tumor immunity, a study demonstrated that using corticosteroids to treat irAEs did not affect the duration of tumor response.36 The most important aspect of caring for individuals who develop irAEs during CTLA-4 blocking therapy is prompt recognition and treatment. Unfortunately there is no clear preventive strategy to avoid irAEs; a randomized, double-blind, placebo controlled trial assessing the role of prophylactic budesonide in reducing ipilimumab associated diarrhea showed no benefit.37
IrAEs are not believed to be true autoimmune diseases as they typically resolve with cessation of ipilimumab and appropriate immunosuppressive therapy. Given this side effect profile, however, ipilimumab has not been used in patients with underlying autoimmune diseases, particularly autoimmune hepatitis and inflammatory bowel diseases. Patients with underlying autoimmune conditions were excluded from clinical trials in the development of ipilimumab, and its safety has not been assessed in this patient population. It is possible that in the future ipilimumab may be explored in clinical trials cautiously for patients with stable autoimmune conditions, but at the present, therapy with these agents is of uncertain safety.
Patients who experience irAEs may be more likely to benefit from anti-CTLA-4 therapy, but serious irAEs are not required for an anti-tumor response.38 The dose of ipilimumab is likely important as more side effects were seen in a phase II trial of patients treated at 10mg/kg than at 3mg/kg or 0.3mg/kg.20
Antibodies that target PD-1 have been associated with some of the same irAEs to CTLA-4 inhibition therapy such as colitis and hypothyroidism.33 Though antibodies directed towards PD-1 have been less evaluated that CTLA-4 therapy, the degree of irAEs has been somewhat milder than those reported in patients undergoing CTLA-4 therapy. PD-1 therapy with MDX-1106 (BMS-936559), however, has been associated with the rare unique side effect of pneumonitis. Monitoring for predisposition to the development of irAEs and attempting to separate the therapeutic benefits of antibodies that target immune checkpoints from irAEs are areas of ongoing investigation.
Importance of Patient Selection and Biomarker Analyses
Considerable efforts have been directed towards understanding immunologic biomarkers associated with disease response to immunologic checkpoint antibodies to ultimately help guide which patients may be expected to be the best candidates for therapy. Monitoring of immunological parameters of patients undergoing therapy with ipilimumab, has therefore been an integral component of completed and ongoing clinical trials. In one retrospective study of 51 patients treated with ipilimumab (10mg/kg), an absolute lymphocyte count (ALC) that exceeded 1000/μL at the time of the third ipilimumab dose (week 7 of therapy) was associated with a six and 12 month survival benefit.39 Additional work has investigated antigen specific immune responses to a number of cancer-related antigens. Specifically, immune responses to the cancer-testis antigen NY-ESO-1 have been the most extensively characterized to correlate with clinical activity following ipilimumab. In one recent study of 144 patients, patients with detectable serum antibody by ELISA prior to or during the course of ipilimumab therapy were more likely to achieve disease control (stable disease or disease response) from ipilimumab than those who did not have serum antibodies to NY-ESO-1.40 Seropositive patients treated at Memorial Sloan-Kettering Cancer Center who also had a detectable NY-ESO-1-specific CD8+ T-cell response showed a significant survival advantage compared to seropositive patients without a detectable CD8+ T-cell response. Though immunity to the cancer antigen NY-ESO-1 correlated with clinical benefit from ipilimumab, NY-ESO-1 specific immunity is likely a surrogate marker for the broader mechanisms of ipilimumab’s anti-tumor effects, rather than a direct mediator. Studies of ALC and NY-ESO-1 as potential biomarkers have been identified in retrospective analyses and prospective validation is an area of active research. Further, the majority of biomarker analyses were conducted in patients receiving ipilimumab at 10mg/kg. Whether they apply to patients treated at 3mg/kg, the commercially available dose of ipilimumab, additionally requires further study.
Since significantly fewer patients have been treated with PD-1 antibody therapy, far less is known about patient characteristics or other biomarkers which predict response to therapy. PD-L1 expression on tumor cells has been associated with an adverse prognosis in a variety of malignancies.41-46 Since PD-L1 expression on the cell surface of tumors possibly reflects a functional escape mechanism from anti-tumor immunity, it is hypothesized that patients with tumors that express PD-L1 will be more likely to respond to PD-1 blocking antibody therapy. In a phase I trial of BMS-936559, tumor biopsies from nine patients were examined to assess the presence of PD-L1 by immunohistochemistry (IHC) and to correlate expression with response. Out of the four patients with PD-L1 expression on their cell surface, three responded. Notably, the one patient who did not respond was treated in the 0.3mg/kg cohort where no other responses were seen. Interestingly, none of the five patients who lacked PD-L1 cell surface expression responded. Despite the limitations of a small sample size, the correlation between cell surface PD-L1 expression on tumor cells and the likelihood of tumor regression following PD-1 blockade suggested potential significance (two-sided P= 0.0476; Fisher’s exact test).33 Patients with melanoma who enroll on the extension arm of the MK-3475 PD-1 blocking antibody trial (NCT01295827) will be required to undergo a tumor biopsy to help further assess PD-L1 as a biomarker predictive of response to PD-1 therapy.
Distinct Response Kinetics Necessitate a Re-Evaluation of Traditional Radiographic Response End-Points
In addition to ipilimumab’s unique side effect profile, ipilimumab is also associated with novel patterns of clinical response, distinct from those observed with traditional cytotoxic chemotherapy. Cytotoxic chemotherapy typically is characterized by prompt responses. The “Response Evaluation Criteria in Solid Tumors” (RECIST) and modified World Health Organization (mWHO) criteria were developed to standardize assessment of responses to cytotoxic chemotherapy in clinical trials. Patients treated with ipilimumab, however, experience alternative response patterns. In some cases, patients may have a period of early apparent disease progression before a profound disease response or even regression of initial lesions despite the development of additional new smaller lesions. An analysis evaluating the novel patterns of disease responses to ipilimumab across three phase II studies determined that improved survival was associated with a variety of radiographic response patterns.47 Consequently, the immune-related response criteria (irRC) were proposed to evaluate the benefits of ipilimumab and other related immunotherapeutic approaches. In general, the irRC considers the patient’s “total tumor burden” and requires confirmation of suspected disease progression in asymptomatic patients with a subsequent radiographic test, approximately four to six weeks later.
The first reported phase I study of the PD-1 blocking agent, BMS-936559 reported response using traditional RECIST 1.0 criteria. Nevertheless, it is possible that response kinetics to PD-1 therapy may mirror those seen with CTLA-4 therapy making the irRC a more accurate assessment of response. In the ongoing trial of PD-1 blockade with CT-011 in melanoma, response is being assessed by the irRC in addition to traditional RECIST criteria, further assessing the irRC’s applicability to PD-1 therapy and providing additional prospective validation.
Combination Strategies
Regardless of the criteria for interpreting response to therapy, there is a clear need to increase the proportion of patients who benefit from immunologic checkpoint targeting agents. Currently, many different possible mechanisms of synergy are being investigated by combining immunologic checkpoint targeting agents with other approaches such as other checkpoint blocking agents, targeted therapy, chemotherapy, and radiotherapy. (Table 1)
Table 1.
Selected Ipilimumab Combination Clinical Trials for Patients with Melanoma (Source: www.clinicaltrials.gov)
| Agent(s) used in Combination with Ipilimumab |
Patient Population | Study ID | Phase | Status |
|---|---|---|---|---|
| Vemurafenib | Unresectable/Metastatic | NCT01400451 | I | Not yet accruing |
| Bevacizumab | Unresectable/Metastatic | NCT00790010 | I | Accruing |
| BMS-936558 (PD-1 Inhibitor) |
Unresectable/Metastatic | NCT01024231 | I | Accruing |
| Temozolomide, cisplatin, interferon alpha-2b, Interleukin-2 (IL-2) |
Unresectable/Metastatic, no prior chemotherapy. |
NCT01409174 | I/II | Not yet accruing |
| Palliative Radiation |
Metastatic melanoma, failed or intolerant to at least one prior line of therapy |
NCT01449279 | Pilot Study |
Accruing |
| Granulocyte Macrophage- Colony Stimulating Factor |
Unresectable/Metastatic | NCT01363206 | II | Accruing |
| Temozolomide | Unresectable/Metastatic, no prior chemotherapy or targeted therapy |
NCT01119508 | II | Ongoing but not accruing |
Preclinical evidence combining PD-1 blockade with CTLA-4 blockade and Flt3, an immunostimulatory ligand, showed increased rejection of tumors in a B16 melanoma murine model than either agent alone.48 A phase I clinical trial combining BMS-936559 (NCT01024231) with ipilimumab is ongoing. Further investigation of this strategy of dual checkpoint blockade is warranted.
Ideally, other agents used to treat melanoma such as targeted therapy, chemotherapy, and radiotherapy that result in tumor cell death may liberate tumor antigens, providing the right fuel for checkpoint blocking antibodies to activate an even more effective anti-tumor immune response. Targeted therapy is an extremely promising treatment for patients with BRAF mutant melanoma that can result in massive tumor destruction. Clinical results involving the BRAF inhibitor, vemurafenib (Zelboraf®, Roche, Basel, Switzerland), have been remarkable, demonstrating a high response rate and an overall survival benefit.49,50 Preclinical data suggest the possibility of synergy between BRAF inhibition and ipilimumab. In one study, treatment of melanoma cell lines with PLX4720, a laboratory BRAF inhibitor reagent, led to increased tumor antigen expression which contributed to enhanced antigen-specific T-cell killing of tumor cell targets.51 Biopsies of melanoma tumors from patients treated with BRAF inhibitors have also shown increases in tumor infiltrating lymphocytes.52 The exciting promise of combining vemurafenib with ipilimumab is being tested in a phase I/II study (NCT01400451). Chemotherapy may also synergize with ipilimumab as demonstrated by the results of a phase II study that showed the combination of ipilimumab and dacarbazine resulted in a higher response rate than ipilimumab alone.53 Whether radiotherapy may also be beneficial concomitantly with ipilimumab is being explored in a pilot study (NCT01449279).
Summary and Future Directions
Immunologic checkpoints are an essential component of the immune system, functioning in an elaborate system of self-regulation to prevent excessively damaging immune responses. Though essential for immunologic homeostasis, in the presence of active malignancy, inhibitory signals may predominate and prevent otherwise effective anti-tumor immune responses. Research over the past two decades has highlighted the important components of several immune regulatory circuits and insight into these mechanisms has led to novel approaches to tumor immunotherapy.
Immune regulatory antibodies that target immunologic checkpoints, such as those directed against CTLA-4 and PD-1, have been developed for clinical use and are revitalizing interest in solid tumor immunotherapy. Continued research into understanding biomarkers predictive of response and the unique side effect profiles of these agents will ultimately lead to improved patient selection and clinical care. Future studies will likely uncover additional promising immunologic checkpoint targets as well. Though many of the agents that target immunologic checkpoints have been developed for patients with melanoma, promising data exists in a variety of other tumors. We expect ongoing and future trials will assess these promising agents alone, and in combination with other immunotherapeutic approaches, targeted therapy, chemotherapy, and radiotherapy, in a variety of additional malignancies.
Footnotes
Conflicts of Interest and Source of Funding: JDW is a consultant for Bristol-Myers Squibb.
For the remaining authors, none were declared.
References
- 1.Nauts HC, Fowler GA, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley’s toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley’s mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med Scand Suppl. 1953;276:1–103. [PubMed] [Google Scholar]
- 2.Coley WB. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus) Proc R Soc Med. 1910;3:1–48. [PMC free article] [PubMed] [Google Scholar]
- 3.Marchand M, Weynants P, Rankin E, et al. Tumor regression responses in melanoma patients treated with a peptide encoded by gene MAGE-3. International journal of cancer Journal international du cancer. 1995;63:883–5. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 4.Wolchok JD, Yuan J, Houghton AN, et al. Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Mol Ther. 2007;15:2044–50. doi: 10.1038/sj.mt.6300290. [DOI] [PubMed] [Google Scholar]
- 5.Bergman PJ, McKnight J, Novosad A, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. 2003;9:1284–90. [PubMed] [Google Scholar]
- 6.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–32. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 7.Hsueh EC, Essner R, Foshag LJ, et al. Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine. J Clin Oncol. 2002;20:4549–54. doi: 10.1200/JCO.2002.01.151. [DOI] [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with anti-tumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–7. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 10.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 11.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. The New England journal of medicine. 2011;364:2119–27. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alegre ML, Noel PJ, Eisfelder BJ, et al. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–70. [PubMed] [Google Scholar]
- 13.van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–43. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 15.Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 16.Linsley PS, Wallace PM, Johnson J, et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–5. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 17.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 18.Leach DR, Krummel MF, Allison JP. Enhancement of anti-tumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 19.Tchekmedyian S, Glasby J, Korman A, et al. MDX-010 (human anti-CTLA4): a phase I trial in malignant melanoma. Proc Am Soc Clin Oncol. 2002:21. Abstr 56. [Google Scholar]
- 20.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 21.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 23.Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–81. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16:1042–8. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 25.A. Ribas AHaRK, et al. Phase III, open-label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide or dacarbazine) in patients with advanced melanoma. J Clin Oncol. 2008:26. Suppl. [Google Scholar]
- 26.Marshall MRA, Huang B. Evaluation of baseline serum C-reactive protein and benefit from tremelimumab compared to chemotherapy in first-line melanoma. J Clin Oncol. 2010;28:2609. [Google Scholar]
- 27.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–96. [PubMed] [Google Scholar]
- 29.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 30.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–43. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 33.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit anti-tumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–52. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–8. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–8. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 38.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–75. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108:16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–66. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 42.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 43.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–7. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 44.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–82. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu MC, Hsiao JR, Chang KC, et al. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 2010;23:1393–403. doi: 10.1038/modpathol.2010.130. [DOI] [PubMed] [Google Scholar]
- 47.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 48.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. The New England journal of medicine. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 52.Wilmott JS, Long GV, Howle JR, et al. Selective BRAF inhibitors induce marked T cell infiltration into human metastatic melanoma. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 53.Hersh EM, O’Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Investigational new drugs. 2011;29:489–98. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]