Abstract
Entry of bacteria from the vagina into the uterus raises the question of uterine epithelial cell (UEC) signaling in response to the presence of bacteria. Our model system helps to define microbially elicited UEC basolateral cytokine release, important in regulating underlying stromal immune cell protection. UECs from adult rats were grown in cell culture inserts to establish a confluent polarized monolayer as was determined by transepithelial resistance (TER). Polarized epithelial cell cultures were treated apically with live or heat-killed Escherichia coli or Lactobacillus rhamnosus prior to collection of basolateral media after 24 h of incubation. Coculture of polarized UECs with live E. coli had no effect on epithelial cell TER. In response to exposure to live E. coli, epithelial cell basolateral release of macrophage inflammatory protein 3α (MIP3α) and tumor necrosis factor alpha (TNF-α) increased at a time when basolateral release of biologically active transforming growth factor β (TGF-β) decreased. Incubation of UECs with heat-killed E. coli resulted in an increased basolateral release of MIP3α and TNF-α, without affecting TER or TGF-β. In contrast to E. coli, live or heat-killed L. rhamnosus had no effect on TER or cytokine release. These studies indicate that polarized rat UECs respond to gram-negative E. coli by releasing the cytokines MIP3α and TNF-α, signals important to both the innate and adaptive immune systems. These findings suggest that UEC responses to bacteria are selective and important in initiating and regulating immune protection in the female reproductive tract.
The immune system in the female reproductive tract has evolved to meet the unique requirements of procreation, protecting mother and fetus against infection, while at the same time ensuring tolerance of the presence of commensal microbes, allogeneic sperm, and the fetal-placental unit(s) (54). Uterine epithelial cells provide a mechanical barrier between the lumen of the uterus and internal body systems (43). Central to immune protection in the female reproductive tract, uterine epithelial cell functions are complex and include antigen presentation (12, 38), immunoglobulin A (IgA) transport (44, 59), the production of antimicrobial agents (13, 55), and the release of cytokines into the lumen and underlying tissues (9, 23, 45, 46, 60).
In women, a healthy vaginal ecosystem is populated by a diverse microbiota dominated by lactobacilli (4), which periodically enter the upper reproductive tract. Movement of sperm along with bacteria occurs as a consequence of sexual intercourse as well as peristaltic contractions, which contribute to the movement of vaginal contents through the cervix into the uterus and subsequently to the Fallopian tubes (27, 37; A. K. Parsons, R. A. Cone, and T. R. Moench, presented at Microbicides 2002, Antwerp, Belgium, 12 to 15 May 2002). In recent studies, a radio-opaque dye was placed in the vaginae of women during the menstrual cycle, while on oral contraception, and following menopause. The dyes were detected in both the uterus and the Fallopian tubes within 3 h (Parsons et al., Microbicides 2002). These studies indicate that irrespective of endocrine balance, the upper reproductive tract is routinely exposed to vaginal microflora as well as to potential pathogens.
In the female genitourinary tract, Escherichia coli, a gram-negative species, is a primary source of urogenital infections (10, 11, 48), including pyometra (18, 34), uterine abscess (14), preterm premature rupture of membranes during pregnancy (3), and urinary tract infections (17). Lactobacillus rhamnosus, formerly designated Lactobacillus casei subsp. rhamnosus, a gram-positive, nonmotile, microaerophilic rod, is a commensal which has been isolated from the human gastrointestinal tract (16, 35) and the lower female reproductive tract (33, 42).
Important in the initiation of an immune response, release of the chemokine macrophage inflammatory protein 3α (MIP3α) has been demonstrated both in human gut epithelial cells (19, 20, 52) and in the human uterine epithelial cell line HHUA (49). As the ligand for the CCR6 receptor, MIP3α is chemotactic for immature CD35+ bone marrow cell-derived dendritic cells as well as B cells and memory T cells (29, 30). Tumor necrosis factor alpha (TNF-α), which is produced by uterine stromal and epithelial cells (50), plays a role in the activation of macrophages, granulocytes, and cytotoxic T cells as well as in the maturation of dendritic cells (7). Helping to maintain a balanced response in the immune system, transforming growth factor β (TGF-β) is generally inhibitory to inflammatory immune responses (28, 31). Within the context of the uterus, TGF-β is associated with successful pregnancy (8). For example, changes in normal TNF-α/TGF-β ratios are associated with fetal loss (2). In other studies, work in our laboratory has recently shown that TGF-β, which is released predominately to the basolateral compartment by uterine epithelial cells, mediates the inhibition of antigen presentation by uterine epithelial cells (58).
The overall goal of this work was to define the pattern of cytokine release by polarized rat uterine epithelial cells in response to the presence of the selected gram-negative and gram-positive bacteria. Specifically, our objectives were (i) to determine whether MIP3α is released by primary uterine epithelial cells, (ii) to ascertain if transepithelial resistance (TER), as a measure of cell integrity, is affected by the presence of bacteria in this system, and (iii) to establish if differential release of MIP3α, TNF-α, and TGF-β by rat uterine epithelial cells occurs in response to E. coli and L. rhamnosus.
MATERIALS AND METHODS
General procedures.
Specific-pathogen-free female Lewis rats weighing 125 to 175 g (Charles River Breeding Laboratories, Kingston, N.Y.) were maintained with an alternating 12-h dark-light cycle and free access to rat chow and water. Following sacrifice by decapitation, uteri were recovered for isolation of uterine epithelial cells. All procedures involving animals were conducted following approval by the Dartmouth College Institutional Animal Care and Use Committee.
Preparation of epithelial cell cultures.
Tissues from adult rats (6 to 14 animals/experiment) at random stages of the reproductive cycle were pooled for each experiment. Uteri from intact rats were removed, rinsed in sterile ice-cold Hanks' balanced salt solution (HBSS) (Gibco, Grand Island, N.Y.), weighed, and then transferred to a pancreatin (Gibco)-trypsin (Sigma, St. Louis, Mo.)-DNase (Worthington, Lakewood, N.J.) digest (400 U of DNase/ml of pancreatin, 46,500 U of trypsin/ml of pancreatin, 19.5 ml of pancreatin/g of uterine tissue) as previously described (23). Under sterile conditions, uterine tissues in the enzyme digest were cut into fine pieces, transferred to 6-well culture plates, and incubated for 1 h at 4°C, followed by an additional 60 min at room temperature. Following digestion, uterine pieces were vortexed prior to passage through a sterile 250-μm-mesh screen. Epithelial cells were recovered by pouring the resulting suspension through a 20-μm-mesh screen. Following an additional rinse, epithelial cells were collected and suspended in F12K medium (American Type Culture Collection [ATCC], Manassas, Va.) plus 10% fetal bovine serum, supplemented with 100 μg of streptomycin/ml and 100 U of penicillin/ml (F12K complete medium), and plated onto growth factor-reduced Matrigel-coated 10-mm NUNC, 0.4-μm-pore-size polycarbonate membrane tissue culture inserts (Nalge Nunc International) at a density of three to four insert wells per rat uterus. In some experiments, epithelial cells prepared as described above were suspended and grown in RPMI 1640 medium (Life Technologies Inc.) containing 25 mM HEPES supplemented with 10% fetal bovine serum (HyClone Laboratories, Inc., Logan, Utah), 5% NCTC109 (BioWhittaker, Inc., Walkersville, Md.), 50 μM 2-mercaptoethanol, 100 μg of streptomycin/ml, 100 U of penicillin/ml, and 2 mM l-glutamine (RPMI complete medium). Cell cultures prepared in RPMI complete medium were plated on growth factor-reduced Matrigel-coated (BD Biosciences) 0.4-μm-pore-size Falcon cell culture inserts (Becton Dickinson Labware). Uterine epithelial cells were cultured at 37°C under 5% CO2. The development of a polarized confluent monolayer of uterine epithelial cells was monitored by measuring TER on an EVOM Voltohmmeter (World Precision Instruments).
Bacterial preparations.
Lyophilized L. rhamnosus (ATCC 7469) and E. coli (ATCC 29839) were obtained from the ATCC. ATCC 29839 is a biosafety level I (nonpathogenic), motile, F+, Strs, Met−, Thr+ strain. L. rhamnosus and E. coli were reconstituted and grown in sterile de Man, Rogosa, and Sharp (MRS) broth or Trypticase soy broth (TSB), respectively (Difco, Sparks, Md.). Frozen stocks of bacteria were prepared by placing 700 μl of bacteria in a culture medium into a sterile cryovial with 400 μl of sterile glycerol. Tubes were inverted to mix the contents and were stored at −80°C. Frozen stocks were used to prepare fresh bacterial cultures for those experiments using live bacteria. To measure bacteria, cultures grown to stationary phase were spun at 500 × g for 10 min, resuspended in sterile saline (0.9%), centrifuged as described above, and, following resuspension in saline, placed on ice for 2 h. Bacterial counts were determined by optical density analysis of the bacterial saline suspension followed by serial dilution, plating on agar, and triplicate plate counting.
To limit bacterial replication in experiments using live bacteria, E. coli and L. rhamnosus were grown to stationary phase, rinsed twice in cold saline, resuspended in saline, and placed on ice for 2 h prior to coculture with rat uterine epithelial cells. In experiments in which heat-killed bacteria were used, L. rhamnosus and E. coli were grown to stationary phase, centrifuged, and rinsed as noted above, suspended in sterile saline, and placed on ice for 2 h. Bacterial suspensions were placed in sterile glass tubes and immersed in a 70°C water bath for 20 min. Following cooling to room temperature, suspensions were aliquoted and frozen at −20°C.
Treatment of epithelial cells with live and heat-killed bacteria.
In experiments with live bacteria, epithelial cells were fed with antibiotic-free complete medium for 24 h prior to bacterial treatment. At the time of bacterial addition, media were removed from the basolateral and apical compartments of polarized epithelial cell cultures. Apical compartments received either a bacterial inoculum (300 μl) or sterile saline (300 μl). In all cases, basolateral compartments received antibiotic-free complete medium (500 μl for NUNC/F12K cultures; 800 μl for Falcon/RPMI cultures). At the end of a 24-h incubation period, basolateral media were collected, centrifuged at 10,000 × g for 5 min, and stored at −20°C until analysis. In experiments with heat-killed microbes, F12K complete medium or RPMI complete medium with 100 μg of streptomycin/ml and 100 U of penicillin/ml was used throughout the experimental period. Heat-killed bacteria in 100 μl of saline were added to 200 μl of apical medium. Control wells were treated with the same proportion of saline and apical medium.
Measurement of MIP3α, TNF-α, and TGF-β.
MIP3α and TNF-α protein levels were determined with an enzyme-linked immunosorbent assay (ELISA) kit for rat MIP3α or rat TNF-α (DuoSet ELISA Development System; R&D Systems, Minneapolis, Minn.). Levels of TGF-β were measured by a bioassay using a mink lung epithelial cell line (MLE) transfected with the plasminogen activator inhibitor-1 (PAI-1) promoter linked to a luciferase reporter gene, as previously described (1, 56). This bioassay is based on the cell line's specific sensitivity to picogram levels of biologically active TGF-β, which induces expression of PAI-1, resulting in dose-dependent luciferase activity. Briefly, transfected MLE cells were seeded onto a 96-well plate at 105/100 μl of medium/well and spun on a Beckman centrifuge at 1,500 rpm for 15 s. Following a 3-h incubation at 37°C, the medium was removed and replaced with 50 μl of fresh medium plus 50 μl of a serially diluted standard (recombinant human TGF-β) or culture medium. Cells were then incubated for an additional 17 h before being washed with HBSS and lysed by adding 50 μl of Cell Culture Lysis Reagent (Promega)/well for 15 min. Luciferase activity was determined by the response of cell lysates to 100 μl of Luciferase Reagent (Promega Corp.) for 10 s/well in a model LB96V Microplate Luminometer (EG&G Berthold, Gaithersburg, Md.).
Statistics.
Data were compared by one-way analysis of variance, followed by a Tukey multiple comparison posttest. Differences with a P value of <0.05 were considered significant.
RESULTS
Cytokine/chemokine release by polarized rat uterine epithelial cells.
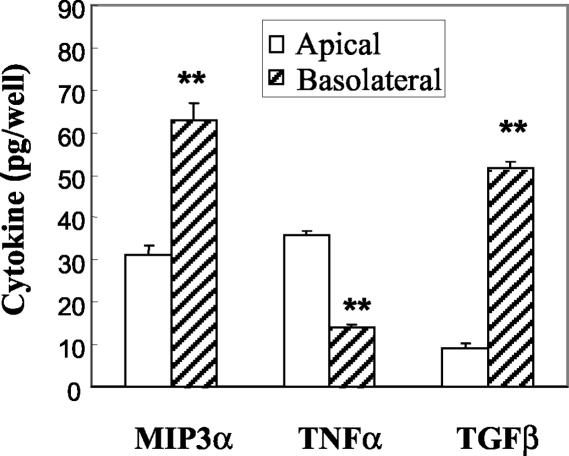
Studies from our laboratory and others have demonstrated that rat uterine epithelial cells in culture produce TNF-α (23) and biologically active TGF-β (58) and release these into both the apical and basolateral compartments of tissue culture inserts. Previously, Wira and colleagues reported the purity of uterine epithelial cells prepared by enzymatic digestion prior to filter capture of epithelial sheets (44, 57). As seen in Fig. 1, when epithelial cells are grown to confluence and form tight junctions, as determined by TER, they produce MIP3α, TNF-α, and TGF-β. Moreover, when cultured in F12K complete medium in NUNC tissue culture inserts, polarized rat uterine epithelial cells release MIP3α and TGF-β preferentially to the basolateral compartment. In contrast, TNF-α is released preferentially to the apical compartment.
FIG. 1.
Constitutive release of MIP3α, TGF-β, and TNF-α by polarized uterine epithelial cells. Rat uterine epithelial cells were grown to confluence for 5 to 6 days in F12K complete medium at 37°C in NUNC cell culture inserts. Following a 24-h incubation in fresh medium, apical and basolateral media were collected for analysis of MIP3α and TNF-α by ELISA or of TGF-β by bioassay. Values shown are mean cytokine levels ± standard errors from five or more wells per group. Results for each cytokine are representative of three or more separate experiments. **, significantly (P < 0.01) different from cytokine level measured in the apical chamber.
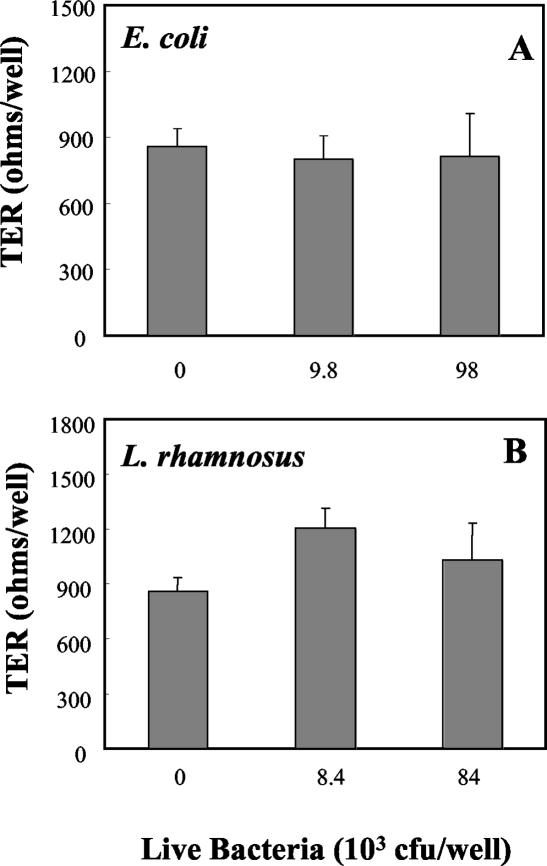
Effect of live bacteria on TER in polarized uterine epithelial cells.
To explore the effect of bacteria on uterine epithelial cell integrity, TER was measured prior to treatment and at the end of a 24-h coculture of cells with bacteria placed in the apical chambers of cell culture inserts. As shown in Fig. 2, when polarized rat uterine epithelial cells were incubated with 9.8 × 103 or 9.8 × 104 live E. coli bacteria in saline, TER was not significantly different from that of saline-treated control wells. Similarly, when uterine epithelial cells were cocultured with 8.4 ×103 or 8.4 ×104 live L. rhamnosus organisms in saline, the integrity of the polarized monolayers as determined by TER was not affected.
FIG. 2.
Influence of live E. coli and live L. rhamnosus on rat uterine epithelial cell TER. Polarized uterine epithelial cells were grown in NUNC cell culture inserts. Apical medium was replaced with live E. coli (A) or live L. rhamnosus (B) in saline. Controls were treated apically with sterile saline. Basolateral compartments were fed with antibiotic-free F12K complete medium. TER (expressed in ohms per well) was measured after 24 h of incubation with bacteria (background TER, 180 Ω/well). Values shown are mean TERs ± standard errors for groups of three to four wells per treatment group. E. coli results are representative of four separate experiments (n = 4), and L. rhamnosus results are representative of six experiments (n = 6).
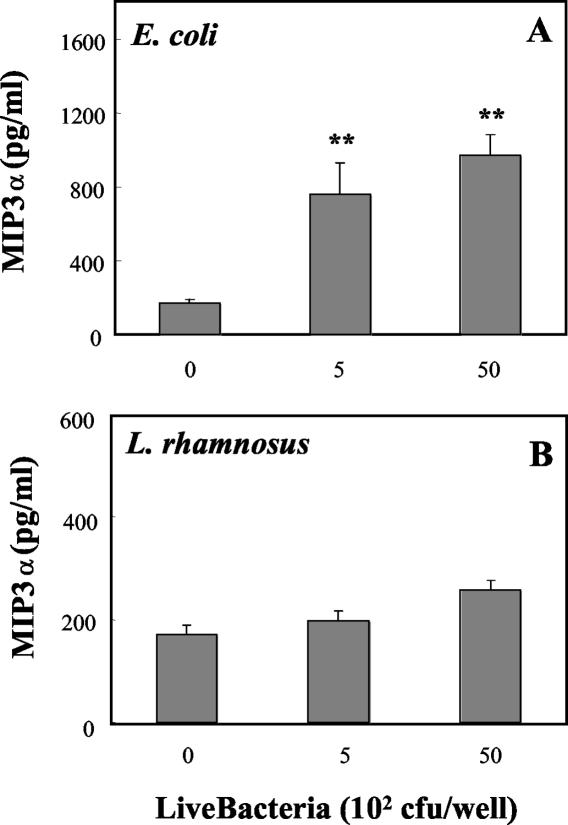
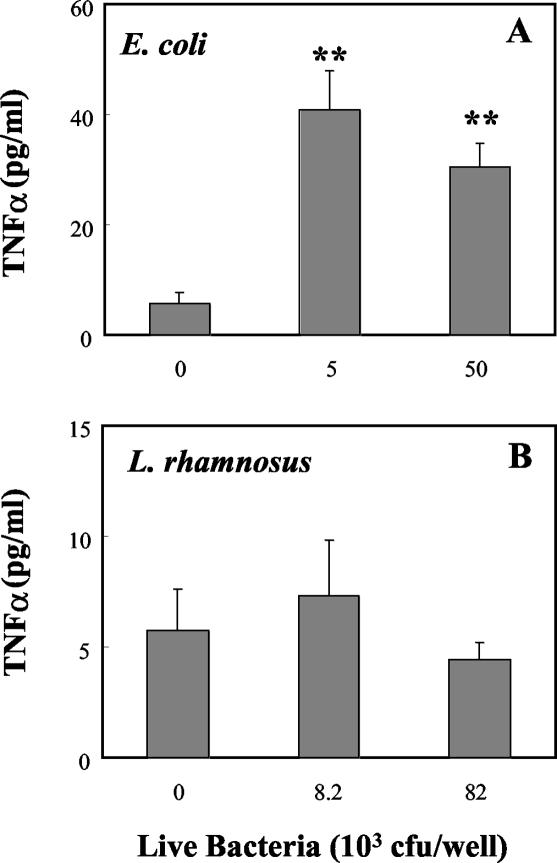
Basolateral release of MIP3α, TNF-α, and TGF-β by polarized uterine epithelial cells in response to bacteria.
To determine the effect of bacteria on basolateral release of the chemokine MIP3α, polarized uterine epithelial cells were grown to confluence prior to coculture with live E. coli or live L. rhamnosus. As seen in Fig. 3A, addition of live E. coli to the apical chamber increased the release of MIP3α in basolateral media. In contrast, the presence of live L. rhamnosus in the apical chamber had no effect on basolateral release of MIP3α relative to that for controls (Fig. 3B). Measurement of TNF-α in basolateral media (Fig. 4A) indicated that E. coli significantly increased the release of TNF-α into the basolateral compartment, whereas the presence of L. rhamnosus had no effect on TNF-α release relative to that for control cultures (Fig. 4B). In other experiments (data not shown), E. coli and L. rhamnosus were cocultured with uterine epithelial cells for 24 h prior to measurement of TNF-α and MIP3α. Under these conditions, the presence of L. rhamnosus had no effect on the stimulatory action of E. coli on TNF-α or MIP3α release into the basolateral chamber.
FIG. 3.
Effect of live E. coli on basolateral MIP3α release by polarized rat uterine epithelial cells. Primary rat uterine epithelial cells were grown to polarized monolayers in NUNC cell culture inserts and treated apically with live E. coli (A) or live L. rhamnosus (B) in saline. Basolateral compartments received antibiotic-free F12K complete medium at the time of bacterial treatment. Basolateral media were collected after 24 h of incubation. Values shown are means ± standard errors from four wells per group. **, significantly (P < 0.01) different from control (n = 3).
FIG. 4.
Effect of live E. coli in physiologic saline on basolateral release of TNF-α by rat uterine epithelial cells. Polarized monolayer cultures of rat uterine epithelial cells were grown in RPMI medium in Falcon cell culture inserts prior to apical inoculation with live E. coli (A) or live L. rhamnosus (B) in saline. Basolateral media from a 24-h incubation were analyzed for TNF-α content. Values shown are means ± standard errors for groups of four wells. **, significantly (P < 0.01) different from controls (n = 5).
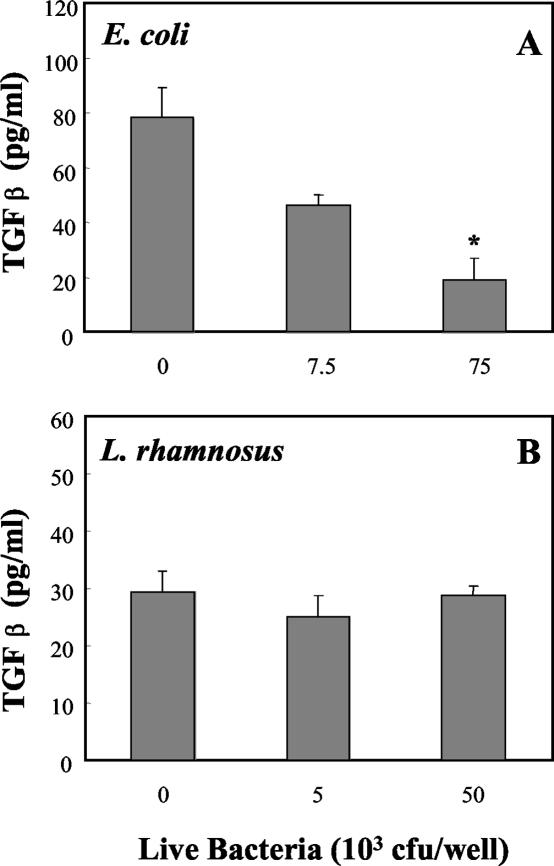
TGF-β must be present in its activated form in order to bind to its receptor (1). To determine if bacteria affect the release of biologically active TGF-β, uterine epithelial cell cultures were grown to confluence in Falcon cell culture inserts prior to treatment with bacteria. Conditioned media from the basolateral compartments were analyzed by a specific and sensitive bioassay, described in Materials and Methods. In this assay a truncated PAI-1 promoter region, fused to a luciferase reporter gene, results in a TGF-β dose-dependent increase in luciferase activity (1). As shown in Fig. 5, the level of biologically active TGF-β decreased when epithelial cells were treated apically with E. coli. In contrast, L. rhamnosus had no effect on the presence of biologically active TGF-β in media from the basolateral compartment.
FIG. 5.
Effect of bacteria on basolateral release of TGF-β by polarized rat uterine epithelial cells. Uterine epithelial cells cultured in RPMI medium in Falcon cell culture inserts were treated apically with live E. coli (A) or live L. rhamnosus (B) in saline and incubated for 24 h. Following collection of basolateral media, levels of biologically active TGF-β were determined by bioassay as described in Materials and Methods. Values shown are mean TGF-β concentrations ± standard errors for groups of three wells. *, significantly different from control. (P < 0.05) (n = 3).
Epithelial cell responses to heat-killed E. coli and L. rhamnosus.
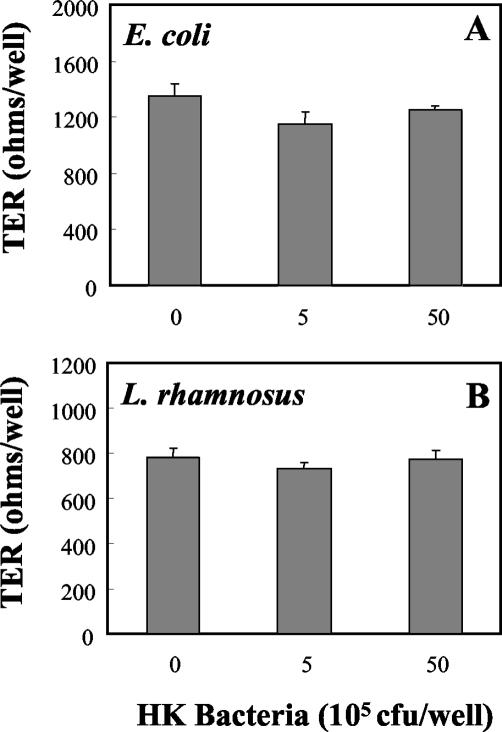
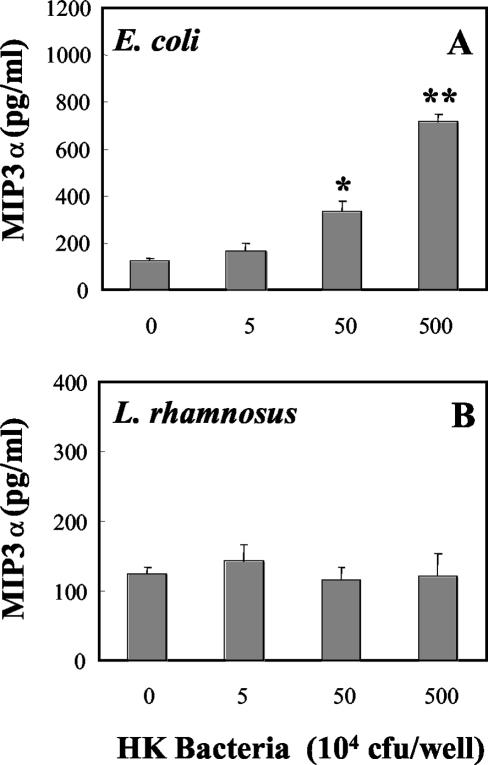
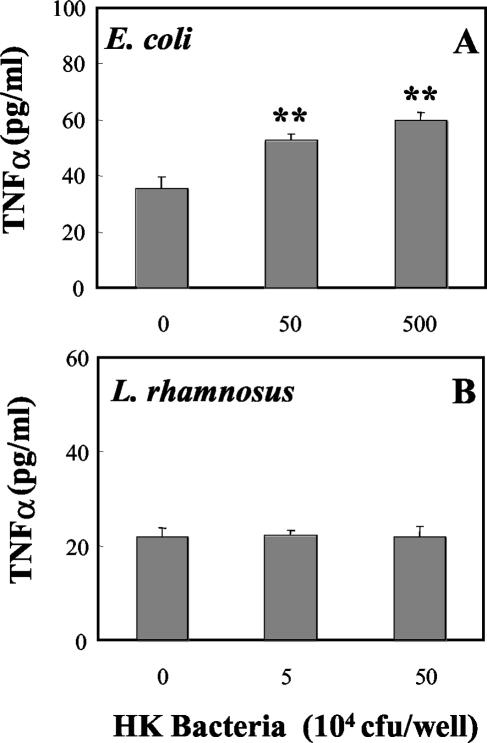
Recognizing that live bacteria, placed in the apical chamber of tissue culture inserts, replicate over a 24-h incubation period, we prepared heat-killed bacteria in order to control the number of bacteria present, as well as to eliminate the possible effects of bacterial metabolites on cell integrity and cytokine production. As seen in Fig. 6, incubation of rat uterine epithelial cells for 24 h with increasing doses of heat-killed E. coli or heat-killed L. rhamnosus had no effect on TER relative to that in control wells. In contrast, heat-killed E. coli increased the basolateral release of both MIP3α (Fig. 7A) and TNF-α (Fig. 8A) in a dose-dependent manner. As seen in Fig. 7B and 8B, in agreement with our findings with live bacteria, heat-killed L. rhamnosus had no effect on the basolateral release of MIP3α or TNF-α by polarized uterine epithelial cells. In other studies (data not shown), we found that basolateral release of TGF-β by epithelial cells was not affected by treatment with heat-killed E. coli or L. rhamnosus.
FIG. 6.
Influence of heat-killed bacteria on TER of polarized uterine epithelial cell cultures. Polarized rat uterine epithelial cells, grown on NUNC cell culture inserts, were fed with F12K complete medium prior to treatment with heat-killed (HK) E. coli (A) or heat-killed L. rhamnosus (B) in the apical compartment (control wells received medium). TER was measured after a 24-h incubation with bacteria. Values shown are mean TERs ± standard errors for groups of three to four wells per treatment group. For E. coli, n = 5; for L. rhamnosus, n = 3.
FIG. 7.
Basolateral release of MIP3α by rat uterine epithelial cells in culture. Polarized uterine epithelial cells, grown in F12K complete medium in NUNC cell culture inserts, were treated apically with either heat-killed (HK) E. coli (A) or heat-killed L. rhamnosus (B). Following a 24-h incubation, basolateral media were harvested and analyzed for the presence of MIP3α. Values are means ± standard errors for three wells per group. * and **, significantly different (P < 0.05 and P < 0.01, respectively) from control. For E. coli, n = 5; for L. rhamnosus, n = 3.
FIG. 8.
Effect of heat-killed (HK) bacteria on basolateral release of TNF-α. Rat uterine epithelial cells, cultured on NUNC cell culture inserts in F12K complete medium, were treated with heat-killed E. coli (A) or L. rhamnosus (B). Basolateral media were removed following a 24-h apical incubation with heat-killed bacteria. Values are means ± standard errors for four wells per group. **, significantly (P < 0.01) different from control (n = 3).
DISCUSSION
The studies presented here demonstrate that epithelial cells from rat uteri are responsive to bacterial challenge. We show that, in response to E. coli exposure at their apical surfaces, epithelial cells release cytokines and chemokines that are known to enhance immune protection through the recruitment and activation of immune cells. That this response is selective is supported by our finding that whereas E. coli increases the basolateral release of MIP3α and TNF-α, L. rhamnosus has no effect. These findings further indicate that under conditions of maintained cell integrity, as determined by high TER, uterine epithelial cells, in response to increasing numbers of live and heat-killed E. coli, exhibit dose dependent increases in the basolateral release of MIP3α and TNF-α at a time when biologically active TGF-β release either is not affected or is decreased.
Polarized uterine epithelial cells release MIP3α and TNF-α into the culture medium in response to E. coli placed on their apical surfaces. To the best of our knowledge, this is the first report that MIP3α is produced by rat uterine epithelial cells. This finding extends the earlier observation by Sun et al., who reported that the human uterine epithelial cell line HHUA produces MIP3α (49). Our findings demonstrate that, in addition to providing a barrier to protect underlying cells, uterine epithelial cells likely play an important role both in recruiting and activating immune cells in the female reproductive tract. Others have shown that MIP3α is chemotactic to B cells and memory T cells as well as to immature bone marrow cell-derived CD35+ dendritic cells, all of which express the CCR6 receptor (29, 30). Similarly, TNF-α plays a central role in the activation of macrophages, granulocytes, and cytotoxic T cells in an acute immune response (15, 39). Using epithelial cells from the gastrointestinal tract and cervical epithelial cells, Kagnoff and colleagues demonstrated the secretion of interleukin-8, GRO alpha, granulocyte-macrophage colony-stimulating factor, and interleukin-6 in response to Chlamydia infection (40). Our findings that MIP3α and TNF-α are released into the basolateral media by uterine epithelial cells, both prior to and following E. coli exposure, extend these findings and suggest that production of cytokines and chemokines by uterine epithelial cells may, in part, account for the presence of a resident population of immune cells in the uteri of intact, noninfected female rats as well as for the increase in immune cells that occurs following intrauterine bacterial challenge (12, 24, 25).
Our studies demonstrate that, in addition to stimulating chemokine and cytokine release, E. coli exposure results in the inhibition of release of biologically active TGF-β by uterine epithelial cells. Production of TGF-β, an antiinflammatory cytokine (29, 32, 53), by uterine epithelial cells has been shown by others to be under hormonal control (51). In response to estrogen treatment, both TGF-β mRNA expression and TGF-β production increase in mouse uterine and vaginal epithelial cells. In other studies, it has been found that estradiol-induced synthesis of biologically active TGF-β mediates the inhibitory effects of estradiol on antigen presentation in the uterus and vagina of the rat (56, 58). Our finding in the present study, to the best of our knowledge, is the first demonstration that E. coli, but not L. rhamnosus, affects uterine epithelial cells so to reduce biologically active TGF-β release at a time when MIP3α and TNF-α production is increased. From the standpoint of immune protection within the reproductive tract, our findings of enhanced MIP3α and TNF-α production, along with suppressed TGF-β production, are consistent with overall protection of this mucosal surface. On the one hand, immune cell recruitment and activation are stimulated by MIP3α and TNF-α; on the other, TGF-β, which is immunosuppressive, is inhibited. The recognition that TNF-α and TGF-β are under hormonal control during the reproductive cycle and throughout pregnancy has led to the identification of a dynamic interaction between these cytokines that is essential for successful pregnancy. For example, when the ratio of TGF-β to TNF-α is high, successful pregnancy ensues (2). In contrast, when the ratio of TGF-β to TNF-α falls, increased fetal absorption occurs in mice. Our findings in the present study suggest that in response to E. coli, the ratio of TGF-β to TNF-α falls to favor immune protection and maternal survival. What remains to be determined is the effect of sex hormones on the cytokine responsiveness of uterine epithelial cells to bacterial challenge.
The presence of lactobacilli in the vagina is known to contribute to host immune defense against infection by competitive exclusion of other bacteria and by producing bacteriocins and lactic acid, which are inhibitory to many pathogens (36, 41). Other studies have documented the importance of hydrogen peroxide (H2O2)-producing strains of lactobacilli in inhibiting microorganisms directly or by reacting with endogenous peroxidase and chloride to form toxic radicals (26). In a study of sex workers in Kenya, a predominance of lactobacilli in vaginal microflora was linked to a decreased prevalence of gonorrhea, Chlamydia infection, and trichomoniasis (32). Interest in the role of lactobacilli for prevention of sexually transmitted diseases prompted us to coculture E. coli with L. rhamnosus in order to determine whether the latter would affect the proinflammatory response of uterine epithelial cells to E. coli. Unexpectedly, we found that coincubation of E. coli with L. rhamnosus had no effect on the E. coli-stimulated release of MIP3α and TNF-α by epithelial cells. What is suggested from this study is that L. rhamnosus is not acting acutely on epithelial cells to alter their responsiveness to E. coli. That L. rhamnosus can have long-term effects on resistance to disease was suggested from studies in which rats received bladder inoculations of L. rhamnosus 3 weeks before challenge with E. coli (42). Animals pretreated with L. rhamnosus had fewer bladder infections when challenged with E. coli than did carrier-pretreated controls. The absence of lactobacilli in treated bladders at the time of challenge suggested that the protective effects of lactobacilli were not related to a direct effect of the lactobacilli on E. coli but were more likely due to underlying influences on the mucosal immune system in the urogenital tract. Whether L. rhamnosus has comparable long-term effects on uterine epithelial cells in our system remains to be determined.
Increases in MIP3α and TNF-α levels following incubation of uterine epithelial cells with live E. coli are comparable to those seen following exposure to heat-killed E. coli. Rather than stimulation occurring as a consequence of logarithmic bacterial growth, which leads to the buildup of metabolites and toxins, the formation of adherent biofilms, and/or the depletion of epithelial substrates (6), our findings suggest that other factors are involved, since heat-killed bacteria are as effective as live bacteria in stimulating MIP3α and TNF-α release. That MIP3α and TNF-α are released in response to E. coli but not to L. rhamnosus suggests a level of specificity in which epithelial cells distinguish between potential gram-negative pathogens and gram-positive commensals. In dose-response studies, in which live and heat-killed L. rhamnosus bacteria were tested, no effect on epithelial MIP3α or TNF-α release was observed. What remains to be done is to identify the mechanism(s) through which E. coli exerts its stimulatory effects on MIP3α and TNF-α release. Based on the pioneering studies of Janeway (21), the innate immune system has been identified as a first line of defense which protects at the onset of infectious microbial challenge (22). This system relies on conserved germ line-encoded receptors and molecules that recognize conserved pathogen-associated molecular patterns (PAMP) found in groups of microorganisms. The pattern recognition receptors (PRR) of the host that recognize PAMP are expressed on many effector cells, including macrophages, dendritic cells, and, as found more recently, epithelial cells (5). One explanation for the increases in MIP3α and TNF-α release in response to E. coli seen in our studies is that lipopolysaccharide, which is a constitutively expressed component of the cell wall of E. coli, binds to Toll-like receptors to directly stimulate cytokine and chemokine production. Alternatively, rather than directly affecting MIP3α, TNF-α, and TGF-β release individually, the effects of E. coli may be indirect. This is suggested from recent studies showing that TNF-α stimulates the release of MIP3α (20) as well as down-regulating the cell surface expression of chemokine receptors (47). Studies to identify the mechanism(s) through which uterine epithelial cells respond to E. coli challenge are under way in our laboratory.
In conclusion, these findings indicate that epithelial cells in the uterus are able to discriminate between potential pathogens and commensal bacteria, which normally reside in the lower reproductive tract and periodically enter the uterus (4, 27). The recognition that sexually transmitted disease agents, including chlamydiae and human immunodeficiency virus type 1, the causative agent of AIDS, rapidly enter the uterus both free and along with sperm following sexual intercourse raises important questions about the role of the epithelial cells that line the entire reproductive tract in conferring protection either directly or indirectly by signaling to underlying immune cells. Our studies indicate that uterine epithelial cells are responsive to potential pathogens. What remains to be identified is the presence of recognition receptors that have evolved to distinguish between different types of bacteria as well as among bacteria, viruses, sperm, and the fetus, all of which are allogeneic. Studies to understand the mechanisms through which uterine epithelial cells respond to bacterial and viral pathogens, as well as the way in which these cells are regulated by hormones and cytokines, are needed to provide the foundation of information essential for the survival and reproductive health of women.
Acknowledgments
We gratefully thank Richard Rossoll, Charu Kaushic, and Ron Taylor for technical assistance that led to the completion of these studies. We also express our appreciation to James Gorham for help in setting up the assay to measure TGF-β.
This work was supported by research grants AI-13541 and AI-51877 from NIH.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abe, M., J. G. Harpel, C. N. Metz, I. Nunes, D. J. Loskutoff, and D. B. Rifkin. 1994. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216:276-284. [DOI] [PubMed] [Google Scholar]
- 2.Arck, P. C., F. S. Merali, J. Manuel, G. Chaouat, and D. A. Clark. 1995. Stress-triggered abortion: inhibition of protective suppression and promotion of tumor necrosis factor-alpha (TNF-α) release as a mechanism triggering resorptions in mice. Am. J. Reprod. Immunol. 33:74-80. [DOI] [PubMed] [Google Scholar]
- 3.Averbuch, B., M. Mazor, I. Shoham-Vardi, W. Chaim, H. Vardi, S. Horowitz, and M. Shuster. 1995. Intra-uterine infection in women with preterm premature rupture of membranes: maternal and neonatal characteristics. Eur. J. Obstet. Gynecol. Reprod. Biol. 62:25-29. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett, J. G., N. E. Moon, P. R. Goldstein, B. Goren, A. B. Onderdonk, and B. F. Polk. 1978. Cervical and vaginal bacterial flora: ecologic niches in the female lower genital tract. Am. J. Obstet. Gynecol. 130:658-661. [DOI] [PubMed] [Google Scholar]
- 5.Becker, M. N., G. Diamond, M. W. Verghese, and S. H. Randell. 2000. CD14-dependent lipopolysaccharide-induced beta-defensin-2 expression in human tracheobronchial epithelium. J. Biol. Chem. 275:29731-29736. [DOI] [PubMed] [Google Scholar]
- 6.Beveridge, T. J., S. A. Makin, J. L. Kadurugamuwa, and Z. Li. 1997. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 20:291-303. [DOI] [PubMed] [Google Scholar]
- 7.Carramolino, L., L. Kremer, I. Goya, R. Varona, J. M. Buesa, J. Gutierrez, A. Zaballos, A. C. Martinez, and G. Marquez. 1999. Down-regulation of the beta-chemokine receptor CCR6 in dendritic cells mediated by TNF-α and IL-4. J. Leukoc. Biol. 66:837-844. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, H. L., S. L. Schneider, C. M. Kane, S. O. Gollnick, C. Grande, D. Thompson, E. Pietrzak, and T. B. Tomasi. 1993. TGF-β2 gene and protein expression in maternal and fetal tissues at various stages of murine development. J. Reprod. Immunol. 25:133-148. [DOI] [PubMed] [Google Scholar]
- 9.De, M., T. R. Sanford, and G. W. Wood. 1992. Interleukin-1, interleukin-6, and tumor necrosis factor alpha are produced in the mouse uterus during the estrous cycle and are induced by estrogen and progesterone. Dev. Biol. 151:297-305. [DOI] [PubMed] [Google Scholar]
- 10.Donders, G. G., E. Bosmans, A. Dekeersmaecker, A. Vereecken, B. Van Bulck, and B. Spitz. 2000. Pathogenesis of abnormal vaginal bacterial flora. Am. J. Obstet. Gynecol. 182:872-878. [DOI] [PubMed] [Google Scholar]
- 11.Ekwempu, C. C., R. V. Lawande, and L. J. Egler. 1981. Microbial flora of the lower genital tract of women in labour in Zaria, Nigeria. J. Clin. Pathol. 34:82-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahey, J. V., R. H. Prabhala, P. M. Guyre, and C. R. Wira. 1999. Antigen-presenting cells in the human female reproductive tract: analysis of antigen presentation in pre- and post-menopausal women. Am. J. Reprod. Immunol. 42:49-57. [DOI] [PubMed] [Google Scholar]
- 13.Fahey, J. V., and C. R. Wira. 2002. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J. Infect. Dis. 185:1606-1613. [DOI] [PubMed] [Google Scholar]
- 14.Fung, T. Y., S. F. Yim, and H. Y. Fung. 1998. Intramyometrial abscess complicating pregnancy. A report of two cases. J. Reprod. Med. 43:1002-1004. [PubMed] [Google Scholar]
- 15.Gounni, A. S., E. Nutku, L. Koussih, F. Aris, J. Louahed, R. C. Levitt, N. C. Nicolaides, and Q. Hamid. 2000. IL-9 expression by human eosinophils: regulation by IL-1β and TNF-α. J. Allerg. Clin. Immunol. 106:460-466. [DOI] [PubMed] [Google Scholar]
- 16.Hessle, C., L. A. Hanson, and A. E. Wold. 1999. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin. Exp. Immunol. 116:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooton, T. M., D. Scholes, A. E. Stapleton, P. L. Roberts, C. Winter, K. Gupta, M. Samadpour, and W. E. Stamm. 2000. A prospective study of asymptomatic bacteriuria in sexually active young women. N. Engl. J. Med. 343:992-997. [DOI] [PubMed] [Google Scholar]
- 18.Inui, A., A. Nitta, A. Yamamoto, S. M. Kang, I. Kanehara, H. Tanaka, S. Nakamura, H. Mandai, and S. Nakao. 1999. Generalized peritonitis with pneumoperitoneum caused by the spontaneous perforation of pyometra without malignancy: report of a case. Surg. Today 29:935-938. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki, A., and B. L. Kelsall. 2000. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191:1381-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izadpanah, A., M. B. Dwinell, L. Eckmann, N. M. Varki, and M. F. Kagnoff. 2001. Regulated MIP-3α/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G710-G719. [DOI] [PubMed] [Google Scholar]
- 21.Janeway, C. A., Jr. 1980. Idiotypes, T-cell receptors, and T-B cooperation. Contemp. Top. Immunobiol. 9:171-203. [DOI] [PubMed] [Google Scholar]
- 22.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 23.Kaushic, C., K. Grant, M. Crane, and C. R. Wira. 2000. Infection of polarized primary epithelial cells from rat uterus with Chlamydia trachomatis: cell-cell interaction and cytokine secretion. Am. J. Reprod. Immunol. 44:73-79. [DOI] [PubMed] [Google Scholar]
- 24.Kaushic, C., A. D. Murdin, B. J. Underdown, and C. R. Wira. 1998. Chlamydia trachomatis infection in the female reproductive tract of the rat: influence of progesterone on infectivity and immune response. Infect. Immun. 66:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushic, C., F. Zhou, A. D. Murdin, and C. R. Wira. 2000. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect. Immun. 68:4207-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klebanoff, S. J., S. L. Hillier, D. A. Eschenbach, and A. M. Waltersdorph. 1991. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J. Infect. Dis. 164:94-100. [DOI] [PubMed] [Google Scholar]
- 27.Kunz, G., D. Beil, H. Deiniger, A. Einspanier, G. Mall, and G. Leyendecker. 1997. The uterine peristaltic pump. Normal and impeded sperm transport within the female genital tract. Adv. Exp. Med. Biol. 424:267-277. [PubMed] [Google Scholar]
- 28.Levings, M. K., R. Bacchetta, U. Schulz, and M. G. Roncarolo. 2002. The role of IL-10 and TGF-β in the differentiation and effector function of T regulatory cells. Int. Arch. Allerg. Immunol. 129:263-276. [DOI] [PubMed] [Google Scholar]
- 29.Liao, F., R. L. Rabin, C. S. Smith, G. Sharma, T. B. Nutman, and J. M. Farber. 1999. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3α. J. Immunol. 162:186-194. [PubMed] [Google Scholar]
- 30.Liao, F., A. K. Shirakawa, J. F. Foley, R. L. Rabin, and J. M. Farber. 2002. Human B cells become highly responsive to macrophage-inflammatory protein-3α/CC chemokine ligand-20 after cellular activation without changes in CCR6 expression or ligand binding. J. Immunol. 168:4871-4880. [DOI] [PubMed] [Google Scholar]
- 31.Marek, A., J. Brodzicki, A. Liberek, and M. Korzon. 2002. TGF-β (transforming growth factor-beta) in chronic inflammatory conditions—a new diagnostic and prognostic marker? Med. Sci. Monit. 8:RA145-RA151. [PubMed] [Google Scholar]
- 32.Martin, H. L., B. A. Richardson, P. M. Nyange, L. Lavreys, S. L. Hillier, B. Chohan, K. Mandaliya, J. O. Ndinya-Achola, J. Bwayo, and J. Kreiss. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis. 180:1863-1868. [DOI] [PubMed] [Google Scholar]
- 33.McGroarty, J. A., L. Tomeczek, D. G. Pond, G. Reid, and A. W. Bruce. 1992. Hydrogen peroxide production by Lactobacillus species: correlation with susceptibility to the spermicidal compound nonoxynol-9. J. Infect. Dis. 165:1142-1144. [DOI] [PubMed] [Google Scholar]
- 34.Mikamo, H., K. Kawazoe, K. Izumi, K. Watanabe, K. Ueno, and T. Tamaya. 1998. Studies on the pathogenicity of anaerobes, especially Prevotella bivia, in a rat pyometra model. Infect. Dis. Obstet. Gynecol. 6:61-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molin, G., B. Jeppsson, M. L. Johansson, S. Ahrne, S. Nobaek, M. Stahl, and S. Bengmark. 1993. Numerical taxonomy of Lactobacillus spp. associated with healthy and diseased mucosa of the human intestines. J. Appl. Bacteriol. 74:314-323. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa, M., K. Shimizu, K. Nomoto, R. Tanaka, T. Hamabata, S. Yamasaki, T. Takeda, and Y. Takeda. 2001. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 68:135-140. [DOI] [PubMed] [Google Scholar]
- 37.Parr, E. L., and M. B. Parr. 1985. Secretory immunoglobulin binding to bacteria in the mouse uterus after mating. J. Reprod. Immunol. 8:71-82. [DOI] [PubMed] [Google Scholar]
- 38.Prabhala, R. H., and C. R. Wira. 1995. Sex hormone and IL-6 regulation of antigen presentation in the female reproductive tract mucosal tissues. J. Immunol. 155:5566-5573. [PubMed] [Google Scholar]
- 39.Rangel, R., L. Rocha, J. L. Ramirez, M. J. Ibarra, G. Solorza, A. Monroy, M. A. Ramirez, A. Herrera, and B. Weiss-Steider. 1995. Generation of memory CD4+, CD8+, CD45RO+ and CD16− lymphocytes activated with IL-2, INF-γ, and TNF-α with specific cytotoxicity against autologous cervical cancer cells in a mixed leukocyte-tumour cell culture. Eur. Cytokine Netw. 6:195-202. [PubMed] [Google Scholar]
- 40.Rasmussen, S. J., L. Eckmann, A. J. Quayle, L. Shen, Y. X. Zhang, D. J. Anderson, J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Investig. 99:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid, G., A. W. Bruce, J. A. McGroarty, K. J. Cheng, and J. W. Costerton. 1990. Is there a role for lactobacilli in prevention of urogenital and intestinal infections? Clin. Microbiol. Rev. 3:335-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reid, G., R. C. Chan, A. W. Bruce, and J. W. Costerton. 1985. Prevention of urinary tract infection in rats with an indigenous Lactobacillus casei strain. Infect. Immun. 49:320-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson, J., C. Kaushic, and C. R. Wira. 1993. Estradiol regulation of secretory component: expression by rat uterine epithelial cells. J. Steroid Biochem. Mol. Biol. 47:143-149. [DOI] [PubMed] [Google Scholar]
- 44.Richardson, J. M., C. Kaushic, and C. R. Wira. 1995. Polymeric immunoglobin (Ig) receptor production and IgA transcytosis in polarized primary cultures of mature rat uterine epithelial cells. Biol. Reprod. 53:488-498. [DOI] [PubMed] [Google Scholar]
- 45.Robertson, S. A., G. Mayrhofer, and R. F. Seamark. 1992. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol. Reprod. 46:1069-1079. [DOI] [PubMed] [Google Scholar]
- 46.Rotello, R. J., R. C. Lieberman, A. F. Purchio, and L. E. Gerschenson. 1991. Coordinated regulation of apoptosis and cell proliferation by transforming growth factor β1 in cultured uterine epithelial cells. Proc. Natl. Acad. Sci. USA 88:3412-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato, K., H. Kawasaki, H. Nagayama, M. Enomoto, C. Morimoto, K. Tadokoro, T. Juji, and T. A. Takahashi. 2000. TGF-β1 reciprocally controls chemotaxis of human peripheral blood monocyte-derived dendritic cells via chemokine receptors. J. Immunol. 164:2285-2295. [DOI] [PubMed] [Google Scholar]
- 48.Sobel, J. D., J. Schneider, D. Kaye, and M. E. Levison. 1981. Adherence of bacteria to vaginal epithelial cells at various times in the menstrual cycle. Infect. Immun. 32:194-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun, B., K. Nasu, J. Fukuda, S. Mine, M. Nishida, and I. Miyakawa. 2002. Expression of macrophage inflammatory protein-3α in an endometrial epithelial cell line, HHUA, and cultured human endometrial stromal cells. Mol. Hum. Reprod. 8:930-933. [DOI] [PubMed] [Google Scholar]
- 50.Tabibzadeh, S. 1991. Ubiquitous expression of TNF-α/cachectin immunoreactivity in human endometrium. Am. J. Reprod. Immunol. 26:1-4. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi, T., B. Eitzman, N. L. Bossert, D. Walmer, K. Sparrow, K. C. Flanders, J. McLachlan, and K. G. Nelson. 1994. Transforming growth factors β1, β2, and β3 messenger RNA and protein expression in mouse uterus and vagina during estrogen-induced growth: a comparison to other estrogen-regulated genes. Cell Growth Differ. 5:919-935. [PubMed] [Google Scholar]
- 52.Tanaka, Y., T. Imai, M. Baba, I. Ishikawa, M. Uehira, H. Nomiyama, and O. Yoshie. 1999. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur. J. Immunol. 29:633-642. [DOI] [PubMed] [Google Scholar]
- 53.Weiss, J. M., C. A. Cuff, and J. W. Berman. 1999. TGF-β downmodulates cytokine-induced monocyte chemoattractant protein (MCP)-1 expression in human endothelial cells. A putative role for TGF-β in the modulation of TNF receptor expression. Endothelium 6:291-302. [DOI] [PubMed] [Google Scholar]
- 54.Wira, C., B. O'Mara, J. Richardson, and R. Prabhala. 1992. The mucosal immune system in the female reproductive tract: influence of sex hormones and cytokines on immune recognition and responses to antigen. Vaccine Res. 1:151-167. [Google Scholar]
- 55.Wira, C. R., and K. Merritt. 1977. Effect of the estrous cycle, castration andpseudopregnancy on E. coli in the uterus and uterine secretions of the rat. Biol. Reprod. 17:519-522. [DOI] [PubMed] [Google Scholar]
- 56.Wira, C. R., M. A. Roche, and R. M. Rossoll. 2002. Antigen presentation by vaginal cells: role of TGFβ as a mediator of estradiol inhibition of antigen presentation. Endocrinology 143:2872-2879. [DOI] [PubMed] [Google Scholar]
- 57.Wira, C. R., and R. M. Rossoll. 1995. Antigen-presenting cells in the female reproductive tract: influence of the estrous cycle on antigen presentation by uterine epithelial and stromal cells. Endocrinology 136:4526-4534. [DOI] [PubMed] [Google Scholar]
- 58.Wira, C. R., and R. M. Rossoll. 2003. Estradiol regulation of antigen presentation by uterine stromal cells: role of TGFβ production by epithelial cells in mediating antigen presenting cell function. Immunology 109:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wira, C. R., D. A. Sullivan, and C. P. Sandoe. 1983. Epithelial cell involvement in the estradiol-stimulated accumulation of IgA in the rat uterus. J. Steroid Biochem. 19:469-474. [DOI] [PubMed] [Google Scholar]
- 60.Yelavarthi, K. K., H. L. Chen, Y. P. Yang, B. D. Cowley, Jr., J. L. Fishback, and J. S. Hunt. 1991. Tumor necrosis factor-α mRNA and protein in rat uterine and placental cells. J. Immunol. 146:3840-3848. [PubMed] [Google Scholar]