Abstract
We followed 8 patients (4 males) with biochemically and/or molecular genetically proven deficiencies of the E1α subunit of the pyruvate dehydrogenase complex (PDC; 3 patients) or respiratory chain complexes I (1 patient), IV (3 patients) or I+IV (1 patient) who received oral dichloroacetate (DCA; 12.5 mg/kg/12 hours) for 9.7 to 16.5 years. All subjects originally participated in randomized controlled trials of DCA and were continued on an open-label chronic safety study. Patients (1 adult) ranged in age from 3.5 to 40.2 years at the start of DCA administration and are currently aged 16.9 to 49.9 years (mean ± SD: 23.5 ± 10.9 years). Subjects were either normal or below normal body weight for age and gender. The 3 PDC deficient patients did not consume high fat (ketogenic) diets. DCA maintained normal blood lactate concentrations, even in PDC deficient children on essentially unrestricted diets. Hematological, electrolyte, renal and hepatic status remained stable. Nerve conduction either did not change or decreased modestly and led to reduction or temporary discontinuation of DCA in 3 patients, although symptomatic worsening of peripheral neuropathy did not occur. We conclude that chronic DCA administration is generally well-tolerated in patients with congenital causes of lactic acidosis and is effective in maintaining normal blood lactate levels, even in PDC-deficient children not consuming strict ketogenic diets.
Keywords: congenital lactic acidosis, dichloroacetate, pyruvate dehydrogenase, respiratory chain, clinical trial
Introduction
1.1.1
Dichloroacetate (DCA) has been administered since at least 1978 (1) to patients with inborn errors of mitochondrial metabolism. Its use has been predicated on its ability to inhibit pyruvate dehydrogenase kinase, thereby maintaining the pyruvate dehydrogenase complex (PDC) in its unphosphorylated, active state, and to increase the stability of the PDC, by unknown mechanisms (2). Oral DCA is rapidly absorbed, crosses the blood-brain barrier and activates the PDC within minutes following administration (2). Consequently, it has been used orally and parenterally for the acute and chronic treatment of acquired (3–5) and congenital (1, 6–12) forms of lactic acidosis, for which it is designated an Orphan Product by the Food and Drug Administration.
1.1.2
A randomized controlled trial (RCT) of 12.5 mg/kg/12h oral DCA for six months in 43 children with PDC deficiency one or more defects in respiratory chain enzymes or a pathological mutation in mitochondrial DNA (mtDNA) found the drug to be as well tolerated as placebo and to induce significant, sustained decreases in venous blood and cerebrospinal fluid lactate concentrations (10). Open label continuation of DCA administration in 36 of the original patients, for a median exposure of 2.4 years (maximum 9.7 years), also demonstrated good tolerability, although some patients developed asymptomatic worsening of lower extremity nerve conduction (12). Nine of 26 patients with a respiratory chain defect or mtDNA mutation died during an approximately 10 year period of treatment, whereas only 1 of 10 PDC deficient children died. In a second RCT, in which seven children and one adult with various forms of congenital lactic acidosis received 12.5 mg/kg/12h oral DCA for three months in a crossover design, the drug was well tolerated and significantly attenuated the blood lactate rise following submaximal treadmill exercise (7).
1.1.3
We now report outcome measures for 5 participants from the RCT of children with congenital lactic acidosis (10) and 3 subjects involved in the aforementioned exercise trial (12).
2.1. Methods
2.1.1. Study Design
This study was approved by the University of Florida Investigational Review Board and by the Scientific Advisory Committee of the Shands Hospital Clinical Research Center. Consent or assent was obtained from all patients or guardians. The trial was also overseen by a Medical Monitor, unaffiliated with the University of Florida, who chaired the Data Safety and Monitoring Board for the initial RCT (10). The Monitor determined whether patients could continue on treatment or whether their dose should be adjusted or discontinued, based on review of clinical, laboratory and nerve conduction findings and the correlative opinion of the neurologist.
2.1.2
The biochemical and/or molecular genetic diagnoses of the patients were based on the diagnostic eligibility criteria required for participating in the two RCTs (4,7) in which they participated. Each patient was admitted to the Clinical Research Center every six months for general physical and neurological examinations, laboratory tests of hematological, electrolyte, hepatic and renal function, serum thyrotropin and fasting venous whole blood glucose and lactate (YSI Instruments, Yellow Springs, OH). Blood for glucose and lactate determinations was obtained at least four hours after feeding in infants and after an overnight fast in older subjects, using minimal tourniquet pressure. Blood was placed immediately into chilled, unstoppered tubes containing EDTA and sodium fluoride, stoppered and inverted slowly several times, returned to the ice bath and analyzed within minutes of blood withdrawal. Upper and lower extremity nerve conduction was measured at each visit, as reported previously (10).
2.1.3
Clinical grade sodium DCA (TCI America, Portland, OR) was formulated as a liquid for oral use as previously described (15) and was administered at a dose of 12.5 mg/kg/12 hr by mouth and was kept refrigerated until use. At each admission patients exchanged remaining drug for a new lot of DCA, the concentration and homogeneity of which had been confirmed before release. Plasma trough DCA and urinary maleylacetone (MA) concentrations were measured, in duplicate, by gas chromatography-mass spectrometry (16) at least once during most admissions.
2.1.4. DNA Isolation, Genotyping and Haplotype Analysis
DNA was isolated from blood samples from 6 children aged 2 to 10 years, with congenital lactic acidosis using Qiagen Gentra Puregene Buccal Cell and blood Kits (Qiagen Inc., CA, USA). DNA samples were genotyped for 3 non-synonymous SNPs: G94>A (rs3177427) Glu→Lys at position 32; G124>A (rs7972) Gly→Arg at position 42; and C245>T (rs1046428) Thr→Met at position 82) in the GSTZ1/MAAI gene by pyrosequencing (11). Haplotypes were inferred by computational methods using the Bayesian haplotype reconstruction program, PHASE version 2.1 (12).
2.1.5. Resequencing and Mutation Discovery
A DNA sample from subject 4 with the EGT/KGT haplotype who showed inordinately high plasma DCA levels was selected for further analysis by resequencing and mutation discovery. The exons and intron/exon boundaries, 5′ and 3′ untranslated regions (UTR) of GSTZ1/MAAI gene were amplified by PCR and the purified PCR products were evaluated by direct sequencing using the Amersham Biosciences ET-terminator chemistry method. Bidirectional DNA sequence data were compiled and polymorphic sites were identified using PolyPhred (13). We did not observe any novel mutation in this DNA sample, but found that the individual is heterozygous for GSTZ1, -1002 G/A (rs7160195) promoter SNP (14). DNA samples from the other individuals in this study were also resequenced to determine whether they carried this mutation.
2.1.6. Diet and supplemental nutrients
Dietary intake was recorded, but no explicit nutritional interventions were recommended, other than ensuring overall nutritional adequacy. The three PDC deficient patients regularly consumed diets in which the fat:carbohydrate + protein ratio varied widely, from 1:1.3 in 2 patients to an extremely high carbohydrate regimen (1:13) in a third patient. The DCA formulation contained 1 mg/kg thiamine • HCl. The diets of patients 1–5 were supplemented by various home-based “cocktails” that included multivitamins, carnitine, fish oils, and coenzyme Q10. In addition, patient 3 received alpha lipoic acid (200 mg/d) and thiamine (250 mg/d), while patient 4 received alpha lipoic acid (100 mg/d) and milk thistle (75 mg/d).
3.1. Results
3.1.1
Table 1 summarizes the clinical characteristics of the patients in relation to treatment. Most patients were less than 10 years old when DCA administration commenced. All subjects were Caucasian except patient 4, who was of mixed race (Asian and Caucasian). Patients 1 and 2 are identical twins whose discordant clinical phenotype has been reported (9) and patients 6 and 7 are brothers. Patients 1–5 demonstrated mild to profound neurodevelopmental delay complicated in patient 5 by epilepsy that was treated with carbamazepine and diazepam and contractures of the extremities that markedly restricted ambulation. Patients 6–8 are cognitively normal. Patient 1 received a progesterone intrauterine device for control of menses. Patient 6 demonstrated mild exercise intolerance, but his younger sibling is athletic. Patient 8’s complications included mild, progressive fatigability, intermittent diarrhea, cramping of calf muscles and numbness and tingling of his feet; serum creatine kinase and liver transaminase levels were normal. Despite his symptoms, patient 8 maintained a rigorous work schedule outside the home in an occupation requiring high cognitive skills and travel. No patient had subjective or objective evidence of heart disease, although echocardiography or other cardiac diagnostic procedures were not performed.
Table 1.
Patient characteristics.
| Patient # | Gender | Diagnosis | DCA Exposure (years) | Current Age (years) | GSTZ1 Haplotype |
|---|---|---|---|---|---|
| Patient 1 | F | PDC Deficiency | 14.8 | 20.3 | EGT/EGT |
| Patient 2 | F | PDC Deficiency | 14.8 | 20.3 | EGT/EGT |
| Patient 3 | F | PDC Deficiency | 13.5 | 16.7 | EGT/KGT |
| Patient 4 | F | Complex I | 11.9 | 23.7 | EGT/KGT |
| Patient 5 | M | Complex I & V | 12.5 | 20.7 | KGT/KGT |
| Patient 6 | M | Complex IV | 10.8 | 18.7 | nd |
| Patient 7 | M | Complex IV | 10.8 | 16.8 | nd |
| Patient 8 | M | mtDNA T9997C | 10.0 | 49.6 | EGT/EGT |
| Mean: 12.4 | |||||
| Total patient yrs: 99.1 | |||||
nd, not determined. These patients were lost to follow-up before genotyping could be performed.
3.1.2
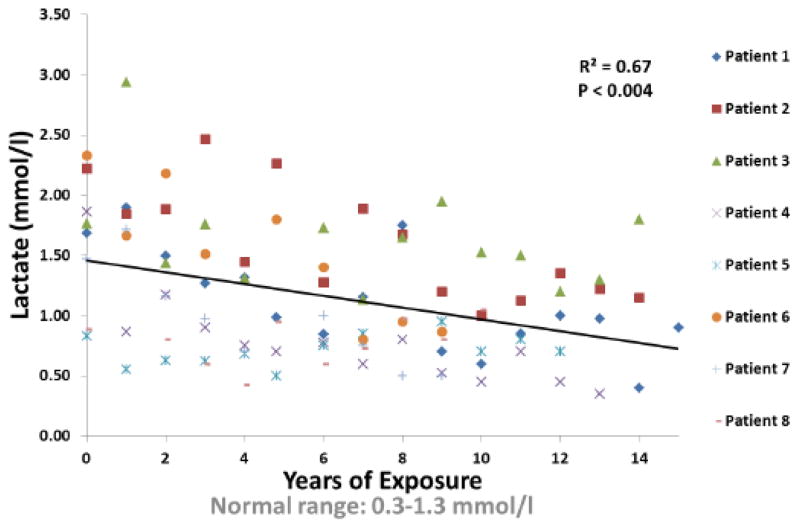
All patients tolerated DCA well. Patients and/or family members did not report changes in eating or sleep habits while receiving DCA or worsening of activities of daily living. In addition, there were no significant changes over time in white blood cell count, hematocrit, hemoglobin or platelets or fasting serum levels of sodium, potassium, chloride, bicarbonate, urea nitrogen, creatinine, triglycerides, total cholesterol, low-density and high-density lipoprotein cholesterol, total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase or thyrotropin. There were no relationships between plasma DCA and blood lactate (p=0.38), or between serum alanine aminotransferase and either blood lactate (p=0.72) or years of DCA exposure (p=0.11). In contrast, there was a highly significant inverse association between venous blood lactate concentrations and DCA exposure (Fig. 1).
Fig. 1.
Blood Lactate vs. Years of Exposure.
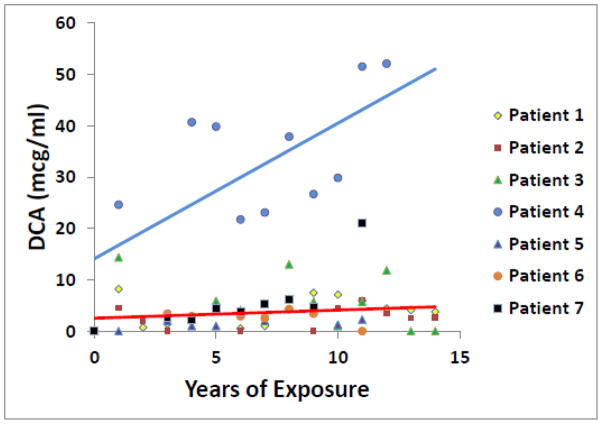
3.1.3
Fig. 2 correlates plasma DCA trough (dosing interval) levels and duration of drug exposure in 7 patients. Overall, no significant change occurred in DCA concentrations over time, except for a progressive rise in drug levels in patient 4. DCA is dehalogenated to glyoxylate, upon which it enters the general carbon pool of the host. This biotransformation is mediated by GSTZ1, a bifunctional enzyme that, as maleylacetoacetate isomerase (MAAI), catalyzes the penultimate step in tyrosine metabolism. DCA inhibits GSTZ1/MAAI, leading in some subjects to plasma and/or urinary accumulation of DCA and MA, a substrate of the isomerase (reviewed in 19). Haplotype variations in the GSTZ1/MAAI gene confer differences in the biotransformation and plasma clearance of DCA that may influence its chronic safety (13). Based on results obtained in healthy subjects administered DCA (20), the 6 subjects who were genotyped in the present study possessed haplotypes that would be predicted to result in rapid metabolism and plasma clearance of DCA (Table 1). Urinary concentrations of MA is these patients ranged from 0.27 to 4.7 mcg/mg creatinine (mean ± SD: 1.6 ± 1.1) [normal: <0.2 μg/ml (below detection limit)], but were not related to plasma DCA trough levels (p ≥ 0.81) or duration of exposure to the drug (p = 0.53). Re-sequencing the DNA sample from patient 4, revealed no novel mutation, but did show that the individual is heterozygous for GSTZ1-1002 G/A (re7160195) promoter SNP, which has been shown in vitro to confer lower GSTZa promoter activity (16). In addition, DNA re-sequencing of samples from the other patients whose GSTZ1/MAAI genotype was known (Table 1) demonstrated patient 3 was also heterozygous for the same promoter SNP.
Fig. 2.
Plasma DCA trough levels vs. years of exposure.
3.1.4
In accordance with recommendations of the Medical Monitor, DCA administration was temporarily discontinued or the dose was decreased 50 percent for up to 1.5–39 months at a time in patients 3 (up to 24 months DCA), 5 (p to 14 months) and 8 because of periodic asymptomatic worsening of lower extremity nerve conduction velocity. This effect persisted longest (up to 39 months before restarting DCA) in patient 8, who, to our knowledge, is the oldest subject to be chronically treated with the drug. Transient deterioration of nerve conduction was not associated in any patient with changes in blood lactate or with any abnormalities in blood chemistries. Of note, patient 8 and his wife repeatedly observed that his fatigue, exercise intolerance and calf muscle cramps increased within 48 hours of discontinuing DCA, but resolved within approximately the same time span once drug administration resumed. Chronic numbness and tingling of his feet were not affected by DCA exposure.
3.1.5
In general, all patients remained in stable health and, except for patient 5 (whose epilepsy and contractures persisted unchanged), achieved developmental milestones and improved home functionality, demonstrated by increased ambulation and exercise tolerance (patients 1, 3, 4 and 6–8), scholastic progression, including beyond high school (patients 1, 3, 4 and 6–8) and menarche (patients 1–4).
4.1. Discussion
4.1.1
There are no proven treatments for any primary mitochondrial disease. However, this study represents the longest formal evaluation of safety of any investigational agent for such conditions. The results indicate that oral DCA, at a dose of 12.5 mg/kg/12h, is generally very well tolerated in patients with mitochondrial diseases, at least from young childhood through early adulthood. This conclusion is consistent with the results of prior open label studies (6, 8, 9, 12) and RCTs (7, 10) in pediatric subjects. These findings contrast with results of a RCT conducted in older adolescents and adults with mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) that ended prematurely because of symptomatic and electrical evidence of exacerbation or new onset of peripheral neuropathy (11). However, DCA metabolism in humans is inversely proportional to age (21) and GSTZ1 haplotype also influences plasma clearance of the drug (13). All the patients who were genotyped in this study were found to be rapid metabolizers of DCA except patient 4, whose GSTZ1 allelic profile was associated with marked plasma drug accumulation over continued exposure. This phenomenon is also illustrated by other healthy (13) or diseased (22) individuals who harbor GSTZ1 alleles that lead to reduced biotransformation of DCA. We first thought that patient 4’s unusually high levels were due to the GSTZ1-1002 G/A (re7160195) promoter SNP, which has been shown in vitro to confer lower GSTZ1 promoter activity (16). However, this SNP was also present in patient 3, whose plasma drug levels were low. Thus, the SNP we discovered in the promoter region in the GSTZ1 gene is unlikely the cause of the plasma DCA accumulation in patient 4; thus, the mechanism accounting for the high plasma trough levels of DCA in this subject remains unknown. However, as more discoveries are made about genetic variations in the coding and noncoding regions of GSTZ1/MAAI, their impact on DCA kinetics, biotransformation and human safety will become clearer. Indeed, recent data obtained in adults with recurrent malignant brain tumors treated with DCA (21) and unpublished observations demonstrate that GSTZ1/MAAI haplotype variability can be important in developing rational and safe dose regimens. Therefore, this study, and the pharmacokinetic investigations in other patient populations (22), emphasize the importance of genotype in determining the dosing and chronic safety of the drug.
4.1.2
This open label study cannot distinguish unequivocally between the relative impact of DCA exposure vs. underlying disease on the frequency or severity of peripheral neuropathy. However, peripheral neuropathy is a common complication of primary mitochondrial diseases in general (22–23) and was prevalent among the participants of both pediatric (10) and MELAS (11) RCTs prior to commencing DCA. In addition, vulnerability to DCA-induced neuropathy in rodent models is directly associated with age (ref. 24 and unpublished observations). Lastly, the occasional evidence of worsening nerve conduction in three patients described in this report was not associated with symptomatic neuropathy, elevated DCA plasma levels or declining overall clinical status. Therefore, we postulate that the observed changes in nerve conduction may reflect the combined and variably impactful effects of technical variability in electrical measurements, the waxing and waning of the underlying disease, chronic DCA exposure and advancing age of the patients. Nevertheless, prospective clinical evaluation of peripheral nerve function is warranted in any future trials of DCA.
4.1.3
Elevated liver enzymes can be a complication of primary mitochondrial diseases (13). Studies in inbred rodent strains clearly show that chronic DCA is hepatotoxic, and mild, asymptomatic and readily reversible increases in serum hepatic transaminases have occasionally been reported in humans during chronic exposure (19). Thus, it is reassuring that we found no evidence for transaminitis in our long-term evaluation of DCA.
4.1.4
DCA maintained normal blood lactate concentrations in all patients of this series, even in the three PDC deficient children who chronically consumed diets relatively liberal in carbohydrates. This finding is consistent with prior data, indicating that the lactate-lowering effect of DCA is sustained over time (12) and that DCA significantly blunts the expected blood lactate rise following carbohydrate ingestion, even in PDC-deficient subjects (10).
4.1.5
The value of blood lactate as a clinically useful biomarker in mitochondrial diseases is controversial. However, worsening lactic acidosis, due to acquired or congenital etiologies, has long been known to portend clinical deterioration and death. In our natural history study of 371 cases of PDC deficiency (25), the maximum blood lactate averaged 7.8 mmol/l among evaluable cases and the magnitude of hyperlactatemia was indistinguishable among etiologies, based on defects in specific subunits or components of the complex. Hyperpyruvatemia was another common biochemical finding, as were increased levels of lactate and pyruvate in CSF. Compared to patients who were alive at the time of case reporting, patients who died had higher maximum blood lactate concentrations that were inversely associated with residual PDC activity. Thus, analysis of a large number of PDC deficient patients suggests that serial blood lactate measurements have prospective value in relation to an individual’s clinical outcome. This relationship is not surprising, given the critical importance of the PDC to both overall cellular energetics and organismal function and its pivotal role in determining the fate of pyruvate and, hence, lactate. Indeed, lactic acidosis and muscle fatigue or failure are linked, not only during voluntary exercise, but also in other rare inborn errors of metabolism in which pathological inhibition of the PDC occurs. Hyperpyruvatemia occurs in PDC deficiency because of an increased rate of glycolysis by PDC deficient cells (2) and a decreased rate of oxidative removal, due to the PDC block. Neither measurement of blood pyruvate per se nor the lactate:pyruvate ratio offer greater prognostic potential than does the blood lactate level alone (1). However, impairment of pyruvate oxidation may lead to a chronic metabolic acidosis that may exacerbate bone demineralization, growth retardation, abnormal glucose tolerance, renal impairment and muscle wasting and contribute to a general pro-oxidant state associated with depletion of anti-oxidant defense mechanisms (3, 4). Given the broad spectrum of pathology associated with acute or chronic lactic acidosis, it is noteworthy that DCA stimulates residual PDC activity and decreases the adverse local tissue and/or systemic effects of lactic acidosis on cardiac or skeletal muscle function associated with exercise, insulin resistance, sepsis, ischemia, high fat feeding, or statin-induced myopathy and may inhibit tissue protein wasting (reviewed in 2, 19, 26). Together, these observations lend particular import to both circulating lactate as a clinically useful and readily obtainable biomarker of disease progression and to the potential utility of DCA in treating PDC deficiency.
4.1.6
In summary, we found long-term DCA administration to be generally safe and well-tolerated by patients with congenital forms of lactic acidosis and to maintain normal circulating lactate levels, regardless of underlying disease or dietary intake. Prior knowledge of GSTZ1 genotype may be important in determining safe dosing regimens for future chronic drug administration.
Highlights.
We describe the chronic safety profile of DCA, an investigational drug for mitochondrial diseases
We examine the influence of pharmacogenetics in drug metabolism and safety
We show DCA is well-tolerated and maintains normal blood lactate levels over several years of exposure
Patient genotype is important in determining future DCA dosing regimens
Acknowledgments
This work was supported in part by the University of Florida Clinical and Translational Science Institute and NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064. We thank Mercy Medical Airlift for facilitating patient transportation and Ms. Candy Caputo for editorial assistance.
Abbreviations
- CSF
cerebrospinal fluid
- DCA
dichloroacetate
- EDTA
ethylenediaminetetraacetic acid
- GSTZ1
glutathione transferase zeta 1
- MA
maleylacetone
- MAAI
maleylacetoacetate isomerase
- MELAS
mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes
- mtDNA
mitochondrial deoxyribonucleic acid
- PDC
pyruvate dehydrogenase complex
- RCT
randomized controlled trial
- TCA
tricarboxyclic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coude FX, Saudubray JM, DeMaugre F, Marsac C, Leroux JP, Charpentier C. Dichloroacetate as treatment for congenital lactic acidosis. N Engl J Med. 1978;299:1365–6. doi: 10.1056/NEJM197812142992414. [DOI] [PubMed] [Google Scholar]
- 2.Stacpoole PW. The pharmacology of dichloroacetate. Metabolism. 1989;38:1124–1144. doi: 10.1016/0026-0495(89)90051-6. [DOI] [PubMed] [Google Scholar]
- 3.Stacpoole PW, Harman EM, Curry SH. Baumgartner TG, Misbin RI: Treatment of lactic acidosis. N Engl J Med. 1984;310:320–321. doi: 10.1056/NEJM198308183090702. [DOI] [PubMed] [Google Scholar]
- 4.Stacpoole PW, Wright EC, Baumgartner TG, Bersin RM, Buchalter S, Curry SH, Duncan CA, Harman EM, Henderson GN, Jenkinson S, Lachin JM, Lorenz A, Schneider SH, Siegel JH, Summer WR, Thompson D, Wolfe C, Zorovich B the DCA-Lactic Acidosis Study Group. A controlled clinical trial of dichloroacetate treatment of lactic acidosis in adults The Dichloroacetate-Lactic Acidosis Study Group. N Engl J Med. 1992;327:1564–1569. doi: 10.1056/NEJM199211263272204. [DOI] [PubMed] [Google Scholar]
- 5.Agbenyega T, Planche T, Bedo-Addo G, Ansong D, Owusu-Ofori A, Bhattaram VA, Nagaraja NV, Shroads AL, Henderson GN, Hutson AD, Derendorf H, Krishna S, Stacpoole PW. Population kinetics, efficacy, and safety of dichloroacetate for lactic acidosis due to severe malaria in children. J Clin Pharmacol. 2003;43:386–396. doi: 10.1177/0091270003251392. [DOI] [PubMed] [Google Scholar]
- 6.Stacpoole PW, Barnes CL, Hurbanis MD, Cannon SL, Kerr DS. Treatment of congenital lactic acidosis with dichloroacetate. Arch Dis Child. 1997;77:535–541. doi: 10.1136/adc.77.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan GE, Perkins LA, Theriaque DW, Neiberger RE, Stacpoole PW. Dichloroacetate therapy attenuates the blood lactate response to submaximal exercise in patients with defects in mitochondrial energy metabolism. J Clin Endocrinol Metab. 2004;89:1733–1738. doi: 10.1210/jc.2003-031684. [DOI] [PubMed] [Google Scholar]
- 8.Barshop BA, Naviaux RK, McGowan KA, Levine F, Nyhan WL, Loupis-Geller A, Haas RH. Chronic treatment of mitochondrial disease patients with dichloroacetate. Mol Genet Metab. 2004;83:138–49. doi: 10.1016/j.ymgme.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Berendzen K, Theriaque D, Shuster J, Stacpoole PW. Therapeutic potential of dichloroacetate for pyruvate dehydrogenase complex deficiency. Mitochondrion. 2006;6:126–135. doi: 10.1016/j.mito.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, Felitsyn NM, Gilmore RL, Greer M, Henderson GN, Hutson AD, Neiberger RE, O’Brien RG, Perkins LE, Quisling RG, Shroads AL, Shuster JJ, Silverstein JH, Theriaque DW, Valenstein E. A controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117:1519–1531. doi: 10.1542/peds.2005-1226. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, Millar WS, Hong X, Gooch CL, Mao X, Pascual JM, Hirano M, Stacpoole PW, DiMauro S, De Vivo DC. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology. 2006;66:324–30. doi: 10.1212/01.wnl.0000196641.05913.27. [DOI] [PubMed] [Google Scholar]
- 12.Stacpoole PW, Gilbert LR, Neiberger RE, Carney PR, Valenstein E, Theriaque DW, Shuster JJ. Evaluation of long-term treatment of children with congenital lactic acidosis with dichloroacetate. Pediatrics. 2008;121:e1223–e1228. doi: 10.1542/peds.2007-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shroads AL, Langaee T, Coats BS, Kurtz TL, Bullock JR, Weithorn D, Gong Y, Wagner DA, Ostrov DA, Johnson JA, Stacpoole PW. Human polymorphisms in the glutathione transferase zeta 1 maleylacetoacetate isomerase gene predict the kinetics and toxicity of dichloroacetate. J Clin Pharmacol. 2012;52:837–49. doi: 10.1177/0091270011405664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nickerson DA, Tobe VO, Taylor SL. PolyPhred: automating the detection and genotyping of single nucleotide substitutions using fluorescence-based resequencing. Nucleic Acids Res. 1997;25:2745–2751. doi: 10.1093/nar/25.14.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang YY, Kashkarov U, Anders MW, Board PG. Polymorphisms in the human glutathione transferase zeta promoter. Pharmacogenet Genomics. 2006;16(5):307–13. doi: 10.1097/01.fpc.0000205000.07054.b3. [DOI] [PubMed] [Google Scholar]
- 17.Henderson GH, Darr RA, Whalen PO, Curry SH, Stacpoole PW. Development of an oral drug formulation for dichloroacetate and thiamine. Drug Devel Indust Pharm. 1994;20:2425–2437. [Google Scholar]
- 18.Yan Z, Henderson GN, James MO, Stacpoole PW. Determination of dichloroacetate and its metabolites in human plasma by gas chromatography-mass spectrometry. J Chromatography B Biomed Sci Appl. 1997;703:75–84. doi: 10.1016/s0378-4347(97)00404-0. [DOI] [PubMed] [Google Scholar]
- 19.Stacpoole PW. The dichloroacetate dilemma: environmental hazard versus therapeutic goldmine—both or neither? Environ Health Perspect. 2011;119:155–8. doi: 10.1289/ehp.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shroads AL, Guo X, Dixit V, Liu H-P, James MO, Stacpoole PW. Age-dependent metabolism of dichloroacetate in rats: possible relevance to human toxicity. J Pharmacol Exper Ther. 2008;324:1163–1171. doi: 10.1124/jpet.107.134593.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stacpoole PW, Dunbar EM, Shroads AL, Coats BS, Langaee T. Predictability of glutathione transferase zeta 1 haplotype on kinetics of dichloroacetate in human trials. 10th International ISSX Meeting; Atlanta, GA. October 16–20, 2011. [Google Scholar]
- 22.Stickler D, Valenstein E, Neiberger RE, Perkins LA, Carney PR, Shuster JJ, Theriaque DW, Stacpoole PW. Peripheral neuropathy in genetic mitochondrial diseases. Pediatr Neurology. 2006;34:127–31. doi: 10.1016/j.pediatrneurol.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann P, Pascual JM, Anziska Y, Gooch CL, Engelstad K, Jhung S, DiMauro S, De Vivo DC. Nerve conduction abnormalities in patients with MELAS and the A3243G mutation. Arch Neurol. 2006;63:746–8. doi: 10.1001/archneur.63.5.746. [DOI] [PubMed] [Google Scholar]
- 24.Calcutt NA, Lopez VL, Bautista AD, Mizisin LM, Torres BR, Shroads AL, Mizisin AP, Stacpoole PW. Peripheral neuropathy in rats exposed to dichloroacetate. J Neuropathol Exp Neurol. 2009;68:985–93. doi: 10.1097/NEN.0b013e3181b40217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel KP, O’Brien TW, Subramony SH, Shuster J, Stacpoole PW. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol Genet Metab. 2012;105(1):34–43. doi: 10.1016/j.ymgme.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallinson JE, Constantin-Teodosiu D, Glaves PD, Martin EA, Davies WJ, Westwood FR, Sidaway JE, Greenhaff PL. Pharmacological activation of the pyruvate dehydrogenase complex reduces statin-mediated upregulation of FOXO gene targets and protects against statin myopathy in rodents. J Physiol. 2012;590(Pt 24):6389–402. doi: 10.1113/jphysiol.2012.238022. [DOI] [PMC free article] [PubMed] [Google Scholar]