Abstract
Background
Antiretroviral treatments (ART) form the basis of adequate clinical control in human immunodeficiency virus-infected patients, and adherence plays a primary role in the grade and duration of the antiviral response. The objectives of this study are: (1) to determine the impact of the implementation of a pharmaceutical care program on improvement of ART adherence and on the immunovirological response of the patients; and (2) to detect possible correlations between different adherence evaluation measurements.
Methods
A 60-month long retrospective study was conducted. Adherence measures used were: therapeutic drug monitoring, a simplified medication adherence questionnaire, and antiretroviral dispensation records (DR). The number of interviews and interventions related to adherence made for each patient in yearly periods was related to the changes in the adherence variable (measured with DR) in these same yearly periods. The dates when the laboratory tests were drawn were grouped according to proximity with the study assessment periods (February–May, 2005–2010).
Results
A total of 528 patients were included in the study. A significant relationship was observed between the simplified medication adherence questionnaire and DR over the 60-month study period (P < 0.01). Improvement was observed in the mean adherence level (P < 0.001), and there was a considerable decrease in the percentage of patients with CD4+ lymphocytes less than 200 cells/mm3 (P < 0.001). A relationship was found between the number of patients with optimum adherence levels and the time that plasma viral load remained undetected. The number of interviews and interventions performed in each patient in the first 12 months from the onset of the pharmaceutical care program (month 6), was related to a significant increase in adherence during this same time period.
Conclusion
The results suggest that the establishment and permanence of a pharmaceutical care program may increase ART adherence, increase permanence time of the patient with undetectable plasma viral loads, and improve patients’ lymphocyte count.
Keywords: pharmaceutical care, antiretroviral treatment adherence, undetectable PVLs, CD4+ lymphocyte count, adherence measures, HIV/AIDS
Introduction
Acquired immunodeficiency syndrome (AIDS) was first identified in the United States in the summer of 1981 when the Centers for Disease Control and Prevention reported the appearance of an unexplained pneumonia due to Pneumocystis jiroveci (previously named P. carinii) and Kaposi’s sarcoma in five previously healthy male homosexuals in New York and Los Angeles.1 Since then, its control has become one of the most important public health challenges because of the nature of this epidemic, its impact on health, economics, and on social and political matters, as well as due to the virus’s epidemiological characteristics. Different solutions have been sought, including the development of a possible vaccine. However, mainly drug treatments have been developed in order to improve and increase the quality of life and expectancy of those infected by the human immunodeficiency virus (HIV) that may lead to the development of AIDS.
Current antiretroviral treatments (ART) form the basis of adequate virological and immunological control in HIV-infected patients. The rates of hospitalization, opportunistic infections, and mortality associated with HIV infection have been reduced. This has given rise to chronification of the infection and to a significant increase in survival.
ART adherence plays a primary role in the grade and duration of the antiviral response. This patient adherence is the result of a complex process developed through acceptance of the diagnosis, perception of the need to correctly carry out the treatment, motivation to do so, disposition and training of the skills to do it, capacity to overcome the difficulties that appear, and maintenance of the achievements reached over time. Several studies have demonstrated that medication adherence is second only to CD4+ count in accurately predicting progression to AIDS and death.2,3
Adherence is not the only determinant of ART failure or success. Other factors include genetic differences in drug metabolism, severe baseline immunosuppression, prior drug resistance, and concurrent opportunistic infections, among others. Adherence to ART, however, is one of few potentially alterable factors determining outcomes for patients with HIV.
The data obtained during the first available combined treatments based on unboosted protease inhibitor (PI) drugs showed that maximum efficacy was obtained with almost perfect adherence, usually superior to 95%.4 However, recent studies suggest that the therapeutic objectives can be achieved in regimens based on ritonavir-boosted PIs (PI/r) or on non-nucleoside reverse transcriptase inhibitors with adherence rates of approximately 75%.5,6 In spite of this controversy, there is general agreement that although viral suppression may be possible with moderate adherence levels, the probability of viral suppression and, more importantly, reduced disease progression and mortality, improves with every increase in adherence level.
According to a study conducted in the Spanish population, adherence in 20% to 50% of the patients receiving ART is inadequate.7 A meta-analysis has recently been published in Spain on ART adherence and concluded that the global percentage of ART adherence is 55%.8 Studies from the United States, Canada, and developed countries in Latin America and Europe have demonstrated similar rates of suboptimal adherence.9–12 The average rate of adherence appears to be approximately 70%. In light of this evidence, global care programs need to be established for the patient. These should include both the evaluation of ART adherence and the elaboration of action strategies aimed at optimizing these results.
Up to now, there is no known intervention method superior to the others to improve patient adherence. However, the best levels of evidence come from randomized and controlled studies. The easiest and most common intervention is based on providing the patients with information and knowledge in an attempt to achieve their maximum commitment with the proposed treatment. This commitment will be possible if the patient understands the purpose of the ART and the importance of adherence; this shows how important it is for the patient to feel like a participant in treatment-related decisions. Pharmacotherapy follow-up of the patient, included within the context of a pharmaceutical care program, comprises follow-up of the patient, the interventions themselves, as well as continuing education, among others. The principal objective of this study was to evaluate the impact of the implementation of a pharmaceutical care program on the evolution of ART adherence over the course of 60 months in a cohort of HIV outpatients from our hospital, and to determine the repercussion of ART adherence on the patients’ virological and immunological evolution. Equally, we hoped to detect possible correlations between the different adherence evaluation measurements used in this study: therapeutic drug monitoring (TDM), a simplified medication adherence questionnaire (SMAQ),13 and ART dispensation records (DRs).
Methods
Patient screening
A 60-month-long retrospective study with data from 528 HIV-infected subjects treated in the outpatient unit of the Pharmacy Service of the University Hospital of Salamanca, Salamanca, Spain was conducted. The patients were invited to participate voluntarily.
Inclusion criteria included patients with confirmed HIV infection and who had been receiving active ART in our hospital for more than 6 months (either naïve or pretreated patients).
Adherence measures
The scientific literature recommends using and combining several adherence evaluation methods. If not, the results obtained only by one of these methods would not be reliable.14 Three strategies were used in our case: TDM (direct method), DR, and SMAQ (the latter two being considered as indirect methods). These three adherence evaluation tools are routinely included in our pharmaceutical care program. There are TDM and SMAQ data for each pharmaceutical interview or clinical control. These records correspond to the measurements obtained around the month of April (February-May) of the year in question (2005–2010) since this is the month that corresponds to the study initiation point. The data for DR were obtained twice a year (April and October of each year).
Antiretroviral plasma concentrations were determined with the high-performance liquid chromatography–ultraviolet validated technique that permits the measurement of atazanavir, darunavir, efavirenz, fosamprenavir, indinavir, lopinavir, nelfinavir, nevirapine, ritonavir, and saquinavir.15,16 The analyzed samples had been drawn when fasting and before the administration of the next medication dose. In every case, the plasma trough concentrations (Cssmin) of the monitored antiretroviral therapy were estimated individually using Bayesian algorithms, based on previously published population pharmacokinetics models using PKS® software (Abbott Diagnostics, Abbott Laboratories, Abbott Park, IL, USA). The Cssmin obtained in this way were classified into three groups – according to whether they were within (therapeutic), low (subtherapeutic), or above (supratherapeutic) the therapeutic range – in accordance with the therapeutic margins collected previously17,18
The SMAQ questionnaire, which was validated in a Spanish population, was used to evaluate treatment adherence. The questionnaire consists of six questions with previously defined short answers that the patients are asked to answer. Based on the patients’ answers, each patient is classified as being either adherent or nonadherent to the pharmacological treatment.13
The DR is an indirect method of measuring adherence based on the assumption that a patient cannot take medication that has not been dispensed and that those that are dispensed to him/her are taken adequately. An adherence calculation was made using the dispensation dates that were included in 6-month periods beginning from the initiation of the study. Adherence was calculated as the number of pills taken during the previous 6 months divided by the number of pills prescribed during the same period. Levels that exceeded 100% were rounded down to 100%. Patients who consumed ≥95% of the medication prescribed were considered “adherent patients.” Adherence values were dichotomized into two levels: ≥95% versus >95%. However, since some studies consider 90% as the cutoff of adherence,8,19–21 this percentage was also considered.
Pharmaceutical care
The comprehensive follow-up program for patients with established HIV includes pharmaceutical care activities, TDM of antiretroviral drugs, and pharmacogenetic analysis. The latter two tools make it possible to individualize the PI, PI/r, and non-nucleoside reverse transcriptase inhibitor doses according to the needs of each patient because these are the antiretroviral drug families that have a demonstrated correlation between plasma concentrations and response.22 This comprehensive follow-up program has been established and developed based on close collaboration among the pharmacists, physicians, and nurses who attend this patient population.
The interviewer always consults the DR adherence data at the onset of pharmaceutical care in order to intervene during the program, if necessary. Furthermore, the adherence results measured by DR are communicated to the prescribers twice a year.
During the interview, the SMAQ questionnaire was used and the evolution of the plasma viral load (PVL), CD4+ lymphocyte count, and antiretroviral Cssmin was monitored. Possible analytic alterations that could be due to adverse drug events and possible interactions of the antiretroviral drugs with other drugs, food, natural products, abuse drugs, and so on were also reviewed.
During the study period, information regarding HIV and its treatment as well as the importance that adherence has in clinical outcomes and the prevention of resistance was specifically provided to the patients. In addition, a personalized dosing schedule was developed with the patient, and strategies on how to manage side effects were made. During follow-up visits, recommendations were made to solve any problems encountered; adherence was verbally reinforced and plans were developed to solve the problems that had appeared up to that time.
In this study, the number of interviews and interventions related to adherence made for each patient was determined in yearly periods (2005–2010), beginning with the implementation of the pharmaceutical care program in month 6, and then related to changes in the adherence variable (measured with DR) in these same periods. Pharmaceutical interventions related to adherence were considered as providing both oral and written information regarding the treatment, giving a weekly medication pill organizer, and making posological adjustments if the patient had supratherapeutic levels associated with adverse drug events that could decrease treatment adherence.
Clinical evolution
CD4+ counts and PVLs are indicators of immune status and HIV viral activity; both are expected to improve with ART adherence. However, the intended effect of ART (that of preventing viral replication) is more directly assessed by the PVL.23–25 We used data extracted from participants’ medical records. Dates at which laboratory tests were obtained were grouped according to proximity to the study assessment periods. For the analysis, we used the CD4+ count and the PVL, which was considered undetectable when the number of copies per milliliter was less than 50.
The number of months in which undetectable PVL levels were maintained in each patient during the study was determined. This information was then contrasted with the patients’ average adherence.
Statistical analysis
The IBM SPSS Statistics for Windows (version 19.0.0.1, released 2010; IBM Corporation, Armonk, NY, USA) was used for the statistical analyses. Chi-square tests were used to compare categorical variables. The Mann–Whitney test was used to assess differences between two independent samples, and the Wilcoxon signed-rank test was used to compare two related samples. The Kruskal–Wallis test was used to compare k >2 independent samples. Spearman’s correlation was used to study the relationship between quantitative or ordinal variables.
Results
A total of 528 patients were included in the study, 259 of whom received pharmacological treatment during the entire study period. The remaining patients were either lost to follow-up during this period (due to transfers, exitus, and so on), and/or they entered the study after the established initiation date. The mean patient permanence time in the study was 3.56 ± 1.68 years. Demographic and baseline clinical characteristics of the total study population are shown in Table 1.
Table 1.
Demographic and clinical characteristics of study population (n = 528)
| Value
|
|
|---|---|
| Mean ± SD or n (%) | |
| Age (years)a | 40.48 ± 8.94 |
| Male | 371 (70.3) |
| Therapy-naïveb | 187 (35.6) |
| Number of years on ARTc | 5.49 ± 3.41 |
| CD4+ counts (cells/mcL)c | 381.63 ± 245.93 |
| Undetectable plasma viral loadc | 251 (83.7) |
Notes:
Age at which the patient entered the study
number of total naïve patients who entered the study
variable measured at onset of the study (n = 332).
Abbreviations: n, number; SD, standard deviation; ART, antiretroviral treatment.
Association among adherence measurements
All three methods were used to collect the adherence data, and the results were grouped into periods of 6 months (DR) and 12 months (SMAQ and TDM) for each of the patients. Not all of the three measurements were available for the entire population at each of the cutoff levels because this was a retrospective study. Associations among the three methods used to measure adherence (TDM, SMAQ, and DR) were analyzed (Table 2).
Table 2.
Association among adherence measurements
| Time (months) | 0 | 12 | 24 | 36 | 48 | 60 |
|---|---|---|---|---|---|---|
| N | 332 | 377 | 398 | 410 | 411 | 398 |
| Adherent patients (%) | ||||||
| TDMa | – | 78.95 | 81.61 | 84.45 | 95.51 | 93.77 |
| SMAQ | – | 65.20 | 75.26 | 80.25 | 84.62 | 90.44 |
| DRb | 64.8 | 70.16 | 76.01 | 73.69 | 78.41 | 76.36 |
| Agreement between adherence measurements (%) | ||||||
| SMAQ–TDMa | – | 53.57 | 69.66 | 75.00 | 83.18 | 88.17 |
| SMAQ–DRb | – | 69.40† | 77.64† | 76.39† | 78.51† | 80.58† |
| TDMa–DRb | – | 60.70 | 72.73 | 68.67 | 81.36 | 76.21 |
Notes:
TDM categorized into subtherapeutic or not subtherapeutic
DR categorized into: <95%; ≥95%
P < 0.01 (Chi-square test and kappa coefficient).
Abbreviations: N, number; TDM, therapeutic drug monitoring; SMAQ, simplified medication adherence questionnaire; DR, dispensation record.
A significant association was found between TDM (therapeutic/subtherapeutic/supratherapeutic) and SMAQ (P = 0.013) in the first 6 months after the pharmaceutical care program was initiated. However, no association was found during this period when TDM was categorized into subtherapeutic and nonsubtherapeutic. No significant correlations were found between these two adherence measurements in the remaining periods analyzed; however, there was a clear tendency for adherent patients to reach therapeutic levels (50.0% in month 12 versus 77.9% in month 60) according to the SMAQ questionnaire.
A significant association (P < 0.01) was found between DR and SMAQ when the cutoff for adherent patients was 95% during the entire study period. Nonetheless, if the cutoff was established at 90%, this association was only observed in some periods. Furthermore, mean adherence obtained by DR was greater in those who were adherent according to the SMAQ than those who were nonadherent during the entire study.
There was no significant association between DR and TDM, regardless of the cutoff level used (90% or 95%) or the TDM variable category (subtherapeutic/therapeutic/supratherapeutic or subtherapeutic/not subtherapeutic).
Antiretroviral treatment adherence
Mean global adherence of the patients during the 60 months of the study was 94.98% when the DR was used to measure adherence.
Mean adherence at the implementation of the pharmaceutical care program was 92.69%; at month 60, adherence was approximately 96.04%, with this difference being very significant (P < 0.001) (Figure 1).
Figure 1.
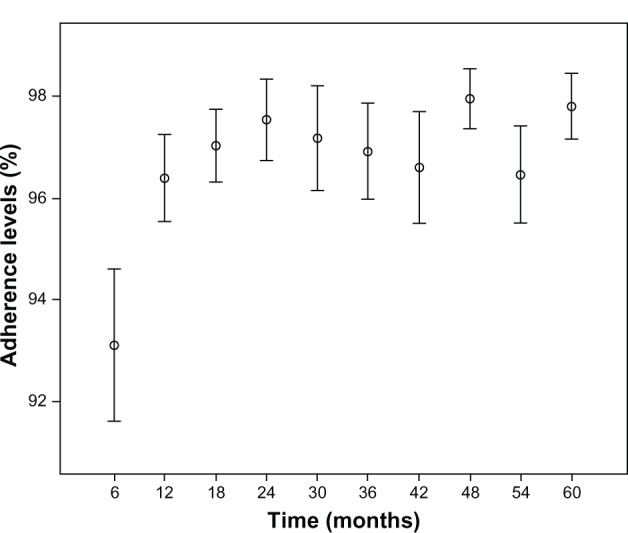
Evolution of the means (95% confidence interval) of antiretroviral treatment adherence during the 60 months of the study.
In addition, statistically significant differences (P < 0.001) were detected in the mean patient adherence level between the first 6 months of the study (prior to the initiation of the pharmaceutical care program) and second 6 months of the study. All the differences were highly significant (P < 0.003) when the first 6 months of the study were compared with the 6-month adherence measurements evaluated during the entire period. Similarly, it stands out that statistically significant differences were not observed when adherences measures after the first 6 months of the study were compared with each other.
There were 107 nonadherent patients in the baseline measurement. The follow-up of this subgroup showed that 43 of them had good adherence at 1 year after the implementation of the pharmaceutical care program, and this number increased to 75 at the end of the study period (while the remainder continued to be nonadherent or were lost to follow-up).
Regarding the evolution of the percentage of patients with optimum adherence (≥95%), this number increased from 64.80% at baseline to 76.36% at the end of the study. The number of patients with adherence levels greater than 90% also increased significantly (73.36% at baseline versus 86.68% at the end of the study).
Plasma viral load (PVL)
The percentage of patients with undetectable PVL remained practically unchanged during the study period with a mean of 76.7%. Statistically significant differences were found between the number of patients with optimum adherence levels and those who did not have these levels in regards to the amount of time they remained with undetectable PVL. This occurred when both the cutoff level of 95% adherence was considered and when adherence was grouped into four different levels (P < 0.002 and P < 0.001, respectively) (Figure 2).26
Figure 2.
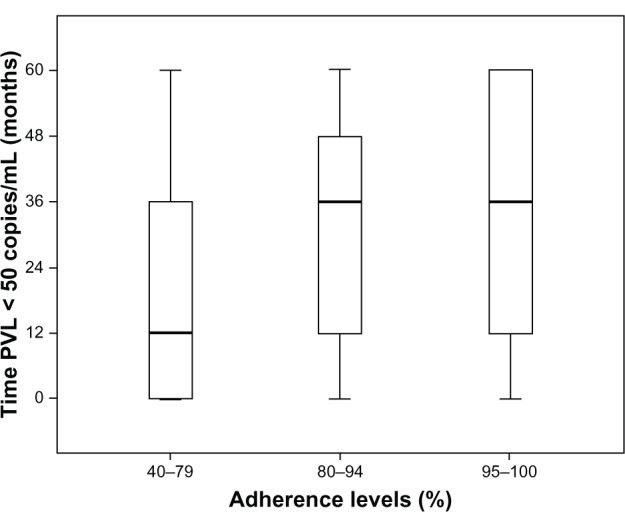
Influence of antiretroviral treatment patient adherence on permanence time in virological suppression.
Note: no patients had adherence <40%.
Abbreviation: PVL, plasma viral load.
CD4+ lymphocyte count
CD4+ lymphocyte levels were recorded at the onset and end of the study. The proportion of patients who had CD4+ < 200 cells/mm3 was compared. Statistically significant differences were found between both values (27.8% versus 14.9%, respectively; P = 1.992 × 10−5). Furthermore, the mean count of CD4+ lymphocytes significantly increased (P < 0.001) from 381.6 cells/mm3 (baseline) to 458.5 cells/mm3 (month 60).
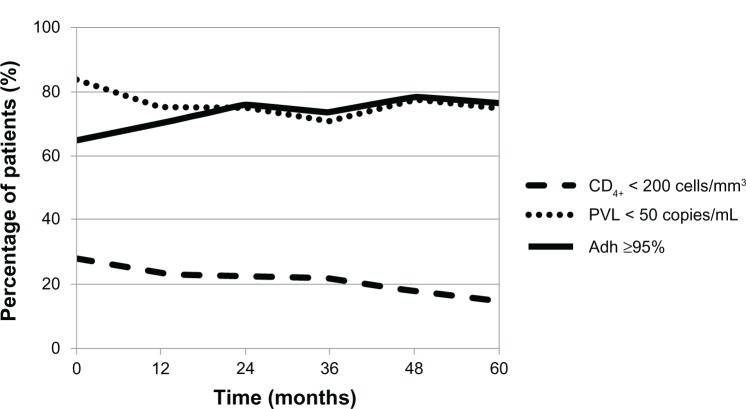
Evolution of adherence, PVL, and CD4+ lymphocyte count over the 60 months of the study is shown in Figure 3. The data are represented as the percentage of patients with adherence levels ≥95%, PVL < 50 copies/mL, and CD4+ lymphocyte count <200 cells/mm3.
Figure 3.
Evolution of patients in terms of adherence, virological status, and immunological status over the 60 months of the study.
Abbreviations: PVL plasma viral load; Adh, adherence to antiretroviral therapy.
Effect of interviews and interventions on adherence
During the study period, 2,199 interviews (4.2 ± 3.3 interviews/patient) and 3,100 interventions related to adherence (5.9 ± 4.4 interventions/patient) were conducted in the 528 patients studied. In accordance with the DR data collected, the number of interviews made for each patient in the first 12 months from the onset of the pharmaceutical care program was significantly related to an increase in his/her adherence levels in this same period (P < 0.05) (Figure 4). The same was found for the number of interventions conducted during the same year (P < 0.002) and the following year (P < 0.05). This relation was not found during the remainder of the study period.
Figure 4.
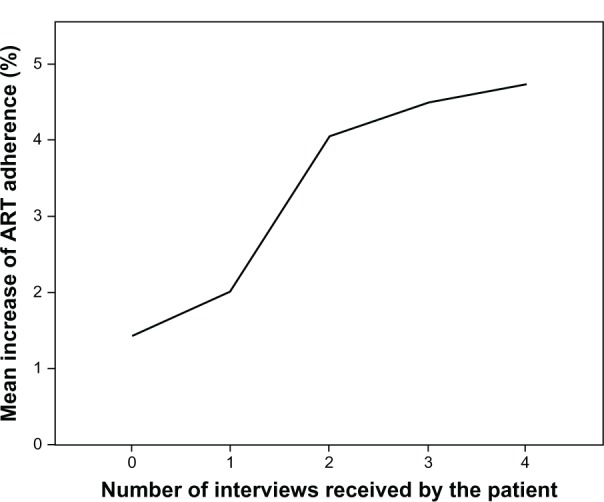
Relation between the mean increase of ART adherence in the first year after the pharmaceutical care program was initiated and the number of interviews made in this period.
Abbreviation: ART, antiretroviral treatment.
In regard to adherence determined by the SMAQ questionnaire, it was observed that the mean number of interviews conducted among the adherent patients was higher than among the nonadherent patients in all of the time periods, although these results are not statistically significant.
Discussion
In regard to the baseline characteristics of the population, it is evident that most of the patients were males (70.3%), a little more than one-third of patients were treatment-naïve when they entered into the study, the mean age was 40 years, and mean time in ART was approximately 5 years.
Association among adherence measurements
Adherence should be evaluated and optimized periodically during the ART in order to achieve the expected clinical response and to be able to make pertinent therapeutic decisions. Many studies have demonstrated that there is no single reliable method for its evaluation;7,27 therefore, consensus has been reached that at least two methods should be used. Included among the most important methods are administering a structured questionnaire during the interview, recording the collection of medication from the pharmacy, and determine the plasma concentrations of the antiretroviral drugs that can be monitored. Some authors have proposed that the immunovirological course of the patient could be considered a direct adherence evaluation method;28 however, subsequent studies have demonstrated that this is a consequence of the adherence grade of the patient with their treatment.29
In relation to the above, it would be advisable to know how the different adherence measurement methods are interrelated to see if there is a correlation between their results, or to see if they are different. For this reason, the present study has applied three different methods and has analyzed the relations between them.
TDM versus DR
DR is an indirect method that correlates positively with virological evolution,20,24,30,31 and it has acceptable specificity and sensitivity.11,32 However, this method requires the dispensation of ART in a single center, as is done in both this study and in Spanish hospitals. However, this centralized dispensation is not also performed in every country. Its principal limitations are that dispensation of the medication is not synonymous with correct adherence and, on the other hand, that mobility of the patients and sharing medication with their close relations may induce biases in the evaluation.
Although TDM is considered to be the most objective method to evaluate adherence, it has many and important limitations. Some of these limitations include elevated inter-and intraindividual variability, the lack of establishment of a standard cutoff to classify the patients as adherent or nonadherent, providing information only from the recent adherence level, and so on. Furthermore, evaluation of compliance could be affected by whether the medication is taken with or without meals, since this considerably affects bioavailability,33,34 as well as the Cssmin reached by the antiretroviral agent. The growing importance of pharmacogenetics in the field of HIV/AIDS must also be mentioned. It has been possible to demonstrate how the presence of a single nucleotide polymorphism in an individual patient causes considerable variation of drugs plasma levels.35,36 Thus, for example, patients who are rapid metabolizers could be classified as nonadherent in spite of having optimum adherence.
Consequently, the limitations of both evaluation methods could be responsible for the fact that a significant relation has not been found between both measurements.
TDM versus SMAQ
Over the 60 months of the study, a statistically significant relation was only found between TDM (subtherapeutic, therapeutic, and supratherapeutic) and SMAQ in the first 6 months after the pharmaceutical care program was initiated (P = 0.013). In the remainder of the study period, no association was observed.
The SMAQ is one of the many questionnaires existing to evaluate treatment adherence.7,13,37–39 In this questionnaire, it is the patient who, based on a reduced number of short response questions – normally dichotomous ones (yes/no) – states if he/she has been adherent in regards to taking the medication as indicated by the health care staff, or if this adherence has been occasional or nonexistent. The results provided by the SMAQ are generally overestimated,39,40 and consequently their reliability will depend on the communication skills of the interviewer.41,42 However, it will also significantly depend on how much confidence the patient has in the interviewer.
It is important to mention that the implementation of the monitoring program of antiretroviral plasma levels (TDM) coincided with the initiation of the pharmaceutical care program. Therefore, the patient was not familiar with this analytic measurement at the onset of the study period. For this reason, many undiscovered adherence problems surfaced with it. Over time and after the patients with low drug concentrations in their blood were warned by the health care personnel, some of the patients decided to take their medication prior to the analytic control to avoid new warnings. However, this was only done in specific situations which, in no case, implied permanent improvements in adherence.43
Due to the previously mentioned limitation of TDM, it is necessary to measure patients’ plasma concentrations of anti-retroviral therapy several times, so as to carry out population pharmacokinetic studies and to have a profound knowledge of those factors affecting the kinetic profile of the drugs to assure that the results obtained with this tool are reliable.
SMAQ versus DR
As commented on in the previous section, the results provided by the SMAQ depend both on the communication ability of the health care worker and of the trust the patient has in health care professionals. This will depend, among other factors, on the continuity of the program and on the interviewer.41–44 Both circumstances were taken into account when analyzing the data in this study.
In relation to the DRs, although it is true that they have some limitations, it seems to be the most widely used method in pharmacy departments to evaluate patient treatment adherence. Furthermore, many publications use it as the reference method, not only to measure adherence, but to also compare it with other variables for which some type of relationship is studied.3,19,24,26,30,45–47 In our study, a significant relation was found between both adherence measurement methods during the 60 months of the study (P < 0.002). For this reason, both the DR and SMAQ seem to be equally reliable in patients with ART.
Evolution of adherence
Given the correlation found between SMAQ and DR, and considering that the latter allows us to quantify adherence easily, DR was used as a reference method to measure the evolution of adherence.
The implementation and continuity of the pharmaceutical care program over the 60 months of the study improved the mean adherence level (92.7% to 96.0%; P < 0.001), increased the percentage of patients with adherence ≥95% (64.8% to 76.4%), as well as increased the percentage of patients with adherence equal to 100% (39.7% to 50.8%). The results reached in the present study are similar to, and even better than, those obtained in previous studies.30,31,48–50
It would be well to comment that mean adherence did not decrease during the study. This may be because the pharmaceutical care program remained in force during this period. In fact, there are many scientific references that establish that adherence is a continuous process, and therefore, motivation and permanent education is also necessary to maintain the adherence levels reached with the first interventions.44,51,52 The above would explain why no statistically significant differences were found between the different adherences obtained after the 6th month.
Indicators of clinical evolution
Different studies have searched for a relationship between adherence and the principal indicators of clinical evolution. Thus, some authors have found a relationship between adherence and PVL,4,20,31,40,53,54 others between adherence and CD4+ lymphocyte count,47,55 and others have found a relationship with both parameters.30 Our study has found significant differences in the virological as well as the immunological evolution of patients (P < 0.002 and P < 0.0001, respectively).
In the case of PVL, statistically significant differences were found between the number of patients with optimum adherence levels and those who did not have these levels in regard to time they remained with undetectable PVL. This indicates a direct correlation between better adherence rates and longer time with undetectable PVL. Although it is true that this correlation is quite logical, it has not always been possible to establish it.56 Furthermore, the patients need to be aware of this reality since this knowledge, by itself, would provide motivation to achieve and maintain optimal adherence rates. Maintenance of virological success may increase the time that the patients continue with the same treatment. This would avoid changes and exposure to new drugs with another profile of adverse effects and toxicity. This aspect gains even more importance in these current times of economic restrictions in health care costs since most of the time, changes in treatment entail a considerable increase in costs.
The evolution of the number of CD4+ lymphocytes over the 60 months of this study was examined in regard to the immunological status of the patients. The cutoff point was considered at 200 cells/mm3 – a limit that was established in agreement with many international guidelines for the follow-up and control of HIV/AIDS patients. These guidelines relate this level as a cutoff point to assure absence of opportunistic diseases.57–59 The outcomes obtained showed a significant decrease in the percentage of patients with CD4+ lymphocytes inferior to 200 cells/mm3 from the onset until the end of the study. This difference is highly significant (P < 0.001). This result demonstrates the successfulness of the pharmaceutical care program implemented, and specifically illustrates the development of a continuous pharmacotherapeutic follow-up. It is very likely that improved adherence has contributed to the virological improvement of the patients which, in turn, has been responsible for the increases observed in the immunological response.
Interviews and interventions
Pharmaceutical care, understood as pharmacotherapy follow-up, is based on carrying out personalized interviews with the patient. In these interviews, the patient is educated and reinforced regarding subject matters related to adherence. Furthermore, when pertinent, interventions can be made. This is why a positive relation between both the number of interviews and interventions received by the patient and improvement in adherence can be expected. This has been evidenced for both variables (interviews and interventions) during the first year of implementation of the pharmaceutical care program. The reason for this may be that the number of patients with poor adherence (35.2%) was greater when the study was being initiated, so that greater opportunities existed to educate the patients and to intervene. On the other hand, interviews and subsequent interventions would not improve adherence in those who were already adherent; however, interviews and interventions would make it possible for the adherence levels to remain elevated over time.
A highly significant change is observed in those patients who entered at the beginning of the study and were classified as nonadherent at that time (n = 107), since 40.2% of them had optimum adherence levels at 6 months after initiation of the pharmaceutical care program; this percentage increased up to 55.1% at the end of the study. On the other hand, 62.8% of those patients who improved their adherence in the first year of follow-up (n = 43) continued with this improved adherence until the end of the study. These results show the need for patients to have access to a pharmacotherapeutical follow-up program where they will be educated and motivated in terms of both their disease and the correct taking of their antiretroviral medication. This program will play a principal role enabling patients to change their behavior and to perceive and appreciate their treatment.51,60
For those patients who did not show improvements in antiretroviral medication adherence during the first 6 months after initiation of the pharmaceutical care program, 45.8% did so during the remaining months of the study. This seems to demonstrate how the continuous interviews and messages patients received in this program allowed some patients with inadequate adherence to become aware of the risks entailed, and they subsequently changed their behavior.
As expected, some individuals need more time than others to be able to internalize concepts, messages, and recommendations. However, everyone should have the opportunity to receive detailed and personalized information adjusted to their sociocultural and educational reality, as well as tailored to their clinical profile and history.
Included within the principal limitations of this investigation are the facts that (1) some patients withdrew during the 60 months of the study due to transfers to other hospital centers, treatment abandonments, exitus, and others; and (2) some patients entered the study after it had been initiated (from transfers, new treatments, and so on). Although it is true that one could consider that treatment abandonments are related to noncompliance, it has been observed that these abandonments are significantly due to toxicity problems related to the ART; in turn, this is directly related to elevated adherence rates. These limitations have complicated the data collection and interpretation. However, it is estimated that of the population studied, 22.5% dropped out and 25.6% entered into it. Thus, this probably has not significantly affected the results and conclusions obtained.
Another limitation to keep in mind is that interprofessional work was begun with the physicians, nurses, and pharmacists in order to optimize treatments when the pharmaceutical care program was established. The purpose of this interprofessional work was to not only optimize the treatment, but to also improve antiretroviral therapy adherence. Therefore, improvements in the levels of adherence observed in this study cannot be exclusively attributed to the pharmaceutical intervention. They should also be attributed to the participation of the remaining members of the health care team.
Finally, improvement in adherence and evolution of the patients may be partially due to the improvements introduced in the medications available during the years of the study. Medications that require fewer daily doses, medications that have fewer side effects, or medications that exhibit better efficacy with lower adherence requirements have been incorporated into the current treatment. This contributes to obtaining better results with less effort by the patient. A more comprehensive study on the relation of these and other variables with the grade of adherence or efficacy of the different treatment regimes is needed, since it must be remembered that treatment adherence is, by definition, multifactorial.57,59
Conclusion
Both DR and SMAQ may be considered as adequate measurements of treatment adherence because of the good correlation observed between them. The SMAQ is the easiest method and, in our opinion, its validity is based on the existence of a long, continuous, and permanent pharmacist– patient relationship over 5 years. This makes it possible for the patient to openly admit the times when his/her adherence has fluctuated. The advantage of DR is that it has more objectivity, which makes it possible to quantify this variable.
The correlation observed between treatment adherence and the two most important indicators of clinical response (number of CD4+ lymphocytes and PVL) shows how adherence rates equal to or greater than 95% make it possible for the patient to maintain undetectable PVLs for a longer period of time, and consequently, patients can have a better lymphocyte count.
Finally, the correlation observed between both the number of interviews and the number of pharmaceutical interventions with adherence should be emphasized. This shows the importance of the establishment and permanence of this pharmaceutical service for HIV/AIDS patients.
Acknowledgments
Thanks to the Europharma Foundation under an agreement of collaboration between the University of Salamanca and the University Austral of Chile. The findings are presented here on behalf of the Tormes Team: Carmen Bustos, Miguel Cordero, Aurelio Fuertes, and Guillermo Luna.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Centers for Disease Control (CDC) Pneumocystis pneumonia – Los Angeles. MMWR Morb Mortal Wkly Rep. 1981;30(21):250–252. [PubMed] [Google Scholar]
- 2.García de Olalla P, Knobel H, Carmona A, Guelar A, López-Colomés JL, Caylà JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30(1):105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 3.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 4.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 6.Maggiolo F, Airoldi M, Kleinloog HD, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials. 2007;8(5):282–292. doi: 10.1310/hct0805-282. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz-Moreno JA, Fumaz CR, Ferrer MJ, et al. SERAD Validation Team Assessing self-reported adherence to HIV therapy by questionnaire: the SERAD (Self-Reported Adherence) Study. AIDS Res Hum Retroviruses. 2007;23(10):1166–1175. doi: 10.1089/aid.2006.0120. [DOI] [PubMed] [Google Scholar]
- 8.Ortego C, Huedo-Medina TB, Vejo J, Llorca FJ. Adherence to highly active antiretroviral therapy in Spain. A meta-analysis. Gac Sanit. 2011;25(4):282–289. doi: 10.1016/j.gaceta.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Barroso PF, Schechter M, Gupta P, Bressan C, Bomfim A, Harrison LH. Adherence to antiretroviral therapy and persistence of HIV RNA in semen. J Acquir Immune Defic Syndr. 2003;32(4):435–440. doi: 10.1097/00126334-200304010-00014. [DOI] [PubMed] [Google Scholar]
- 10.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269–277. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- 11.Murri R, Ammassari A, Gallicano K, et al. Patient-reported nonadherence to HAART is related to protease inhibitor levels. J Acquir Immune Defic Syndr. 2000;24(2):123–128. doi: 10.1097/00126334-200006010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Pinheiro CA, de-Carvalho-Leite JC, Drachler ML, Silveira VL. Factors associated with adherence to antiretroviral therapy in HIV/AIDS patients: a cross-sectional study in Southern Brazil. Braz J Med Biol Res. 2002;35(10):1173–1181. doi: 10.1590/s0100-879x2002001000010. [DOI] [PubMed] [Google Scholar]
- 13.Knobel H, Alonso J, Casado JL, et al. GEEMA Study Group Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study. AIDS. 2002;16(4):605–613. doi: 10.1097/00002030-200203080-00012. [DOI] [PubMed] [Google Scholar]
- 14.Knobel H. Cómo y por qué debe monitorizarse la adherencia al tratamiento antirretroviral en la actualidad [How and why should adherence to antiretroviral therapy] Enferm Infecc Microbiol Clin. 2002;20(10):481–483. doi: 10.1016/s0213-005x(02)72849-8. Spanish. [DOI] [PubMed] [Google Scholar]
- 15.Colombo S, Guignard N, Marzolini C, Telenti A, Biollaz J, Decosterd LA. Determination of the new HIV-protease inhibitor atazanavir by liquid chromatography after solid-phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;810(1):25–34. doi: 10.1016/j.jchromb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Hirano A, Takahashi M, Kinoshita E, et al. High performance liquid chromatography using UV detection for the simultaneous quantification of the new non-nucleoside reverse transcriptase inhibitor etravirine (TMC-125), and 4 protease inhibitors in human plasma. Biol Pharm Bull. 2010;33(8):1426–1429. doi: 10.1248/bpb.33.1426. [DOI] [PubMed] [Google Scholar]
- 17.La Porte CJL, Back DJ, Blaschke T, et al. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Reviews in Antiviral Therapy. 2006;3:4–14. [Google Scholar]
- 18.Higgins N, Tseng A, Sheehan NL, la Porte CJ. Antiretroviral therapeutic drug monitoring in Canada: current status and recommendations for clinical practice. Can J Hosp Pharm. 2009;62(6):500–509. doi: 10.4212/cjhp.v62i6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitahata MM, Reed SD, Dillingham PW, et al. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. Int J STD AIDS. 2004;15(12):803–810. doi: 10.1258/0956462042563666. [DOI] [PubMed] [Google Scholar]
- 20.Bisson GP, Gross R, Bellamy S, et al. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5(5):e109. doi: 10.1371/journal.pmed.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danel C, Gabillard D, Inwoley A, et al. Medium-term probability of success of antiretroviral treatment after early warning signs of treatment failure in West African adults. AIDS Res Hum Retroviruses. 2009;25(8):783–793. doi: 10.1089/aid.2009.0018. [DOI] [PubMed] [Google Scholar]
- 22.Aymard G, Legrand M, Trichereau N, Diquet B. Determination of twelve antiretroviral agents in human plasma sample using reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2000;744(2):227–240. doi: 10.1016/s0378-4347(00)00225-5. [DOI] [PubMed] [Google Scholar]
- 23.Duong M, Piroth L, Peytavin G, et al. Value of patient self-report and plasma human immunodeficiency virus protease inhibitor level as markers of adherence to antiretroviral therapy: relationship to virologic response. Clin Infect Dis. 2001;33(3):386–392. doi: 10.1086/321876. [DOI] [PubMed] [Google Scholar]
- 24.Fairley CK, Permana A, Read TR. Long-term utility of measuring adherence by self-report compared with pharmacy record in a routine clinic setting. HIV Med. 2005;6(5):366–369. doi: 10.1111/j.1468-1293.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- 25.Oette M, Kroidl A, Göbels K, et al. Predictors of short-term success of antiretroviral therapy in HIV infection. J Antimicrob Chemother. 2006;58(1):147–153. doi: 10.1093/jac/dkl189. [DOI] [PubMed] [Google Scholar]
- 26.Lima VD, Harrigan R, Murray M, et al. Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS. 2008;22(17):2371–2380. doi: 10.1097/QAD.0b013e328315cdd3. [DOI] [PubMed] [Google Scholar]
- 27.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knobel H, Codina C, Miró JM, et al. Recomendaciones GESIDA/SEFH/PNS para mejorar la adherencia al tratamiento antirretroviral [The recommendations of GESIDA/SEFH/PNS for improving adherence to antiretroviral treatment. AIDS Study Group of the Spanish Society of Hospital Pharmacy and the National Plan on AIDS of the Minister of Health and Consumers] Enferm Infecc Microbiol Clin. 2000;18(1):27–39. Spanish. [PubMed] [Google Scholar]
- 29.Panel de expertos de Secretaría del Plan Nacional sobre el Sida (SPNS) Sociedad Española de Farmacia Hospitalaria (SEFH) Grupo de Estudio del Sida (GESIDA) Mejorar la adherencia al tratamiento antirretroviral. Recomendaciones de la SPNS/SEFH/GESIDA [Improving adhesion to antiretroviral treatment] Farm Hosp. 2008;32(6):349–357. doi: 10.1016/s1130-6343(08)76284-6. Spanish. [DOI] [PubMed] [Google Scholar]
- 30.Ma A, Chen DM, Chau FM, Saberi P. Improving adherence and clinical outcomes through an HIV pharmacist’s interventions. AIDS Care. 2010;22(10):1189–1194. doi: 10.1080/09540121003668102. [DOI] [PubMed] [Google Scholar]
- 31.Frick P, Tapia K, Grant P, Novotny M, Kerzee J. The effect of a multidisciplinary program on HAART adherence. AIDS Patient Care STDS. 2006;20(7):511–524. doi: 10.1089/apc.2006.20.511. [DOI] [PubMed] [Google Scholar]
- 32.Gao X, Nau DP, Rosenbluth SA, Scott V, Woodward C. The relationship of disease severity, health beliefs and medication adherence among HIV patients. AIDS Care. 2000;12(4):387–398. doi: 10.1080/09540120050123783. [DOI] [PubMed] [Google Scholar]
- 33.Le Tiec C, Barrail A, Goujard C, Taburet AM. Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir. Clin Pharmacokinet. 2005;44(10):1035–1050. doi: 10.2165/00003088-200544100-00003. [DOI] [PubMed] [Google Scholar]
- 34.Kakuda TN, Falcon RW. Effect of food and ranitidine on saquinavir pharmacokinetics and gastric pH in healthy volunteers. Pharmacotherapy. 2006;26(8):1060–1068. doi: 10.1592/phco.26.8.1060. [DOI] [PubMed] [Google Scholar]
- 35.Ribaudo HJ, Liu H, Schwab M, et al. Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS Clinical Trials Group study. J Infect Dis. 2010;202(5):717–722. doi: 10.1086/655470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubomirov R, di Iulio J, Fayet A, et al. Swiss HIV Cohort Study ADME pharmacogenetics: investigation of the pharmacokinetics of the anti-retroviral agent lopinavir coformulated with ritonavir. Pharmacogenet Genomics. 2010;20(4):217–230. doi: 10.1097/FPC.0b013e328336eee4. [DOI] [PubMed] [Google Scholar]
- 37.Holstad MM, Foster V, Diiorio C, McCarty F, Teplinskiy I. An examination of the psychometric properties of the Antiretroviral General Adherence Scale (AGAS) in two samples of HIV-infected individuals. J Assoc Nurses AIDS Care. 2010;21(2):162–172. doi: 10.1016/j.jana.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ventura-Cerdá JM, Mínguez-Gallego C, Fernández-Villalba EM, Alós-Almiñana M, Andrés-Soler J. Escala simplificada para detectar problemas de adherencia (ESPA) al tratamiento antirretroviral [Simplified scale for medication adherence related problems in anti-retroviral therapy] Farm Hosp. 2006;30(3):171–176. doi: 10.1016/s1130-6343(06)73968-x. Spanish. [DOI] [PubMed] [Google Scholar]
- 39.Pearson CR, Simoni JM, Hoff P, Kurth AE, Martin DP. Assessing anti-retroviral adherence via electronic drug monitoring and self-report: an examination of key methodological issues. AIDS Behav. 2007;11(2):161–173. doi: 10.1007/s10461-006-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33(8):1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murri R, Antinori A, Ammassari A, et al. Physician estimates of adherence and the patient-physician relationship as a setting to improve adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S158–S162. doi: 10.1097/00126334-200212153-00015. [DOI] [PubMed] [Google Scholar]
- 42.Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Intern Med. 2004;19(11):1096–1103. doi: 10.1111/j.1525-1497.2004.30418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials. 2008;9(4):238–246. doi: 10.1310/hct0904-238. [DOI] [PubMed] [Google Scholar]
- 44.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farley J, Hines S, Musk A, Ferrus S, Tepper V. Assessment of adherence to antiviral therapy in HIV-infected children using the Medication Event Monitoring System, pharmacy refill, provider assessment, caregiver self-report, and appointment keeping. J Acquir Immune Defic Syndr. 2003;33(2):211–218. doi: 10.1097/00126334-200306010-00016. [DOI] [PubMed] [Google Scholar]
- 46.Abaasa AM, Todd J, Ekoru K, et al. Good adherence to HAART and improved survival in a community HIV/AIDS treatment and care programme: the experience of The AIDS Support Organization (TASO), Kampala, Uganda. BMC Health Serv Res. 2008;8:241. doi: 10.1186/1472-6963-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chalker JC, Andualem T, Gitau LN, et al. INRUD-IAA Measuring adherence to antiretroviral treatment in resource-poor settings: the feasibility of collecting routine data for key indicators. BMC Health Serv Res. 2010;10:43. doi: 10.1186/1472-6963-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holstad MM, DiIorio C, Kelley ME, Resnicow K, Sharma S. Group motivational interviewing to promote adherence to antiretroviral medications and risk reduction behaviors in HIV infected women. AIDS Behav. 2011;15(5):885–896. doi: 10.1007/s10461-010-9865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Codina Jané C, Tuset Creus M, Ibarra Barrueta O, et al. Grupo VIH de la SEFH Evaluación de un programa de atención farmacéutica dirigido a mejorar la adherencia al tratamiento antirretroviral [Evaluation of a pharmaceutical care program to improve adherence to antiretroviral therapy] Farm Hosp. 2004;28(6 Suppl 1):19–26. Spanish. [PubMed] [Google Scholar]
- 50.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rueda S, Park-Wyllie LY, Bayoumi AM, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006;3:CD001442. doi: 10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berg KM, Litwin AH, Li X, Heo M, Arnsten JH. Lack of sustained improvement in adherence or viral load following a directly observed antiretroviral therapy intervention. Clin Infect Dis. 2011;53(9):936–943. doi: 10.1093/cid/cir537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quiros-Roldan E, Torti C, Lapadula G, et al. RADAR-Master Study Group Adherence and plasma drug concentrations are predictors of confirmed virologic response after 24-week salvage highly active antiretroviral therapy. AIDS Patient Care STDS. 2007;21(2):92–99. doi: 10.1089/apc.2005.0037. [DOI] [PubMed] [Google Scholar]
- 54.Ford N, Darder M, Spelman T, Maclean E, Mills E, Boulle A. Early adherence to antiretroviral medication as a predictor of long-term HIV virological suppression: five-year follow up of an observational cohort. PLoS ONE. 2010;5(5):e10460. doi: 10.1371/journal.pone.0010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cocohoba JM, Althoff KN, Cohen M, et al. Pharmacist counseling in a cohort of women with HIV and women at risk for HIV. Patient Prefer Adherence. 2012;6:457–463. doi: 10.2147/PPA.S30797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooperman NA, Heo M, Berg KM, et al. Impact of adherence counseling dose on antiretroviral adherence and HIV viral load among HIV-infected methadone maintained drug users. AIDS Care. 2012;24(7):828–835. doi: 10.1080/09540121.2011.644231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Panel de expertos de GESIDA y Plan Nacional sobre el Sida Documento de consenso de GESIDA/Plan Nacional sobre el Sida respecto al tratamiento antirretroviral en adultos infectados por el virus de la inmunodeficiencia humana (actualización enero 2011) [National consensus document by GESIDA/National Aids Plan on antiretroviral treatment in adults infected by the human immunodeficiency virus (January 2011 update)] Enferm Infecc Microbiol Clin. 2011;29(3):209. doi: 10.1016/j.eimc.2010.12.004. Spanish. [DOI] [PubMed] [Google Scholar]
- 58.Clumeck N, Pozniak A, Raff F, EACS Executive Committee . HIV Med. 2. Vol. 9. European AIDS Clinical Society; 2008. (EACS) guidelines for the clinical management and treatment of HIV-infected adults; pp. 65–71. [DOI] [PubMed] [Google Scholar]
- 59.Aidsinfonihgov [homepage on the Internet] Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents Washington, DC: Department of Health and Human Services; [updated February 12, 2013]. Available from: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdfAccessed April 12, 2013 [Google Scholar]
- 60.Johnson MO, Charlebois E, Morin SF, Remien RH, Chesney MA, National Institute of Mental Health Healthy Living Project Team Effects of a behavioral intervention on antiretroviral medication adherence among people living with HIV: the healthy living project randomized controlled study. J Acquir Immune Defic Syndr. 2007;46(5):574–580. doi: 10.1097/qai.0b013e318158a474. [DOI] [PMC free article] [PubMed] [Google Scholar]