Abstract
A novel antigen that induces cross-reactive bactericidal antibodies against a number of Neisseria meningitidis strains is described. This antigen, a ∼28-kDa lipoprotein called LP2086, was first observed within a complex mixture of soluble outer membrane proteins (sOMPs) following a series of fractionation, protein purification, and proteomics steps. Approximately 95 different neisserial isolates tested positive by Western blotting and PCR screening methods for the presence of the protein and the gene encoding LP2086. The strains tested included isolates of N. meningitidis serogroups A, B, C, W135, and Y, Neisseria gonorrhoeae, and Neisseria lactamica. To better understand the microheterogeneity of this protein, the 2086 genes from 63 neisserial isolates were sequenced. Two different subfamilies of LP2086 were identified based on deduced amino acid sequence homology. A high degree of amino acid sequence similarity exists within each 2086 subfamily. The highest degree of genetic diversity was seen between the two subfamilies which share approximately 60 to 75% homology at the nucleic acid level. Flow cytometry (fluorescence-activated cell sorting) analyses and electron microscopy indicated that the LP2086 is localized on the outer surface of N. meningitidis. Antiserum produced against a single protein variant was capable of eliciting bactericidal activity against strains expressing different serosubtype antigens. Combining one recombinant lipidated 2086 (rLP2086) variant from each subfamily with two rPorA variants elicited bactericidal activity against all strains tested. The rLP2086 family of antigens are candidates worthy of further vaccine development.
Neisseria meningitidis is an obligate human pathogen that inhabits the upper respiratory tract, from which it occasionally disseminates, causing disease. Invasion results in bacteremia, with possible progression to sepsis, meningitis, and death. Two distinct age groups show peak incidence of disease: children less than 2 years of age and older teenagers. Even in apparently healthy individuals, the onset of flu-like symptoms can rapidly progress into a life-threatening condition. In a meningococcal epidemic, death rates can approach 10 to 15% (39). Serogroup A and C epidemics often last 1 to 3 years, while serogroup B epidemics last 5 to 10 years (43, 45). During epidemics, attack rates increase and the age distribution broadens, particularly in the 4- to 19-year age group (8). While early diagnosis and antibiotic treatment greatly enhance survival, prevention through vaccination would appear the best way to limit meningococcal disease (17, 31, 45). Vaccines based on capsular polysaccharide have been developed against serogroups A, C, Y, and W135. Unfortunately, these vaccines are not effective in children under 2 years of age. Polysaccharide conjugated to a carrier protein is expected to enhance efficacy, particularly in younger children, as exemplified by the meningococcal serogroup C glycoconjugate vaccines (9, 21) recently licensed in Europe and Canada. A dramatic reduction in serogroup C disease has occurred since these vaccines were introduced (32). Conjugate vaccines for serogroups A, Y, and W135 are currently in development and likely will prove similarly effective. However, developing a glycoconjugate vaccine against serogroup B disease is challenging, because the polysialic acid polysaccharide expressed by N. meningitidis serogroup B is poorly immunogenic in humans. Furthermore, its α-2,8-linked N-acetylneuraminic acid (polysialic acid) has structural similarity to human neural antigens (10, 15), raising potential safety concerns should N. meningitidis serogroup B glycoconjugate vaccines be used. These concerns have prompted examination of noncapsular vaccine approaches. Serogroup B outer membrane proteins (OMPs) in complexes and in vesicles have been developed as alternative vaccine antigens (23, 26, 40). One example of an OMP vaccine approach targets the serosubtype antigen PorA protein. Given PorA protein variability, a multivalent vaccine consisting of five or more serosubtypes will be needed to obtain >50% coverage against potential serogroup B disease in the United States (33, 42). Alternatively, highly conserved N. meningitidis serogroup B antigens have been sought and evaluated as vaccine candidates. Neisserial surface protein A (NspA) is a highly conserved membrane protein which elicits serum bactericidal antibodies that confer passive protection in animal models (22). Differences in surface expression of NspA, however, may limit anti-NspA complement-mediated bacteriolysis (27) of some N. meningitidis serogroup B strains. Genome-derived antigen, GNA33, a highly conserved lipoprotein with similarity to transglycosylase A from Escherichia coli (30), has been shown to elicit bactericidal antibody responses. Unfortunately, these appear to be the result of cross-reactivity with a variable loop of PorA (12). NadA, another novel surface antigen of N. meningitidis serogroup B, also has been evaluated as a vaccine candidate. Although NadA induces strong bactericidal antibodies and is protective in an infant rat model, the encoding gene is present in only 50% of N. meningitidis serogroup B isolates (7). Obviously, identification of an antigen that is present in every N. meningitidis serogroup B strain and that elicits broader cross-protection against multiple serosubtypes is a highly desirable goal for serogroup B vaccine development.
Here, we report the identification of a neisserial outer membrane lipoprotein found in all N. meningitidis serogroup B strains tested. We describe the cloning, expression, and purification of recombinant lipoprotein LP2086 (rLP2086). A gene encoding one variant of LP2086 was identified in our analysis of the Sanger Institute N. meningitidis serogroup A Z2491 early release of genomic sequence in contig form. Recently, Masignani et al. reported similar findings with their genome-derived neisserial antigen, GNA1870 (24). We now show the existence of two distinct subfamilies of LP2086 based on amino acid sequence diversity derived from sequencing the LP2086 genes from 63 neisserial isolates. We demonstrate the utility of recombinant forms of this protein as immunogens which elicit antibodies capable of inducing bactericidal activity against many N. meningitidis strains expressing different serosubtype antigens. Recombinant LP2086 (rLP2086) antigens elicit broad cross-reactivity against multiple serosubtype strains and will simplify vaccine development for N. meningitidis serogroup B disease.
(A portion of our work on the discovery of this novel N. meningitidis vaccine candidate was presented at the International Pathogenic Neisseria Conference held in Oslo, Norway, in September 2002 [4, 11].)
MATERIALS AND METHODS
Neisserial isolates.
The 95 neisserial strains described in this study were kindly provided by a number of investigators and were isolated from at least five different countries (United Kingdom, United States, Chile, The Netherlands, and Norway) between the 1960s and 1990s. All isolates have been stored as frozen glycerol stocks at −70°C. The strains were obtained from the following seven institutions as indicated in Table 1: Manchester Public Health Laboratory (MPHL), Manchester, United Kingdom; RIVM, Bilthoven, The Netherlands; University of Iowa, Iowa City, Iowa; Walter Reed Army Institute of Research, Washington, D.C.; Centers for Disease Control and Prevention (CDC), Atlanta, Ga.; University of North Carolina at Chapel Hill; and University of Rochester, Rochester, N.Y.
TABLE 1.
Description and origins of Neisserial strains used in this studya
| Strain | Serogroup type:subtype | 2086 subfamily | Source | Location (country) | 2086 expression on Western blot | Phylogenetic cluster |
|---|---|---|---|---|---|---|
| M98 250670 | B:1:P1.4 | B | MPHL | UK | + | B8 |
| M98 250024 | B:1:P1.4 | B | MPHL | UK | + | B5 |
| M97 253524 | B:1:P1.4 | B | MPHL | UK | + | B8 |
| M97 252060 | B:1:P1.4 | B | MPHL | UK | + | B5 |
| M97 251870 | B:4z:P1.4 | B | MPHL | UK | + | B5 |
| M97 251854 | B:4z:P1.4 | A | MPHL | UK | + | A1 |
| M97 251836 | B:4z:P1.4 | B | MPHL | UK | + | B5 |
| M97 251830 | B:4z:P1.4 | B | MPHL | UK | + | B5 |
| M97 251905 | B:4z:P1.4 | B | MPHL | UK | + | B5 |
| M97 251898 | B:4z:P1.4 | B | MPHL | UK | + | B5 |
| M97 251885 | B:4z:P1.4 | B | MPHL | UK | + | B8 |
| M97 251876 | B:4z:P1.4 | B | MPHL | UK | + | B5 |
| M97 251994 | B:4z:P1.4 | B | MPHL | UK | + | B5 |
| M97 251985 | B:4z:P1.4 | B | MPHL | UK | + | B5 |
| M97 251957 | B:4z:P1.4 | B | MPHL | UK | + | B5 |
| M97 251926 | B:4z:P1.4 | B | MPHL | UK | + | B8 |
| M97 252045 | B:4z:P1.4 | B | MPHL | UK | + | ND |
| M97 252038 | B:4z:P1.4 | B | MPHL | UK | + | ND |
| M97 252026 | B:4z:P1.4 | B | MPHL | UK | + | ND |
| M97 252010 | B:4z:P1.4 | B | MPHL | UK | + | ND |
| M97 252098 | B:4z:P1.4 | B | MPHL | UK | + | ND |
| M97 252083 | B:4z:P1.4 | B | MPHL | UK | + | ND |
| M97 252078 | B:4z:P1.4 | B | MPHL | UK | + | ND |
| M98 250735 | B:4z:P1.15 | B | MPHL | UK | + | ND |
| M98 250797 | B:4z:P1.15 | B | MPHL | UK | + | ND |
| M98 250768 | B:4z:P1.15 | B | MPHL | UK | + | ND |
| M98 250622 | B:2b:P1.10 | A | MPHL | UK | + | A1 |
| M98 250572 | B:2b:P1.10 | A | MPHL | UK | + | A1 |
| M98 250716 | B:2b:P1.10 | A | MPHL | UK | + | A1 |
| M98 250699 | B:4z:P1.10 | B | MPHL | UK | + | ND |
| M98 250393 | B:4z:P1.10 | B | MPHL | UK | + | ND |
| M98 250173 | B:4z:P1.10 | B | MPHL | UK | + | ND |
| M98 250771 | B:4z:P1.22,14 | A | MPHL | UK | + | A12 |
| M98 250732 | B:4z:P1.22,14-1 | A | MPHL | UK | + | A12 |
| CDC-1343 | B:NT:P1.22,14 | B | CDC | US | + | B1 |
| CDC-2369 | B:NT:P1.22-1,14-1 | A | CDC | US | + | A7 |
| 6557 | B:7:P1.22-1,14 | A | RIVM | US | + | A3 |
| M97 253462 | B:4z:P1.14 | B | MPHL | UK | + | ND |
| CDC-1127 | B:NT:P1.7,16 | B | CDC | US | + | ND |
| CDC-982 | B4:P1.7,16 | B | CDC | US | + | ND |
| CDC-1359 | B4:P1.7,16 | B | CDC | US | + | ND |
| M98 250762 | B:15:P1.7,16 | B | MPHL | UK | + | ND |
| M98 250610 | B:15:P1.7,16 | B | MPHL | UK | + | ND |
| M98 250626 | B:15:P1.16 | B | MPHL | UK | + | ND |
| M98 250809 | B:15:P1.7,16 | A | MPHL | UK | + | A2 |
| M97 253248 | B:15:P1.7,16 | A | MPHL | UK | + | A2 |
| M97 252029 | B:15:P1.7,16 | B | MPHL | UK | + | ND |
| M97 251875 | B:15:P1.7,16-10 | B | MPHL | UK | + | ND |
| CDC-798 | B:15:P1.7,16 | B | CDC | US | + | ND |
| CDC-1078 | B:15:P1.7,16 | B | CDC | US | + | ND |
| CDC-1614 | B:15:P1.7,16 | B | CDC | US | + | ND |
| CDC-1658 | B:15:P1.7,16 | B | CDC | US | + | ND |
| CDC-937 | B:15:P1.7,16 | B | CDC | US | + | B4 |
| H44/76 | B:15:P1.7,16 | B | RIVM | Norway | + | B4 |
| M97 250571 | B:15:P1.16 | B | MPHL | UK | + | B4 |
| M97 252097 | B:15:P1.16 | B | MPHL | UK | + | B4 |
| M97 253092 | B:1:P1.6 | B | MPHL | UK | + | ND |
| M97 252697 | B:1:P1.18,25,6 | A | MPHL | UK | + | A3 |
| M97 252988 | B:4:P1.18,25,6 | A | MPHL | UK | + | A3 |
| M97 252976 | B:4:P1.18,25,6 | A | MPHL | UK | + | A1 |
| M97 252153 | B:4:P1.18,25,6 | A | MPHL | UK | + | A11 |
| 6940 | B:NT:P1.18,25,6 | B | RIVM | US | + | B2 |
| CDC-1610 | B:4:P1.18-7,16-4 | A | CDC | US | + | A3 |
| CDC-1521 | B:2b:P1.6 | A | CDC | US | + | A1 |
| CDC-1985 | B:4:P1.7,13 | B | CDC | US | + | ND |
| CDC-1034 | B:4:P1.7 | A | CDC | US | + | A5 |
| L6 M992 | B:NT:P1.7,1 | ND | Walter Reed | + | ND/PICK> | |
| L8 M978 | B:15:P1.7,1 | A | Walter Reed | + | A3 | |
| CDC-1492 | B:4:P1.7,1 | A | CDC | US | + | A3 |
| CDC-1573 | B:4:P1.7-1,1 | B | CDC | US | + | B9 |
| L7 6155 | B:4:P1.7,1 | B | Walter Reed | + | ND | |
| 8529 | B:15:P1.7-2,3 | B | RIVM | Chile | + | B4 |
| 880049 | B:4:P1.7-2,4 | B | RIVM | Netherlands | + | B8 |
| 870446 | B:4:P1.12-1,13 | A | RIVM | Netherlands | + | A2 |
| CDC-2367 | B:4:P1.15 | B | CDC | US | + | B4 |
| H355 | B:15:P1.19,15 | B | RIVM | Norway | + | B4 |
| M982 | B:9:P1.22,9 | B | RIVM | US | + | B6 |
| 870227 | B:4:P1.5-3,10 | B | RIVM | Netherlands | + | B4 |
| B40 | A:NT:P1.5-3,10 | B | RIVM | + | B5 | |
| 5315 | B:4:P1.5-3,10 | B | RIVM | + | B5 | |
| 2996 | B:2b:P1.5-1,2-2 | A | RIVM | US | + | A1 |
| NMB | B:2b:P1.5-1,2-2 | A | UIOWA | US | + | A1 |
| CDC-983 | B:2a:P1.5,2 | B | CDC | US | + | B3 |
| L3 6275 | B:2a:P1.5,2 | A | Walter Reed | + | A8 | |
| CDC-852 | B:NT:P1.5,2 | B | CDC | US | + | B7 |
| B16B6 | B:2a:P1.5,2 | A | RIVM | + | A4 | |
| L4 891 | C:NT:P1.21,16 | A | Walter Reed | + | A6 | |
| L5 M981 | B:4:P1.21-6,1 | A | Walter Reed | + | A1 | |
| CDC-1135 | B:NT | A | CDC | US | + | A9 |
| A4 | A:NT | B | + | B5 | ||
| C11 | C:16,P1.7-1,1 | A | CDC | Germany | + | A4 |
| Y | Y: | A | Walter Reed | + | A4 | |
| W135 | W135: | A | Walter Reed | + | A4 | |
| Ng-FA1090 | A | UNC | US | + | A10 | |
| Nl-UR5 | A | UR | + | ND | ||
| Ns-UR4 | — | UR | − | ND |
Strains are arbitrarily arranged by PorA serosubtype. The 2086 gene subfamily was determined by PCR screening as described in Materials and Methods. A dash indicates negative results; ND indicates not done. Country or origin of each neisserial isolate is listed where known. UK, United Kingdom; US, United States. The 2086 gene was sequenced from those strains where a phylogenetic cluster number based on amino acid sequence homology is indicated (see Fig. 1).
Genomic analysis.
The contig genomic sequence of N. meningitidis serogroup A strain Z2491 (http://www.sanger.ac.uk/) was analyzed for open reading frames (ORFs) using both GLIMMER (34) and proprietary software. An ORF was defined as having one of three potential start site codons, ATG, GTG, or TTG, one of three potential stop codons, TAA, TAG, or TGA, and a minimum of 74 amino acids. The predicted protein sequences were further analyzed using BLAST, Pfam, TopPred, Psort, and SignalP software to help with putative annotation, function, and localization (45). Proprietary hidden Markov models were used to predict lipoproteins and OMPs. BLAST analysis of the 2086 gene against available sequence databases found no homologs outside the genus Neisseria.
Amplification of the 2086 gene from neisserial isolates.
Initial identification of the full-length ∼860-bp ORF 2086 from N. meningitidis serogroup B strain 8529 relied on PCR using the primer pair 2086NDE and 3STP2086 (Table 2 describes all primer sequences), based on the Sanger Institute N. meningitidis serogroup A Z2491 genomic sequence (29) and primary internal amino acid sequence data from a protein isolated from strain 8529 (4). Thereafter, an approximately 900-bp section of the genome including the 2086 gene and immediate flanking regions was amplified from a variety of N. meningitidis serogroup B strains by using a pair of primers, 5UNI2086 and 3UNI2086
TABLE 2.
Primers used in PCR amplification experiments
| Primer name | Sequence | Restriction site (underlined) |
|---|---|---|
| 2086NDE | 5′-CTATTCTGCATATGACTAGGAGC-3′ | NdeI |
| 3STP2086 | 5′-GCGCGGATCCTTACTGCTTGGCGGCAAGACC-3′ | BamHI |
| 5UNI2086 | 5′-CTATTCTGCGTATGACTAG-3′ | NAa |
| 3UNI2086 | 5′-GTCCGAACGGTAAATTATCGTG-3′ | NA |
| 5INT2086 | 5′-TGCCGATGCACTAACCGCACC-3′ | NA |
| 3INT2086 | 5′-CGTTTCGCAACCATCTTCCCG-3′ | NA |
| 5OUT2086 | 5′-GACAGCCTGATAAACC-3′ | NA |
| 3OUT2086 | 5′-GATGCCGATTTCGTGAAC-3′ | NA |
| N2086ILE | 5′-GCGCAGATCTCATATGAGCAGCGGAGGGGGTGGTGTCGCCGCCGAYATWGGTGCGGGGCTTGCCG-3′ | BglII/NdeI |
| 20863STP | 5′-GAGATCTCACTCACTCATTACTGCTTGGCGGCAAGACCGATATG-3′ | BglII |
| N1573PCR | 5′-GAGATCTCATATGAGCAGCGGAGGCGGCGGAAGC-3′ | BglII |
| N29963STP | 5′-GAGATCTCACTCACTCACTACTGTTTGCCGGCGATGCCGATTTC-3′ | BglII |
| 5LIP2086 | 5′-GCGGATCCAGCGGAGGGGGTGGTGTCGCC-3′ | BamHI |
| 3SPH2086 | 5′-GCGCATGCTTACTGCTTGGCGGCAAGACCGATATG-3′ | SphI |
| 5LIP1573 | 5′-GCGGATCCAGCGGAGGCGGCGGAAGC-3′ | BamHI |
| 5LIP2996 | 5′-GCGGATCCAGCGGAGGCGGCGGTGTCGCC-3′ | BamHI |
| N2996SPH | 5′-GCGCATGCCTACTGTTTGCCGGCGATG-3′ | SphI |
| 5LIP771 | 5′-GCGGATCCAGCGGAAGCGGAAGCGGAGGCGGCGGTGTCGCC-3′ | BamHI |
| PorABg12Fwd | 5′-CGCGAGATCTCATATGGATGTCAGCCTATACGGCGAAATCAAAGCCGGCGTGGAAGGCAGGAACTACCAG-3′ | BglII/NdeI |
| PorABg12Ter | 5′-CGCGAGATCTTCAGTCACTCATTAGAATTTGTGGCGCAAACCGACG-3′ | BglII |
NA, not applicable.
These primers were based on regions of homology identified in alignments of intergenic regions directly flanking the 2086 gene homologs found in the Sanger Institute N. meningitidis serogroup A Z2491 genome and The Institute for Genomic Research N. meningitidis serogroup B MC58 genomic sequence (41). Oligonucleotide primers 5INT2086 and 3INT2086 designed to be specific for the subfamily B type 2086 gene amplified a 333-bp internal fragment of the 2086 gene. Oligonucleotide primers 5OUT2086 and 3OUT2086 designed to be specific for the subfamily A type 2086 gene amplified a 393-bp internal fragment of the 2086 gene. Amplification reactions were performed in volumes of 20 or 50 μl containing 18 or 45 μl of ReddyMix PCR Master mix (ABgene House, Surrey, United Kingdom), a 1 μM concentration of each primer, and a stab of frozen cells. Reactions were performed in a GeneAmp PCR system 2400 (Applied Biosystems, Foster City, Calif.) with a hot start of 95°C for 5 min and then 30 cycles of denaturation at 95°C for 50 s, annealing at 55°C for 50 s, and extension at 72°C for 50 s. A final extension step was carried out at 72°C for 7 min.
Cloning, sequence analysis, and phylogenetic analysis of 2086 genes.
The amplified PCR products were cloned into the pCR2.1-TOPO vector (Invitrogen Co., Carlsbad, Calif.). DNA sequencing was carried out using BigDye terminator chemistry with the specifically designed primers on an Applied Biosystems 377 automated DNA sequencer (Applied Biosystems). Sequencher 4.0.5 software (Gene Codes Corp., Ann Arbor, Mich.) was used to analyze the DNA sequences. The DNA sequences of the 2086 genes were translated into amino acid sequences using the LASERGENE software (DNAStar, Inc., Madison, Wis.). Each deduced amino acid sequence was aligned using ClustalW (16) within the DNAStar MegAlign suite of tools. The phylogenetic trees were constructed using neighbor joining (35), the unweighted pair group method with arithmetic means analysis (38), and ClustalW within DNAStar MegAlign.
Retrieved sequences.
Homologs of the 2086 gene were identified within the available sequenced neisserial genomes and are listed with strain name, ORF designation, and database accession numbers as follows: strain Z2491, ORF NMA0586, accession no. AL162753; strain MC58, ORF NMB1870, no. AE002537; FA1090, University of Oklahoma unpublished designation [http://dna1.chem.ou.edu/gono.html].
Amplification, cloning, and expression of nonlipidated protein 2086 (rP2086) in E. coli.
The 2086 gene from strain 8529 was PCR amplified as described above using AmpliTaq (Applied Biosystems) and primers N2086ILE and 20863STP (Table 2). For strain CDC-1573, primers N1573PCR and 20863STP were used. For strain 2996, primers N2086ILE and 29963STP were used. Features of these primers include a synthetic BglII restriction site in each and a synthetic NdeI restriction site in N2086ILE and N1573PCR. Termination codons in all three reading frames are present in 20863STP and 29963STP. Primers N2086ILE and N1573PCR amplify the 2086 gene with an ATG (Met) fused to the second amino-terminal codon (ACG), representing a single amino acid substitution (replaces TGC Cys) of the mature 2086 polypeptide. BglII-cleaved 2086 amplified gene fragments were cloned into the BamHI site of vector pET9a (Novagen, Madison, Wis.). This configuration results in a T7-tag fusion to the amino terminus of rP2086. Deletion of the NdeI fragment removes the T7-tag DNA sequences and links the 2086 gene directly to the ATG start codon provided by the pET9a vector. rP2086 lacking the T7 fusion was expressed in E. coli strain BLR(DE3)pLysS (Novagen) grown in HySoy broth (Sheffield Products, Norwich, N.Y.) supplemented with 1% sterile glucose and 50 μg of kanamycin (Sigma, St. Louis, Mo.)/ml. The cell lines containing the rP2086 genes were grown to mid-log phase and induced to express the recombinant proteins by adding isopropyl β-d-thiogalactopyranoside (IPTG) to a 1 mM concentration and grown for an additional 3 h.
Amplification, cloning, and expression of rLP2086 in E. coli.
The 2086 gene from each of nine different N. meningitidis strains was cloned behind the P4 lipoprotein signal sequence of nontypeable Haemophilus influenzae (13). Amplification reactions were performed as described above using ReddyMix PCR Master mix (ABgene House). Four subfamily B 2086 genes were amplified from the genomes of 8529, M982, 880049, and CDC-1573 strains. Oligonucleotides used for the amplification (Table 2) were as follows: for strains 8529, M982, and 880049, 5LIP2086 and 3SPH2086 were used; for strain CDC-1573, 5LIP1573 and 3SPH2086 were used. Five subfamily A 2086 genes were amplified from the genomes of 2996, M97 252988, C11, 870446, and M98 250771 strains. Oligonucleotides used for the amplification of 2086 genes from strains 2996, M97 252988, C11, and 870446 were 5LIP2996 and N2996SPH; for strain M98 250771 the oligonucleotides were 5LIP771 and N2996SPH. The restriction site BamHI was incorporated into the 5′ end of each NH2-terminal primer and resulted in the insertion of a glycine residue in the mature protein at amino acid position +2. The reverse primers were designed to be homologous to the COOH-terminal end of the 2086 gene and included the stop codon as well as an SphI site for cloning purposes. Each amplified fragment was cloned into a pBAD18-Cm vector (14) modified to contain the P4 leader sequence (M2pLP339) using the BamHI and SphI restriction sites (all restriction enzymes were supplied by New England Biolabs, Inc., Beverly, Mass.).
rLP2086 was expressed in E. coli strain BLR (Novagen) grown in HySoy broth supplemented with 1% sterile glucose and 30 μg of chloramphenicol (Sigma)/ml. The cell lines containing the rLP2086 genes were grown to mid-log phase and induced to express the recombinant proteins by adding arabinose (Sigma) to a 0.2% concentration and grown for an additional 3 h.
rPorA expression.
The mature PorA gene sequences from meningococcal strains 6557 and NMB were PCR amplified using primers PorABgl2Fwd and PorABlg2Ter. The BglII and NdeI sites are shown in Table 2. Amplified products were cloned directly into the pCR2.1-TOPO vector (Invitrogen). A BglII fragment containing the porA gene was cleaved by restriction endonuclease digestion and cloned into the BamHI site of the pET9a (Novagen) vector. Deletion of the NdeI fragment was performed as described above. The recombinant plasmids were introduced into the E. coli expression strain BLR(DE3)pLysS (Novagen) by transformation. The T7 expression system was then used to overexpress the mature form of rPorA. The BLR(DE3)/pET9a strains were grown overnight at 37°C in HySoy broth supplemented with 30 μg of kanamycin/ml and 2% glucose. Overnight cultures were diluted 20-fold in HySoy broth with 30 μg of kanamycin/ml and 1% glycerol and grown at 37°C for 1 h. These cultures were induced by the addition of IPTG to a final concentration of 1 mM, grown for an additional 2 to 3 h, and then harvested.
Gene 2086 knockout mutants.
The 2086 gene of N. meningitidis serogroup B strain 8529 together with 500 bp of its flanking sequences was PCR amplified. The PCR product was cloned into the pBAD TOPO vector (Invitrogen) and then cleaved at the unique PstI restriction site located at the predicted signal sequence cleavage site of the 2086 gene. A kanamycin resistance expression cassette was inserted into the PstI site, and the resulting plasmid was used to insertionally inactivate the chromosomal 2086 gene of naturally competent meningococcal strains 880049, CDC-1573, and NMB. Each mixture was spread on a GC agar plate (Difco Laboratories, Detroit, Mich.) containing 50 μg of kanamycin/ml and incubated at 36°C in 5%CO2 overnight. Potential knockout strains were identified by failure to detect LP2086 expression using subfamily-specific polyclonal antibody in Western blot analysis of whole-cell lysates and by fluorescence-activated cell sorter (FACS) analysis.
Purification of rLP2086 and rP2086 variants.
Frozen pellets of E. coli BLR cells expressing rLP2086 or rP2086 variants were generally suspended in 10 mM HEPES-NaOH (pH 7.4), 1 mM Na2EDTA containing 1 μg of Pefabloc SC (Roche Diagnostics)/ml and lysed by passage through a 110Y microfluidizer (Microfluidics Corp.) equipped with a ceramic disruption chamber at 18,000 lb/in2. The cell lysate was centrifuged at 150,000 to 300,000 × g for 1 h. The pellet was washed and centrifuged twice with the same buffer and frozen overnight. The membrane pellet was extracted with 10 mM HEPES-NaOH (pH 7.4), 1 to 5 mM MgCl2, and 1% Triton X-100 or 1% reduced Triton X-100 for 30 min, followed by centrifugation at 150,000 × g for 30 min. This was repeated three times. All but one of the rLP2086 variants were solubilized with the Triton detergents. Only rLP2086-8529 required further extraction with Zwittergent 3-12 and 3-14 detergents (Calbiochem) prior to solubilization with 1% N-lauroylsarcosine (sodium salt; Sigma) in 50 mM Tris-HCl (pH 8), 5 mM Na2EDTA. The rLP2086 variants were purified using a combination of anion exchange (either Poros HQ [Applied Biosystems] or Fractogel EMD TMAE [Merck]) and either cation exchange (S Fractogel) and/or size exclusion chromatography (S-12; Amersham Biosciences). The combination of anion exchange with either cation exchange and/or size exclusion chromatography was individualized for each variant. The outcomes of these processes were very reproducible. The solubilized rLP2086 was adjusted to 1% Zwittergent 3-12 prior to adsorption on the anion exchange column and then eluted with a gradient of NaCl. Cation exchange chromatography was performed generally in 30 mM sodium phosphate buffer containing 1% Zwittergent 3-12, eluting with a pH gradient between 6 and 8. Minor pH variations were necessary to ensure binding to the anion and/or cation exchange columns and were related to the pI of each variant. Size exclusion chromatography utilized HEPES-NaOH or sodium phosphate buffers at pH 7.4 containing 150 mM NaCl and 1% Zwittergent 3-12. Nonlipidated rP2086 variants were located in the cytoplasmic fractions and purified essentially as the lipidated variants, except that the buffers did not contain detergents. Purity was accessed by laser densitometry following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining. Seven of the nine rLP2086 variants and two of the three rP2086 variants achieved protein purity of ≥90% by these processes.
Purification of rPorA.
The rPorA was solubilized from E. coli inclusion bodies with 50 mM Tris-HCl (pH 8.0), 5 mM Na2EDTA containing 8 M urea. The denatured inclusion body solution was adjusted to 1% Zwittergent 3-14, 250 mM NaCl and refolded by dialysis against 50 mM Tris-HCl (pH 8.0), 5 mM Na2EDTA, 250 mM NaCl, 1% Zwittergent 3-14, without urea. The refolded rPorA was concentrated by tangential flow filtration using a Pellicon XL 50-cm2 filter with 10K MWCO (Millipore, Billerica, Mass.), and the buffer was exchanged to 20 mM sodium phosphate (pH 6.0), 5 mM EDTA, 50 mM NaCl, 0.1% Zwittergent 3-14 using Sephadex G-25 (Amersham Biosciences) gel filtration. The rPorA was further purified by cation exchange chromatography in S Fractogel equilibrated in buffer F using 1 M NaCl for elution.
Mass spectral analysis by MALDI-TOF.
Mass spectrometry of rP2086 and rLP2086 was carried out on a Perseptive Biosystems Voyager DE-sSTR matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometer as described by the manufacturer. Sinapinic acid or α-cyano-4-hydroxycinnamic acid were used as matrices.
Mouse immunization.
Formulations with purified rP2086, rLP2086, and rPorA proteins contained 10 μg of each protein and 20 μg of QS-21(Antigenics, Inc., Framingham, Mass.). For all immunizations, a 0.2-ml dose was administered by subcutaneous (rump) injection to 6- to 8-week-old female Swiss-Webster mice at weeks 0 and 4. Blood was collected at weeks 0, 4, and 6.
SDS-PAGE.
Proteins were separated by SDS-PAGE using a 10-to-20% precast gel (Zaxis or PAGEr Gold; Cambrex Bioscience Inc., Rockland, Maine) and the Laemmli system (19). Proteins were then stained with Coomassie brilliant blue.
Whole-cell ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were performed as described previously (1), using an alkaline phosphatase detection system. Endpoint titers were determined as the dilution of the antiserum that resulted in an optical density of 0.1.
Bactericidal assays.
Bactericidal assays were performed as previously described (28) with human serum from individual donors as the complement source. Complement-mediated antibody-dependent bactericidal titers for the serum bactericidal assay (BC) were expressed as the reciprocal of the highest dilution of test serum that killed ≥50% of the target cells introduced into the assay mixtures (BC50 titer).
Western blot reactivity of meningococcal cell lysates with polyclonal rLP2086 antiserum.
Frozen aliquots of meningococcal strains were streaked out on GC agar plates containing 1% Kellogg's supplement and incubated at 36°C in 5% CO2 overnight. Cells were scraped from plates and transferred to 500 ml of phosphate-buffered saline (PBS). The cell suspension was then heat killed by incubation in a 65°C water bath for 45 min. Total protein was determined using the bicinchoninic acid assay (Pierce Biotechnology, Inc., Rockford, Ill.). Gradient SDS-PAGE (10 to 20%; PAGEr Gold) was performed with 30 to 40 μg of total protein of each cell lysate as described above. Western blot analysis was performed according to standard procedures (19), using either antisera pooled by subfamily or antisera homologous to the LP2086 of the neisserial strain being tested. The LP2086 subfamily A antiserum pool contained a mixture of anti-rLP2086-250771 (1/500 dilution), anti-rLP2086-870446 (1/1,000 dilution), and anti-rLP2086-2996 (1/1,000 dilution) polyclonal antibodies, while the LP2086 subfamily B antiserum pool contained a mixture of anti-rLP2086-880049 (1/500 dilution), anti-rLP2086-M982 (1/500 dilution), anti-rLP2086-8529 (1/1,000 dilution), and anti-rLP2086-1573 (1/500 dilution) polyclonal antibodies. Antibody binding was detected using a secondary antibody, alkaline phosphate-labeled goat anti-mouse (Biosource International, Camarillo, Calif.). Western blots were developed using the BCIP/NBT substrate system (Kirkegaard & Perry Laboratories, Gaithersburg, Md.).
FACS analysis.
Meningococcal strains were grown overnight in 5% CO2 at 37°C on GC agar plates containing 1% Kellogg's supplement (18). The cells were scraped from plates and resuspended in 1 ml of 1× Dulbecco's PBS without Mg2+ and Ca2+, 0.5% bovine serum albumin (BSA), and 0.1% fish gelatin (FLUKA, Milwaukee, Wis.) (PBS-BSA-FG) at a dilution of ∼108 cells/ml. Anti-rLP2086 antiserum was added to 99 μl of cells in a 1/100 dilution and incubated on ice for 1 h. The cells were washed twice with 1 ml of PBS-BSA-FG and resuspended in 99 μl of PBS-BSA-FG. Secondary antibody conjugated to Alexa Fluor 488 (Molecular Probes, Eugene, Oreg.) was added at 1/100 dilution and incubated on ice for 30 min. The cells were washed twice as above and resuspended in 100 μl of PBS with 0.4% formaldehyde for 30 min at room temperature or overnight at 4°C. Fixed cells were washed in 1 ml of PBS-BSA-FG. The cells in 1 ml of PBS-BSA-FG were analyzed on a Becton Dickinson FACS Vantage SE apparatus.
Immunoelectron microscopy.
N. meningitidis serogroup B strain H44/76 cells were fixed for 60 min at room temperature in 4% paraformaldehyde plus 0.05% glutaraldehyde in PBS, pH 7.2. Whole-cell, negative-stain, immunogold labeling was performed using a modified procedure developed by Slot and Geuze (37). Following fixation, droplets of cells were placed on Parafilm. Formvar carbon-coated gold grids were placed face down on each droplet. Excess fluid was wicked off, and blocking was accomplished in two stages using PBS containing 1% BSA (PBS-BSA) for 5 min and, later, PBS containing 1% cold water fish gelatin (FLUKA) for 10 min. Excess aldehyde was quenched using 0.02 M glycine in PBS for 5 min. Grids with cells were inverted over rLP2086 antibody derived from strain 8529 and diluted 1:50 in PBS-BSA for 1 h in a humidified chamber. Grids were rinsed five times for 1 min in PBS-BSA. Antigen was detected by incubation for 60 min with goat anti-mouse immunoglobulin G plus immunoglobulin M conjugated to 12-nm colloidal gold beads diluted 1:5 in PBS-BSA (Jackson ImmunoResearch Labs, West Grove, Pa.). Rinsing took place in PBS (four times for 1 min). Grids with cells were stabilized with 1% glutaraldehyde in PBS (3 min). Each sample was rinsed in distilled water (five times for 1 min). Grids were negatively stained (30 s) using a vanadium-based stain (Nanoprobes, Inc., Stony Brook, N.Y.). Control samples were incubated either with preimmune sera (week 0) or in the absence of primary antibody. All studies used a Zeiss 10C transmission electron microscope operating at 100 kV. Photographic prints were processed from the electron micrographs.
Nucleotide sequence accession number.
The nucleotide sequences of the mature 2086 coding region from 63 neisserial isolates and a partial nucleotide sequence for the 2086 gene from N. lactamica were deposited in the GenBank database. Their accession numbers (isolate identification) are as follows: AY330352 (L3 6275), AY330353 (CDC-2369), AY330354 (CDC-1034), AY330355 (L4 891), AY330356 (B16B6), AY330357 (W135), AY330358 (C11), AY330359 (Y), AY330360 (M98 250732), AY330361 (M98 250771), AY330362 (CDC-1135), AY330363 (M97 252153), AY330364 (CDC-1610), AY330365 (CDC-1492), AY330366 (L8 M978), AY330367 (M97 252988), AY330368 (M97 252697), AY330369 (6557), AY330370 (2996), AY330371 (M97 252976), AY330372 (M97 251854), AY330373 (CDC-1521), AY330374 (M98 250622), AY330375 (870446), AY330376 (M97 253248), AY330377 (M98 250809), AY330378 (L5 M981), AY330379 (NMB), AY330380 (M98 250572), AY330381 (A4), AY330382 (M97 251836), AY330383 (M97 251957), AY330384 (M97 251985), AY330385 (M97 252060), AY330386 (M97 251870), AY330387 (M97 251994), AY330388 (M98 250024), AY330389 (M97 251905), AY330390 (M97 251876), AY330391 (M97 251898), AY330392 (M97 251830), AY330393 (CDC-937), AY330394 (M97 252097), AY330395 (870227), AY330396 (H355), AY330397 (H44/76), AY330398 (8529), AY330399 (6940), AY330400 (M982), AY330401 (880049), AY330402 (M97 253524), AY330403 (M97 251885), AY330404 (M97 251926), AY330405 (M98 250670), AY330406 (CDC-1573), AY330407 (CDC-852), AY330408 (CDC-983), AY330409 (CDC-1343), AY330410 (CDC-2367), AY330411 (M97 250571), AY330412 (B40), AY330413 (CDC-5315), AY330414 (M98 250716), AY330415 (UR5).
RESULTS
PCR screening and identification of 2086 subfamilies.
A large number of N. meningitidis serogroup B isolates (n = 86) were screened by PCR for the presence of a 2086 homolog (Table 1). Four additional disease-associated serogroups (A, C, Y, and W135) and N. gonorrhoeae were also included in this study. One strain each of the commensals N. lactamica and Neisseria sicca were tested as well. To facilitate screening a large number of strains, internal 2086 primers homologous to the Sanger Institute N. meningitidis serogroup A Z2491 sequence were designed and synthesized (Table 2). Approximately 70% of N. meningitidis strains tested were positive in a PCR screen using these primers. The intergenic regions surrounding the 2086 gene from the Sanger Institute serogroup A Z2491 and The Institute for Genomic Research serogroup B MC58 N. meningitidis genomes were examined and aligned. Universal primers based on shared homology were designed to correspond with regions directly flanking the 2086 gene. These primers were used to amplify greater-than-full-length 2086 genes from a variety of N. meningitidis strains for sequence comparison. Unexpectedly, the PCR amplification of strain 6557 resulted in a low yield of PCR product. Cloning and sequencing results from this PCR product indicated a new type of 2086 gene with greater sequence variability. The 2086 gene from strain 6557 shared ∼75% identity at the amino acid level to the 2086 gene sequences we had previously identified. Interestingly, strain 6557 was one of the ∼30% of strains that had initially tested negative by PCR screening using the original internal primer set. Internal primers specific to the COOH-terminal variable regions within strain 6557 were therefore designed. All available neisserial strains (n = 95) were screened by PCR with the newly identified internal 2086 primers. Only the ∼30% of N. meningitidis strains that had previously tested negative by PCR for 2086 were positive in this screen. These variants comprise a second family of 2086 genes and are designated as subfamily A. The 2086 genes identified in the initial PCR screen, representing ∼70% of strains tested, are designated as subfamily B. Two sets of internal primers based on the COOH-terminal variable regions of the 2086 genes were utilized to discriminate between subfamily A and B gene sequences (data not shown). With the exception of N. sicca, all neisserial isolates tested by this PCR screening method yielded a single amplified product of the expected size. Additionally, whole-cell lysates of the 95 neisserial isolates were each tested for polyclonal antibody reactivity by Western blot analysis to detect the presence of a 2086 protein (Table 1) using LP2086 subfamily-specific pooled antisera. All neisserial isolates expressed an immunoreactive 2086 protein, except for N. sicca.
Amplification and sequencing of 2086 genes.
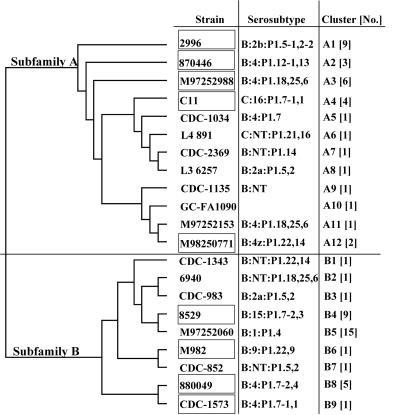
The coding regions of 2086 genes from 63 neisserial isolates were amplified by PCR and cloned, and their sequences were determined. Computer analysis of the deduced 2086 amino acid sequences using DNAStar MegAlign divided the proteins into two subfamilies. Analysis of the 63 deduced LP2086 amino acid sequences indicated clusters of identity within the subfamily branches. The LP2086 amino acid sequences within each cluster were ≥99.6% identical to each other. Based on amino acid sequence identity and clustering of the 2086 proteins, 21 unique sequences were identified (Fig. 1). The greatest degree of sequence conservation was seen within subfamilies. Amino acid sequences within subfamily A were 84.3 to 99.2% identical. Amino acid identity was 87.1 to 99.2% within subfamily B. The amino acid sequence diversity was greatest between the two subfamilies, ranging from 59.2 to 74.4% identity. The group, type, and subtype of each neisserial isolate in the phylogenetic tree are indicated along with the cluster identification and the number of isolates within each cluster. As shown in Table 1 and Fig. 1, cluster A4 contained one isolate each of N. meningitidis serogroups B, C11, Y, and W135. One N. meningitidis serogroup A isolate was located in cluster B5. The 2086 homolog from the N. meningitidis serogroup A strain Z2491 is also contained within this cluster. GNA1870 from N. meningitidis serogroup B strain MC58 would be contained within cluster B4. The subfamily A branch of the phylogenetic tree contains a gonococcal 2086 protein sequence (FA1090-GC) in cluster A10. The presence of this gonococcal sequence within the tree may indicate genetic exchange within this family of neisserial genes. Interestingly, the FA1090-GC gene from N. gonorrhoeae did not contain a lipoprotein signal sequence.
FIG. 1.
Phylogenetic tree showing the strain clustering according to 2086 protein distances. Subfamily A and B grouping is indicated on the main branches. The tree was constructed with LASERGENE software using ClustalW and was based on mature protein sequences of 254 to 262 amino acids in length. One representative strain and the respective group, type, and serosubtype from each cluster are shown. Boxes indicate 2086 strains chosen for expression studies. The group, type, and subtype of each neisserial isolate is indicated where available. Cluster identity for each of the 63 neisserial 2086 genes sequenced is listed in Table 1. Numbers in square brackets indicate the number of strains with ≥99.6% sequence identity present in each branch of the tree.
Analysis of 2086 sequences.
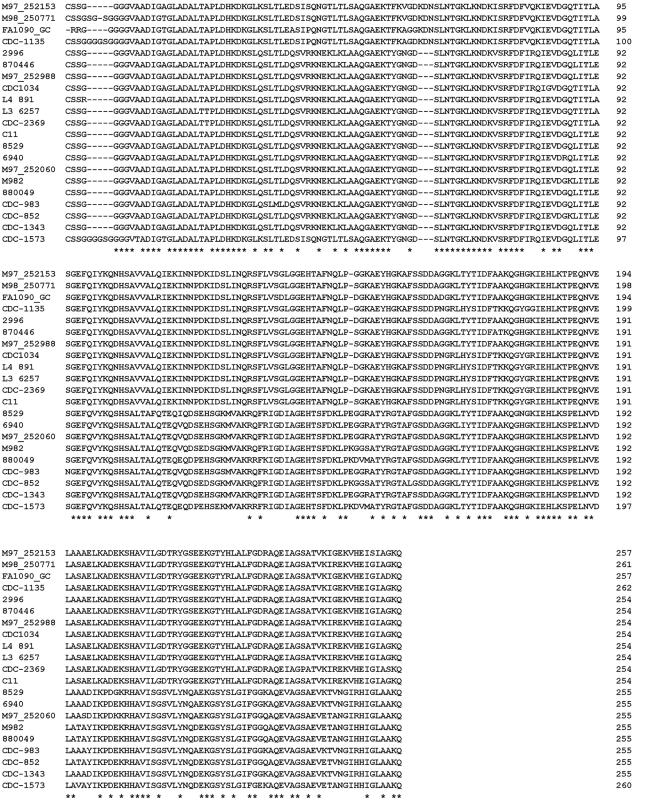
The deduced 2086 mature amino acid sequences of the 21 unique proteins were aligned and compared using ClustalW (Fig. 2). The amino acid alignment indicated that the two subfamilies of proteins were similar at their NH2-terminal ends but contained considerable variation in the carboxy-terminal ∼154 amino acids, with 48 to 57% identity. Although there is significant variability between LP2086 subfamilies, within a subfamily the proteins are highly conserved, >86%, among different strains. To further characterize the diversity among the 21 unique 2086 genes from the various neisserial species, a sequence distances matrix was generated from the amino acid alignment (data not shown). The amino acid sequence homology between the 21 unique proteins ranged from 99.2 to 59.2% identity. Interestingly, 3 of the 21 unique proteins (M98 250771, CDC-1135, and CDC-1573) contained a four- to five-glycine-rich amino acid insertion near the NH2-terminal end (Fig. 2). Two of these proteins, LP2086-CDC1135 and LP2086-M98250771, and two additional proteins in the A subfamily contained a three-amino-acid insert, K-D-N, approximately 67 amino acids from the N-terminal of the mature protein. This insertion was only seen in a subset of the A subfamily.
FIG. 2.
Comparison of neisserial 2086 sequences. The amino acid sequences of 21 unique 2086 proteins were deduced from the nucleotide sequences and aligned with ClustalW. Dashes indicate gaps. Identical amino acid sequences are indicated by asterisks.
Protein expression, purification, and characterization.
Nine of the 21 unique proteins described above were selected for recombinant lipoprotein expression studies based on their 2086 subfamily, PorA serosubtype, and sequence diversity. These strains included C11, M98 250771, M97 252988, 2996, 870446, 8529, M982, 880049, and CDC-1573. The 2086 protein from each of these strains was expressed behind the P4 lipidation signal sequence. For expression studies, three of the nine proteins listed above, 8529, 2996, and CDC-1573, were developed in parallel as nonlipidated proteins. Whole-cell lysates of E. coli expressing the rLP2086 and rP2086 proteins were analyzed on SDS-PAGE gels. rP2086 was expressed at levels comparable to those of the rLP2086 proteins. The lipidated and nonlipidated forms of the 2086 protein were expressed at approximately 5 to 8% of total cellular protein. rP2086 was soluble and localized in the cytoplasm, while rLP2086 was associated with the membrane fractions and was solubilized with detergent.
The presence of a lipoprotein signal sequence motif [-Leu-(Ala/Val)−4-Leu−3-Ala-(Ser)−2-Gly(Ala)−1-Cys+1-(sub-scripts denote residue numbers on the mature protein) (39)] and the use of the P4 lipid signal sequence in our recombinant plasmid constructs led to the production of lipoproteins. NH2-terminal amino acid sequencing of purified native LP2086 and rLP2086 indicated that the NH2-terminal amino acid residue was blocked.
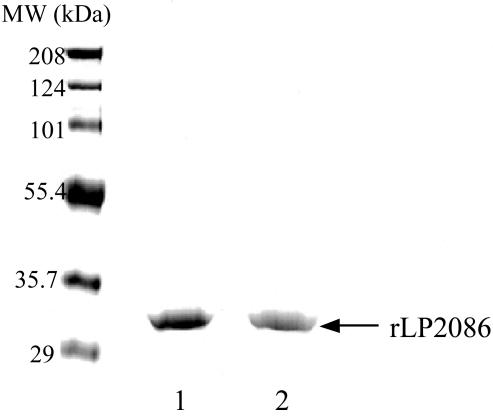
Purified rLP2086-8529 and -2996 proteins migrated at ∼32 kDa as analyzed by SDS-PAGE, which was slightly larger than their theoretical molecular masses (Fig. 3). To more accurately define the molecular weight, the mass of several rLP2086 proteins was determined by MALDI-TOF mass spectral analysis. The theoretical molecular weights without the N-terminal lipidation of P2086-8529 and P2086-2996 are 26,963, and 27,128, respectively. The mass of each rLP2086 differed from the theoretical weight of rP2086 by 679 to 823, which is within the range of the mass of the NH2-terminal lipid modification common to bacterial lipoproteins.
FIG. 3.
Coomassie-stained gel after SDS-PAGE (10 to 20% gradient polyacrylamide). Lane 1, purified rLP2086-8529; lane 2, purified rLP2086-2996.
Expression and surface exposure of native LP2086.
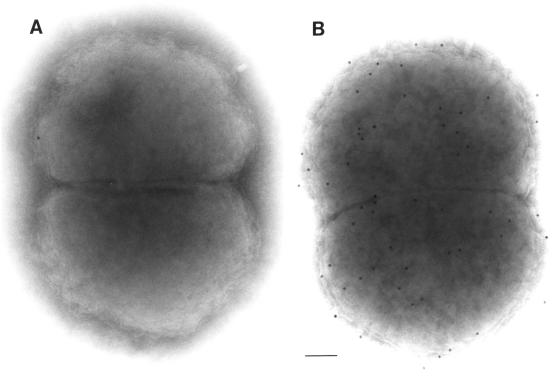
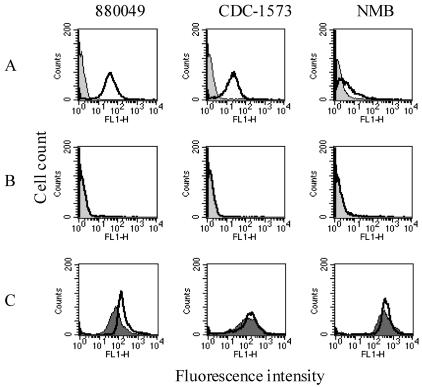
Antisera produced against two purified rLP2086 variants demonstrated strong surface reactivities to 13 out of 15 strains tested by whole-cell ELISA (Table 3). Whole-cell ELISA titers ranged from >1,458,000 to <2,000, depending on the strain and antiserum used. Serogroup B strains 870446 and H44/76 had some of the highest ELISA titers, while strains 6557 and NMB had lower titers. In general, the whole-cell ELISA titers were higher against strains within a subfamily. Both electron microscopy and FACS analysis (Fig. 4 and 5) confirmed that the LP2086 is exposed on the surface of intact N. meningitidis organisms. FACS analyses indicated high, medium, and low levels of LP2086 surface exposure as represented by N. meningitidis serogroup B strains 880049, CDC-1573, and NMB, respectively (Fig. 5A). LP2086 knockout mutants of each of these strains were also probed with the homologous rLP2086 antisera (Fig. 5B) and PorA-specific monoclonal antibodies (Fig. 5C). The LP2086 knockout mutants were each recognized by their serosubtype-specific PorA monoclonal antibodies (Fig. 5C), but not by their homologous rLP2086-specific antiserum (Fig. 5B). The lack of reactivity of LP2086 deletion mutants by FACS analysis (Fig. 5B) further demonstrated the specificity of the rLP2086 antiserum. Western blot analysis of 95 strains showed that the protein was expressed by all neisserial strains tested, with the exception of N. sicca. Some cross-reactivity between 2086 subfamilies was also observed. Serogroup A N. meningitidis strain A4 contains a 2086 subfamily B protein which reacted well with both polyclonal subfamily A and B antiserum pools by Western blot analysis, as did serogroup B strain 870446, which contains a 2086 subfamily A protein (data not shown). Interestingly, the commensal strain N. lactamica UR5 appears to be in the 2086 subfamily A group based on PCR and partial sequence identity. N. lactamica whole-cell lysates also reacted well with the polyclonal subfamily A antiserum pool by Western blot analysis (data not shown).
TABLE 3.
Whole-cell ELISA and bactericidal titers of mouse antibodies raised against rLP2086 tested against multiple serogroup B meningococcal strains
| Test strain | Test strain serosubtype | rLP2086-8529 anti- sera (subfamily B)
|
rLP2086-2996 anti- sera (subfamily A)
|
||
|---|---|---|---|---|---|
| Whole-cell ELISA titera | BC50 titerb | Whole-cell ELISA titera | BC50 titerb | ||
| Subfamily B | |||||
| 539 | P1.7-2,3 | >1,458,000 | 3,200 | — | — |
| H44/76 | P1.7,16 | >1,458,000 | 3,200 | 56,386 | <25 |
| H355 | P1.19,15 | >1,458,000 | 3,200 | — | — |
| CDC-937 | P1.7-2,3-4 | >1,458,000 | >800 | — | — |
| M97 252097 | P1.7-2,16 | >1,458,000 | >800 | — | — |
| 6940 | P1.18,25,6 | 900,162 | >800 | 11,157 | — |
| M982 | P1.22,9 | 435,909 | 200 | 6,424 | — |
| 880049 | P1.7-2,4 | 349,912 | 400 | 10,588 | — |
| CDC-1573 | P1.7-1,1 | 102,508 | 25 | 7,871 | — |
| Subfamily A | |||||
| 870446 | P1.12-1,13 | 389,829 | 800 | >1,458,000 | >800 |
| M98 250771 | P1.22,14 | 139,397 | <25 | 447,867 | 800 |
| M98 250732 | P1.22,14-1 | 23,928 | — | 241,510 | >800 |
| M97 252697 | P1.18,25,6 | 99,580 | — | 320,732 | >800 |
| 6557 | P1.22-1,14 | 25,590 | <25 | 77,319 | <25 |
| NMB | P1.5-1,2-2 | <2,000 | <25 | 8,979 | <25 |
Endpoint titers expressed as the reciprocal of the dilution at absorbance = 0.1.
Bactericidal (BC50) titers are represented as the reciprocal of the dilution of antiserum that reduced viable cell count by 50%. Week 0 normal mouse sera had BC50 titers of <25. A dash indicates samples were not tested.
FIG. 4.
Electron micrographs of N. meningitidis serogroup B strain H44/76 showing a whole cell. (A) Negative control; (B) immunogold labeling with rLP2086-8529-derived antiserum. Bar, 100 nm.
FIG. 5.
FACS analysis of wild-type and knockout strains of N. meningitidis serogroup B labeled with homologous antiserum to rLP2086. (A) Labeling of three wild-type strains with homologous rLP2086 antiserum, with the negative control (week 0) shown as the light gray histogram and the positive antiserum (week 6) shown as a bold unfilled histogram. (B) Labeling of the knockout strains with the same antiserum and the same labels as for panel A, with a light gray histogram for the negative control and a bold line for the anti-rLP2086 serum. (C) Labeling of wild-type (dark gray, filled histogram) and knockout strains (bold unfilled histogram) with the homologous PorA monoclonal antibody specific for each serosubtype.
rLP2086 induces bactericidal antibodies.
Bactericidal activity was detected against 13 of 15 strains tested, including 8 of 9 strains expressing heterologous serosubtype antigens, PorAs (Table 3). The rLP2086 proteins consistently elicited greater BC titers than the nonlipidated forms, often 10-fold higher (Table 4). Additionally, purified rLP2086 from a variety of subfamily A or subfamily B strains elicited high levels of bactericidal activity, directed almost exclusively against the members of their respective subfamilies (Table 5). One strain in the A subfamily, 870446, was susceptible to killing with sera raised against B subfamily proteins.
TABLE 4.
rLP2086 elicits greater bactericidal antibody than rP2086 in mice
| Antigen | Test strain titera
|
|
|---|---|---|
| H44/76 | M97 252697 | |
| rP2086-8529 (nonlipidated) | 200 | — |
| rLP2086-8529 (lipidated) | 3,200 | — |
| rP2086-1573 (nonlipidated) | <25 | — |
| rLP2086-1573 (lipidated) | 200 | — |
| rP2086-2996 (nonlipidated) | — | <25 |
| rLP2086-2996 (lipidated) | — | >800 |
Bactericidal (BC50) titers represented as the reciprocal of the dilution of antiserum that reduced the viable cell count by 50%. Week 0 normal mouse serum had BC50 titers of <50. A dash indicates samples were not tested.
TABLE 5.
Purified rLP2086 from subfamily A or subfamily B elicits bactericidal antibodies in mice which are bactericidal against several strains of serogroup B N. meningitidis
| Antigen | Titera
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subfamily B test strains
|
Subfamily A test strains
|
||||||||
| H44/76 | 880049 | 1573 | 6940 | M982 | 870446 | M98 250771 | M98 250732 | M97 252697 | |
| Subfamily B | |||||||||
| rLP2086-8529 | 3,200 | 400 | <25 | >800 | 200 | 800 | <25 | — | — |
| rLP2086-880049 | 400 | >800 | 100 | 400 | 200 | 400 | — | — | — |
| rLP2086-1573 | 200 | >800 | 800 | 200 | 100 | 50 | — | — | — |
| rLP2086-M982 | >800 | >800 | <25 | 200 | >800 | 400 | — | — | — |
| Subfamily A | |||||||||
| rLP2086-2996 | <25 | — | — | — | — | >800 | 800 | >800 | >800 |
| rLP2086-870446 | — | — | — | — | — | >800 | 400 | 400 | 400 |
| rLP2086-250771 | — | — | — | — | — | >800 | >800 | >800 | >800 |
| rLP2086-252988 | — | — | — | — | — | >800 | >800 | >800 | >800 |
| rLP2086-C11 | — | — | — | — | — | >800 | 200 | 200 | 400 |
Bactericidal (BC50) titers represented as the reciprocal of the dilution of antiserum that reduced viable cell count by 50%. Week 0 normal mouse sera had BC50 titers of <50. A dash indicates samples were not tested.
rLP2086 mixed with rPorA induces complementary bactericidal antibodies.
N. meningitidis serogroup B strains 6557 and NMB express LP2086 but are resistant to killing in a BC assay with antiserum raised against either their homologous rLP2086 or a mixture of rLP2086 variants representing subfamilies A and B (Tables 3 and 6). However, strains 6557 and NMB were sensitive to the BC activity of antisera directed against their respective rPorA proteins (Table 6, column 3), whether used alone or in combination with rLP2086 antisera (data not shown). Therefore, we investigated whether the proteins could be mixed to broaden the coverage of a potential vaccine preparation. A combination of rLP2086s (rLP2086-8529 and rLP2086-2996) with rPorAs (P1.22-1,14 [6557] and P1.5-1,2-2 [NMB]) elicited complementary BC activity against all 10 strains tested (Table 6, column 5).
TABLE 6.
Antisera from mice immunized with mixtures of rLP2086 variants and rPorA are bactericidal against multiple strains of serogroup B meningococcal strains
| Test strain | Bactericidal titer
|
|||
|---|---|---|---|---|
| rLP2086 monovalent controla | rPorA monovalent controlb | rLP2086-8529 + rLP2086-2996 | rLP2086-8529 + rLP2086-2996 + rP1.22-1,14 + rP1.5-1,2-2 | |
| H44/76 | >800 | — | >800 | >800 |
| 6940 | >800 | — | >800 | 800 |
| 880049 | 200 | — | 200 | 100 |
| M982 | 400 | — | 400 | 200 |
| 870446 | >800 | — | >800 | >800 |
| M98 250771 | 800 | — | 800 | 400 |
| M98 250732 | >800 | — | >800 | 400 |
| M97 252697 | >800 | — | >800 | >800 |
| 6557 | <25 | 800 | <25 | 200 |
| NMB | <25 | >800 | — | >800 |
The monovalent control for subfamily B strains H44/76, 6940, 880049, and M982 was mouse antiserum raised against rLP2086-8529, and the monovalent control for subfamily A strains 870446, M98 250771, M98 250732, M97 252697, 6557, and NMB was mouse antiserum raised against rLP2086-2996.
The rPorA monovalent control for strain 6557 was mouse antiserum raised against rP1.22-1,14-1, and the monovalent control for NMB was mouse antiserum raised against rP1.5-1,2-2. A dash indicates samples were not tested.
DISCUSSION
Although N. meningitidis serogroup B causes ∼50% of invasive meningococcal disease in developed countries (2), most approaches to date have been directed towards epidemic control. Many of the vaccine candidates currently under investigation are only efficacious against isolates representing a small number of serosubtypes. Recent reports on serogroup B meningococcal vaccine candidates in clinical trials have focused on complex mixtures or OMP preparations containing many different proteins (6, 36, 40). The complex nature of these vaccines makes obtaining correlates of protection challenging, because it is difficult to determine which antigen(s) is responsible for the protective immune response. While complexity would not necessarily prevent an efficacious vaccine from being used, it would be preferable to utilize identifiable antigens capable of inducing complement-mediated bactericidal antibodies against strains representing a variety of serosubtypes.
To better understand the immunogenicity of LP2086, the amino acid sequences of 63 neisserial isolates were analyzed. Phylogenetic analysis of the deduced 2086 protein sequences led to the identification of two distinct subfamilies, represented by 21 unique amino acid sequences. Approximately 70% of the neisserial isolates in our collection contained a subfamily B 2086 gene. To gain further insight into the epidemiological distribution of 2086 subfamily isolates, additional PCR screening studies are planned. Although the 21 unique LP2086 sequences vary in amino acid homology (59 to 99.2% identity) within a subfamily, the C-terminal ∼154 amino acids share considerable homology and appear to be responsible for the subfamily grouping. The conserved N-terminal region (∼100 amino acids) appears to define family membership and in at least one strain (870446) appears to be exposed at the bacterial surface. Amino acid alignments of full-length 2086 proteins indicated the presence of at least four different native 2086 lipoprotein signal sequences. To minimize potential recombinant protein expression problems in E. coli due to message instability and/or incompatibility, the nontypeable H. influenzae P4 lipoprotein signal sequence (13) was used to express each gene of interest. In the pBAD vector system, the P4 signal sequence improved the expression of rLP2086 compared with genes expressed using a native 2086 lipoprotein signal sequence, starting with the methionine at position −25 (data not shown).
Our data suggest that the level of LP2086 expression and/or surface exposure may vary among the different neisserial isolates. We have observed 10- to 200-fold differences in the level of LP2086 surface reactivity between isolates as detected by whole-cell ELISA (Table 3). Additionally, there were significant differences in surface reactivities between isolates as seen by FACS analyses (Fig. 5). Isolates considered negative by FACS and with low whole-cell ELISA titers were shown to express LP2086 when their whole-cell lysates were tested for Western blot reactivity with the appropriate pooled antisera (data not shown). The presence, absence, or variation of other surface components (e.g., OMPs, LOS, etc.) may alter the accessibility of LP2086 epitopes (3, 25). Alternatively, there may be differences in the transport of LP2086 variants to the bacterial cell surface. Additional experiments to gain a better understanding of the differences between levels of LP2086 expression and/or surface reactivity, utilizing monoclonal antibodies, are planned for the future.
Our results suggest that purified rLP2086 may significantly reduce the number of proteins required to provide adequate vaccine coverage of the serosubtypes responsible for group B meningococcal disease. While the epidemiological relationships between serosubtypes of PorAs and the 2086 subfamilies will need further study, the sequence variation within a 2086 subfamily has less impact on the ability of antisera to support bactericidal activity than is seen with PorAs. Nine of the 21 unique LP2086 variants were selected for further study based on their sequence diversity and potential to complement a PorA vaccine preparation. Antisera prepared to these nine proteins supported bactericidal activity against strains within each of the two 2086 subfamilies. Antisera to three nonlipidated rP2086 variants also supported bactericidal activity, although the lipidated versions consistently elicited a greater immune response. These results are consistent with the propensity of lipidation to increase or modify the immunological response to proteins (5, 20).
Bactericidal activity was detected against representatives of strains known to cause group B meningococcal disease throughout western Europe, the Americas, Australia, and New Zealand. One variant from each subfamily of rLP2086 could be enough to elicit immunity against most strains of meningococcus.
The family of rLP2086 antigens are candidates for clinical vaccine testing either alone or in combination with other antigens, such as rPorA. A vaccine that includes multiple antigens could reduce the possibility of pathogenic neisserial escape mutants. The addition of rLP2086 proteins to individual conjugate vaccines for serogroups A, C, Y, and W135 might also be beneficial and provide protection against capsule switching.
Acknowledgments
We thank the following individuals and institutions for supplying neisserial strains: Ray Borrow, MPHL; Gloria Ajello, CDC; Mike Apicella, UIOWA; N. A. Vedros, Walter Reed; and Jan Poolman, RIVM. We thank Rasappa Arumugham for his encouragment during the early phase of this work. We also thank Ben Metcalf, Sub Pillai, Bruce Green, and Susan Hoiseth for helpful discussions, David Russell for technical assistance with illustrations, and Tom Baroody for technical assistance with fermentation.
Editor: J. N. Weiser
REFERENCES
- 1.Abdillahi, H., and J. T. Poolman. 1987. Whole cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol. Lett. 48:367-371. [PubMed] [Google Scholar]
- 2.Ashton, F. E., L. Mancino, A. J. Ryan, J. T. Poolman, H. Abdillahi, and W. D. Zollinger. 1991. Serotypes and subtypes of Neisseria meningitidis serogroup B strains associated with meningococcal disease in Canada, 1977-1989. Can. J. Microbiol. 37:613-617. [DOI] [PubMed] [Google Scholar]
- 3.Bentley, A. T., and P. E. Klebba. 1988. Effect of lipopolysaccharide structure on reactivity of antiporin monoclonal antibodies with the bacterial cell surface. J. Bacteriol. 170:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernfield, L., L. Fletcher, A. Howell, J. Farley, R. Zagursky, M. Knauf, and G. Zlotnick. 2002. Identification of a novel vaccine candidate for group B Neisseria meningitidis, p. 116. In D. A. Caugant and E. Wedege (ed.), Abstracts of the Thirteenth International Pathogenic Neisseria Conference. National Institute of Public Health, Oslo, Norway.
- 5.Bessler, W. G., and G. Jung. 1992. Synthetic lipopeptides as novel adjuvants. Res. Immunol. 143:548-553. [DOI] [PubMed] [Google Scholar]
- 6.Bjune, G., E. A. Hiby, J. K. Grnnesby, and O. Arnesen. 1991. Effect of an outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 7.Comanducci, M., S. Bambini, B. Brunelli, J. Adu-Bobie, B. Aricò, B. Capecchi, M. M. Giuliani, V. Masignani, L. Santini, S. Savino, D. M. Granoff, D. A. Caugant, M. Pizza, R. Rappouli, and M. Mora. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diermayer, M., K. Hedberg, F. Hoesley, M. Fischer, B. Perkins, M. Reeves, and D. Fleming. 1999. Epidemic serogroup B meningococcal disease in Oregon. The evolving epidemiology of the ET-5 strain. JAMA 281:1493-1497. [DOI] [PubMed] [Google Scholar]
- 9.English, M., J. M. MacLennan, J. M. Bowen-Morris, J. Deeks, M. Boardman, K. Brown, S. Smith, J. Buttery, J. Clarke, S. Quataert, S. Lockhart, and E. R. Moxon. 2001. A randomised, double-blind, controlled trial of the immunogenicity and tolerability of a meningococcal group C conjugate vaccine in young British infants. Vaccine 19:1232-1238. [DOI] [PubMed] [Google Scholar]
- 10.Finne, J., D. Bitter-Suermann, C. Goridis, and U. Finne. 1987. An IgG monoclonal antibody to group B meningococci crossreacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J. Immunol. 138:4402-4407. [PubMed] [Google Scholar]
- 11.Fletcher, L., J. Farley, L. Bernfield, A. Howell, M. Knauf, P. Weise, X. Xie, and Y. Zhang. 2002. Characterization, cloning and expression of different subfamilies of the ORF 2086 gene from Neisseria meningitidis, p. 124. In D. A. Caugant and E. Wedege (ed.), Abstracts of the Thirteenth International Pathogenic Neisseria Conference. National Institute of Public Health, Oslo, Norway.
- 12.Granoff, D. M., G. R. Moe, M. M. Giuliani, J. Adu-Bobie, L. Santini, B. Brunelli, F. Piccinetti, P. Zuno-Mitchell, S. S. Lee, P. Neri, L. Bracci, L. Lozzi, and R. Rappuoli. 2001. A novel mimetic antigen eliciting protective antibody to Neisseria meningitidis. J. Immunol. 167:6487-6496. [DOI] [PubMed] [Google Scholar]
- 13.Green, B. A., J. E. Farley, T. Quinn-Dey, R. A. Deich, and G. W. Zlotnick. 1991. The e (P4) outer membrane protein of Haemophilus influenzae: biologic activity of anti-e serum and cloning and sequencing of the structural gene. Infect. Immun. 59:3191-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayrinen, J., H. Jennings, H. V. Raff, G. Rougon, N. Hanai, R. Gerardy-Schahn, and J. Finne. 1995. Antibodies to polysialic acid and its N-propyl derivative: binding properties and interaction with human embryonal brain glycopeptides. J. Infect. Dis. 171:1481-1490. [DOI] [PubMed] [Google Scholar]
- 16.Higgins, D. G., and R. Sharp. 1989. Fast and sensitive multiple sequence alignments on a microcomputer. Comput. Appl. Biosci. 5:151-153. [DOI] [PubMed] [Google Scholar]
- 17.Jòdar, L., I. M. Feavers, D. Salisbury, and D. M. Granoff. 2002. Development of vaccines against meningococcal disease. Lancet 359:1499-1508. [DOI] [PubMed] [Google Scholar]
- 18.Kellogg, D. S., W. L. Peacock, W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Lex, A., K. H. Wiesmuller, G. Jung, and W. G. Bessler. 1986. A synthetic analogue of Escherichia coli lipoprotein, tripalmitoyl pentapeptide, constitutes a potent immune adjuvant. J. Immunol. 137:2676-2681. [PubMed] [Google Scholar]
- 21.MacLennan, J. M., F. Shackley, P. T. Heath, J. J. Deeks, C. Flamank, M. Herbert, H. Griffiths, E. Hatzmann, C. Goilav, and E. R. Moxon. 2000. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococcal conjugate vaccine in infants: a randomized controlled trial. JAMA 283:2795-2801. [DOI] [PubMed] [Google Scholar]
- 22.Martin, D., N. Cadieux, J. Hamel, and B. R. Brodeur. 1997. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J. Exp. Med. 185:1173-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin, S. L., R. Borrow, P. van der Ley, M. Dawson, A. J. Fox, and K. A. V. Cartwright. 2000. Effect of sequence variation in meningococcal PorA outer membrane protein on the effectiveness of a hexavalent PorA outer membrane vesicle vaccine. Vaccine 18:2476-2481. [DOI] [PubMed] [Google Scholar]
- 24.Masignani, V., M. Comanducci, M. M. Giuliani, S. Bambini, J. Adu-Bobie, B. Aricò, B. Brunelli, A. Pieri, L. Santini, S. Savino, D. Serruto, D. Litt, S. Kroll, J. A. Welsch, D. M. Granoff, R. Rappuoli, and M. Pizza. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeil, G., and M. Virji. 1997. Phenotypic variants of meningococci and their potential in phagocytic interactions: the influence of opacity proteins in pili, PilC and surface sialic acids. Microb. Pathog. 22:295-304. [DOI] [PubMed] [Google Scholar]
- 26.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. A. Melles, V. S. D. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 62:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moe, G. R., S. Tan, and D. M. Granoff. 1999. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect. Immun. 67:5664-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mountzouros, K. T., and A. P. Howell. 2000. Detection of complement-mediated antibody-dependent bactericidal activity in a fluorescence-based serum bactericidal assay for group B Neisseria meningitidis. J. Clin. Microbiol. 38:2878-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkhill, J., M. Achtman, K. C. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, E. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M.-A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 30.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliana, B. Aricò, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 31.Poolman, J. T. 1995. Development of a meningococcal vaccine. Infect. Agents Dis. 4:13-28. [PubMed] [Google Scholar]
- 32.Ramsay, M. E., N. Andrews, E. B. Kaczmarski, and E. Miller. 2001. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 357:195-196. [DOI] [PubMed] [Google Scholar]
- 33.Sacchi, C. T., A. M. Whitney, T. Popovic, D. S. Beall, M. W. Reeves, B. D. Plikaytis, N. E. Rosenstein, B. A. Perkins, M. L. C. Tondella, and L. W. Mayer. 2000. Diversity and prevalence of PorA types in Neisseria meningitidis serogroup B in the United States, 1992-1998. J. Infect. Dis. 182:1169-1176. [DOI] [PubMed] [Google Scholar]
- 34.Salzberg, S. L., A. L. Delcher, S. Kasif, and O. White. 1998. Microbial gene identification using interpolated Markov models. Nucleic Acids Res. 26:544-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for constructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 36.Sierra, G. V. G., H. C. Campa, and N. W. Varcacel. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195-207. [PubMed] [Google Scholar]
- 37.Slot, J. W., and H. J. Geuze. 1984. Gold markers for single and double immunolabeling of ultrathin cryosections, p. 129. In J. M. Polak and I. M. Varndell (ed.), Immunolabeling for electron microscopy. Elsevier, Amsterdam, The Netherlands.
- 38.Sneath, P. H. A., and R. R. Sokal. 1973. Numerical taxonomy—the principles and practice of numerical taxonomy. Freeman Press, San Francisco, Calif.
- 39.Stephens, D. S., and S. M. Zimmer. 2002. Pathogenesis, therapy, and prevention of meningococcal sepsis. Curr. Infect. Dis. Rep. 4:377-386. [DOI] [PubMed] [Google Scholar]
- 40.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Hoiby, J. Holst, H. Nokleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer membrane protein meningococcal vaccines. A randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 41.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 42.Tondella, M. L. C., T. Popovic, N. E. Rosenstein, D. B. Lake, G. M. Carlone, L. W. Mayer, B. A. Perkins, and The Active Bacterial Core Surveilance Team. 2000. Distribution of Neisseria meningitidis serosubtypes and serotypes circulating in the United States. J. Clin. Microbiol. 38:3323-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wenger, J. D. 1999. Serogroup B meningococcal disease: new outbreaks, new strategies. JAMA 281:1541-1543. [DOI] [PubMed] [Google Scholar]
- 44.Wu, H. C. 1996. Biosynthesis of lipoproteins, p. 1005-1014. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 45.Zagursky, R. J., and D. Russell. 2003. Bioinformatics: use in bacterial vaccine discovery, p. 261-285. In S. M. Brown (ed.), BioComputing: computer tools for biologists. Eaton Publishing, Westborough, Mass.