Abstract
Macrophages from A/J and BALB/c mice were more susceptible to Coxiella burnetii phase II infection than were those from C57BL/6, C57BL/10, B10.A, C3H/HePas, and Swiss mice. Resistant macrophages effectively controlled the development of large replication vacuoles (LRVs), which accounted for the restriction of bacterial multiplication within the cultures. However, compared to fibroblasts, all macrophages controlled bacterial multiplication within LRVs.
Coxiella burnetii, a gram-negative obligate intracellular bacterium, is the etiological agent of Q fever (6, 13). Two phases of the bacteria have been described: highly virulent phase I and nonvirulent phase II. The C. burnetii phase II Nine Mile strain contains a 26-kb deletion in the genome, and although it is avirulent in animals, it infects cultured cells efficiently (5, 7, 13). After internalization of permissive host cells, the small phagosomes containing bacteria fuse with each other and with endocytic and phagocytic vesicles, forming one large replication vacuole (LRV) that displays phagolysosomal characteristics and supports C. burnetii multiplication (1, 2, 6, 8, 12, 16, 17, 19). In contrast, mouse primary macrophages, which are more restrictive than fibroblasts or continuous cell cultures, are able to control both bacterial multiplication and LRV formation (3, 17, 18). As LRVs are a privileged niche for C. burnetii multiplication, control of their formation is an important host cell defense mechanism.
It was previously demonstrated that inbred mouse strains display differential susceptibilities to C. burnetii phase I infection in vivo (14). C57BL/6 mice were highly resistant, whereas the 50% lethal dose observed for A/J mice was 1,000-fold smaller (14). In this context, it was important to investigate whether the differences observed with infection of inbred mice correlates with an inherent ability of macrophages to control intracellular C. burnetii infection. In the studies described herein, several inbred strains of mice were used to evaluate the natural resistance of their macrophages to C. burnetii phase II infection.
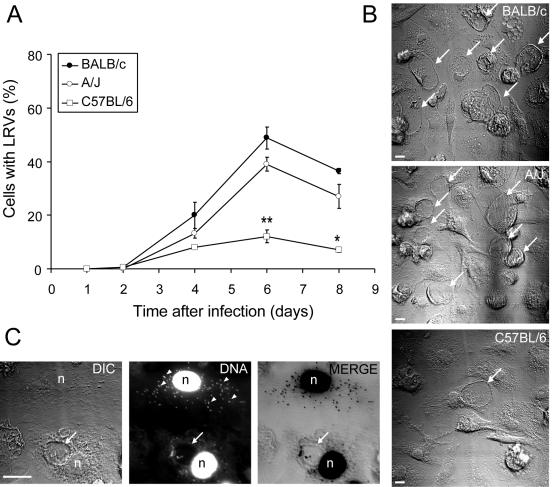
Bone marrow-derived macrophages (BMMs) were generated as previously described (18) in order to investigate whether primary macrophages from A/J, C57BL/6, and BALB/c mice differed in the ability to control the formation of LRVs. Figure 1A shows that there are significant differences in the proportions of LRVs found in cultures of C57BL/6 BMMs and LRVs found in BMMs from either BALB/c or A/J mice. These differences are illustrated in the images shown in Fig. 1B; a higher proportion of cells containing LRVs can be observed in the macrophage cultures from BALB/c and A/J mice compared to those from C57BL/6 cultures. Figure 1C shows, by confocal microscopy, two infected cells stained with 4′,6′-diamidino-2-phenylindole (DAPI), one of which displays an LRV whereas the other contains clusters of intracellular bacteria in small vacuoles dispersed throughout the cytoplasm. Although previous studies have demonstrated that C. burnetii within these small vacuoles does not appear either to be multiplying or being degraded (17, 18), further investigation is required to characterize bacterial viability, membrane markers, and the pH of these small vacuoles. Therefore, macrophages from C57BL/6 mice may be useful host cells.
FIG. 1.
Formation of LRVs in macrophages from C57BL/6, BALB/c, and A/J mice. Parallel cultures of macrophages were infected for 24 h with approximately 100 infective C. burnetii phase II bacteria per cell. At different times, the cultures were fixed (for 1 h with 3.5% formaldehyde containing 7.5% sucrose) and stained with 3.5 μM DAPI. About 500 cells per coverslip were scored with an inverted microscope (40× objective) for the presence or absence of LRV. (A) Percentage of cells with LRVs as a function of the duration of infection. Closed circles, BALB/c; open circles, A/J; open squares, C57BL/6. Asterisks indicate statistically significant differences between C57BL/6 macrophages and A/J or BALB/c macrophages (*, P < 0.05; **, P < 0.01). (B) Nomarski differential interference contrast images of representative fields of BALB/c, A/J, and C57BL/6 macrophage cultures infected for 6 days with C. burnetii phase II. (C) Confocal images of two macrophages (from a C57BL/6 culture) infected for 6 days; one cell (lower) displays the LRV, and the other (upper) does not. DIC, Nomarski differential interference contrast; DNA, DAPI fluorescence of the same field; MERGE, superimposed confocal images of DAPI fluorescence (converted to black) and Nomarski differential interference contrast (gray). Arrows, LRVs; arrowheads, intracellular C. burnetii in small vacuoles dispersed throughout the cytoplasm; n, cell nucleus; bars, 10 μm. The experiments were performed in triplicate sets of wells; the values shown are averages of triplicates from one representative experiment out of five performed.
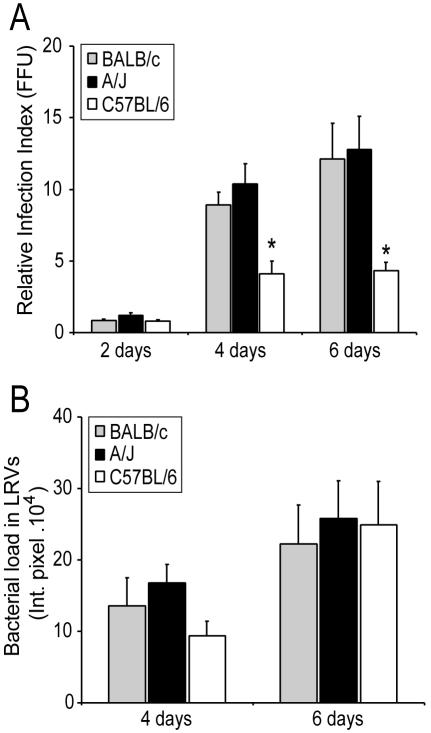
Since LRVs are important sites for C. burnetii multiplication, it was important to investigate whether a higher frequency of LRVs in BALB/c and A/J macrophages is correlated with an increased bacterial load in these cultures. Figure 2A shows that the total number of infective bacteria recovered from cultures infected for 4 and 6 days was higher in macrophages from BALB/c and A/J mice compared to those from C57BL/6 mice. The absence of LRVs on the second day after infection may explain the similar amounts of viable bacteria recovered from macrophages infected for 2 days (Fig. 2A).
FIG. 2.
C. burnetii loads in macrophages from C57BL/6, BALB/c, and A/J mice. Macrophages were infected for 24 h with approximately 100 infective bacteria/cell. At different times, the cultures were processed to determine the relative bacterial loads. (A) Relative amounts of viable bacteria recovered from cultures infected for 2, 4, or 6 days were determined as previously described (18). FFU, focus-forming units. (B) Relative bacterial loads in LRVs from cells infected for 4 or 6 days were determined as previously described (16). Gray bars, BALB/c; black bars, A/J; open bars, C57BL/6. Asterisks indicate statistically significant differences between infective bacteria recovered from C57BL/6 macrophages and those from A/J and BALB/c macrophages (P < 0.05). The experiments were performed in triplicate sets of wells; the values shown are averages of triplicates from one representative experiment out of three performed. Int. pixel., integrated pixel value (16).
To investigate if the LRVs found in macrophages from BALB/c, A/J, or C57BL/6 mice contained similar amounts of C. burnetii, images of LRVs (DAPI stained) were acquired by confocal microscopy and bacterial fluorescence was quantified by image processing as previously described (16). Figure 2B shows that the bacterial loads in LRVs of macrophages from BALB/c, A/J, and C57BL/6 mice were not statistically significantly different. These results indicate that the smaller amount of viable bacteria recovered from C57BL/6 cells compared to BALB/c or A/J cultures (Fig. 2A) was caused by a restriction in LRV development (Fig. 1A) and not by limiting bacterial multiplication within mature LRVs (Fig. 2B).
The results presented so far are consistent with previous observations of the susceptibility of inbred mice to C. burnetii phase I (14). However, previous studies have reported differences in the intracellular trafficking of C. burnetii phases I and II in human macrophages (4). Thus, the bacterial phase used in these studies may be a limitation to the appropriate correlation of data presented here with mouse infection. In this context, Yoshiie and colleagues showed by flow cytometry that lung alveolar macrophages from C57BL/6 mice displayed relatively greater loads of C. burnetii phase I than did similarly infected phagocytes from A/J mice (15). Therefore, these studies also support the hypothesis that differences in mouse susceptibility are related to inherent differences in the abilities of their macrophages to control the infection.
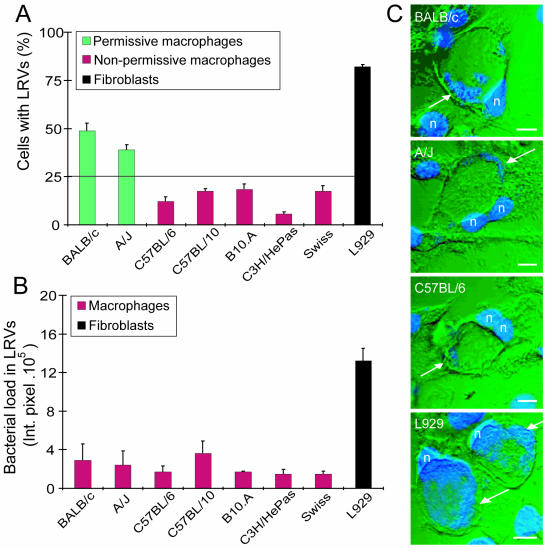
To test the natural resistance of macrophages from other inbred mouse strains, an experiment was performed with six inbred mouse strains (A/J, BALB/c, C57BL/6, C57BL/10, B10.A, and C3H/HePas) and one outbred mouse strain (Swiss). Mouse-derived L929 fibroblasts were included because these cells are known to be very permissive for C. burnetii phase II infection (17). To avoid inaccurate estimation of the proportion of LRVs, cultures were irradiated prior to infection. The small dose of radiation used (1,000 rads in a 137Cs gamma irradiator) did not appear to affect L929 microbicidal activities against C. burnetii, since the bacterial loads within LRVs were similar in irradiated cells and untreated cells (17). After 6 days of infection, about 80% of the L929 cells displayed LRVs, whereas less than 50% of the permissive BALB/c and A/J macrophages developed LRVs. Macrophages from all of the other mouse strains displayed a restrictive phenotype similar to that of C57BL/6 cells (Fig. 3A). Statistical analyses of these data support the classification of macrophages into two groups: nonpermissive and permissive. Macrophages were defined as permissive when during the course of infection, the proportion of cells with LRVs reached more than 25% (Fig. 3A). Figure 3B shows that the bacterial loads inside LRVs were similar among the macrophages studied (as shown in Fig. 2B for BALB/c, A/J, and C57BL/6 cells). In contrast, the bacterial load inside the LRVs formed in fibroblasts was severalfold greater than that in macrophages (Fig. 3B). Figure 3C illustrates the restriction of bacterial multiplication in LRVs in macrophages compared to those formed in fibroblasts. Interestingly, compared to fibroblasts, both sensitive and resistant macrophages were able to restrict C. burnetii phase II multiplication within LRVs. Thus, the mechanisms by which macrophages control C. burnetii multiplication inside LRVs may be similar in different inbred macrophages. In contrast, the factor(s) responsible for the restriction of large-vacuole formation appears to be either expressed at lower levels or absent or deficient in A/J and BALB/c macrophages.
FIG. 3.
C. burnetii phase II infection of fibroblasts and macrophages. Parallel cultures of L929 fibroblasts and macrophages from BALB/c, A/J, C57BL/6, C57BL/10, B10.A, C3H/HePas, and Swiss mice were infected for 24 h with C. burnetii at a multiplicity of infection of 100 bacteria/cell. Six days after infection, the cultures were fixed and processed to determine LRV frequency and bacterial loads in LRVs. (A) Percentage of cells with LRVs. Green bars, permissive macrophages; red bars, nonpermissive macrophages; black bar, L929 fibroblasts. (B) Relative bacterial loads in LRVs from cells infected for 6 days. Red bars, macrophages; black bar, L929 fibroblasts; Int. pixel., integrated pixel value (16). (C) Superimposed confocal images of DAPI fluorescence (blue) and Nomarski differential interference contrast (green) of LRVs formed in macrophages (BALB/c, A/J, and C57BL/6) or fibroblasts (L929). Greater bacterial loads can be observed inside LRVs from fibroblasts than in those from macrophages (arrows). n, cell nucleus; bars, 10 μm. The experiments were performed in triplicate sets of wells; the values shown are averages of triplicates from one representative experiment out of three performed.
In conclusion, these studies show that macrophages from A/J and BALB/c mice are more susceptible to C. burnetii phase II infection than are those from C57BL/6, C57BL/10, B10.A, C3H/HePas, and Swiss mice. Resistance is associated with preventing the formation of the large parasitophorous vacuoles in which C. burnetii multiplies. These data provide a background for further studies involving C. burnetii pathogenesis in murine models. Specifically, it will be important for studies involving the infection of primary cells obtained from genetically disrupted mice (10). Indeed, distinct inbred strains of mice that differ in susceptibility to specific pathogens are invaluable for dissecting the complex patterns in host-pathogen interactions. The inbred strains of mice have allowed the identification of several host resistance loci that regulate natural and acquired immunity in response to several pathogens (reviewed in reference 9). The host resistance phenotype definition presented here is the first step in the identification and cloning of host genes associated with resistance against C. burnetii (11).
Acknowledgments
I thank Michel Rabinovitch for mentoring, constructive criticisms, and helpful suggestions during the course of this work. I also thank Regina A. de Paula for technical assistance, Samithamby Jeyaseelan for helpful discussions, and Jude Wilson and Annie Neild for critical review of the manuscript.
Part of this work was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil) grants 96/9850-0 (Michel Rabinovitch) and 99/10742-5 (D.S.Z.).
Editor: J. T. Barbieri
REFERENCES
- 1.Baca, O. G., Y. P. Li, and H. Kumar. 1994. Survival of the Q fever agent Coxiella burnetii in the phagolysosome. Trends Microbiol. 2:476-480. [DOI] [PubMed] [Google Scholar]
- 2.Beron, W., M. Gutierrez, M. Rabinovitch, and M. I. Colombo. 2002. Coxiella burnetii phase II localizes in a Rab-7-labeled compartment with autophagic characteristics. Infect. Immun. 70:5816-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downs, C. M. 1968. Phagocytosis of Coxiella burnetii, phase I and phase II by peritoneal monocytes from normal and immune guinea pigs and mice. Zentbl. Bakteriol. 206:329-343. [PubMed] [Google Scholar]
- 4.Ghigo, E., C. Capo, C. H. Tung, D. Raoult, J. P. Gorvel, and J. L. Mege. 2002. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-γ mediates its restoration and bacterial killing. J. Immunol. 169:4488-4495. [DOI] [PubMed] [Google Scholar]
- 5.Hackstadt, T. 1996. Biosafety concerns and Coxiella burnetii. Trends Microbiol. 4:341-342. [DOI] [PubMed] [Google Scholar]
- 6.Heinzen, R. A., T. Hackstadt, and J. E. Samuel. 1999. Developmental biology of Coxiella burnetii. Trends Microbiol. 7:149-154. [DOI] [PubMed] [Google Scholar]
- 7.Hoover, T. A., D. W. Culp, M. H. Vodkin, J. C. Williams, and H. A. Thompson. 2002. Chromosomal DNA deletions explain phenotypic characteristics of two antigenic variants, phase II and RSA 514 (Crazy), of the Coxiella burnetii Nine Mile strain dagger. Infect. Immun. 70:6726-6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howe, D., J. Melnicakova, I. Barak, and R. A. Heinzen. 2003. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell. Microbiol. 5:469-480. [DOI] [PubMed] [Google Scholar]
- 9.Kramnik, I., and V. Boyartchuk. 2002. Immunity to intracellular pathogens as a complex genetic trait. Curr. Opin. Microbiol. 5:111-117. [DOI] [PubMed] [Google Scholar]
- 10.Lengeling, A., K. Pfeffer, and R. Balling. 2001. The battle of two genomes: genetics of bacterial host/pathogen interactions in mice. Mamm. Genome 12:261-271. [DOI] [PubMed] [Google Scholar]
- 11.Malo, D., and E. Skamene. 1994. Genetic control of host resistance to infection. Trends Genet. 10:365-371. [DOI] [PubMed] [Google Scholar]
- 12.Maurin, M., A. M. Benoliel, P. Bongrand, and D. Raoult. 1992. Phagolysosomes of Coxiella burnetii-infected cell lines maintain an acidic pH during persistent infection. Infect. Immun. 60:5013-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 14:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott, G. H., J. C. Williams, and E. H. Stephenson. 1987. Animal models in Q fever: pathological responses of inbred mice to phase I Coxiella burnetii. J. Gen. Microbiol. 133:691-700. [DOI] [PubMed] [Google Scholar]
- 15.Yoshiie, K., S. Matayoshi, T. Fujimura, N. Maeno, and H. Oda. 1999. Induced production of nitric oxide and sensitivity of alveolar macrophages derived from mice with different sensitivity to Coxiella burnetii. Acta Virol. 43:273-278. [PubMed] [Google Scholar]
- 16.Zamboni, D. S., R. A. Mortara, and M. Rabinovitch. 2001. Infection of Vero cells with Coxiella burnetii phase II: relative intracellular bacterial load and distribution estimated by confocal laser scanning microscopy and morphometry. J. Microbiol. Methods 43:223-232. [DOI] [PubMed] [Google Scholar]
- 17.Zamboni, D. S., E. Freymuller, R. A. Mortara, and M. Rabinovitch. 2002. Mouse resident peritoneal macrophages partially control in vitro infection with Coxiella burnetii phase II. Microbes Infect. 4:591-598. [DOI] [PubMed] [Google Scholar]
- 18.Zamboni, D. S., and M. Rabinovitch. 2003. Nitric oxide partially controls Coxiella burnetii phase II infection in mouse primary macrophages. Infect. Immun. 71:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamboni, D. S., S. McGrath, M. Rabinovitch, and C. Roy. 2003. Coxiella burnetii express type IV secretion system proteins that function similarly to components of the Legionella pneumophila Dot/Icm system. Mol. Microbiol. 49:965-976. [DOI] [PubMed] [Google Scholar]